The Co-Expression Pattern of Calcium-Binding Proteins with γ-Aminobutyric Acid and Glutamate Transporters in the Amygdala of the Guinea Pig: Evidence for Glutamatergic Subpopulations
Abstract
:1. Introduction
2. Results
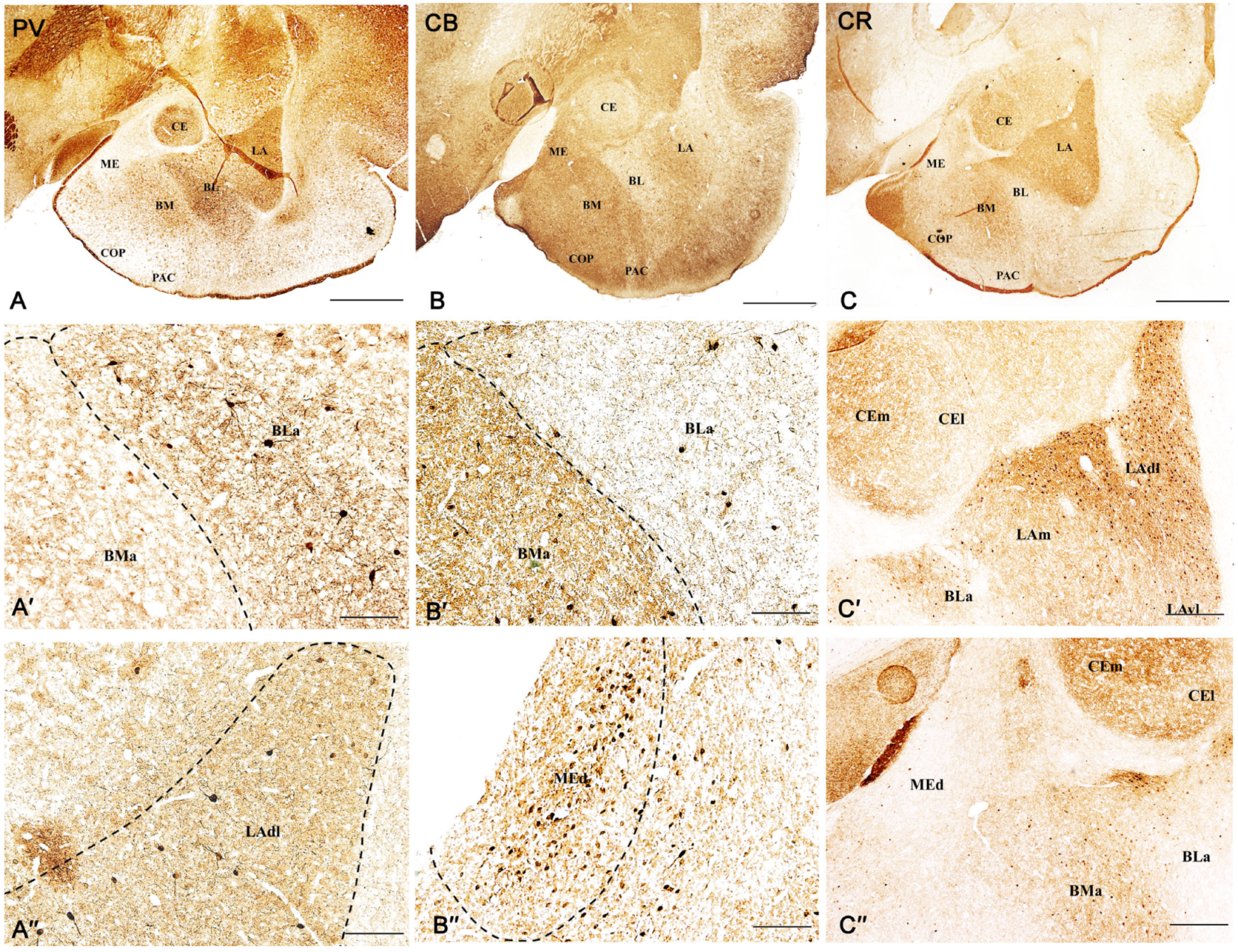
2.1. Distribution Pattern of PV, CB, and CR Neurons in the Amygdala of the Guinea Pig
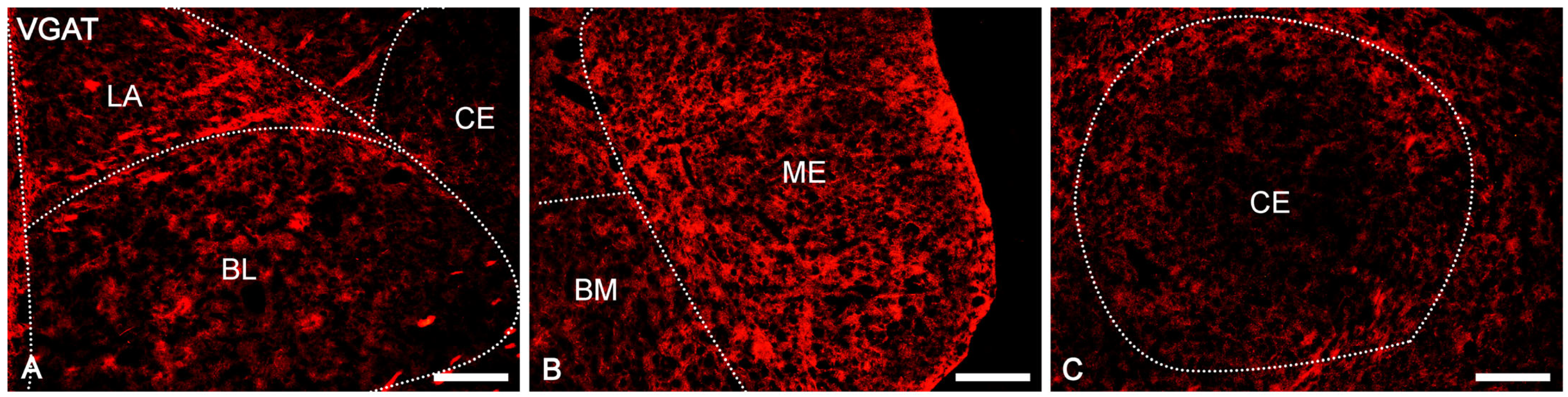
2.2. Distribution Pattern of VGAT and VGLUT2 Immunoreactivity in the Amygdala of the Guinea Pig

2.3. Co-Expression Pattern of PV, CB, and CR Neurons with VGAT and VGLUT2 in the Amygdala of the Guinea Pig
2.3.1. Parvalbumin Neurons
| Parvalbumin | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VGAT | VGLUT2 | |||||||||||||||||
| PV | PV/VGAT | % | PV | PV/VGLUT2 | % | |||||||||||||
| LA | 120.3 | ± | 6.7 | 106.3 | ± | 4.2 | 88.0 | ± | 1.9 | 128.0 | ± | 3.5 | 1.0 | ± | 0.2 | 1.5 | ± | 0.1 |
| BL | 130.7 | ± | 9.9 | 114.7 | ± | 3.3 | 87.7 | ± | 2.9 | 127.7 | ± | 6.9 | 1.3 | ± | 0.2 | 1.6 | ± | 0.4 |
| BM | 110.0 | ± | 4.4 | 96.0 | ± | 2.9 | 86.2 | ± | 0.1 | 124.0 | ± | 2.0 | 2.0 | ± | 0.2 | 1.5 | ± | 0.2 |
| CO | 151.0 | ± | 13.5 | 131.3 | ± | 2.9 | 87.5 | ± | 1.6 | 142.3 | ± | 5.7 | 2.7 | ± | 0.2 | 2.4 | ± | 0.1 |
| CE | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| ME | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Mean | 128 | ± | 8.6 | 112.1 | ± | 3.3 | 87.4 | ± | 1.6 | 130.5 | ± | 4.5 | 1.8 | ± | 0.2 | 1.7 | ± | 0.2 |
2.3.2. Calbindin Neurons
| Calbindin | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VGAT | VGLUT2 | |||||||||||||||||
| CB | CB/VGAT | % | CB+ | CB/VGLUT2 | % | |||||||||||||
| LA | 117.3 | ± | 3.3 | 91.7 | ± | 4.1 | 77.2 | ± | 1.4 | 121.7 | ± | 0.4 | 3.0 | ± | 0.6 | 3.6 | ± | 0.5 |
| BL | 160.0 | ± | 2.1 | 121.0 | ± | 1.0 | 75.6 | ± | 1.1 | 192.3 | ± | 3.7 | 7.3 | ± | 0.4 | 4.4 | ± | 0.2 |
| BM | 133.7 | ± | 5.3 | 106.3 | ± | 2.4 | 77.2 | ± | 1.9 | 146.0 | ± | 4.9 | 4.0 | ± | 0.4 | 3.5 | ± | 0.4 |
| CO | 176.0 | ± | 4.5 | 145.0 | ± | 8.0 | 80.8 | ± | 2.8 | 159.0 | ± | 7.3 | 3.3 | ± | 0.8 | 3.0 | ± | 0.3 |
| CE | 131.0 | ± | 8.5 | 105.0 | ± | 6.5 | 79.2 | ± | 1.2 | 142.7 | ± | 3.3 | 4.3 | ± | 0.4 | 4.6 | ± | 0.1 |
| ME | 263.3 | ± | 21.6 | 213.0 | ± | 8.0 | 79.1 | ± | 3.8 | 230.0 | ± | 1.6 | 10.7 | ± | 0.4 | 6.6 | ± | 1.0 |
| Mean | 163.6 | ± | 7.6 | 130.3 | ± | 5.0 | 78.2 | ± | 2.0 | 165.3 | ± | 3.5 | 5.4 | ± | 0.5 | 4.3 | ± | 0.4 |
2.3.3. Calretinin Neurons
| Calretinin | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VGAT | VGLUT2 | |||||||||||||||||
| CR | CR/VGAT | % | CR | CR/VGLUT2 | % | |||||||||||||
| LA | 306.0 | ± | 15.5 | 152.0 | ± | 6.1 | 52.6 | ± | 2.9 | 235.3 | ± | 4.1 | 6.3 | ± | 0.4 | 2.4 | ± | 0.0 |
| BL | 251.3 | ± | 21.2 | 118.7 | ± | 1.6 | 49.2 | ± | 3.7 | 152.7 | ± | 9.8 | 5.0 | ± | 0.8 | 4.2 | ± | 0.2 |
| BM | 207.7 | ± | 18.8 | 119.0 | ± | 18.0 | 57.0 | ± | 2.7 | 161.0 | ± | 3.7 | 2.7 | ± | 0.5 | 2.3 | ± | 0.1 |
| CO | 205.7 | ± | 13.1 | 114.7 | ± | 3.8 | 57.6 | ± | 3.7 | 166.3 | ± | 3.3 | 6.0 | ± | 0.4 | 5.5 | ± | 0.2 |
| CE | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| ME | 230.7 | ± | 16.7 | 102.3 | ± | 16.3 | 47.6 | ± | 4.4 | 201.3 | ± | 1.2 | 2.7 | ± | 0.4 | 2.4 | ± | 0.1 |
| Mean | 240.3 | ± | 17.1 | 121.3 | ± | 9.2 | 52.8 | ± | 3.5 | 183.3 | ± | 4.4 | 4.5 | ± | 0.5 | 3.4 | ± | 0.1 |
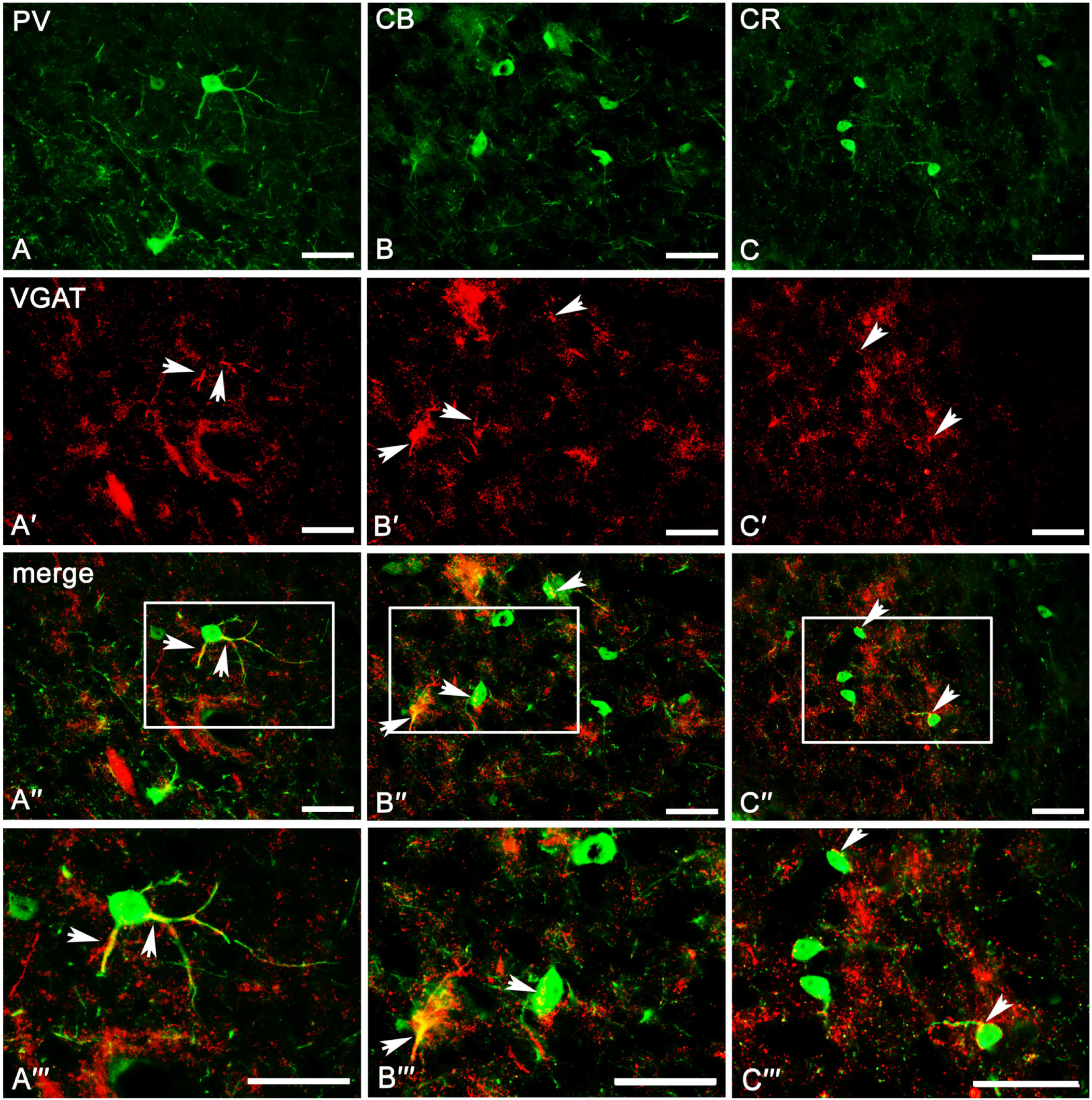
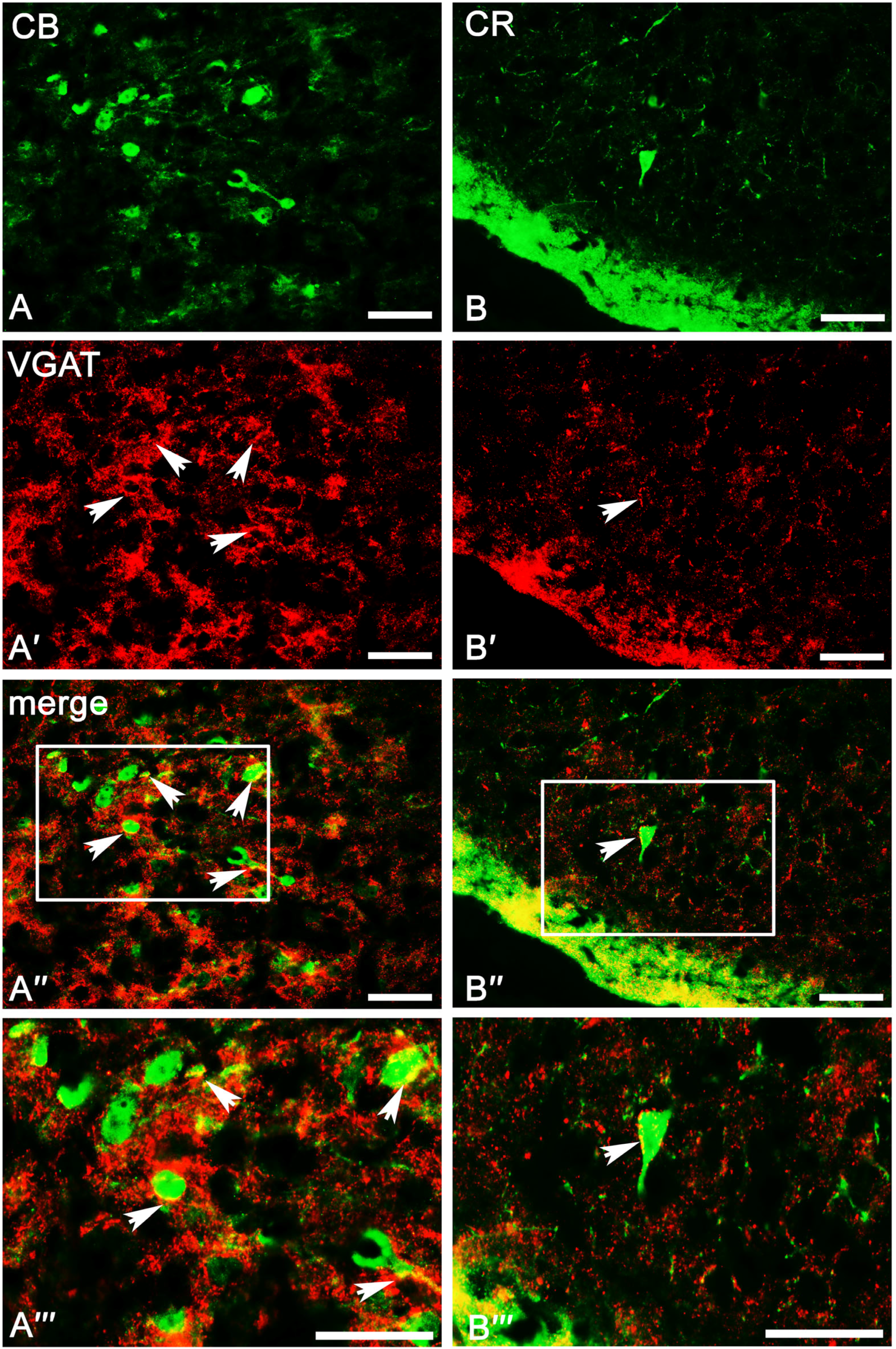
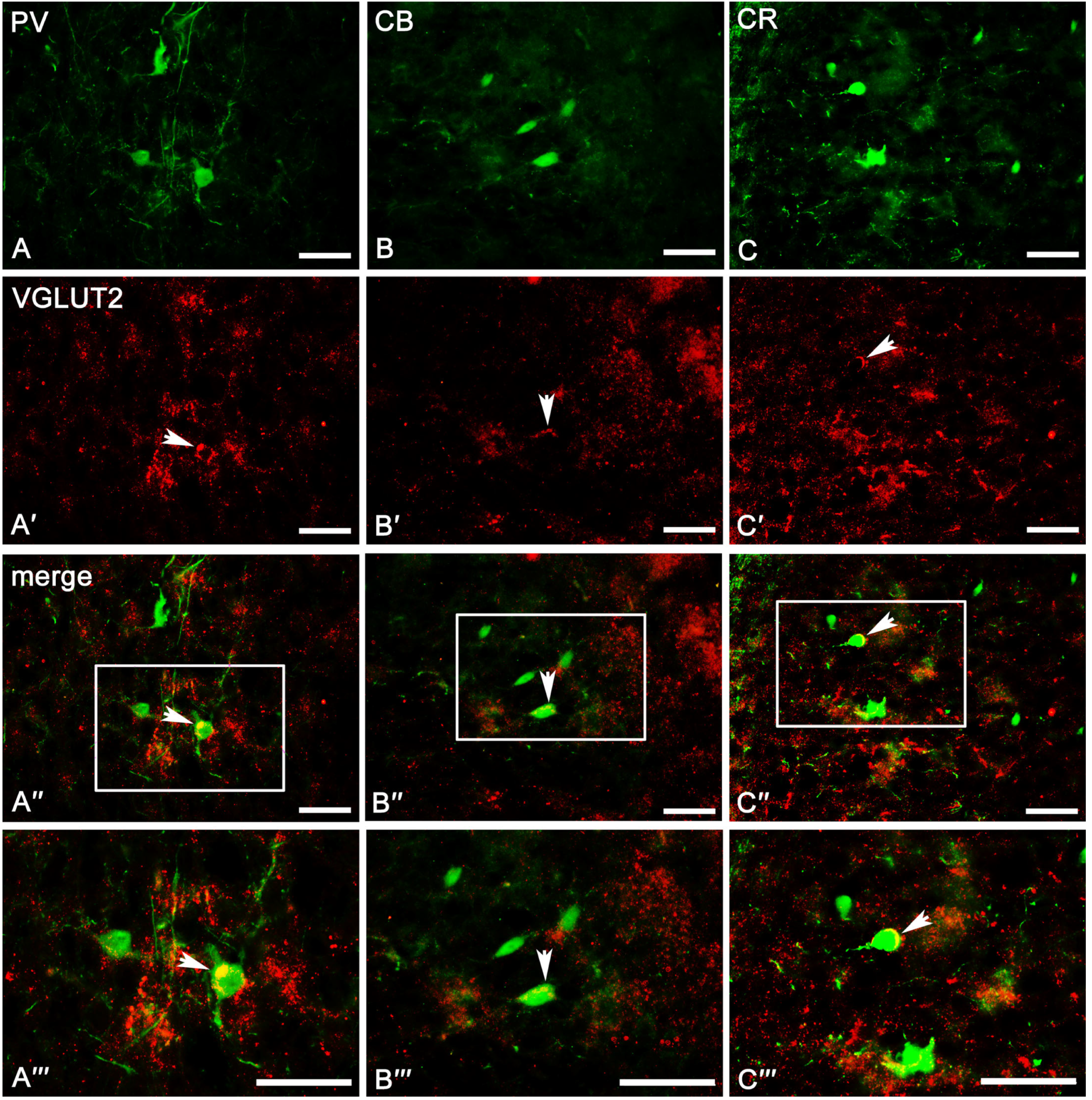
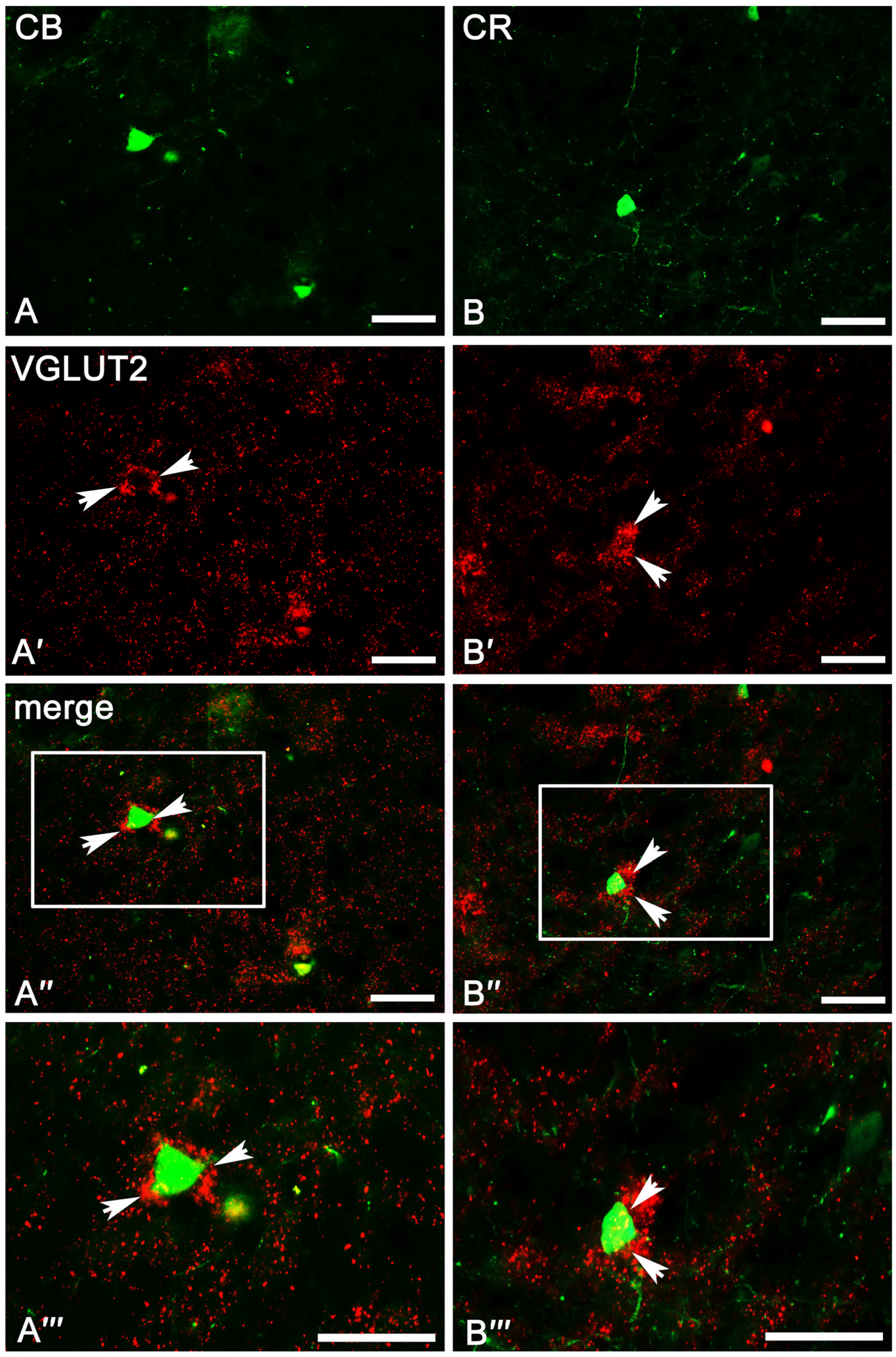
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Tissue Preparation
4.3. Immunofluorescence
4.4. Controls
4.5. Cell Counts
4.6. Volume Density Counting
4.7. Photomicrographic Production
4.8. Statistic
