Graphitic Carbon Nitride/Zinc Oxide-Based Z-Scheme and S-Scheme Heterojunction Photocatalysts for the Photodegradation of Organic Pollutants
Abstract
:1. Introduction
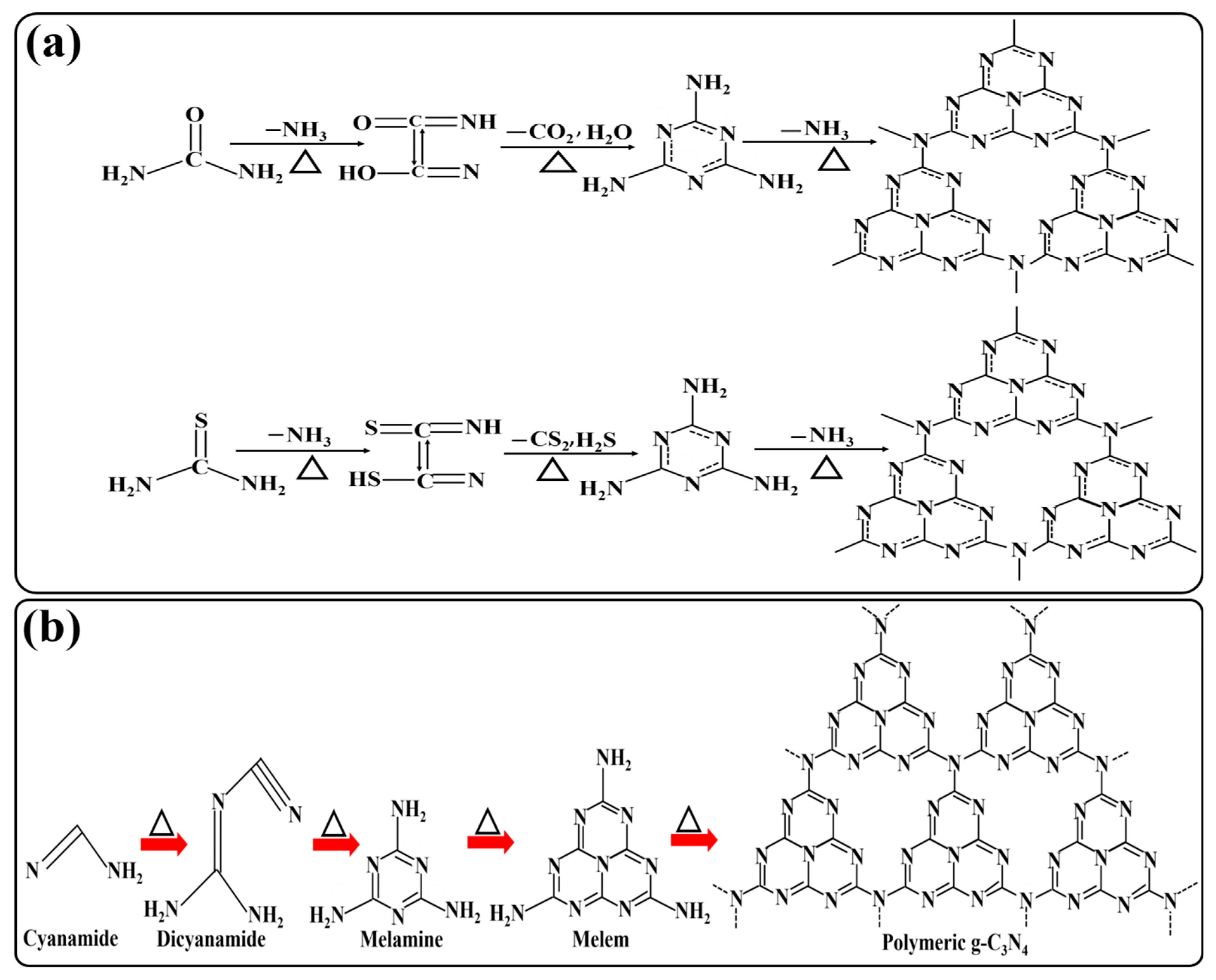
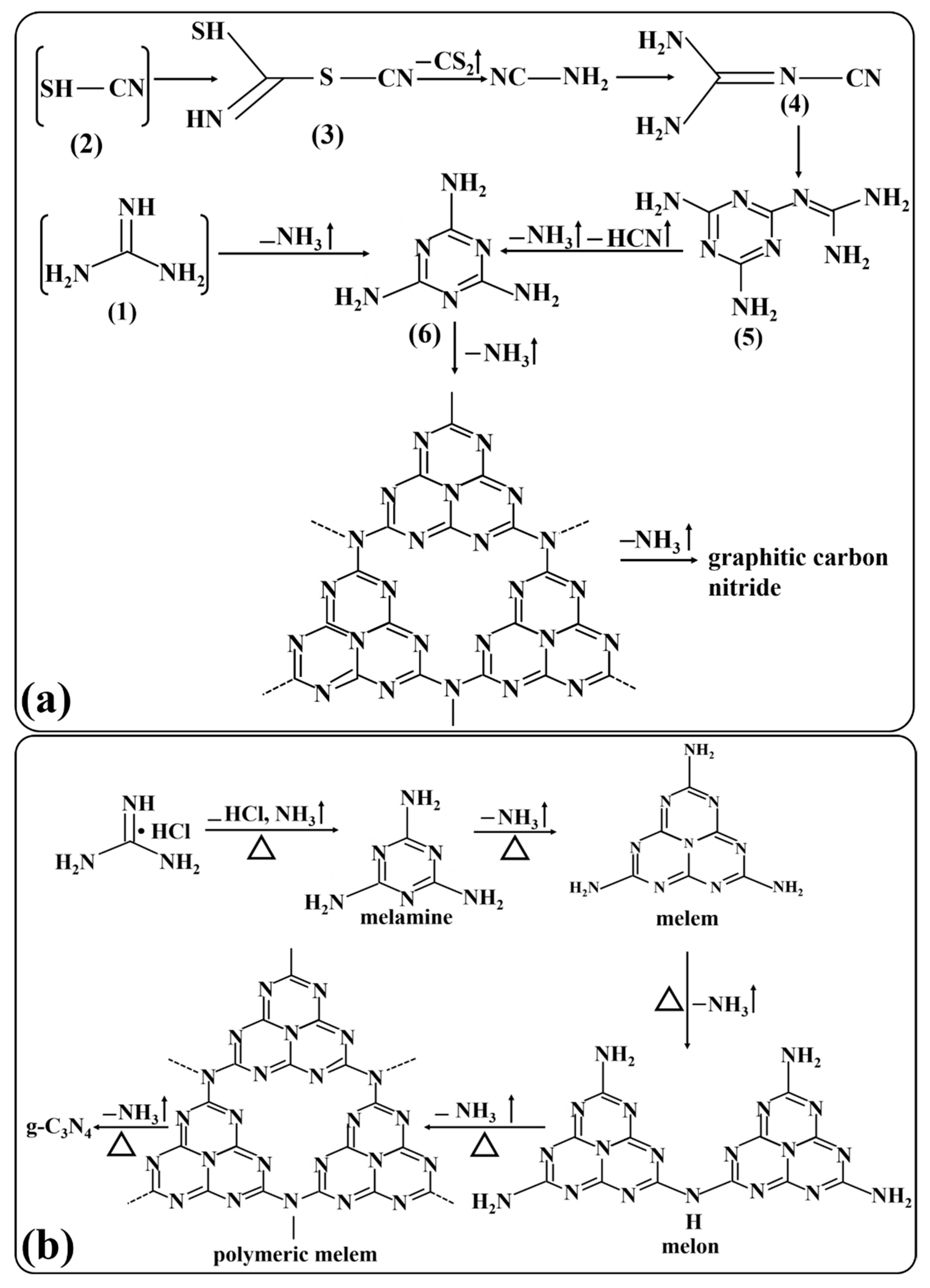
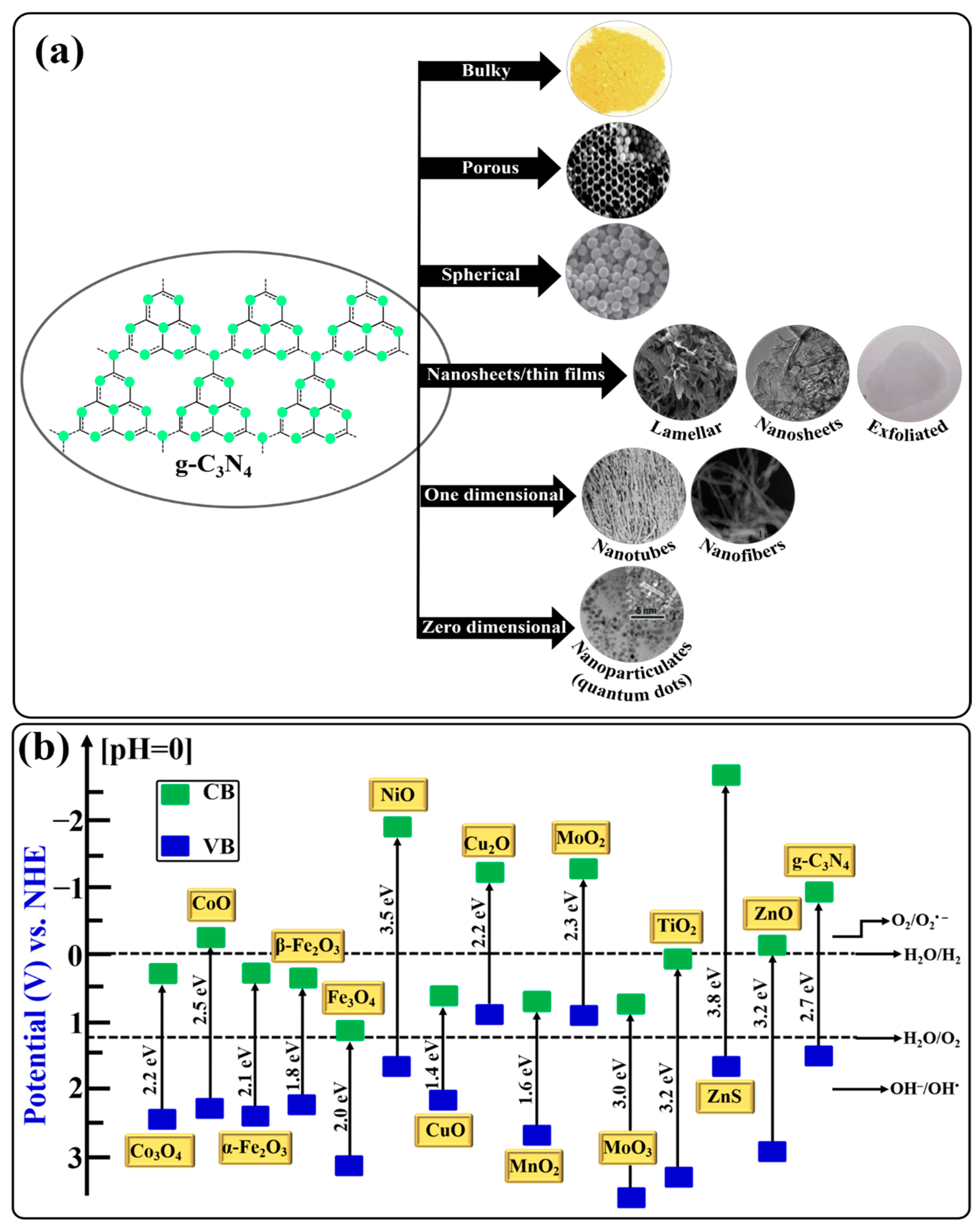
2. Structure of g-C3N4
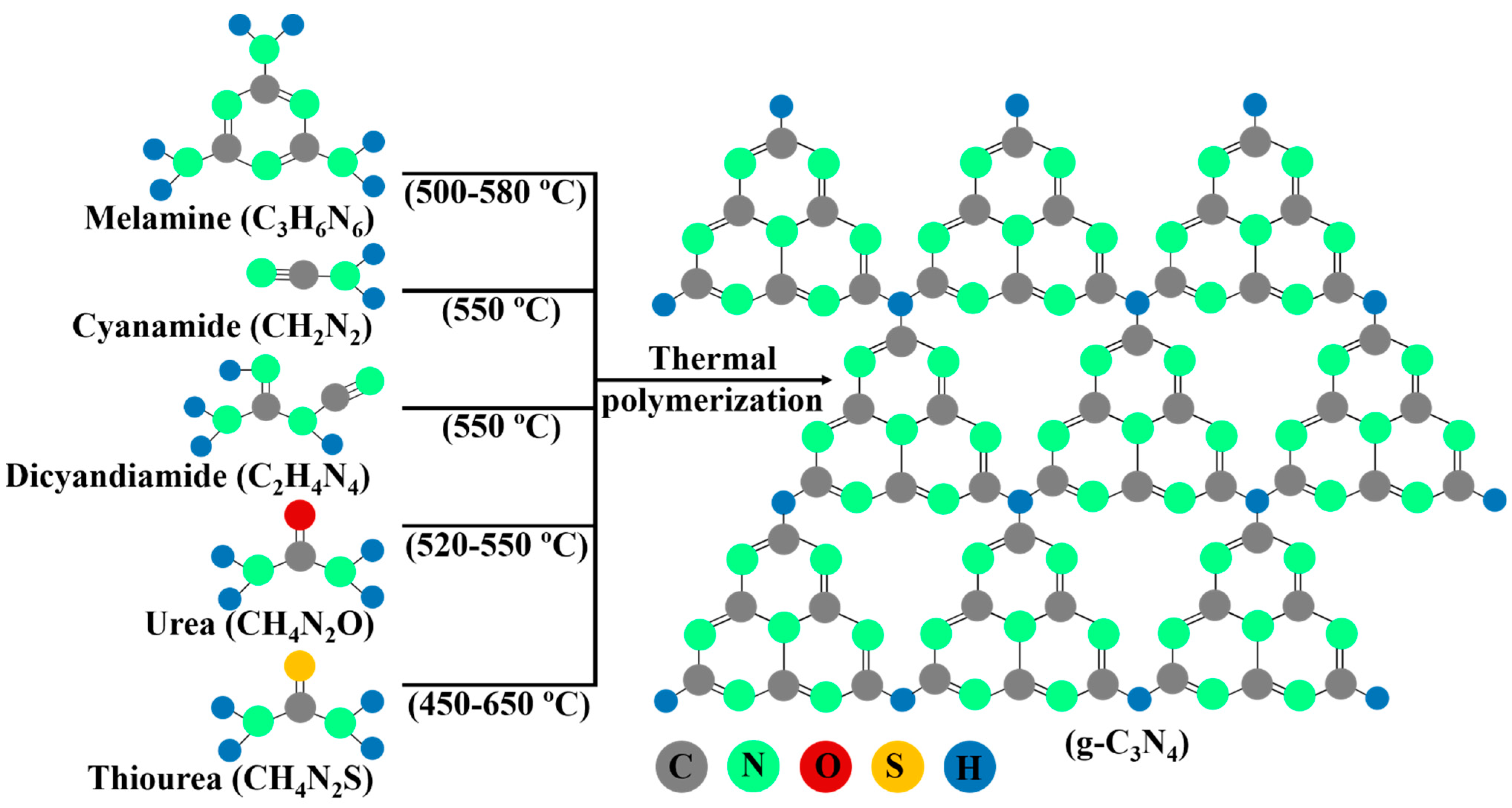
3. Synthesis of g-C3N4
Synthetic Pathways of g-C3N4
4. Morphologies of g-C3N4
4.1. Bulky g-C3N4
4.2. Porous g-C3N4
4.3. Spherical g-C3N4
4.4. Nanosheets and Nanofilms of g-C3N4
4.5. One-Dimensional g-C3N4 Nanostructures
4.6. Zero-Dimensional g-C3N4 Nanostructures
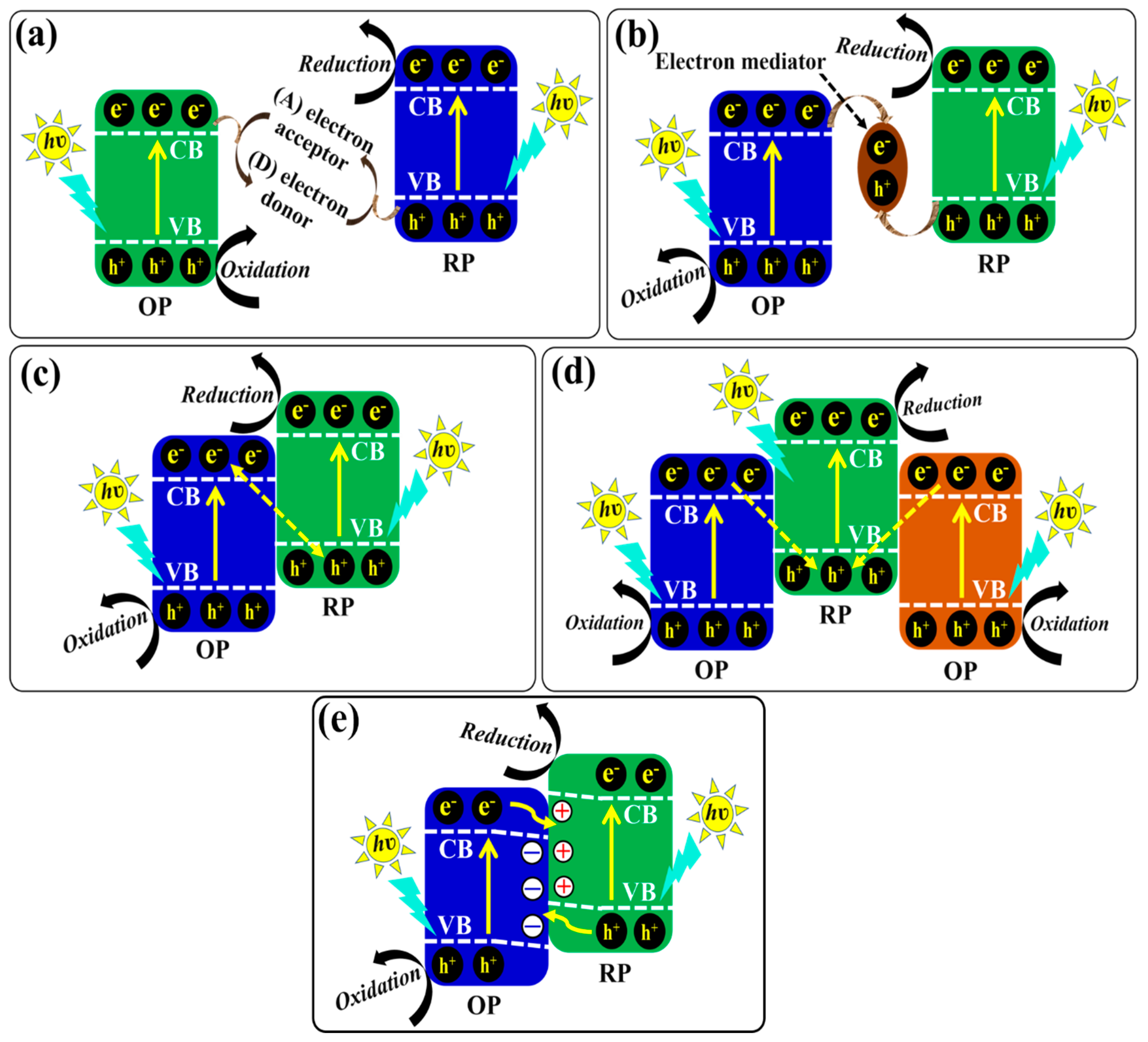
5. Heterojunction Photocatalysts
5.1. Z-Scheme Heterojunction Photocatalysts
5.1.1. Traditional Z-Scheme Heterojunction Photocatalysts
5.1.2. All-Solid-State Z-Scheme Heterojunction Photocatalysts
5.1.3. Direct Z-Scheme Heterojunction Photocatalysts
5.1.4. Double Z-Scheme Heterojunction Photocatalysts
5.2. Step-Scheme (S-Scheme) Heterojunction Photocatalysts
6. Formation of g-C3N4/ZnO-Based Z-Scheme Heterojunction Photocatalysts
6.1. Formation of g-C3N4/ZnO-Based All-Solid-State Z-Scheme Heterojunction Photocatalysts
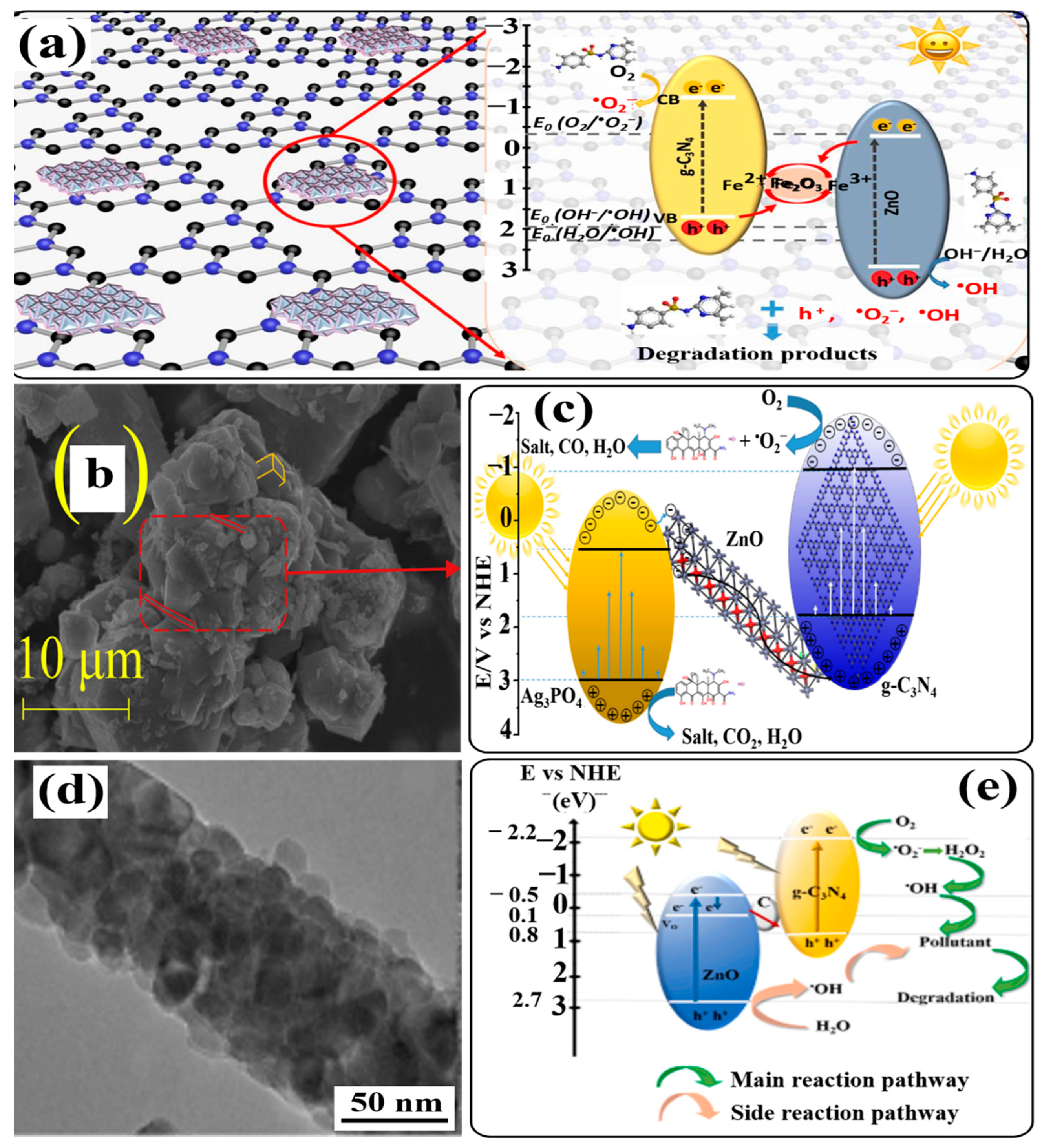
6.2. Formation of g-C3N4/ZnO-Based Direct Z-Scheme Heterojunction Photocatalysts
6.2.1. Formation of Binary g-C3N4/ZnO-Based Direct Z-Scheme Heterojunction Photocatalysts

6.2.2. Formation of Ternary g-C3N4/ZnO-Based Direct Z-Scheme Heterojunction Photocatalysts

6.2.3. Formation of Metal/Non-Metal-Doped g-C3N4/ZnO-Based Direct Z-Scheme Heterojunction Photocatalysts