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeDoux, J. The Emotional Brain, Fear, and the Amygdala. Cell. Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Rajmohan, V.; Mohandas, E. The Limbic System. Indian J. Psychiatry 2007, 49, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Sah, P.; Faber, E.S.L.; Lopez De Armentia, M.; Power, J. The Amygdaloid Complex: Anatomy and Physiology. Physiol. Rev. 2003, 83, 803–834. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.J. Sexual Differentiation of Pheromone Processing: Links to Male-Typical Mating Behavior and Partner Preference. Horm. Behav. 2009, 55, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Vining, C.; Denski, K. Regulation of Chronic Stress-Induced Changes in Hypothalamic-Pituitary-Adrenal Activity by the Basolateral Amygdala. Ann. N. Y. Acad. Sci. 2004, 1032, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Durbak, L.; Chan, J.; Smithies, O.; Gustafsson, J.-Å.; Korach, K.S.; Pfaff, D.W.; Ogawa, S. Genotype/Age Interactions on Aggressive Behavior in Gonadally Intact Estrogen Receptor β Knockout (ΒERKO) Male Mice. Horm. Behav. 2002, 41, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Pape, H.-C.; Pare, D. Plastic Synaptic Networks of the Amygdala for the Acquisition, Expression, and Extinction of Conditioned Fear. Physiol. Rev. 2010, 90, 419–463. [Google Scholar] [CrossRef]
- Swanson, L.W.; Petrovich, G.D. What Is the Amygdala? Trends Neurosci. 1998, 21, 323–331. [Google Scholar] [CrossRef]
- Pabba, M. Evolutionary Development of the Amygdaloid Complex. Front. Neuroanat. 2013, 7, 27. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From Circuits to Behaviour in the Amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef]
- Martinez-Garcia, F.; Martinez-Marcos, A.; Lanuza, E. The Pallial Amygdala of Amniote Vertebrates: Evolution of the Concept, Evolution of the Structure. Brain Res. Bull. 2002, 57, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Równiak, M.; Szteyn, S.; Robak, A. A Comparative Study of the Mammalian Amygdala: A Golgi Study of the Basolateral Amygdala. Folia Morphol. 2003, 62, 331–339. [Google Scholar]
- Puelles, L.; Kuwana, E.; Puelles, E.; Bulfone, A.; Shimamura, K.; Keleher, J.; Smiga, S.; Rubenstein, J.L. Pallial and Subpallial Derivatives in the Embryonic Chick and Mouse Telencephalon, Traced by the Expression of the Genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J. Comp. Neurol. 2000, 424, 409–438. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L. Comments on the Updated Tetrapartite Pallium Model in the Mouse and Chick, Featuring a Homologous Claustro-Insular Complex. Brain. Behav. Evol. 2017, 90, 171–189. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Glutamate and Aspartate Immunoreactive Neurons of the Rat Basolateral Amygdala: Colocalization of Excitatory Amino Acids and Projections to the Limbic Circuit. J. Comp. Neurol. 1996, 365, 367–379. [Google Scholar] [CrossRef]
- Mascagni, F.; Muly, E.C.; Rainnie, D.G.; McDonald, A.J. Immunohistochemical Characterization of Parvalbumin-Containing Interneurons in the Monkey Basolateral Amygdala. Neuroscience 2009, 158, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J.; Mascagni, F. Colocalization of Calcium-Binding Proteins and GABA in Neurons of the Rat Basolateral Amygdala. Neuroscience 2001, 105, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.F.; Mascagni, F.; McDonald, A.J. Coupled Networks of Parvalbumin-Immunoreactive Interneurons in the Rat Basolateral Amygdala. J. Neurosci. 2005, 25, 7366–7376. [Google Scholar] [CrossRef]
- Kemppainen, S.; Pitkänen, A. Distribution of Parvalbumin, Calretinin, and Calbindin-D28k Immunoreactivity in the Rat Amygdaloid Complex and Colocalization with γ-Aminobutyric Acid. J. Comp. Neurol. 2000, 426, 441–467. [Google Scholar] [CrossRef]
- McDonald, A.J.; Pearson, J.C. Coexistence of GABA and Peptide Immunoreactivity in Non-Pyramidal Neurons of the Basolateral Amygdala. Neurosci. Lett. 1989, 100, 53–58. [Google Scholar] [CrossRef]
- Spampanato, J.; Polepalli, J.; Sah, P. Interneurons in the Basolateral Amygdala. Neuropharmacology 2011, 60, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Kim, D.-W.; Anderson, D.J. Antagonistic Control of Social versus Repetitive Self-Grooming Behaviors by Separable Amygdala Neuronal Subsets. Cell 2014, 158, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Batten, T.F.; Henderson, Z. A GABAergic Projection from the Central Nucleus of the Amygdala to the Nucleus of the Solitary Tract: A Combined Anterograde Tracing and Electron Microscopic Immunohistochemical Study. Neuroscience 2000, 99, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-O.; Funderburk, S.C.; Bhatti, D.L.; Motard, L.E.; Newbold, D.; Girven, K.S.; McCall, J.G.; Krashes, M.; Sparta, D.R.; Bruchas, M.R. A GABAergic Projection from the Centromedial Nuclei of the Amygdala to Ventromedial Prefrontal Cortex Modulates Reward Behavior. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 10831–10842. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Immunohistochemical Identification of Gamma-Aminobutyric Acid-Containing Neurons in the Rat Basolateral Amygdala. Neurosci. Lett. 1985, 53, 203–207. [Google Scholar] [CrossRef]
- Pitkänen, A.; Amaral, D.G. The Distribution of GABAergic Cells, Fibers, and Terminals in the Monkey Amygdaloid Complex: An Immunohistochemical and in Situ Hybridization Study. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 2200–2224. [Google Scholar] [CrossRef] [PubMed]
- Równiak, M.; Bogus-Nowakowska, K.; Robak, A. The Densities of Calbindin and Parvalbumin, but Not Calretinin Neurons, Are Sexually Dimorphic in the Amygdala of the Guinea Pig. Brain Res. 2015, 1604, 84–97. [Google Scholar] [CrossRef]
- Prager, E.M.; Bergstrom, H.C.; Wynn, G.H.; Braga, M.F.M. The Basolateral Amygdala GABAergic System in Health and Disease. J. Neurosci. Res. 2016, 94, 548–567. [Google Scholar] [CrossRef]
- Leranth, C.; Kiss, J. A Population of Supramammillary Area Calretinin Neurons Terminating on Medial Septal Area Cholinergic and Lateral Septal Area Calbindin-Containing Cells Are Aspartate/Glutamatergic. J. Neurosci. 1996, 16, 7699–7710. [Google Scholar] [CrossRef]
- Krzywkowski, P.; Jacobowitz, D.M.; Lamour, Y. Calretinin-Containing Pathways in the Rat Forebrain. Brain Res. 1995, 705, 273–294. [Google Scholar] [CrossRef]
- McDonald, A.J.; Mascagni, F.; Zaric, V. Subpopulations of Somatostatin-Immunoreactive Non-Pyramidal Neurons in the Amygdala and Adjacent External Capsule Project to the Basal Forebrain: Evidence for the Existence of GABAergic Projection Neurons in the Cortical Nuclei and Basolateral Nuclear Complex. Front. Neural Circuits 2012, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Jinno, S.; Kosaka, T. Parvalbumin Is Expressed in Glutamatergic and GABAergic Corticostriatal Pathway in Mice. J. Comp. Neurol. 2004, 477, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Wouterlood, F.G.; Canto, C.B.; Aliane, V.; Boekel, A.J.; Grosche, J.; Härtig, W.; Beliën, J.A.M.; Witter, M.P. Coexpression of Vesicular Glutamate Transporters 1 and 2, Glutamic Acid Decarboxylase and Calretinin in Rat Entorhinal Cortex. Brain Struct. Funct. 2007, 212, 303–319. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Projection Neurons of the Basolateral Amygdala: A Correlative Golgi and Retrograde Tract Tracing Study. Brain Res. Bull. 1992, 28, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Washburn, M.S.; Moises, H.C. Muscarinic Responses of Rat Basolateral Amygdaloid Neurons Recorded in Vitro. J. Physiol. 1992, 449, 121–154. [Google Scholar] [CrossRef] [PubMed]