Formation of Metal-Doped g-C3N4/ZnO-Based Direct Z-Scheme Heterojunction Photocatalysts
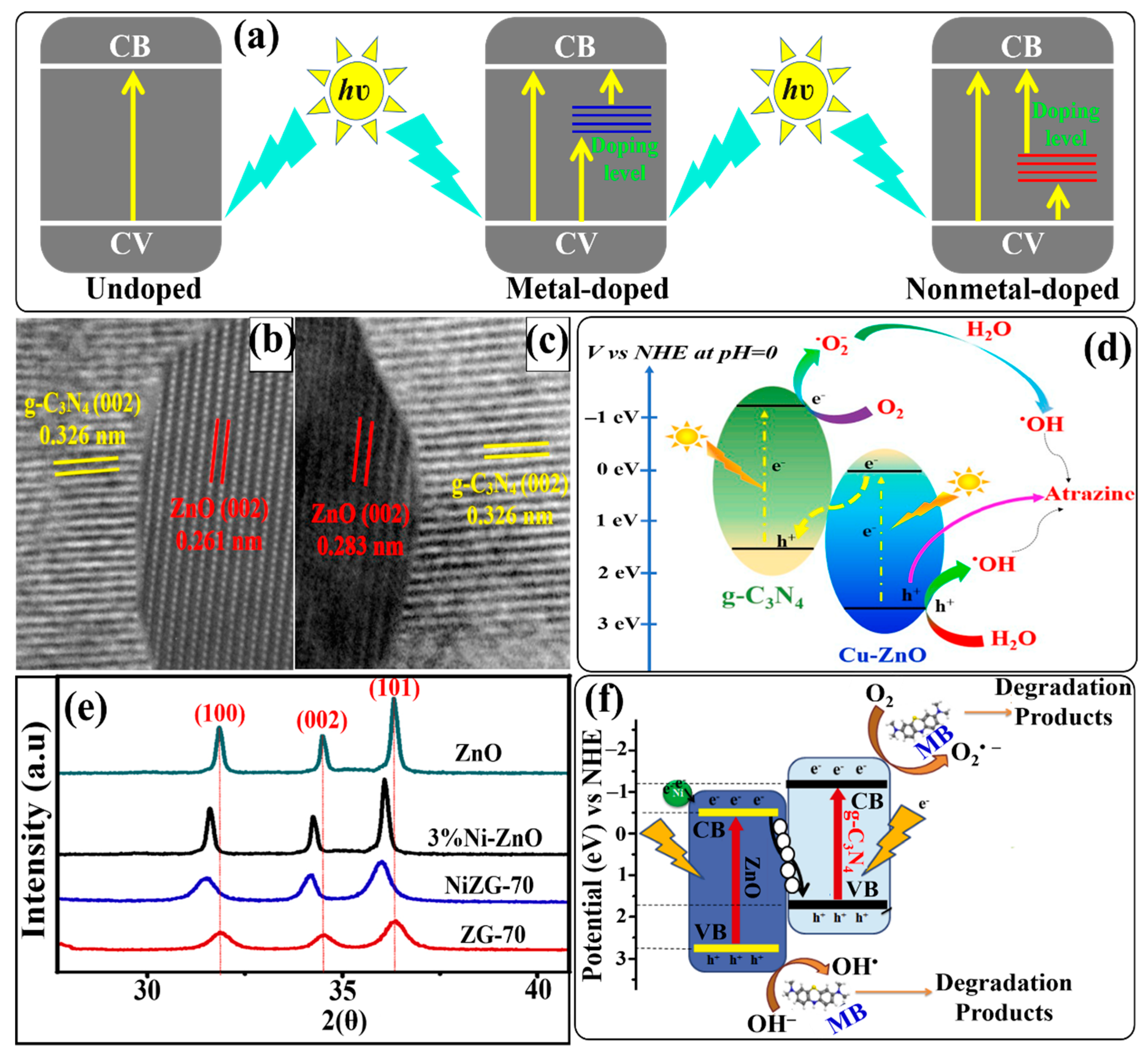
Formation of Non-Metal-Doped g-C3N4/ZnO-Based Direct Z-Scheme Heterojunction Photocatalysts
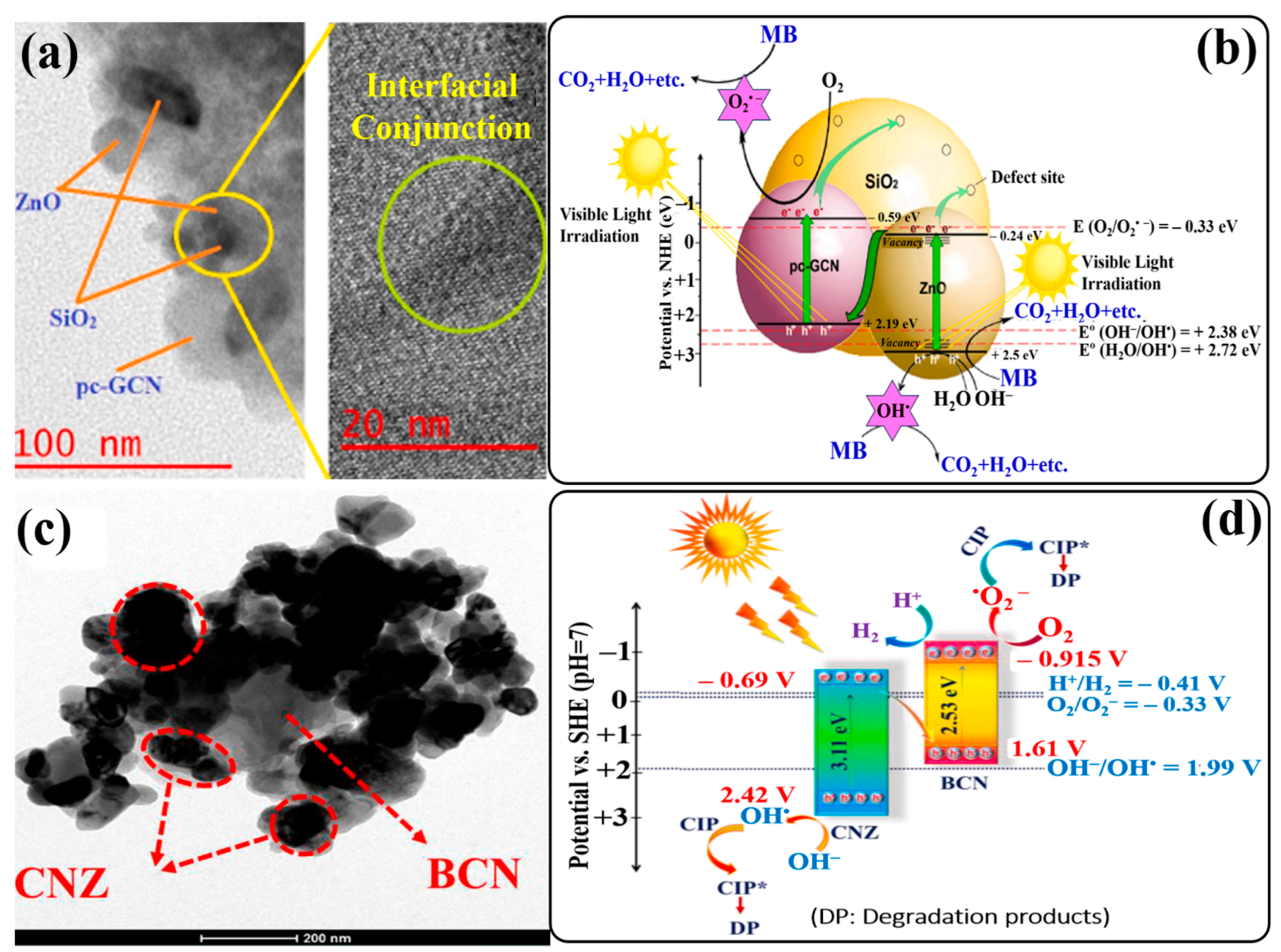
7. Formation of g-C3N4/ZnO-Based Double Z-Scheme Heterojunction Photocatalysts
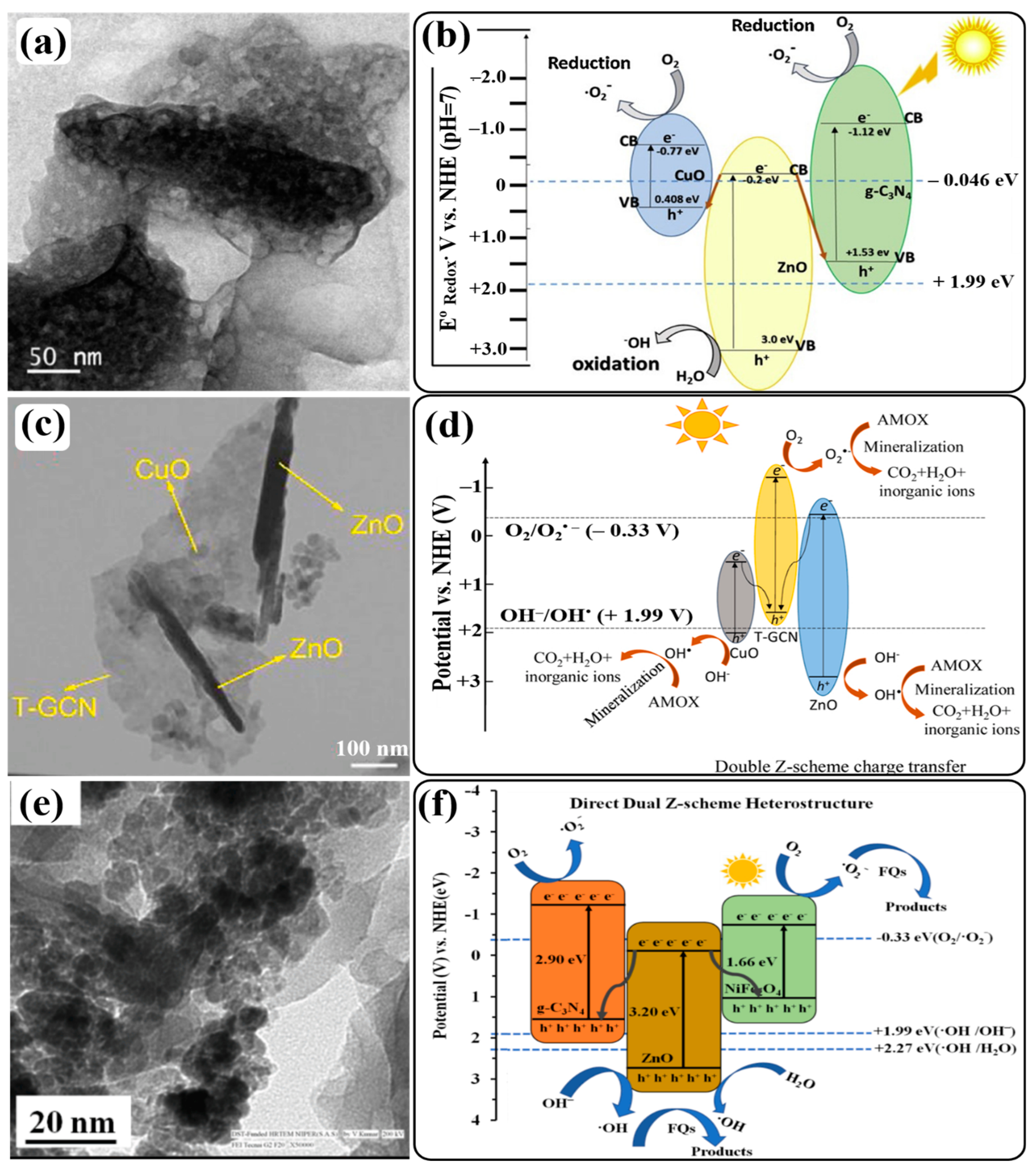
8. Formation of g-C3N4/ZnO-Based S-Scheme Heterojunction Photocatalysts
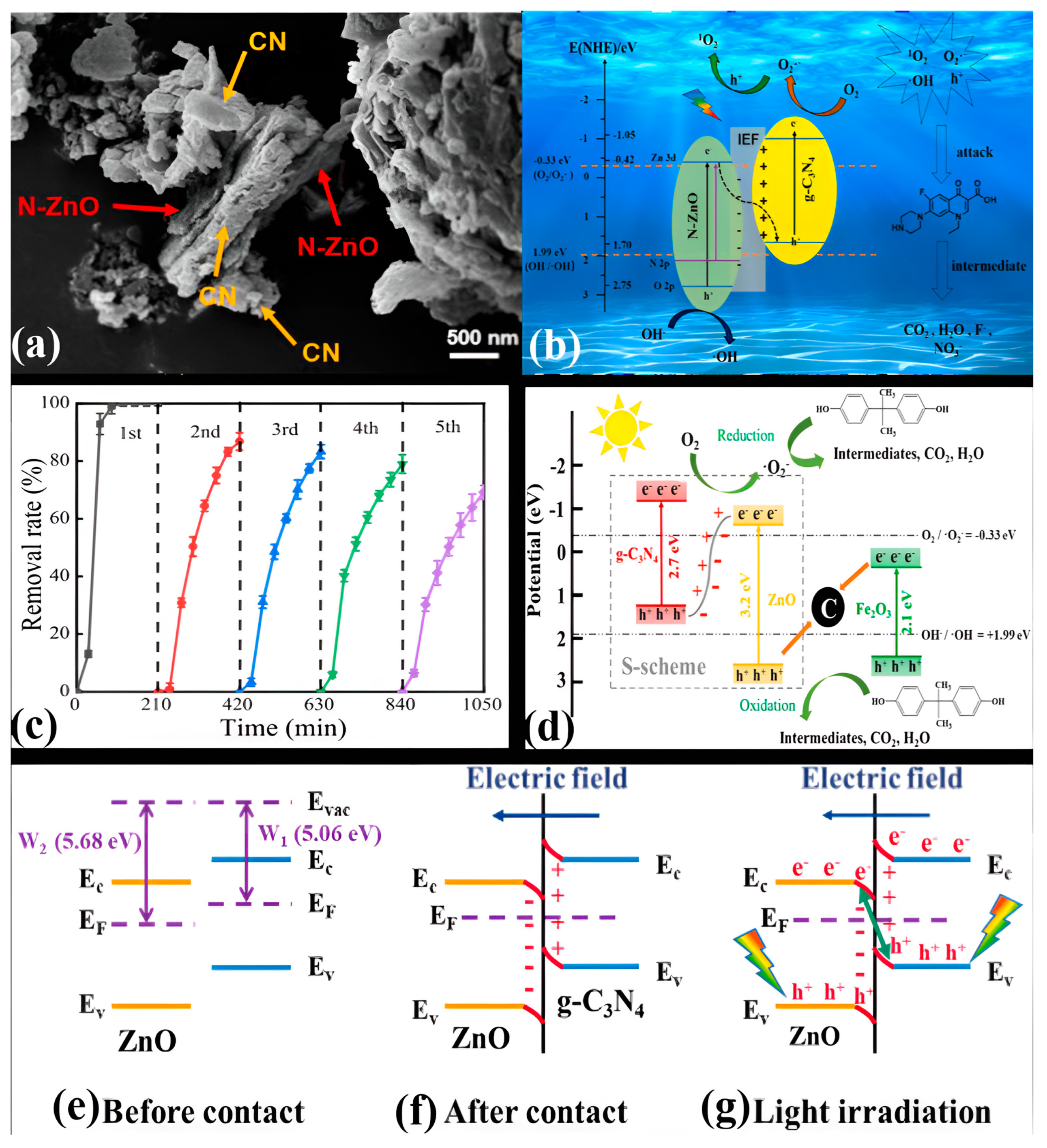
9. Conclusions and Future Perspective
- i.
- The majority of the reports have shown that g-C3N4 can be facilely synthesized by the thermal polymerization of nitrogen-rich precursors. But the resultant g-C3N4 with a bulky structure is disadvantageous to the photocatalytic efficiency due to a low surface area, limited surface reactive sites, and the inadequate utilization of visible light. These shortcomings can be alleviated by selecting appropriate g-C3N4 precursors, optimizing the reaction temperature and condensation period, applying an exfoliation-assisted strategy, and following template-free methods to obtain highly porous g-C3N4 and controlled morphology.
- ii.
- Many studies in the literature have reported that g-C3N4 nanosheets act as anchoring sites for ZnO nanostructures or other components to form nanocomposites. But the contact of nanostructures on the surface of g-C3N4 and their uniform distribution without aggregation on the surface of g-C3N4 are very challenging. Consequently, an appropriate heterojunction interface between g-C3N4 and nanostructures might not be formed, which hinders the effective charge transport/separation. Therefore, functionalization of the g-C3N4 surface with specific functional groups could be the best alternative for strengthening the anchoring ability of g-C3N4 and enhancing the light absorption properties of heterojunctions.
- iii.
- Photocatalytic activities of g-C3N4/ZnO-based heterojunction photocatalysts have thus far mainly been used for the photodegradation of organic contaminants in laboratory samples. Hence, further studies need to focus on real water samples (i.e., from the laboratory to the real field).
- iv.
- As discussed in this review, the visible-light response, redox ability, electron–hole mobility, and surface dynamic heterostructure at the interface of g-C3N4/ZnO-based Z-scheme/S-scheme heterojunction photocatalysts can be increased by metal/non-metal doping or forming ternary composites with g-C3N4 and ZnO. In addition, MXenes (2D few-atom-thick layers of transition-metal carbides and nitrides) could be promising alternatives to form ternary composites. Cost-effective MXenes possessing high conductivity can function as electron sinks, which accelerate the migration of photogenerated charge carriers and their effective separation.
- v.
- The charge-transfer mechanism is the key basis for understanding the likely reaction process occurring on the surface of a photocatalyst. However, in the case of g-C3N4/ZnO-based heterojunction systems, the charge-transfer mechanism seems inconsistent due to the variation in the reported CB/VB potentials of g-C3N4 and ZnO. Therefore, extensive studies should be focused on the DFT, radical-trapping tests, XPS analysis, etc., to understand the exact mechanism.