- Rainnie, D.G.; Asprodini, E.K.; Shinnick-Gallagher, P. Intracellular Recordings from Morphologically Identified Neurons of the Basolateral Amygdala. J. Neurophysiol. 1993, 69, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Gritti, I.; Manns, I.D.; Mainville, L.; Jones, B.E. Parvalbumin, Calbindin, or Calretinin in Cortically Projecting and GABAergic, Cholinergic, or Glutamatergic Basal Forebrain Neurons of the Rat. J. Comp. Neurol. 2003, 458, 11–31. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Reimer, R.J.; Bellocchio, E.E.; Danbolt, N.C.; Osen, K.K.; Edwards, R.H.; Storm-Mathisen, J. The Vesicular GABA Transporter, VGAT, Localizes to Synaptic Vesicles in Sets of Glycinergic as Well as GABAergic Neurons. J. Neurosci. 1998, 18, 9733–9750. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Wang, H.-L.; Li, X.; Ng, T.H.; Morales, M. Mesocorticolimbic Glutamatergic Pathway. J. Neurosci. 2011, 31, 8476–8490. [Google Scholar] [CrossRef]
- Herzog, E.; Bellenchi, G.C.; Gras, C.; Bernard, V.; Ravassard, P.; Bedet, C.; Gasnier, B.; Giros, B.; Mestikawy, S.E. The Existence of a Second Vesicular Glutamate Transporter Specifies Subpopulations of Glutamatergic Neurons. J. Neurosci. 2001, 21, RC181. [Google Scholar] [CrossRef]
- Ziegler, D.R.; Cullinan, W.E.; Herman, J.P. Distribution of Vesicular Glutamate Transporter MRNA in Rat Hypothalamus. J. Comp. Neurol. 2002, 448, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Kawano, M.; Hayashi, S.; Kawano, H.; Hisano, S. Expression and Immunohistochemical Localization of Vesicular Glutamate Transporter 2 in the Migratory Pathway from the Rat Olfactory Placode. Eur. J. Neurosci. 2004, 20, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Henny, P.; Brischoux, F.; Mainville, L.; Stroh, T.; Jones, B.E. Immunohistochemical Evidence for Synaptic Release of Glutamate from Orexin Terminals in the Locus Coeruleus. Neuroscience 2010, 169, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Abbott, G.; Mair, J.; Prescott, M.; Campbell, R.E. Mapping GABA and Glutamate Inputs to Gonadotrophin-Releasing Hormone Neurones in Male and Female Mice. J. Neuroendocrinol. 2018, 30, e12657. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Ozawa, H.; Yamaguchi, S.; Hamaguchi, S.; Ueda, S. Calbindin-Positive Neurons Co-Express Functional Markers in a Location-Dependent Manner Within the A11 Region of the Rat Brain. Neurochem. Res. 2021, 46, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; McKinney, K.; Liu, L.; Lakhlani, S.; Jennes, L. Distribution of Vesicular Glutamate Transporter-2 Messenger Ribonucleic Acid and Protein in the Septum-Hypothalamus of the Rat. Endocrinology 2003, 144, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Pompolo, S.; Pereira, A.; Scott, C.J.; Fujiyma, F.; Clarke, I.J. Evidence for Estrogenic Regulation of Gonadotropin-Releasing Hormone Neurons by Glutamatergic Neurons in the Ewe Brain: An Immunohistochemical Study Using an Antibody against Vesicular Glutamate Transporter-2. J. Comp. Neurol. 2003, 465, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.; Csaba, Z.; Csáki, A.; Halász, B. Demonstration of Estrogen Receptor α Protein in Glutamatergic (Vesicular Glutamate Transporter 2 Immunoreactive) Neurons of the Female Rat Hypothalamus and Amygdala Using Double-Label Immunocytochemistry. Exp. Brain Res. 2013, 226, 595–602. [Google Scholar] [CrossRef]
- Filippi, A.; Mueller, T.; Driever, W. Vglut2 and Gad Expression Reveal Distinct Patterns of Dual GABAergic versus Glutamatergic Cotransmitter Phenotypes of Dopaminergic and Noradrenergic Neurons in the Zebrafish Brain. J. Comp. Neurol. 2014, 522, 2019–2037. [Google Scholar] [CrossRef]
- Bogus-Nowakowska, K.; Robak, A.; Kalinowski, D.; Kozłowska, A.; Równiak, M. GABAergic and Glutamatergic Phenotypes of Neurons Expressing Calcium-Binding Proteins in the Preoptic Area of the Guinea Pig. Int. J. Mol. Sci. 2022, 23, 7963. [Google Scholar] [CrossRef]
- Równiak, M. The Amygdala in the Guinea Pig Is Sexually Dimorphic—A Morphometric Study. Brain Res. 2013, 1524, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Kemppainen, S. Comparison of the Distribution of Calcium-Binding Proteins and Intrinsic Connectivity in the Lateral Nucleus of the Rat, Monkey, and Human Amygdala. Pharmacol. Biochem. Behav. 2002, 71, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Sorvari, H.; Miettinen, R.; Soininen, H.; Pitkanen, A. Parvalbumin-Immunoreactive Neurons Make Inhibitory Synapses on Pyramidal Cells in the Human Amygdala: A Light and Electron Microscopic Study. Neurosci. Lett. 1996, 217, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Sorvari, H.; Soininen, H.; Pitkänen, A. Calretinin-Immunoreactive Cells and Fibers in the Human Amygdaloid Complex. J. Comp. Neurol. 1996, 369, 188–208. [Google Scholar] [CrossRef]
- Sorvari, H.; Soininen, H.; Pitkänen, A. Calbindin-D28k-Immunoreactive Cells and Fibres in the Human Amygdaloid Complex. Neuroscience 1996, 75, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, A.R.; Sah, P. Networks of Parvalbumin-Positive Interneurons in the Basolateral Amygdala. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 553–563. [Google Scholar] [CrossRef]
- Pitkänen, A.; Amaral, D.G. Distribution of Calbindin-D 28k Immunoreactivity in the Monkey Temporal Lobe: The Amygdaloid ComplexWe. J. Comp. Neurol. 1993, 331, 199–224. [Google Scholar] [CrossRef]
- Sorvari, H.; Soininen, H.; Paljärvi, L.; Karkola, K.; Pitkänen, A. Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. J. Comp. Neurol. 1995, 360, 185–212. [Google Scholar] [CrossRef]
- Tanahira, C.; Higo, S.; Watanabe, K.; Tomioka, R.; Ebihara, S.; Kaneko, T.; Tamamaki, N. Parvalbumin Neurons in the Forebrain as Revealed by Parvalbumin-Cre Transgenic Mice. Neurosci. Res. 2009, 63, 213–223. [Google Scholar] [CrossRef]
- McDonald, A.J.; Mascagni, F. Immunohistochemical Characterization of Somatostatin Containing Interneurons in the Rat Basolateral Amygdala. Brain Res. 2002, 943, 237–244. [Google Scholar] [CrossRef]
- Muller, J.F.; Mascagni, F.; McDonald, A.J. Serotonin-Immunoreactive Axon Terminals Innervate Pyramidal Cells and Interneurons in the Rat Basolateral Amygdala. J. Comp. Neurol. 2007, 505, 314–335. [Google Scholar] [CrossRef] [PubMed]
- Cobb, S.R.; Buhl, E.H.; Halasy, K.; Paulsen, O.; Somogyi, P. Synchronization of Neuronal Activity in Hippocampus by Individual GABAergic Interneurons. Nature 1995, 378, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Miles, R.; Tóth, K.; Gulyás, A.I.; Hájos, N.; Freund, T.F. Differences between Somatic and Dendritic Inhibition in the Hippocampus. Neuron 1996, 16, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Moryś, J.; Berdel, B.; Kowiański, P.; Majak, K.; Tarnawski, M.; Wisniewski, H.M. Relationship of Calcium-Binding Protein Containing Neurons and Projection Neurons in the Rat Basolateral Amygdala. Neurosci. Lett. 1999, 259, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, A.I.; Hájos, N.; Freund, T.F. Interneurons Containing Calretinin Are Specialized to Control Other Interneurons in the Rat Hippocampus. J. Neurosci. 1996, 16, 3397–3411. [Google Scholar] [CrossRef] [PubMed]
- Lübkemann, R.; Eberhardt, J.; Röhl, F.-W.; Janitzky, K.; Nullmeier, S.; Stork, O.; Schwegler, H.; Linke, R. Identification and Characterization of GABAergic Projection Neurons from Ventral Hippocampus to Amygdala. Brain Sci. 2015, 5, 299–317. [Google Scholar] [CrossRef]