Author Contributions
Funding
Conflicts of Interest
Acronyms
| 0D | Zero-dimensional |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 4-CP | 4-Chlorophenol |
| ALD | Atomic layer deposition |
| AMOX | Amoxicillin |
| BCN | B-doped g-C3N4 |
| BPA | Bisphenol A |
| CB | Conduction band |
| CBM | Conduction band maxima |
| CFZ | Cefazolin |
| CIP | Ciprofloxacin |
| CNT | Carbon nanotube |
| CNZ | g-C3N4/ZnO composite |
| CZg | ZnO/CuO/g-C3N4 heterostructure |
| CZN | g-C3N4/ZnO/NiFe2O4 heterostructure |
| DCDA | Dicyandiamide |
| DFT | Density functional theory |
| ESR | Electron spin resonance |
| FQs | Fluoroquinolone |
| FZCCN | Fe2O3-ZnO@C/g-C3N4 heterojunction |
| GA | Graphene aerogel |
| HNTs | Halloysite nanotubes |
| HRTEM | High-resolution transmission electron microscopy |
| ICP | Inductive Couple Plasma Emission Spectrometer |
| KCC | KAUST catalysis center |
| KIT | Korea Advanced Institute of Science and Technology |
| LED | Light-emitting diode |
| LSPR | Localized surface-plasmon resonance |
| MG | Malachite green |
| MO | Methyl orange |
| MOF | Metal–organic framework |
| MV | Methyl violet |
| NHE | Normal hydrogen electrode |
| NiZG | Ni/ZnO/g-C3N4 composite |
| NOR | Norfloxacin |
| OD | Oxygen defect |
| OP | Oxidation photocatalyst |
| RP | Reduction photocatalyst |
| OV | Oxygen vacancies |
| pc- GCN | P, C-codoped g-C3N4 |
| PET | Polyester fiber |
| Ppm | Parts per million |
| QDs | Quantum dots |
| RGO, rGO | Reduced graphene |
| RhB | Rhodamine B |
| ROS | Reactive oxygen species |
| SBA-15 | Santa Barbara Amorphous-15 |
| SEM | Scanning electron microscopy |
| SMZ− | Negatively charged sulfonamide |
| SPR | Surface-plasmon resonance |
| S-scheme | Step-scheme |
| TC | Tetracycline |
| TEM | Transmission electron microscopy |
| TGA | Thermogravimetric analysis |
| UV light | Ultraviolet light |
| VB | Valence band |
| VBM | Valence band maxima |
| WFFDBD | Water falling film dielectric barrier discharge |
| WLLI | White LED light irradiation |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| ZIF-8 | Zeolitic imidazolate framework-8 |
| ZPC | Zeta potential charge |
References
- Motelica, L.; Oprea, O.-C.; Vasile, B.-S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Catalytic Activity Against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Panthi, G.; Ranjit, R.; Kim, H.Y.; Mulmi, D.D. Size dependent optical and antibacterial properties of Ag3PO4 synthesized by facile precipitation and colloidal approach in aqueous solution. Optik 2018, 156, 60–68. [Google Scholar] [CrossRef]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Mania, E.N. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci. Afr. 2019, 5, e00116. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M.; Kim, H.Y.; Park, S.J. Electrospun Ag-CoF doped PU nanofibers: Effective visible light catalyst for photodegradation of organic dyes. Macromol. Res. 2014, 22, 895–900. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.M.; Park, M.; Kim, H.Y.; Park, S.J. Fabrication of PdS/ZnS NPs doped PVAc hybrid electrospun nanofibers: Effective and reusable catalyst for dye photodegradation. J. Ind. Eng. Chem. 2015, 21, 298–302. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M. Electrospun carbon nanofibers decorated with Ag3PO4 nanoparticles: Visible-light driven photocatalyst for the photodegradation of methylene blue. Photochem 2021, 1, 345–357. [Google Scholar] [CrossRef]
- Lyulyukin, M.; Kovalevskiy, N.; Bhukhtiyarov, A.; Kozlov, D.; Selishchev, D. Kinetic aspects of benzene degradation over TiO2-N and composite Fe/Bi2WO6/TiO2-N photocatalysts under irradiation with visible light. Int. J. Mol. Sci. 2023, 24, 5693. [Google Scholar] [CrossRef] [PubMed]
- Panthi, G.; Park, M. Approaches for enhancing the photocatalytic activities of barium titanate: A review. J. Energy Chem. 2022, 73, 160–188. [Google Scholar] [CrossRef]
- Carey, J.; Lawrence, J.; Tosine, H. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous solutions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.R.; Sun, Y.; Colmenares, J.C.; Xu, Y.J. One dimension-based spatially order architectures for solar energy conversion. Chem. Soc. Rev. 2015, 44, 5053–5075. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; O’Hare, D.; Zhang, T. Layered double hydroxide nanostructured photocatalysts for renewable energy production. Adv. Energy Mater. 2016, 6, 1501974. [Google Scholar] [CrossRef]
- Huo, H.; Li, Y.; Wang, S.; Tan, S.; Li, X.; Yi, S.; Gao, L. Construction of highly active Zn3In2O6 (110)/g-C3N4 system by low temperature solvothermal for efficient degradation of tetracycline under visible light. Int. J. Mol. Sci. 2022, 23, 13221. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Zeng, G.; Chen, X.; Wu, Z.; Liang, J.; Zhang, J.; Wang, H.; Wang, H. Phosphorus- and sulfur-codoped g-C3N4: Facile preparation, mechanism insight, and application as efficient photocatalyst for tetracycline and methyl orange degradation under visible light irradiation. ACS Sustain. Chem. Eng. 2017, 5, 5831–5841. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Panthi, G.; Kwon, O.H.; Kuk, Y.S.; Gyawali, K.R.; Park, Y.W.; Park, M. Ternary composite of Co-Doped CdSe@electrospun carbon nanofibers: A novel reusable visible light-driven photocatalyst with enhanced performance. Catalysts 2020, 10, 348. [Google Scholar] [CrossRef]
- Zhurenok, E.A.; Vasilchenko, D.B.; Kozlova, E.A. Comprehensive review on g-C3N4-based photocatalysts for the photocatalytic hydrogen production under visible light. Int. J. Mol. Sci. 2023, 24, 346. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, Á.; Pastrana-Martinez, L.M.; Pérez-Poyatos, L.T.; Morales-Torres, S.; Maldonado-Hódar, F.J. One-Pot Thermal Synthesis of g-C3N4/ZnO Composites for the Degradation of 5-Fluoruracil Cytostatic Drug Under UV-LED Irradiation. Nanomaterials 2022, 12, 340. [Google Scholar] [CrossRef] [PubMed]
- Rashtizadeh, A.; Delnavaz, M.; Samadi, A.; Heidarzadeh, N. Photodegradation of POPs-containing wastewater using sunlight driven Ce-doped-ZnO/g-C3N4 photocatalyst: Optimization, and cost-efficiency analysis. Chem. Phys. Lett. 2023, 811, 140253. [Google Scholar] [CrossRef]
- Kroke, E.; Schwarz, M.; Horath-Bordon, E.; Kroll, P.; Noll, B.; Norman, A.D. Tri-s-triazine derivatives. Part I. From trichloro-tri-s-triazine to graphitic C3N4 structures. New J. Chem. 2002, 26, 508–512. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, X.; Tang, H.; Li, G.; Zhou, Z. Origin of photoactivity in graphitic carbon nitride and strategies for enhancement of photocatalytic efficiency: Insights from first principles computations. Phys. Chem. Chem. Phys. 2015, 17, 6280–6288. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Solis, R.R.; Quintana, M.A.; Martin-Lara, M.A.; Pérez, A.; Calero, M.; Muñoz-Batista, M.J. Boosted activity of g-C3N4/UiO-66-NH2 heterostructures for the photocatalytic degradation of contaminants in water. Int. J. Mol. Sci. 2022, 23, 12871. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, X.; Pan, L.; Shi, C.; Zheng, X.; Zou, J. Heterogeneous photocatalytic organic transformation reactions using conjugated polymers-based materials. ACS Catal. 2020, 10, 12256–12283. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Huang, L.; Zaman, S.; Lei, K.; Yue, Y.; Li, Z.; You, B.; Xia, B. Engineering 2D photocatalysts toward carbon dioxide reduction. Adv. Energy Mater. 2021, 11, 2003159. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, R.; Ng, Y.; Zhang, P.; Xiang, Q.; Li, X. A review on 2D MoS2 cocatalysts in photocatalytic H2 production. J. Mater. Sci. Technol. 2020, 56, 89–121. [Google Scholar] [CrossRef]
- Panthi, G.; Park, M.; Kim, H.Y.; Lee, Y.S.; Park, S.J. Electrospun ZnO hybrid nanofibers for photodegradation of wastewater containing organic dyes: A review. J. Ind. Eng. Chem. 2015, 21, 26–35. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, A.-V.; Ficai, D.; Oprea, O.-C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Proterties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar]
- Shim, M.; McDaniel, H.; Oh, N. Prospects for strained type-II nanorod heterostructures. J. Phys. Chem. Lett. 2011, 2, 2722–2727. [Google Scholar] [CrossRef]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride-based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Xing, W.; Tu, W.; Han, Z.; Hui, Y.; Meng, Q.; Chen, G. Template-induced high-crystalline g-C3N4 nanosheets for enhanced photocatalytic H2 evolution. ACS Energy Lett. 2018, 3, 514–519. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Teter, D.M.; Hemley, R.J. Low-compressibility carbon nitrides. Science 1996, 271, 53–55. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Nishihara, Y.; Lu, D.; Antonietti, M.; Domen, K. Photocatalytic Activities of graphitic carbon nitride powder for water reduction and oxidation under visible light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Graphitic carbon nitride-based catalysts and their applications: A review. Nano-Struct. Nano-Objects 2020, 24, 100577. [Google Scholar] [CrossRef]
- Malina, B.; Sansores, L.E. Electronic structure of six phases of C3N4: A theoretical approach. Mod. Phys. Lett. B 1999, 13, 193–201. [Google Scholar] [CrossRef]
- Martin, D.J.; Reardon, P.J.T.; Moniz, S.J.A.; Tang, J. Visible light-driven pure water splitting by a nature-inspired organic semiconductor-based system. J. Am. Chem. Soc. 2014, 136, 12568–12571. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Li, G.S.; Lian, Z.C.; Wang, W.C.; Zhang, D.Q.; Li, H.X. Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy 2016, 19, 446–454. [Google Scholar] [CrossRef]
- Shi, L.; Wang, T.; Zhang, H.; Chang, K.; Ye, J. Electrostatic self-assembly of nanosized carbon nitride nanosheet onto a zirconium metal-organic framework for enhanced photocatalytic CO2 reduction. Adv. Funct. Mater. 2015, 25, 5360–5367. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Chen, X.; Takanabe, K.; Domen, K.; Hou, Y.; Fu, X.; Antonietti, M. Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light. J. Am. Chem. Soc. 2009, 131, 1680–1681. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yang, D.; Li, Z.; Nan, Y.; Ding, F.; Shen, Y.; Jiang, Z. Thylakoid-inspired multi-shell g-C3N4 nanocapsules with enhanced visible-light harvesting and electron transfer properties for high-efficiency photocatalysis. ACS Nano 2017, 11, 1103–1112. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Kanazawa, S.; Sugano, Y.; Tsukamoto, D.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Highly selective production of hydrogen peroxide on graphitic carbon nitride (g-C3N4) photocatalyst activated by visible light. ACS Catal. 2014, 4, 774–780. [Google Scholar] [CrossRef]
- Bai, X.; Yan, S.; Wang, J.; Wang, L.; Jiang, W.; Wu, S.; Sun, C.; Zhu, Y.A. Simple and Efficient strategy for the Synthesis of a chemically tailored g-C3N4 material. J. Mater. Chem. A 2014, 2, 17521–17529. [Google Scholar] [CrossRef]
- Liang, Q.H.; Li, Z.; Huang, Z.H.; Kang, F.Y.; Yang, Q.H. Holey graphitic carbon nitride nanosheets with carbon vacancies for highly improved photocatalytic hydrogen production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Zhang, H.; Meng, X.; Hao, D.; Chang, K.; Li, P.; Kako, T.; Ye, J. Nature-inspired environmental phosphorylation boosts photocatalytic H2 production over carbon nitride nanosheets under visible-light irradiation. Angew. Chem. Int. Ed. 2015, 127, 13765–13769. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Chai, S.P.; Yong, S.T. Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene-g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem. Commun. 2015, 51, 858–861. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Duan, L.; Li, X.; Ji, L.; Geng, Z.; Huang, K.; Lu, L.; Zhou, L.; Liu, Z.; et al. A graphene-like oxygenated carbon nitride material for improved cycle-life lithium/sulfur batteries. Nano Lett. 2015, 15, 5137–5142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Xiao, J.; Xie, Y.; Nawaz, F.; Wang, Y.; Du, P.; Cao, H. Dramatic coupling of visible light with ozone on honeycomb-like porous g-C3N4 towards superior oxidation of water pollutants. Appl. Catal. B 2016, 183, 417–425. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.T.; Wang, X.; Xue, B.; Li, Y.X.; Cao, Y. A new and environmentally benign precursor for the synthesis of mesoporous g-C3N4 with tunable surface area. Chem. Chem. Phys. 2013, 15, 4510–4517. [Google Scholar] [CrossRef]