- Mascagni, F.; McDonald, A.J. Immunohistochemical Characterization of Cholecystokinin Containing Neurons in the Rat Basolateral Amygdala. Brain Res. 2003, 976, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Równiak, M. The Neurons Expressing Calcium-Binding Proteins in the Amygdala of the Guinea Pig: Precisely Designed Interface for Sex Hormones. Brain Struct. Funct. 2017, 222, 3775–3793. [Google Scholar] [CrossRef]
- Meskenaite, V. Calretinin-Immunoreactive Local Circuit Neurons in Area 17 of the Cynomolgus Monkey, Macaca fascicularis. J. Comp. Neurol. 1997, 379, 113–132. [Google Scholar] [CrossRef]
- Melchitzky, D.S.; Lewis, D.A. Dendritic-Targeting GABA Neurons in Monkey Prefrontal Cortex: Comparison of Somatostatin- and Calretinin-Immunoreactive Axon Terminals. Synapse 2008, 62, 456–465. [Google Scholar] [CrossRef]
- Gonchar, Y.; Burkhalter, A. Connectivity of GABAergic Calretinin-Immunoreactive Neurons in Rat Primary Visual Cortex. Cereb. Cortex 1999, 9, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, R.; Gulyás, A.I.; Baimbridge, K.G.; Jacobowitz, D.M.; Freund, T.F. Calretinin Is Present in Non-Pyramidal Cells of the Rat Hippocampus—II. Co-Existence with Other Calcium Binding Proteins and GABA. Neuroscience 1992, 48, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Wouterlood, F.G.; van Denderen, J.C.; van Haeften, T.; Witter, M.P. Calretinin in the Entorhinal Cortex of the Rat: Distribution, Morphology, Ultrastructure of Neurons, and Co-Localization with Gamma-Aminobutyric Acid and Parvalbumin. J. Comp. Neurol. 2000, 425, 177–192. [Google Scholar] [CrossRef]
- Gulyás, A.I.; Hájos, N.; Katona, I.; Freund, T.F. Interneurons Are the Local Targets of Hippocampal Inhibitory Cells Which Project to the Medial Septum. Eur. J. Neurosci. 2003, 17, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Waclaw, R.R.; Ehrman, L.A.; Pierani, A.; Campbell, K. Developmental Origin of the Neuronal Subtypes That Comprise the Amygdalar Fear Circuit in the Mouse. J. Neurosci. 2010, 30, 6944–6953. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, S.A.; Sidhu, H.; Patel, R.R.; Kreifeldt, M.; D’Ambrosio, S.R.; Contet, C.; Roberto, M. Molecular, Morphological, and Functional Characterization of Corticotropin-Releasing Factor Receptor 1-Expressing Neurons in the Central Nucleus of the Amygdala. eNeuro 2019, 6, e0087. [Google Scholar] [CrossRef] [PubMed]
- Fremeau, R.T.; Troyer, M.D.; Pahner, I.; Nygaard, G.O.; Tran, C.H.; Reimer, R.J.; Bellocchio, E.E.; Fortin, D.; Storm-Mathisen, J.; Edwards, R.H. The Expression of Vesicular Glutamate Transporters Defines Two Classes of Excitatory Synapse. Neuron 2001, 31, 247–260. [Google Scholar] [CrossRef]
- Vigneault, É.; Poirel, O.; Riad, M.; Prud’homme, J.; Dumas, S.; Turecki, G.; Fasano, C.; Mechawar, N.; El Mestikawy, S. Distribution of Vesicular Glutamate Transporters in the Human Brain. Front. Neuroanat. 2015, 9, 23. [Google Scholar] [CrossRef]
- Somogyi, J.; Baude, A.; Omori, Y.; Shimizu, H.; El Mestikawy, S.; Fukaya, M.; Shigemoto, R.; Watanabe, M.; Somogyi, P. GABAergic Basket Cells Expressing Cholecystokinin Contain Vesicular Glutamate Transporter Type 3 (VGLUT3) in Their Synaptic Terminals in Hippocampus and Isocortex of the Rat. Eur. J. Neurosci. 2004, 19, 552–569. [Google Scholar] [CrossRef]
- Blanco-Centurion, C.; Bendell, E.; Zou, B.; Sun, Y.; Shiromani, P.J.; Liu, M. VGAT and VGLUT2 Expression in MCH and Orexin Neurons in Double Transgenic Reporter Mice. IBRO Rep. 2018, 4, 44–49. [Google Scholar] [CrossRef]
- Root, D.H.; Zhang, S.; Barker, D.J.; Miranda-Barrientos, J.; Liu, B.; Wang, H.-L.; Morales, M. Selective Brain Distribution and Distinctive Synaptic Architecture of Dual Glutamatergic-GABAergic Neurons. Cell Rep. 2018, 23, 3465–3479. [Google Scholar] [CrossRef] [PubMed]