- Chung, Y.J.; Lee, B.I.; Ko, J.W.; Park, C.B. Photoactive g-C3N4 nanosheets for light-induced suppression of alzheimer’s beta-amyloid aggregation and toxicity. Adv. Healthc. Mater. 2016, 5, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Hu, Y.; Bai, Z.; Zheng, M.; Wei, C. In situ preparation of g-C3N4/bismuth-based oxide nanocomposites with enhanced photocatalytic activity. Appl. Catal. B 2016, 188, 1–12. [Google Scholar] [CrossRef]
- Long, B.H.; Lin, J.L.; Wang, X.C. Thermally-induced desulfurization and conversion of guanidine thiocyanate into graphitic carbon nitride catalysts for hydrogen photosynthesis. J. Mater. Chem. A 2014, 2, 2942–2951. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, Q.; Xu, B.; Liu, S.; Wang, J.; Xie, J.; Zhang, M.; Ohno, T. A new precursor to synthesize g-C3N4 with superior visible light absorption for photocatalytic application. Catal. Sci. Technol. 2017, 7, 1826–1830. [Google Scholar] [CrossRef]
- Hao, Q.; Jia, G.; Wei, W.; Vinu, A.; Wang, Y.; Arandiyan, H.; Ni, B.-J. Graphitic carbon nitride with different dimensionalities for energy and environmental applications. Nano Res. 2020, 13, 18–37. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Li, Q.; Bai, X.; Wang, J. Metal single-atom coordinated graphitic carbon nitride as an efficient catalyst for CO oxidation. Nanoscale 2020, 12, 364–371. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Schnick, W. Thermal conversion of guanylurea dicyanamide into graphitic carbon nitride via prototype CNx precursors. Chem. Mater. 2005, 17, 3976–3982. [Google Scholar] [CrossRef]
- Shi, L.L.; Wang, L.; Ma, F.; Sun, J. Polycondensation of guanidine hydrochloride into a graphitic carbon nitride semiconductor with a large surface area as a visible light photocatalyst. J. Catal. Sci. Technol. 2014, 4, 3235–3243. [Google Scholar] [CrossRef]
- Cui, L.; Liu, Y.; Fang, X.; Yin, C.; Li, S.; Sun, D.; Kang, S. Scalable and clean exfoliation of graphitic carbon nitride in NaClO solution: Enriched surface-active sites for enhanced photocatalytic H2 evolution. Green Chem. 2018, 20, 1354–1361. [Google Scholar] [CrossRef]
- Jun, Y.S.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From melamine-cyanuric acid supramolecular aggregates to carbon nitride hollow spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Shalom, M.; Inal, S.; Fettkenhauer, C.; Neher, D.; Antonietti, M. Antonietti, Improving carbon nitride photocatalysis by supramolecular preorganization of monomers. J. Am. Chem. Soc. 2013, 135, 7118–7121. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.Q.; Xie, C.; Long, J.; Wang, Y.; Wei, L.; Yang, X. Preparation of g-C3N4 with high specific surface area and photocatalytic stability. J. Electron. Mater. 2021, 50, 1067–1074. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Yan, H. Soft-templating synthesis of mesoporous graphitic carbon nitride with enhanced photocatalytic H2 evolution under visible light. Chem. Commun. 2012, 48, 3430–3432. [Google Scholar] [CrossRef]
- Pandiaraj, S.; Aiyappa, H.B.; Banerjee, R.; Kurungot, S. Post modification of MOF derived carbon via g-C3N4 entrapment for an efficient metal-free oxygen reduction reaction. Chem. Commun. 2014, 50, 3363–3366. [Google Scholar] [CrossRef]
- Gibot, P.; Schnell, F.; Spitzer, D. Enhancement of the graphitic carbon nitride surface properties from calcium salts as templates. Micropor. Mesopor. Mater. 2016, 219, 42–47. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-dimensional porous g-C3N4 for highly efficient photocatalytic overall water splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Zhang, M.; Antonietti, M.; Fu, X.; Wang, X. Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles. Nat. Commun. 2012, 3, 1139. [Google Scholar] [CrossRef]
- Huang, J.; Antonietti, M.; Liu, J. Bio-inspired carbon nitride mesoporous spheres for artificial photosynthesis: Photocatalytic cofactor regeneration for sustainable enzymatic synthesis. J. Mater. Chem. A 2014, 2, 7686–7693. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Yang, C.; Wang, X. Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and Separation at a soft photocatalytic interface. Adv. Mater. 2014, 26, 4121–4126. [Google Scholar] [CrossRef]
- Gu, Q.; Liao, Y.; Yin, L.; Long, J.; Wang, X.; Xue, C. Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability. Appl. Catal. B 2015, 165, 503–510. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Cui, Y.; Tang, Y.; Wang, X. Template-free synthesis of graphitic carbon nitride hollow spheres for photocatalytic degradation of organic pollutants. Mater. Lett. 2015, 161, 197–200. [Google Scholar] [CrossRef]
- Ma, T.Y.; Tang, Y.; Dai, S.; Qiao, S.Z. Proton-functionalized two-dimensional graphitic carbon nitride nanosheet: An excellent metal-/label-free biosensing platform. Small 2014, 10, 2382. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule self-assembly synthesis of porous few-layer carbon nitride for highly efficient photoredox catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Deng, S.; Yang, Z.; Lv, G.; Zhu, Y.; Li, H.; Wang, F.; Zhang, X. WO3 nanosheets/g-C3N4 nanosheets’ nanocomposite as an effective photocatalyst for degradation of rhodamine B. Appl. Phys. A 2019, 125, 44. [Google Scholar] [CrossRef]
- Zhang, J.H.; Hou, Y.J.; Wang, S.J.; Zhu, X.; Zhu, C.Y.; Wang, Z.; Li, C.J.; Jiang, J.J.; Wang, H.P.; Pan, M.; et al. A facile method for scalable synthesis of ultrathin g-C3N4 nanosheets for efficient hydrogen production. J. Mater. Chem. A 2018, 6, 10369. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 245–2456. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chen, Z.P.; Moehwald, H.; Fiechter, S.; van de Krol, R.; Wen, L.; Jiang, L.; Antonietti, M. Microcontact-printing-assisted access of graphitic carbon nitride films with favorable textures toward photoelectrochemical application. Adv. Mater. 2015, 27, 712–718. [Google Scholar] [CrossRef]
- Jia, L.; Wang, H.; Dhawale, D.; Anand, C.; Wahab, M.A.; Ji, Q.; Ariga, K.; Vinu, A. Highly ordered macro-mesoporous carbon nitride film for selective detection of acidic/basic molecules. Chem. Commun. 2014, 50, 5976–5979. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Dontsova, D.; Antonietti, M. Facile synthesis of carbon nitride micro-/nanoclusters with photocatalytic activity for hydrogen evolution. RSC Adv. 2013, 3, 22988–22993. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Ye, X.; Guo, F.; Wang, X. Helical graphitic carbon nitrides with photocatalytic and optical activities. Angew. Chem. Int. Ed. 2014, 53, 11926–11930. [Google Scholar] [CrossRef]
- Li, X.H.; Zhang, J.; Chen, X.; Fischer, A.; Thomas, A.; Antonietti, M.; Wang, X. Condensed graphitic carbon nitride nanorods by nanoconfinement: Promotion of crystallinity on photocatalytic conversion. Chem. Mater. 2011, 23, 4344–4348. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Wang, T.; Zhang, P.; Li, A.; Gong, J. Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J. Mater. Chem. A 2014, 2, 2885–2890. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Miao, J.; Wang, Z.; Su, D.; Liu, S.; Cai, W.; Zhang, L.; Hao, S.; Liu, B. Sulfur-mediated self-templating synthesis of tapered C-PAN/g-C3N4 composite nanotubes toward efficient photocatalytic H2 evolution. ACS Energy Lett. 2016, 1, 969–975. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Sun, Y.; Nan, Y.; Jiang, Z. Tubular g-C3N4 isotype heterojunction: Enhanced visible-light photocatalytic activity through cooperative manipulation of oriented electron and hole transfer. Small 2016, 12, 4093–4101. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Cao, C.; Mahmood, N.; Butt, F.K.; Mahmood, A.; Idrees, F.; Hussain, S.; Tanveer, M.; Ali, Z.; Aslam, I. Multifunctional g-C3N4 nanofibers: A template-free fabrication and enhanced optical, electrochemical, and photocatalyst properties. ACS Appl. Mater. Interfaces 2013, 6, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Groenewolt, M.; Antonietti, M. Synthesis of g-C3N4 nanoparticles in mesoporous silica host matrices. Adv. Mater. 2005, 17, 1789–1792. [Google Scholar] [CrossRef]
- Barman, S.; Sadhukhan, M. Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J. Mater. Chem. 2012, 22, 21832–21837. [Google Scholar] [CrossRef]
- Cao, X.; Ma, J.; Lin, Y.; Yao, B.; Li, F.; Weng, W.; Lin, X. A facile microwave-assisted fabrication of fluorescent carbon nitride quantum dots and their application in the detection of mercury ions. Spectrochim. Acta A 2015, 151, 875–880. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, C.-y. A low-temperature solid-phase method to synthesize highly fluorescent carbon nitride dots with tunable emission. Chem. Commun. 2013, 49, 8605–8607. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Zeng, M.; Xu, J.; Wang, X.; Hu, W. Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nanoscale 2014, 6, 4157–4162. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chen, J.; Wang, A.J.; Bao, N.; Feng, J.J.; Wang, W.; Shao, L. Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury (II) detection and bioimaging. J. Mater. Chem. C 2015, 3, 73–78. [Google Scholar] [CrossRef]
- Wang, W.; Jimmy, C.Y.; Shen, Z.; Chan, D.K.; Gu, T. g-C3N4 quantum dots: Direct synthesis, upconversion properties and photocatalytic application. Chem. Commun. 2014, 50, 10148–10150. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wang, H.X.; Wang, H.; Zhang, Q.; Xie, J.F.; Tian, Y.P.; Wang, J.; Xie, Y. Single-layered graphitic-C3N4 quantum dots for two-photon fluorescence imaging of cellular nucleus. Adv. Mater. 2014, 26, 4438–4443. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nanophotocatalytic materials: Possibilities and challenges. Adv Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-art progress in diverse heterostructured photocatalysts toward promoting photocatalytic performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J. Photochem. 1997, 10, 59–75. [Google Scholar] [CrossRef]
- Feng, J.; Ran, X.; Wang, L.; Xiao, B.; Lei, L.; Zhu, J.; Liu, Z.; Xi, X.; Feng, G.; Dai, Z. The synergistic effect of adsorption-photocatalysis for removal of organic pollutants on mesoporous Cu2V2O7/Cu3V2O8/g-C3N4 heterojunction. Int. J. Mol. Sci. 2022, 23, 14264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, G.; Zhang, L.; Lian, R.; Huang, J.; She, H.; Liu, C.; Wang, Q. Construction of TiO2-covalent organic framework Z-scheme hybrid through coordination bond for photocatalytic CO2 conversion. J. Energy Chem. 2022, 64, 85–92. [Google Scholar] [CrossRef]
- Maeda, K. Z-scheme water splitting using two different semiconductor photocatalysts. ACS Catal. 2013, 3, 1486–1503. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All solid-state Z-scheme photocatalytic system. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS-Au-TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y. Transfer process of WO3-metal-gC3N4 (metal = Cu, Ag, Au). ACS Appl. Mater. Interfaces 2016, 8, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-scheme photocatalytic system for promoting photocatalytic performance: Recent progress and future challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, X.; Lu, X.; Zou, S.; Li, Z.; Yao, C.; Ni, C. Photo-assisted selective catalytic reduction of NO by Z-scheme natural clay based photocatalyst: Insight into the effective graphene coupling. J. Catal. 2018, 357, 59–68. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced graphene oxide as a solid-state electron mediator in Z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Interaction of materials synthesis, characterization technique and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, B.; Yu, J. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure. Phys. Chem. Chem. Phys. 2016, 18, 31175–31183. [Google Scholar] [CrossRef]
- Liang, Y.C.; You, S.Y.; Chen, B.Y. Crystal design and photoactivity of TiO2 nanorod template decorated with nanostructured Bi2S3 visible light sensitizer. Int. J. Mol. Sci. 2022, 23, 12024. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Chai, J.; Xue, S.; Wang, R.; Jiang, L.; Wang, J.; Zhang, Z.; Dionysiou, D.D. Construction of novel symmetric double Z-scheme BiFeO3/CuBi2O4/BaTiO3 photocatalyst with enhanced solar-light driven photocatalytic performance for degradation of norfloxacin. Appl. Catal. B Environ. 2020, 272, 119017. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. S-scheme heterojunction photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent advances in g-C3N4 based heterojunction photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step scheme H2-production photocatalyst. Appl. Catal. B 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Yu, C.; Yang, K.; Li, X.; Dai, W.; Guan, J.; Shu, Q.; Huang, W. Construction of S-scheme g-C3N4 heterostructures for enhancing photocatalytic disposal of pollutants and electrocatalytic hydrogen evolution. Dyes Pigments 2020, 180, 108525. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Jia, X.; Zhang, N.; Xia, R.; Zhang, X.; Wang, Z.; Yu, M. In situ fabrication of a novel S-scheme heterojunction photocatalysts Bi2O3/P-C3N4 to enhance levofloxacin removal from water. Sep. Purif. Technol. 2021, 268, 118691. [Google Scholar] [CrossRef]
- Mei, F.; Dai, K.; Zhang, J.; Li, W.; Liang, C. Construction of Ag SPR-promoted step scheme porous g-C3N4/Ag3VO4 heterojunction for improving photocatalytic activity. Appl. Surf. Sci. 2019, 488, 151–160. [Google Scholar] [CrossRef]
- Jia, X.; Han, Q.; Zheng, M.; Bi, H. One-pot milling route to fabricate step-scheme AgI/I-BiOAc photocatalyst: Energy band structure optimized by the formation of solid solution. Appl. Surf. Sci. 2019, 489, 409–419. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Zhang, H.; Zhu, J.; Qiu, Y.; Yin, D.; Küppers, S. Visible-light degradation of sulfonamides by Z-scheme ZnO/g-C3N4 heterojunctions with amorphous Fe2O3 as electron mediator. J. Colloid Interf. Sci. 2018, 538, 256–266. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Mei, J.Y.; Yi, S.S.; Guan, X.X. Constructing of Z-scheme 3D g-C3N4-ZnO@graphene aerogel heterojunctions for high-efficient adsorption and photodegradation of organic pollutants. Appl. Surf. Sci. 2019, 492, 808–817. [Google Scholar] [CrossRef]
- Bai, X.; Li, H.; Zhang, Z.; Zhang, X.; Wang, C.; Xu, J.; Zhu, Y. Carbon nitride nested-tube with graphene as dual electron mediator in Z-scheme photocatalytic deoxynivalenol degradation. Catal. Sci. Technol. 2019, 19, 1680–1690. [Google Scholar] [CrossRef]
- Zhu, P.; Hu, M.; Duan, M.; Xie, L.; Zhao, M. High visible light response Z-scheme Ag3PO4/g-C3N4/ZnO composite photocatalyst for efficient degradation of tetracycline hydrochloride: Preparation, properties and mechanism. J. Alloys Compd. 2020, 840, 155714. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.M.; Hazma, A.M.; Unnithan, A.R.; Motlak, M.; Khalil, K.A.; Shin, Y.S.; Kim, H.Y. Polyaniline-poly(vinyl acetate) electrospun nanofibers mats as novel organic semiconductor material. Sci. Adv. Mater. 2012, 4, 1–9. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.M.; Unnithan, A.R.; Al-Deyab, S.S.; Pant, B.; Nam, K.T.; Kim, H.Y. Influence of gelatin on the wettability and mechanical properties of nylon-6 electrospun nanofibers: Novel mats for biomedical applications. J. Nanoeng. Nanomanuf. 2012, 2, 286–290. [Google Scholar] [CrossRef]
- Panthi, G.; Ranjit, R.; Khadka, S.; Gyawali, K.R.; Kim, H.Y.; Park, M. Characterization and antibacterial activity of rice grain-shaped ZnS nanoparticles immobilized inside the polymer electrospun nanofibers. Adv. Compos. Hybrid Mater. 2020, 3, 8–15. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.M.; Al-Deyab, S.S.; El-Newehy, M.; Pandeya, D.R.; Kim, H.Y. Interior synthesizing of ZnO nanoflakes inside nylon-6 electrospun nanofibers. J. Appl. Polym. Sci. 2013, 127, 2025–2032. [Google Scholar] [CrossRef]
- Panthi, G.; Park, S.J.; Chung, H.J.; Park, M.; Kim, H.Y. Silver nanoparticles decorated Mn2O3 hybrid nanofibers via electrospinning; towards the development of new bactericides with synergistic effect. Chem. Phys. 2017, 189, 70–75. [Google Scholar] [CrossRef]
- Naseri, A.; Samadi, M.; Pourjavadi, A.; Ramakrishna, S.; Moshfegh, A.J. Enhanced photocatalytic activity of ZnO/g-C3N4 nanofibers constituting carbonaceous species under simulated sunlight for organic dye removal. Ceram. Int. 2021, 147, 26185–26196. [Google Scholar] [CrossRef]
- Bajiri, M.A.; Hezam, A.; Namratha, K.; Al-Maswari, B.M.; BhojyaNaik, H.S.; Byrappa, K.; Al-Zaqri, N.; Alsalme, A.; Alasmari, R. Non-noble metallic Cu with three different roles in a Cu doped ZnO/Cu/g-C3N4 heterostructure for enhanced Z-scheme photocatalytic activity. New J. Chem. 2021, 45, 13499–13511. [Google Scholar] [CrossRef]
- An, X.; Wang, H.; Dong, C.; Jian, P.; Wu, Z.; Yu, B. Core-shell P-laden biochar/ZnO/g-C3N4 composite for enhanced photocatalytic degradation of atrazine and improved P slow-release performance. J. Colloid Interface Sci. 2022, 608, 2539–2548. [Google Scholar] [CrossRef]
- Wu, J.; Hu, H.; Qian, H.; Li, J.; Yang, R.; Qu, L. NiCo/ZnO/g-C3N4 Z-scheme heterojunction nanoparticles with enhanced photocatalytic degradation oxytetracycline. Diam. Relat. Mater. 2022, 121, 108738. [Google Scholar] [CrossRef]
- Shanthini, K.; Manivannan, V.; Govindaraju, K.M.; Collins Arun Prakash, V.; Lekshmi, G.S.; Govindan, R. Fabrication of highly efficient g-C3N4/ZnO/Fe2O3 ternary composite with enhanced photocatalytic activity under visible light irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 15393–15407. [Google Scholar] [CrossRef]
- Vignesh, K.; Kang, S.; Kwak, B.S.; Kang, M. Meso-porous ZnO nano-triangles @ graphitic-C3N4 nano-foils: Fabrication and Recyclable photocatalytic activity. Sep. Purif. Technol. 2015, 147, 257–265. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W.; Kong, L.; Cui, H. Z-scheme 2D/3D g-C3N4@ZnO with enhanced photocatalytic activity for cephalexin oxidation under solar light. Chem. Eng. J. 2018, 352, 412–422. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.T.; Shin, E.W. Interactions between ZnO nanoparticles and amorphous g-C3N4 nanosheets in thermal formation of g-C3N4/ZnO composite materials: The annealing temperature effect. Appl. Surf. Sci. 2018, 458, 369–381. [Google Scholar] [CrossRef]
- Murugesan, P.; Girichandran, N.; Narayanan, S.; Manickam, M. Structural, optical and photocatalytic properties of visible light driven zinc oxide hybridized two-dimensional p-conjugated polymeric gC3N4 composite. Opt. Mater. 2018, 75, 431–441. [Google Scholar] [CrossRef]
- Tan, X.; Wang, X.; Hang, H.; Zhang, D.; Zhang, N.; Xiao, Z.; Tao, H. Self-assembly method assisted synthesis of g-C3N4/ZnO heterostructure nanocomposites with enhanced photocatalytic performance. Opt. Mater. 2019, 96, 109266. [Google Scholar] [CrossRef]
- Fang, Q.; Li, B.; Li, Y.Y.; Huang, W.Q.; Peng, W.; Fan, X.; Huang, G.F. 0D/2D Z-scheme heterojunctions of g-C3N4 quantum dots/ZnO nanosheets as a highly efficient visible-light photocatalyst. Adv. Powder Technol. 2019, 30, 1576–1583. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.T.; Shin, E.W. Effect of g-C3N4 precursors on the morphological structures of g-C3N4/ZnO composite photocatalysts. J. Alloys Compd. 2019, 788, 1084–1092. [Google Scholar] [CrossRef]
- Zhang, S.; Su, C.; Ren, H.; Li, M.; Zhu, L.; Ge, S.; Wang, M.; Zhang, Z.; Li, L.; Cao, X. In-situ fabrication of g-C3N4/ZnO nanocomposites for photocatalytic degradation of methylene blue: Synthesis procedure does matter. Nanomaterials 2019, 9, 215. [Google Scholar] [CrossRef]
- Guo, X.; Duan, J.; Li, C.; Zhang, Z.; Wang, W. Highly efficient Z-scheme g-C3N4/ZnO photocatalysts constructed by co-melting-recrystallizing mixed precursors for wastewater treatment. J. Mater. Sci. 2020, 55, 2018–2031. [Google Scholar] [CrossRef]
- Nandi, P.; Das, D. Synthesis of cost-effective g-C3N4/ZnO heterostructure photocatalyst for methyl orange (MO) dye degradation. AIP Conf. Proc. 2020, 2220, 140018. [Google Scholar]
- Ramachandra, M.; Devi Kalathiparambil Rajendra Pai, S.; Resnik Jaleel, U.C.J.; Pinheiro, D. Improved photocatalytic activity of g-C3N4/ZnO: A Potential direct Z-scheme nanocomposite. ChemistrySelect 2020, 5, 11986–11995. [Google Scholar] [CrossRef]
- Ngullie, R.C.; Alaswad, S.O.; Bhuvaneswari, K.; Shanmugam, P.; Pazhanivel, T.; Arunachalam, P. Synthesis and characterization of efficient ZnO/g-C3N4 nanocomposites photocatalyst for photocatalytic degradation of methylene blue. Coatings 2020, 10, 500. [Google Scholar] [CrossRef]
- Shemeena, M.; Binitha, N.N. Visible light active ZnO-g-C3N4 photocatalyst for dye pollutant degradation. Mater. Today Proc. 2020, 25, 107–110. [Google Scholar] [CrossRef]
- Du, Y.; Yang, C.; Han, H.; Zhao, Q.; Jiang, T. One-pot preparation of binary photocatalyst ZnO/g-C3N4 nanosheets with enhanced photocatalytic activity in dye degradation. ChemistrySelect 2022, 7, e202103478. [Google Scholar] [CrossRef]
- Gayathri1, K.; Teja1, Y.N.; Prakash, R.M.; Hossain, M.S.; Alsalme, A.; Sundaravadive, E.; Sakar, M. In situ-grown ZnO particles on g-C3N4 layers: A direct Z-scheme-driven photocatalyst for the degradation of dye and pharmaceutical pollutants under solar irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 9774–9784. [Google Scholar] [CrossRef]
- Brasileiro, I.L.O.; Madeira, V.S.; Lopes-Moriyama, A.L.; Ramalho, M.L.R.A. Addition of g-C3N4 to ZnO and ZnFe2O4 to improve photocatalytic degradation of emerging organic pollutants. Ceram. Int. 2023, 49, 4449–4459. [Google Scholar] [CrossRef]
- Silva, F.F.; Silva, R.B.; Silva, T.R.; Macedo, D.A.; Su, B. Boosting the photocatalytic activity of g-C3N4/ZnO heterojunctions through optimal control of mass ratio. Solid State Sci. 2023, 138, 107128. [Google Scholar] [CrossRef]
- Girish, Y.R.; Udayabhanu; Byrappa, N.M.; Alnaggar, G.; Hezam, A.; Nagaraju, G.; Pramoda, K.; Byrappa, K. Rapid and facile synthesis of Z-scheme ZnO/g-C3N4 heterostructure as efficient visible light-driven photocatalysts for dye degradation and hydrogen evolution reaction. J. Hazard. Mater. Adv. 2023, 9, 100230. [Google Scholar] [CrossRef]
- Mamari, S.A.; Khudaish, E.; Kim, Y.; Khraisheh, M.; Selvaraj, R. Lotus-bud like hexagonal ZnO/g-C3N4 composites for the photodegradation of benzene present in aqueous solution. Inorg. Chem. Commun. 2023, 150, 110539. [Google Scholar] [CrossRef]
- An, H.; Huong, L.M.; Dat, N.M.; Hai, N.D.; Cong, C.Q.; Nam, N.T.H.; Tai, L.T.; Thi, D.N.M.; Nghi, H.B.; Huyen, N.T.T.; et al. Photocatalytic degradation of organic dyes using zinc oxide-decorated graphitic carbon nitride composite under visible light. Diam. Relat. Mater. 2023, 131, 109583. [Google Scholar] [CrossRef]
- Skuta, R.; Kostura, B.; Ritz, M.; Foniok, K.; Pavlovský, J.; Matýsek, D. Comparing the photocatalytic performance of GO/ZnO and g-C3N4/ZnO composites prepared using metallurgical waste as a source of zinc. Inorg. Chem. Commun. 2023, 152, 110728. [Google Scholar] [CrossRef]
- Azimi, E.B.; Badiei, A.; Sadr, M.H. Dramatic visible photocatalytic performance of g-C3N4-based nanocomposite due to the synergistic effect of AgBr and ZnO semiconductors. J. Phys. Chem. Solids 2018, 122, 174–183. [Google Scholar] [CrossRef]
- Thang, N.Q.; Sabbah, A.; Chen, L.C.; Chen, K.H.; Thi, C.M.; Viet, P.V. Highly efficient photocatalytic degradation of commercial drugs for pharmaceutical wastewater treatment prospects: A case study of Ag/g-C3N4/ZnO nanocomposite materials. Chemosphere 2021, 282, 130971. [Google Scholar] [CrossRef] [PubMed]
- Balu, S.; Velmurugan, S.; Palanisamy, S.; Chen, S.W.; Velusamy, V.; Yang, T.C.K.; El-Shafey, E.I. Synthesis of α-Fe2O3 decorated g-C3N4/ZnO ternary Z-scheme photocatalyst for degradation of tartrazine dye in aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 99, 258–267. [Google Scholar] [CrossRef]
- Hashem, E.M.; Hamza, M.A.; El-Shazly, A.N.; El-Rahman, S.A.A.; El-Tanany, E.M.; Mohamed, R.T.; Allam, N.K. Novel Z-Scheme/Type-II CdS@ZnO/g-C3N4 ternary nanocomposites for the durable photodegradation of organics: Kinetic and mechanistic insights. Chemosphere 2021, 277, 128730. [Google Scholar] [CrossRef]
- Vignesh, S.; Eniya, P.; Srinivasan, M.; Sundar, J.K.; Li, H.; Jayavel, S.; Pandiaraman, M.; Manthrammel, M.A.; Shkir, M.; Palanivel, B. Fabrication of Ag/Ag2O incorporated graphitic carbon nitride based ZnO nanocomposite for enhanced Z-scheme photocatalytic performance of various organic pollutants and bacterial disinfection. J. Environ. Chem. Eng. 2021, 9, 105996. [Google Scholar] [CrossRef]
- Sher, M.; Khan, S.A.; Shahid, S.; Javed, M.; Qamar, M.A.; Chinnathambi, A.; Almoallim, H.S. Synthesis of novel ternary hybrid g-C3N4@Ag-ZnO nanocomposite with Z-scheme enhanced solar light-driven methylene blue degradation and antibacterial activities. J. Environ. Chem. Eng. 2021, 9, 105366. [Google Scholar] [CrossRef]
- Madhushree, R.; UC, J.R.J.; Pinheiro, D.; Renuka, N.K.; Kr, S.D.; Park, J.; Manickam, S.; Choi, M.Y. Architecture of visible-light induced Z-scheme MoS2/g-C3N4/ZnO ternary photocatalysts for malachite green dye degradation. Environ. Res. 2022, 214, 113742. [Google Scholar]
- Asadi, A.; Daglioglu, N.; Hasani, T.; Farhadian, N. Construction of Mg-doped ZnO/g-C3N4@ZIF-8 multi-component catalyst with superior catalytic performance for the degradation of illicit drug under visible light. Colloids Surf. A Physiochem. Eng. Asp. 2022, 650, 129536. [Google Scholar] [CrossRef]