- White, L.E.; Hodges, H.D.; Carnes, K.M.; Price, J.L.; Dubinsky, J.M. Colocalization of Excitatory and Inhibitory Neurotransmitter Markers in Striatal Projection Neurons in the Rat. J. Comp. Neurol. 1994, 339, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Boulland, J.-L.; Qureshi, T.; Seal, R.P.; Rafiki, A.; Gundersen, V.; Bergersen, L.H.; Fremeau, R.T.; Edwards, R.H.; Storm-Mathisen, J.; Chaudhry, F.A. Expression of the Vesicular Glutamate Transporters during Development Indicates the Widespread Corelease of Multiple Neurotransmitters. J. Comp. Neurol. 2004, 480, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Pelkey, K.A.; Calvigioni, D.; Fang, C.; Vargish, G.; Ekins, T.; Auville, K.; Wester, J.C.; Lai, M.; Mackenzie-Gray Scott, C.; Yuan, X.; et al. Paradoxical Network Excitation by Glutamate Release from VGluT3+ GABAergic Interneurons. eLife 2020, 9, e51996. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, X.; Xie, L.; Huang, L.; Ke, Z.; Zheng, J.; Lu, H.; Hu, J. Glucocorticoids/Glucocorticoid Receptors Effect on Dopaminergic Neurotransmitters in ADHD Rats. Brain Res. Bull. 2017, 131, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, A.; Hochgerner, H.; Lönnerberg, P.; Johnsson, A.; Memic, F.; van der Zwan, J.; Häring, M.; Braun, E.; Borm, L.E.; La Manno, G.; et al. Molecular Architecture of the Mouse Nervous System. Cell 2018, 174, 999–1014.e22. [Google Scholar] [CrossRef] [PubMed]
- Mullen, R.J.; Buck, C.R.; Smith, A.M. NeuN, a Neuronal Specific Nuclear Protein in Vertebrates. Development 1992, 116, 201–211. [Google Scholar] [CrossRef]
- Airaksinen, M.S.; Eilers, J.; Garaschuk, O.; Thoenen, H.; Konnerth, A.; Meyer, M. Ataxia and Altered Dendritic Calcium Signaling in Mice Carrying a Targeted Null Mutation of the Calbindin D28k Gene. Proc. Natl. Acad. Sci. USA 1997, 94, 1488–1493. [Google Scholar] [CrossRef]
- McIntire, S.L.; Reimer, R.J.; Schuske, K.; Edwards, R.H.; Jorgensen, E.M. Identification and Characterization of the Vesicular GABA Transporter. Nature 1997, 389, 870–876. [Google Scholar] [CrossRef]
- Zimmermann, L.; Schwaller, B. Monoclonal Antibodies Recognizing Epitopes of Calretinins: Dependence on Ca2+-Binding Status and Differences in Antigen Accessibilityin Colon Cancer Cells. Cell Calcium 2002, 31, 13–25. [Google Scholar] [CrossRef]
- Drexel, M.; Preidt, A.P.; Kirchmair, E.; Sperk, G. Parvalbumin Interneurons and Calretinin Fibers Arising from the Thalamic Nucleus Reuniens Degenerate in the Subiculum after Kainic Acid-Induced Seizures. Neuroscience 2011, 189, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Mészár, Z.; Girard, F.; Saper, C.B.; Celio, M.R. The Lateral Hypothalamic Parvalbumin-Immunoreactive (PV1) Nucleus in Rodents. J. Comp. Neurol. 2012, 520, 798–815. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.V.; Wan, Y.; Wang, X.; Cohen, S.; Wetsel, W.C.; Greenberg, M.E.; Kenny, P.J.; Calakos, N.; West, A.E. MeCP2 Phosphorylation Limits Psychostimulant-Induced Behavioral and Neuronal Plasticity. J. Neurosci. 2014, 34, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Stansfield, K.H.; Ruby, K.N.; Soares, B.D.; McGlothan, J.L.; Liu, X.; Guilarte, T.R. Early-Life Lead Exposure Recapitulates the Selective Loss of Parvalbumin-Positive GABAergic Interneurons and Subcortical Dopamine System Hyperactivity Present in Schizophrenia. Transl. Psychiatry 2015, 5, e522. [Google Scholar] [CrossRef] [PubMed]
- Rojek, K.O.; Krzemień, J.; Doleżyczek, H.; Boguszewski, P.M.; Kaczmarek, L.; Konopka, W.; Rylski, M.; Jaworski, J.; Holmgren, L.; Prószyński, T.J. Amot and Yap1 Regulate Neuronal Dendritic Tree Complexity and Locomotor Coordination in Mice. PLoS Biol. 2019, 17, e3000253. [Google Scholar] [CrossRef] [PubMed]