- Nguyen, T.X.Q.; Chen, S.S.; Pasawan, M.; Chang, H.M. Enhanced photocatalytic activity of g-C3N4-n-p type flower like ZnO/BiOBr heterojunction for hexavalent chromium and dye wastewater degradation. Environ. Technol. Innov. 2023, 31, 103154. [Google Scholar] [CrossRef]
- Panthi, G.; Hassan, M.; Kuk, Y.S.; Kim, J.Y.; Chung, H.J.; Hong, S.T.; Park, M. Enhanced antibacterial property of sulfate-doped Ag3PO4 nanoparticles supported on PAN electrospun nanofibers. Molecules 2020, 25, 1411. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Samolia, P.; Romanitan, C. Nd-doped ZnO nanostructures with enhanced photocatalytic performance for environmental protection. Int. J. Mol. Sci. 2023, 24, 6436. [Google Scholar] [CrossRef] [PubMed]
- Truc, N.T.T.; Duc, D.S.; Thuan, D.V.; Tahtamouni, T.A.; Pham, T.D.; Hanh, N.T.; Tran, D.T.; Nguyen, M.V.; Dang, N.M.; Chi, N.T.P.L.; et al. The advanced photocatalytic degradation of atrazine by direct Z-scheme Cu doped ZnO/g-C3N4. Appl. Surf. Sci. 2019, 489, 875–882. [Google Scholar] [CrossRef]
- Neena, D.; Humayun, M.; Bhattacharyya, D.; Fu, D. Hierarchical Sr-ZnO/g-C3N4 heterojunction with enhanced photocatalytic activities. J. Photochem. Photobiol. A Chem. 2020, 396, 112515. [Google Scholar]
- Sher, M.; Javed, M.; Shahid, S.; Iqbal, S.; Qamar, M.A.; Bahadur, A.; Qayyum, M.A. The controlled synthesis of g-C3N4/Cd-doped ZnO nanocomposites as potential photocatalysts for the disinfection and degradation of organic pollutants under visible light irradiation. RSC Adv. 2021, 11, 2025. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Bahadur, A.; AL-Anazy, M.M.; Laref, A.; Li, D. Designing of highly active g-C3N4/Ni-ZnO photocatalyst nanocomposite for the disinfection and degradation of the organic dye under sunlight radiations. Colloids Surf. A Physiochem. Eng. Asp. 2021, 614, 126176. [Google Scholar] [CrossRef]
- Shen, J.H.; Chiang, T.H.; Tsai, C.K.; Jiang, Z.W.; Horng, J.J. Mechanistic insights into hydroxyl radical formation of Cu-doped ZnO/g-C3N4 composite photocatalysis for enhanced degradation of ciprofloxacin under visible light: Efficiency, kinetics, products identification and toxicity evaluation. J. Environ. Chem. Eng. 2022, 10, 107352. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, Y.; Lv, X.; Huang, L.; Fang, L.; Wang, R. Creation of direct Z-scheme Al/Ga co-doping biphasic ZnO/g-C3N4 heterojunction for the sunlight-driven photocatalytic degradations of methylene blue. J. Sol-Gel Sci. Technol. 2022, 103, 876–889. [Google Scholar] [CrossRef]
- Albadri, A.E.A.E.; Aissa, M.A.B.; Modwi, A.; Saleh, S.M. Synthesis of mesoporous Ru-ZnO@g-C3N4 nanoparticles and their photocatalytic activity for methylene blue degradation. Water 2023, 15, 481. [Google Scholar] [CrossRef]
- Malik, M.; Ibrahim, S.M.; Nazir, M.A.; Tahir, A.A.; Tufail, M.K.; Shah, S.S.A.; Anum, A.; Wattoo, M.A.; Rehman, A. Engineering of a hybrid g-C3N4/ZnO-W/Cox heterojunction photocatalyst for the removal of methylene blue dye. Catalysts 2023, 13, 813. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Zain, M.F.M.; Minggu, L.J.; Kassim, M.B.; Jaafar, J.; Amin, N.A.S.; Mastuli, M.S.; Wu, H.; Wong, R.J.; Ng, Y.H. Bio-inspired hierarchical hetero-architectures of in-situ C-doped g-C3N4 grafted on C, N co-doped ZnO micro-flowers with booming solar photocatalytic activity. J. Ind. Eng. Chem. 2019, 77, 393–407. [Google Scholar] [CrossRef]
- Kalisamy, P.; Lallimathi, M.; Suryamathi, M.; Palanivel, B.; Venkatachalam, M. ZnO-embedded S-doped g-C3N4 heterojunction: Mediator-free Z-scheme mechanism for enhanced charge separation and photocatalytic degradation. RSC Adv. 2020, 10, 28365. [Google Scholar] [CrossRef] [PubMed]
- Kalisamy, P.; Hossain, M.S.; Macadangdang, R.R., Jr.; Madhubala, V.; Palanivel, B.; Venkatachalam, M.; Massoud, E.E.S.; Sreedevi, G. ZnO coupled F-doped g-C3N4: Z-scheme heterojunction for visible-light driven photocatalytic degradation reaction. Inorg. Chem. Commun. 2022, 135, 109102. [Google Scholar] [CrossRef]
- Sareshkeh, A.T.; Some-Saraee, R.B.; Rasoulifard, M.H.; Seyed-Dorraji, M.S.; Hosseini, S.F. P and C co-modified g-C3N4/SiO2/ZnO Z-scheme based heterogeneous nanocomposite as a highly boosted visible-light-driven photocatalytic system. J. Alloys Compd. 2022, 923, 166392. [Google Scholar] [CrossRef]
- Kumar, S.; Baruah, A.; Tonda, S.; Kumar, B.; Shanker, V.; Sreedhar, B. Cost-effective and eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core-shell nanoplates with excellent visible-light responsive photocatalysis. Nanoscale 2014, 6, 4830. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhou, H.; Li, T.; Zhang, L. A Z-scheme mechanism of N-ZnO/g-C3N4 for enhanced H2 evolution and photocatalytic degradation. Appl. Surf. Sci. 2019, 466, 133–140. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Yao, Z.; Yang, Z.; Xu, H. Enhanced visible-light-driven photocatalytic hydrogen evolution and NO photo-oxidation capacity of ZnO/g-C3N4 with N dopant. Colloids Surf. A 2020, 599, 124869. [Google Scholar] [CrossRef]
- Alhanash, A.M.; Al-Namshah, K.S.; Mohamed, S.K.; Hamdy, M.S. One-pot synthesis of the visible light sensitive C-doped ZnO@gC3N4 for high photocatalytic activity through Z-scheme mechanism. Optik 2019, 186, 34–40. [Google Scholar] [CrossRef]
- Behera, P.; Ray, A.; Tripathy, S.P.; Acharya, L.; Subudhi, S.; Parida, K. ZIF-8 derived porous C, N co-doped ZnO modified B-g-C3N4: A Z-Scheme charge dynamics approach operative towards photocatalytic hydrogen evolution and ciprofloxacin degradation. J. Photochem. Photobiol. A 2023, 436, 114415. [Google Scholar] [CrossRef]
- Bajiri, M.A.; Hezam, A.; Namratha, K.; Viswanath, R.; Drmosh, Q.A.; Naik, H.S.B.; Byrappa, K. CuO/ZnO/g-C3N4 heterostructures as efficient visible light-driven photocatalysts. J. Environ. Chem. Eng. 2019, 7, 103412. [Google Scholar] [CrossRef]
- Wang, M.; Tan, G.; Ren, H.; Xia, A.; Liu, Y. Direct double Z-scheme O-g-C3N4/Zn2SnO4N/ZnO ternary heterojunction photocatalyst with enhanced visible photocatalytic activity. Appl. Surf. Sci. 2019, 492, 690–702. [Google Scholar] [CrossRef]
- Yaghoot-Nezhad, A.; Moradi, M.; Rostami, M.; Danaee, I.; Khosravi-Nikou, M.R. Dual Z-scheme CuO-ZnO@Graphitic carbon nitride ternary nanocomposite with improved visible light-induced catalytic activity for ultrasound-assisted photocatalytic desulfurization. Energy Fuels 2020, 34, 13588–13605. [Google Scholar] [CrossRef]
- Moradi, M.; Hasanvandian, F.; Isari, A.A.; Hayati, F.; Kakavandi, B.; Setayesh, S.R. CuO and ZnO co-anchored on g-C3N4 nanosheets as an affordable double Z-scheme nanocomposite for photocatalytic decontamination of amoxicillin. Appl. Catal. B Environ. 2021, 285, 119838. [Google Scholar] [CrossRef]
- Garg, T.; Renu; Kaur, J.; Kaur, P.; Nitansh; Kumar, V.; Tikoo, K.; Kaushik, A.; Singhal, S. An innovative Z-scheme g-C3N4/ZnO/NiFe2O4 heterostructure for the concomitant photocatalytic removal and real-time monitoring of noxious fluoroquinolones. Chem. Eng. J. 2022, 443, 136441. [Google Scholar] [CrossRef]
- Aghababaei, N.; Abdouss, M.; Hosseini-Monfared, H.; Ghanbari, F. Photocatalytic degradation of diclofenac using a novel double Z-scheme catalyst (O-g-C3N4/ZnO/TiO2@halloysite nanotubes): Degradation mechanism, identification of by-products and environmental implementation. J. Water Process. Eng. 2023, 53, 103702. [Google Scholar] [CrossRef]
- Liu, X.Y.; Li, J. S-scheme heterojunction ZnO/g-C3N4 shielding polyester fiber composites for the degradation of MB. Semicond. Sci. Technol. 2021, 36, 045025. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, M.; Xu, Z.; Xiong, W.; Yang, Z.; Cao, J.; Peng, H.; Xu, H.; Xiang, Y.; Jing, Y. Constructing 2D/2D N-ZnO/g-C3N4 S-scheme heterojunction: Efficient photocatalytic performance for norfloxacin degradation. Chem. Eng. J. 2021, 430, 132652. [Google Scholar] [CrossRef]
- Elavarasan, N.; Vignesh, S.; Srinivasan, M.; Venkatesh, G.; Palanisamy, G.; Ramasamy, P.; Palanivel, B.; Al-Enizi, A.M.; Ubaidullah, M.; Reddy, V.R.M.; et al. Synergistic S-scheme mechanism insights of g-C3N4 and rGO combined ZnO-Ag heterostructure nanocomposite for efficient photocatalytic and anticancer activities. J. Alloys Compd. 2022, 906, 164255. [Google Scholar] [CrossRef]
- Lee, J.T.; Lee, S.W.; Wey, M.Y. S-scheme g-C3N4/ZnO heterojunction photocatalyst with enhanced photodegradation of azo dye. J. Taiwan Inst. Chem. Eng. 2022, 134, 104357. [Google Scholar] [CrossRef]
- Chai, H.; Yang, C.; Xu, P.; Wang, P.; Qu, J.; Zhang, G. Enhanced visible-light photocatalytic activity with Fe2O3-ZnO@C/g-C3N4 heterojunction: Characterization, kinetics, and mechanisms. J. Clean. Prod. 2022, 377, 134511. [Google Scholar] [CrossRef]
- Sert, B.; Bilici, Z.; Ocakoglu, K.; Dizge, N.; Rad, T.S.; Khataee, A. Preparation of S-scheme g-C3N4/ZnO heterojunction composite for highly efficient photocatalytic destruction of refractory organic pollutant. Catalysts 2023, 13, 485. [Google Scholar] [CrossRef]
- Feyzi, L.; Rahemi, N.; Allahyari, S. Efficient degradation of tetracycline in aqueous solution using a coupled S-scheme ZnO/g-C3N4/zeolite P supported catalyst with water falling film plasma reactor. Process Saf. Environ. Prot. 2022, 161, 827–847. [Google Scholar] [CrossRef]
- Leelavathi, H.; Muralidharan, R.; Abirami, N.; Tamizharasan, S.; Sankeetha, S.; Kumarasamy, A.; Arulmozhi, R. Construction of step-scheme g-C3N4/Co/ZnO heterojunction photocatalyst for aerobic photocatalytic degradation of synthetic wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130449. [Google Scholar] [CrossRef]
- Shi, S.; Jia, M.; Li, M.; Zhou, S.; Zhao, Y.; Zhong, J.; Dai, D.; Qiu, J. ZnO@g-C3N4 S-scheme photocatalytic membrane with visible-light response and enhanced water treatment performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 667, 131259. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Bhatti, H.N. Dual S-scheme ZnO-g-C3N4-CuO heterosystem: A photocatalyst for H2 evolution and wastewater treatment. React. Chem. Eng. 2023, 8, 1159. [Google Scholar] [CrossRef]

| Photocatalysts/Dosage | Synthesis Method | Light Used | Organic Pollutant | Performance | Ref. |
|---|---|---|---|---|---|
| ZnO/Fe2O3/g-C3N4 heterojunction/ (25 mg in 50 mL) | Hydrothermal treatment, followed by low-temperature calcination | Visible and sunlight | Sulfamethazine (5 mg L−1) | 100% in 8 h | [127] |
| 3Dg-C3N4/ZnO@graphene aerogel (g-C3N4/ZnO@GA)30% heterojunction formed with 30 wt% g-C3N4/ (5 mg in 25 mL) | Hydrothermal self-assembly combined with freeze-drying | UV and visible light | Rh B, MO, MV, and MB dyes (20 mg L−1) | Rh B dye in 120 min UV light: 81% Visible light: 82.7% MO dye in 120 min UV light: 50% Visible light: 46.9% | [128] |
| ZnO/g-C3N4/RGO photocatalyst/ (25 mg in 50 mL) | Calcination | Visible light and UV light | Deoxynivalenol (10 ppm) | Visible light: 90% in 5 h UV light: 90% in 120 min | [129] |
| Ag3PO4/g-C3N4/ZnO ternary composite/ (0.6 g/L, 50 mL) | Ultrasound-assisted precipitation | Visible light and sunlight | Tetracycline hydrochloride (30 mg/L) | Visible light: 88.48% Sunlight: 89.95% in 120 min | [130] |
| ZnO/C/g-C3N4 composite ENFs (containing 0.25 wt% g-C3N4)/ (10 mg in 10 mL) | Electrospinning and annealing under N2 atmosphere | Simulated sunlight | MB dye (10−5 M) | 91.8% in 120 min | [136] |
| Cu-doped ZnO/Cu/g-C3N4 heterostructure (g200: prepared by adding 0.02 g of prepared g-C3N4)/(0.4 g/L for MB and 0.5 g/L for Rh B dye) | Calcination–hydrothermal | Sunlight | MB dye and Rh B dye (0.01 g in 100 mL) | MB dye: 98% in 20 min Rh B dye: 99% in 60 min | [137] |
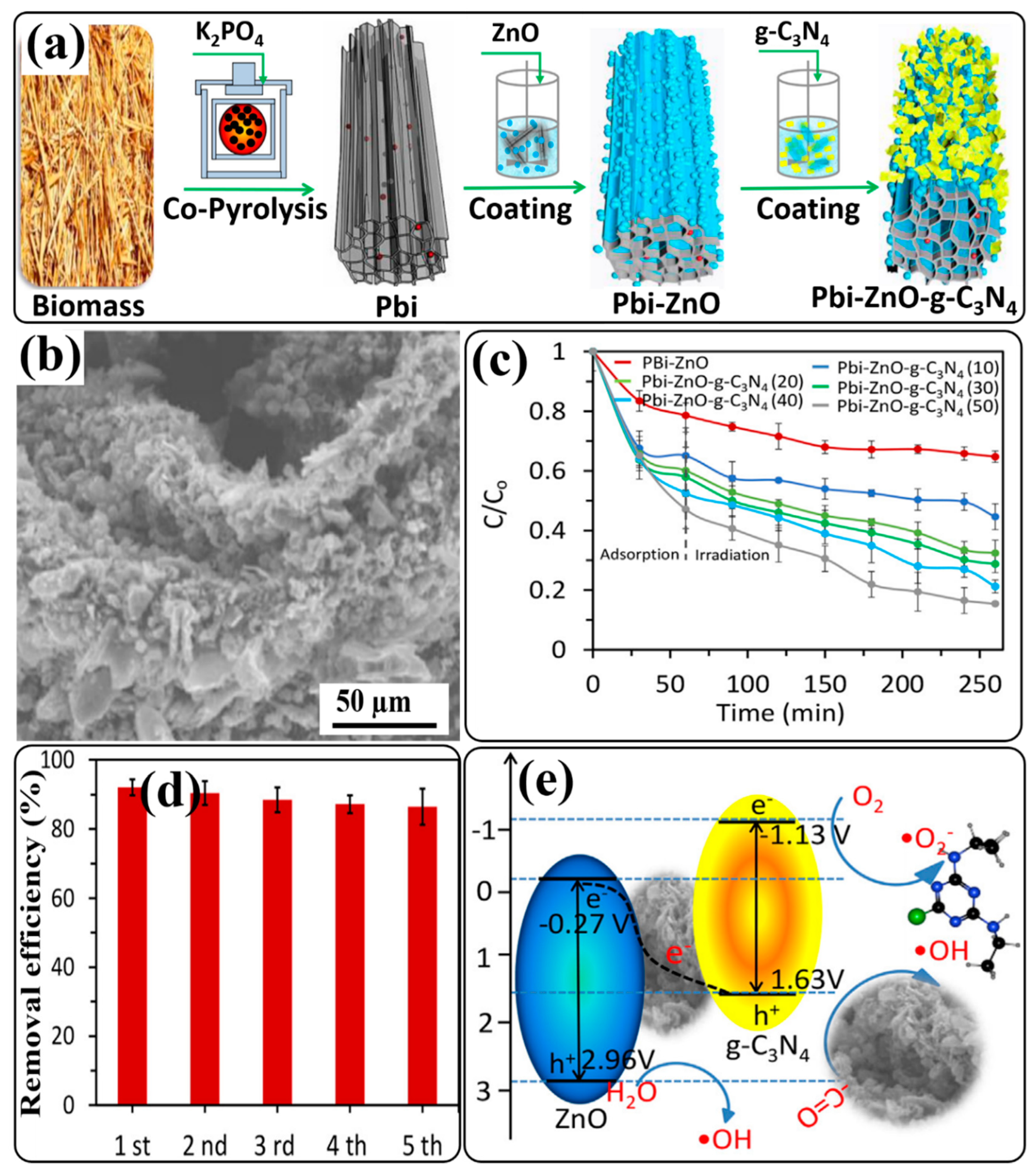
| P-laden biochar/ZnO/g-C3N4 core–shell composite [Pbi-ZnO-g-C3N4 (50)], in which mass% of g-C3N4 to Pbi-ZnO is 50)/ (1 g in 100 mL) | Thermal polymerization, copyrolysis, and annealing under N2 atmosphere | Simulated sunlight | Atrazine (10 mg/L) | 85.3% in 260 min | [138] |
| Nanocomposite of (NiCo/ZnO/g-C3N4)/ (20 mg in 100 mL) | Thermal condensation, in situ hydrothermal treatment, ultrasonic decomposition | Visible light | Oxytetracycline and tetracycline (10 mg/L) | Oxytetracycline: 71.3% Tetracycline: 81.29% in 50 min | [139] |
| g-C3N4//ZnO/Fe2O3 ternary composite/ (50 mg in 50 mL, pH 11) | Calcination | Visible light | MB dye (30 mL/L) | 94% in 120 min | [140] |
| Photocatalysts/Dosage | Synthesis Method | Light Used | Organic Pollutant | Performance | Ref. |
|---|---|---|---|---|---|
| ZnO nano-triangle@g-C3N4 nanofoils (20%)/(1.25 g/L) | Sono-chemical impregnation | Solar light | Rh B dye (10 ppm) | 100% in 60 min | [141] |
| g-C3N4 (10 wt%)/O-defective ZnO (OD-ZnO) nanorods/ (0.1 g/100 mL) | Solution conversion, heating, and ultrasonication | Visible light | 4-CP (10−4 mol L−1) | 95% in 60 min | [142] |
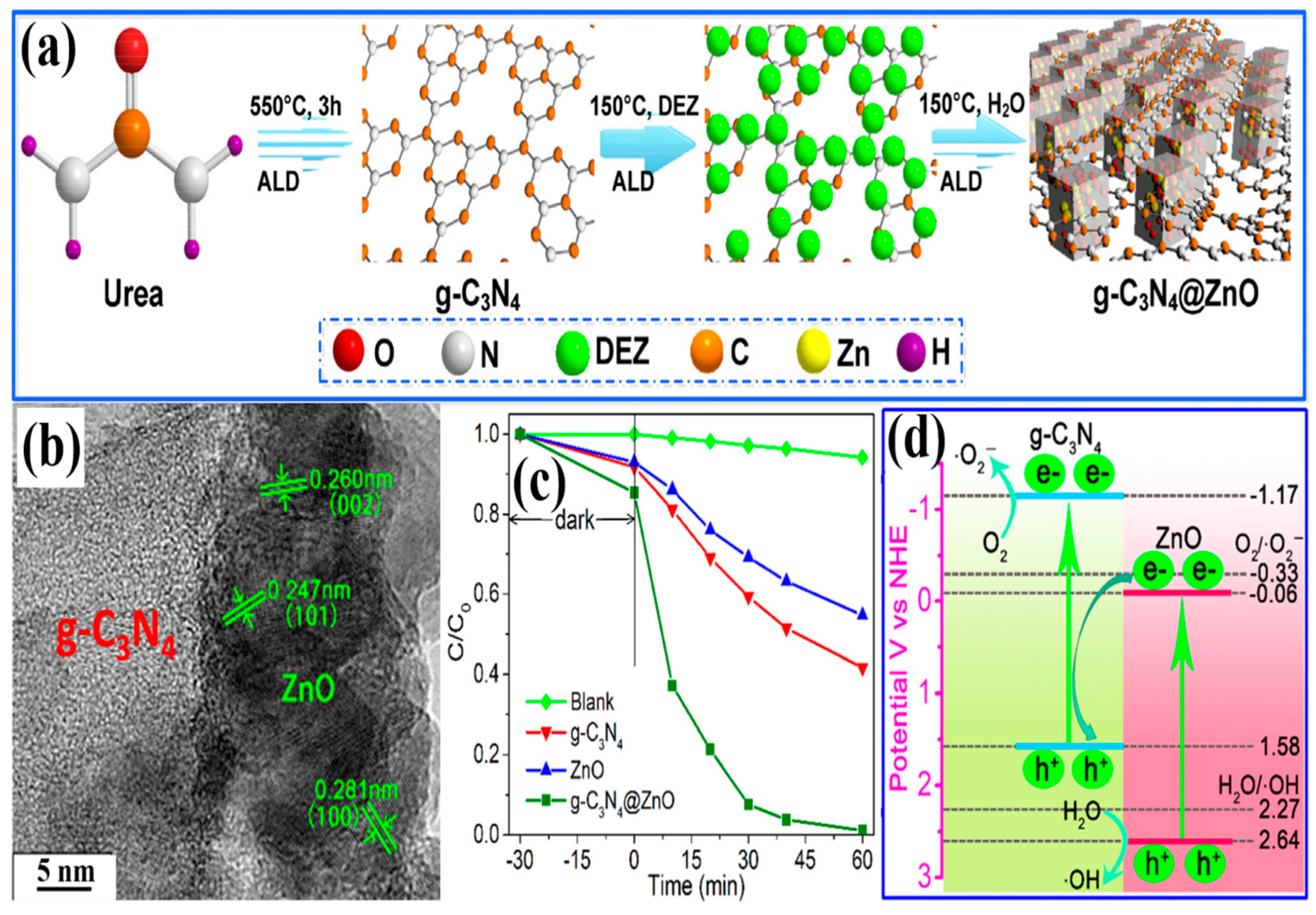
| 2D/3D g-C3N4@ZnO heterostructure/(0.3 g/L) | Thermal atomic layer deposition | Simulated sunlight | Cephalexin (10 mg/L) | 98.9% in 60 min | [143] |
| g-C3N4/ZnO composite@500 °C (CNZ-500)/(10 mg/L) | Thermal treatment | Visible light | MB dye (10 mg/L) | Rate constant: 2.88 × 10−2 min−1 | [144] |
| ZnO/g-C3N4 composite (2 g/L) | One-step calcination | Visible light | MB dye (10 ppm) | 98.83 in 60 min | [145] |
| ZnO/g-C3N4 nanocomposite (7 g-C3N4 /ZnO: 7 wt% of g-C3N4 relative to ZnO)/(0.1 g in 400 mL) | Electrostatic self-assembly combined with low-temperature precipitation | Simulated sunlight | MB dye (10 mL/L) | 93% in 5 h | [146] |
| 0D/2D g-C3N4 quantum dots/ZnO with oxygen vacancies [(CNQDs/OV-ZnO)]/ (10 mg in 80 mL) | Mixing and calcination | Visible light | MB dye and BPA (10 mL/L) | MB dye: 71% in 4 h and BPA: 61% in 12 h | [147] |
| Dicyanadiamine-derived core–shell composite of g-C3N4/ZnO (DCDA-CNZ)/ (10 mg in 50 mL) | Thermal polymerization | Visible light | MB dye (10 ppm) | Rate constant: 2.39 × 10−2 min−1 | [148] |
| g-C3N4/ZnO nanocomposite/ (50 mg in 100 mL) | Calcination | Sunlight UV light | MB dye (1 × 10−5 mol/L) | Sunlight 100% UV light <100% (in 60 min) | [149] |
| g-C3N4/ZnO composite formed by 1:30 precursor mass ratio/ (100 mg in 100 mL) | Co-melting-recrystallization | Visible light | MO dye and levofloxacin (10 mg L−1) | MO dye: 62% in 240 min Levofloxacin: 66.7% in 210 min | [150] |
| g-C3N4/ZnO heterojunction/ (50 mg in 60 mL) | Thermal decomposition | Visible light | MO dye (2 × 10−5 M) | Rate constant: ~0.0117 min−1 | [151] |
| g-C3N4/ZnO heterostructure/ (0.1 g in 50 mL) | Exfoliation process | Visible light | MG dye (10 ppm) | 84.3% in 60 min | [152] |
| 75 wt% ZnO/g-C3N4 nanocomposite/(0.1 g in 100 mL) | Pyrolysis and hydrothermal | Visible light | MB dye (50 mg/L) | 98% in 120 min | [153] |
| ZnO-g-C3N4 nanocomposite/ (25 mg in 50 mL) | Sol–gel-assisted route | Visible light | Congo red (10 mg/L) | More than 80% in 30 min | [154] |
| ZnO doped with 20% g-C3N4 (ZnO-g-C3N4-20) nanosheet/ (50 mg in 50 mL) | Hydrothermal | Visible light | MO dye (20 mg.dm−3) | 98.5% in 150 min | [155] |
| g-C3N4/ZnO composite/ (20 mg in 100 mL) | Hydrothermal | Solar irradiation | MB dye, Rh B dye and Ciprofloxacin (10 ppm) | MB dye: 100% in 50 min Rh B dye: 98% in 100 min Ciprofloxacin: 96% in 180 min | [156] |
| ZnO/g-C3N4 heterojunction (50-Zn/gCN)/ (25 mg in 250 mL) | Mixing, sonication, and thermal treatment | Simulated solar light | CFZ, and RB5 dye/(10 mg L−1) | CFZ: 78% RB5 dye: 95% (in 120 min) | [157] |
| g-C3N4/ZnO heterojunction/ (100 mg in 50 mL) | Impregnation and ultrasonic treatment | Visible light | Rh B dye (10 ppm) | 75% in 250 min | [158] |
| ZnO-g-C3N4 heterostructure/ (40 mg in 100 mL) | Solution mixing | Visible light | MB dye (5 ppm) | 91.5% in 120 min | [159] |
| 40% ZnO-g-C3N4 lotus bud-like composite/ (100 mg in 250 mL) | One-pot pyrolysis | Visible light | Benzene (20 mg/L) | 98.6% in 270 min | [160] |
| ZnO/g-C3N4 composite/ (50 mg in 50 mL, pH 9) | Calcination | Visible light | MB dye, MG dye, and MO dye (10 mg/L) | MB dye: 99.16% MG dye: 96.42% MO dye: 57.57% (in 180 min) | [161] |
| g-C3N4/ZnO composite/ (0.05 g in 150 cm−3) | Annealing | UV light | Acid orange 7 (25 mg in dm−3) | 51.5% in 120 min | [162] |
| Photocatalysts/Dosage | Synthesis Method | Light Used | Organic Pollutant | Performance | Ref. |
|---|---|---|---|---|---|
| g-C3N4/AgBr/ZnO 30 ternary composite (30 is the wt. ratio)/ (0.04 g in 100 mL) | Sonication-assisted deposition technique | Visible light | MB dye (5 mg L−1) | 96.3% in 80 min | [163] |
| Ag/g-C3N4/ZnO nanorod-nanocomposite (0.089 g/L) | Solvothermal, polycondensation, stirring | Visible light | Paracetamol, Cefalexin, and Amoxicillin (40 mg/L) | Paracetamol: 85.3% Cefalexin: 71.74% Amoxicillin: 41.36% in 180 min | [164] |
| α-Fe2O3 decorated g-C3N4/ZnO (g-C3N4/ZnO@α-Fe2O3) ternary nanocomposite/ (0.05 g in 100 mL) | Direct pyrolysis and sol–gel | Visible light | Tetrazine dye (10 mg L−1) | 99.34% in 35 min | [165] |
| CdS@ZnO-g-C3N4 ternary nanocomposite/ (1 g L−1 at pH 6) | One-pot room-temperature ultrasonic route | UV light and visible light | Rh B dye (1 × 10−5 M) | UV light: 93.34% in 120 min Visible light: 90% in 180 min | [166] |
| Ag/Ag2O combined g-C3N4/ZnO (g-C3N4/ZnO-Ag2O) ternary composite/ (50 mg in 100 mL) | Calcination and hydrothermal | Visible light | MB dye (30 ppm) 4-CP (10 ppm) | MB dye: 96.5% 4-CP: 85.7% in 120 min | [167] |
| 55% g-C3N4@Ag-ZnO hybrid nanocomposite/ (0.01 g in 100 mL) | Physical mixing | Solar light | MB dye (100 mg in 100 mL) | 98% in 80 min | [168] |
| MoS2/g-C3N4/ZnO ternary nanocomposite/ (0.1 g in 50 mL) | Hydrothermal and exfoliation | Visible light | MG dye (10 ppm) | 97% in 60 min | [169] |
| Mg-ZnO/g-C3N4@ZIF-8 multicomponent nanocomposite/((0.5 g/L) in presence of NaBH4 at pH 9) | Chemical precipitation | Visible light | Illicit drug (50 mg/L) | 100% in 10 min | [170] |
| g-C3N4-ZnO/BiOBr heterojunction photocatalyst/ (0.05 g in 1 L) | Hydrothermal | UV light | MO dye (20 mg/L) | 99.26% in 130 min | [171] |
| Photocatalysts/Dosage | Synthesis Method | Light Used | Organic Pollutant | Performance | Ref. |
|---|---|---|---|---|---|
| Cu-doped ZnO/g-C3N4 photocatalyst/ (0.5 g in 500 mL) | Autoclave heating and calcination | Visible radiation | Atrazine (100 ppm) | 90% in 180 min | [174] |
| Sr-ZnO/g-C3N4 heterojunction/(0.5 g in 80 mL) | One-pot facile method | UV-vis irradiation | Methylene green dye (10 mg/L) | 96% in 20 min | [175] |
| g-C3N4/(Cd-ZnO) nanocomposite/ (0.01 g in 188 mL water and 12 mL dye solution) | Co-precipitation | Visible light | MB dye (10 mg in 100 mL water) | 95% in 90 min | [176] |
| Ni/ZnO/g-C3N4 nanocomposite [3% Ni/ZnO,70% g-C3N4 (NiZG-70)]/ (200 mg in 200 mL) | Chemical co-precipitation | Sunlight | MB dye (10 mg L−1) | 100% in 70 min | [177] |
| Cu-doped ZnO/g-C3N4 composite/ (100 mg in 200 mL) | Hydrothermal treatment followed by calcination | Visible light | CIP (5 mg/L) | 95% in 360 min | [178] |
| Al/Ga-codoped ZnO/g-C3N4 heterojunction (AGZ/CN 560: where g-C3N4 was prepared at 560 °C)/(20 mg in 30 mL) | Thermal decomposition and single-phase dispersion | Visible light | MB dye (10 mg/L) | 95.4% in 150 min | [179] |
| Ru-ZnO@g-C3N4 mesoporous nanocomposite/ (5 mg in 100 mL, pH 10) | Ultrasonic technique | UV light | MB dye (30 ppm) | 92.2% in 60 min | [180] |
| Hybrid g-C3N4/ZnO-W/Co(0.010) heterojunction/(0.05 mg) | Precipitation method | Visible light | MB dye (10 ppm) | 90% in 90 min | [181] |
| C-doped g-C3N4 grafted on C, N-codoped ZnO (BT-CCN@ZnO) microflowers/ | Bio-templated hydrothermal | Simulated solar irradiation | BPA | 92.5% in 180 min | [182] |
| ZnO-embedded S-doped g-C3N4 (ZnO-SCN) heterojunction/ (50 mg in 100 mL) | Sol–gel-assisted calcination | Visible light | MB dye and Rh B dye (10 ppm) | 93% in 80 min | [183] |
| ZnO-coupled F-doped g-C3N4 (Fe@g-C3N4/ZnO) heterojunction/(50 mg) | Simple wet-chemical | UV-vis and direct sunlight | Rh B dye (10 ppm) | (In 75 min) UV-vis light: 97% Direct sunlight: 98% | [184] |
| P, C-GCN/15 wt% SiO2/5 wt% ZnO (PC-GCN/15-SiO2/5-ZnO) heterogeneous nanocomposite/[100 mL (500 mg L−1)] | Calcination | LED 200 W | MB dye (20 mg L−1) | 100% in 90 min | [185] |
| N-doped ZnO/g-C3N4 core–shell nanoplates with 5 wt% loaded g-C3N4 (CNZON5)/ (0.025 g in 100 mL) | Ultrasonic dispersion | Visible light | Rh B dye (5 mg L−1) | Rate constant: 0.0679 min−1 | [186] |
| N-ZnO/g-C3N4 composite/ (0.1 g in 100 mL) | High-temperature calcination | Visible radiation | MB dye (20 mg/L) | 95% in 90 min | [187] |
| ZnO/g-C3N4 with N dopant (nitrogen-rich ZnO/g-C3N4 composite)/(100 mg) | Rotation-evaporation and calcination route | Visible light | NO gas (600 ppb) | More than 87% in 6 min | [188] |
| C-doped ZnO@ g-C3N4 composite with ZnO loading 50% (Zn-50)/ (0.1 g in 50 mL) | Thermal treatment | Visible light | Methyl green dye (20 mg/L) | 98% in 60 min | [189] |
| C, N-codoped ZnO modified B-doped g-C3N4 [CNZ/BCN (1:1)] nanocomposite/ (30 mg in 20 mL) | In situ calcination | Simulated solar light | CIP (20 ppm) | 86.7% in 60 min | [190] |
| g-C3N4/ZnO-Based Double Z-Scheme Heterojunction Photocatalysts | |||||
|---|---|---|---|---|---|
| Photocatalysts/Dosage | Synthesis Method | Light Used | Organic Pollutant | Performance | Ref. |
| CuO/ZnO/g-C3N4 ternary heterostructure/ (0.04 g in 100 mL) | Solution combustion route | Visible light | MB dye (10 mg/L) | 98% in 45 min | [191] |
| O-g-C3N4/Zn2SnO4N/ZnO heterojunction/ (50 mg in 50 mL) | UV-light irradiation | Visible light | Rh B dye (5 mg L−1) | 96% in 60 min | [192] |
| CuO-ZnO@g-C3N4 nanocomposite/(0.2 g/L with trace amount of H2O2 (250 ppm)) | Ultrasound-assisted hydrothermal | Visible light + ultrasonic wave | Dibenzothiophene (DBT) 250 ppm | 99.1% in 60 min | [193] |
| CuO nanoparticles and ZnO nanorods co-anchored on g-C3N4 nanosheets (CZ@T-GCN)/ (0.9 g L−1 in 250 mL) | Isoelectric-point-mediated method | Simulated sunlight | AMOX (60 mg L−1) | 100% in 120 min | [194] |
| g-C3N4/ZnO-NiFe2O4 (weight ratio of CN/ZnO: NiFe2O4 is 2:1) [CZN1]/(0.5 g/L) | Simple sonication–calcination strategy | Visible light | Levofloxacin: 30 Ofloxacin: 15 Ciprofloxacin: 10 (mg/L) | Levofloxacin: 90% Ofloxacin: 88% Ciprofloxacin: 82% (in 90 min) | [195] |
| [(O-g-C3N4)/ZnO-TiO2@HNTs]/ (1 g in 40 mL) | Calcination and sol–gel | UV light | Diclofenac (10 mg/L) | 100% in 50 min | [196] |
| g-C3N4/ZnO-based S-scheme heterojunction photocatalysts | |||||
| ZnO/g-C3N4@PET composite/ (6 cm × 3 cm in 50 mL) | Hydrothermal | Visible light | MB dye (15 mg L−1) | 92.5% in 120 min | [197] |
| 2D/2D N-ZnO/g-C3N4 composite (15% NZCN)/(0.2 g L−1) | Calcination, ultrasonication, and self-assembly | Visible light | Norfloxacin (5 mg L−1) | 96.4% in 90 min | [198] |
| g-C3N4/rGO/ZnO-Ag heterostructure nanocomposite/ (60 mg in 100 mL) | Hydrothermal | Visible light | Mixed dye (Rh B + MB) (40 ppm) | 90.4% in 100 min | [199] |
| g-C3N4/ZnO-450 heterojunction composite/ (5 mg in 50 mL) | ZIF8 template | Visible light | AR1 (10 mg/L) | 95% in 1 h | [200] |
| Fe2O3-ZnO@C/g-C3N4 (FZCCN-4) heterojunction/ (0.8 g in 250 mL) | Precipitation and calcination | Visible light | BPA (10 mg/L) | 100% in 60 min | [201] |
| g-C3N4/ZnO heterojunction composite/(0.10 g in 100 mL) | Calcination | UV light | CV dye (10 mg/L) | 95.9% in 120 min | [202] |
| ZnO/g-C3N4/zeolite nanocomposite | Hydrothermal | Plasma discharge | TC (50 ppm) | 95.5% in 100 min | [203] |
| g-C3N4/Co/ZnO heterojunction nanocomposite/ (20 mg in 100 mL) | Ultrasonic and sol–gel | Visible light and Sunlight | MB, CV, Rh B dyes (15 ppm) Rh B dye (15 ppm) | MB dye: 96.3% CV dye: 74.5% Rh B dye: 75.4% in 80 min 91.5% in 80 min | [204] |
| ZnO@g-C3N4 composite membrane (size: 10.75 cm−2) (Water flux: 336.8 L• m−2 • bar−1 • h−1) | Vacuum-assisted filtration and in situ growth | Visible light | MB dye (5 mg/L) | 94.4% in 150 min | [205] |
| g-C3N4-ZnO-CuO heterojunction photocatalyst/ (20 mg in 30 mL) | Simple solution combustion approach | Visible light | MB dye and Rh B dye (10 mg/L) | MB dye: 100% Rh B dye: 90% (in 35 min) | [206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panthi, G.; Park, M. Graphitic Carbon Nitride/Zinc Oxide-Based Z-Scheme and S-Scheme Heterojunction Photocatalysts for the Photodegradation of Organic Pollutants. Int. J. Mol. Sci. 2023, 24, 15021. https://doi.org/10.3390/ijms241915021
Panthi G, Park M. Graphitic Carbon Nitride/Zinc Oxide-Based Z-Scheme and S-Scheme Heterojunction Photocatalysts for the Photodegradation of Organic Pollutants. International Journal of Molecular Sciences. 2023; 24(19):15021. https://doi.org/10.3390/ijms241915021
Chicago/Turabian StylePanthi, Gopal, and Mira Park. 2023. "Graphitic Carbon Nitride/Zinc Oxide-Based Z-Scheme and S-Scheme Heterojunction Photocatalysts for the Photodegradation of Organic Pollutants" International Journal of Molecular Sciences 24, no. 19: 15021. https://doi.org/10.3390/ijms241915021
APA StylePanthi, G., & Park, M. (2023). Graphitic Carbon Nitride/Zinc Oxide-Based Z-Scheme and S-Scheme Heterojunction Photocatalysts for the Photodegradation of Organic Pollutants. International Journal of Molecular Sciences, 24(19), 15021. https://doi.org/10.3390/ijms241915021