- Celio, M.R.; Baier, W.; Schärer, L.; de Viragh, P.A.; Gerday, C. Monoclonal Antibodies Directed against the Calcium Binding Protein Parvalbumin. Cell Calcium 1988, 9, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Schwaller, B.; Brückner, G.; Celio, M.R.; Härtig, W. A Polyclonal Goat Antiserum against the Calcium-Binding Protein Calretinin Is a Versatile Tool for Various Immunochemical Techniques. J. Neurosci. Methods 1999, 92, 137–144. [Google Scholar] [CrossRef]
- Härtig, W.; Riedel, A.; Grosche, J.; Edwards, R.H.; Fremeau, R.T.; Harkany, T.; Brauer, K.; Arendt, T. Complementary Distribution of Vesicular Glutamate Transporters 1 and 2 in the Nucleus Accumbens of Rat: Relationship to Calretinin-Containing Extrinsic Innervation and Calbindin-Immunoreactive Neurons. J. Comp. Neurol. 2003, 465, 1–10. [Google Scholar] [CrossRef]
- Hassani, O.K.; Henny, P.; Lee, M.G.; Jones, B.E. GABAergic Neurons Intermingled with Orexin and MCH Neurons in the Lateral Hypothalamus Discharge Maximally during Sleep. Eur. J. Neurosci. 2010, 32, 448–457. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiao, Y.-Y.; Sun, Q.-Q. Developmental Maturation of Excitation and Inhibition Balance in Principal Neurons across Four Layers of Somatosensory Cortex. Neuroscience 2011, 174, 10–25. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Cesca, F.; Schiavo, G.; Benfenati, F.; Baldelli, P. Kidins220/ARMS Is a Novel Modulator of Short-Term Synaptic Plasticity in Hippocampal GABAergic Neurons. PLoS ONE 2012, 7, e35785. [Google Scholar] [CrossRef] [PubMed]
- Michalski, D.; Härtig, W.; Krügel, K.; Edwards, R.H.; Böddener, M.; Böhme, L.; Pannicke, T.; Reichenbach, A.; Grosche, A. Region-Specific Expression of Vesicular Glutamate and GABA Transporters under Various Ischaemic Conditions in Mouse Forebrain and Retina. Neuroscience 2013, 231, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, Q.-Q. Characterization of Axo-Axonic Synapses in the Piriform Cortex of Mus Musculus. J. Comp. Neurol. 2012, 520, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Aracri, P.; Amadeo, A.; Pasini, M.E.; Fascio, U.; Becchetti, A. Regulation of Glutamate Release by Heteromeric Nicotinic Receptors in Layer V of the Secondary Motor Region (Fr2) in the Dorsomedial Shoulder of Prefrontal Cortex in Mouse. Synapse 2013, 67, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Sathyanesan, A.; Ogura, T.; Lin, W. Automated Measurement of Nerve Fiber Density Using Line Intensity Scan Analysis. J. Neurosci. Methods 2012, 206, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nakajima, S.; Shiraga, N.; Atsumi, H.; Yoshida, S.; Koller, T.; Gerig, G.; Kikinis, R. Three-Dimensional Multi-Scale Line Filter for Segmentation and Visualization of Curvilinear Structures in Medical Images. Med. Image Anal. 1998, 2, 143–168. [Google Scholar] [CrossRef]
- Meijering, E. FeatureJ: An ImageJ Plugin Suite for Image Feature Extraction. Available online: https://imagescience.org/meijering/software/featurej/ (accessed on 27 May 2022).
| Antigen | Code | Clonality | Host Species | Dilution | Supplier | Location |
|---|---|---|---|---|---|---|
| Primary antibodies | ||||||
| NEUN | MAB377 | monoclonal | Mouse | 1:1000 | Millipore | Temecula, CA/USA |
| CB | 300 | monoclonal | Mouse | 1:4000 | SWANT | Bellinzona/Switzerland |
| PV | P3088 | monoclonal | Mouse | 1:6000 | Sigma Aldrich | St. Louis, MO/USA |
| CR | 6B3 | monoclonal | Mouse | 1:2000 | SWANT | Bellinzona/Switzerland |
| VGAT | AB5062P | polyclonal | Rabbit | 1:6000 | Millipore | Temecula, CA/USA |
| VGLUT2 | 135 402 | polyclonal | Rabbit | 1:2000 | Synaptic Systems | Goettingen/Germany |
| Secondary antibodies | ||||||
| ALEXA Fluor 488 nm | A-21202 | polyclonal | Anti-Mouse | 1:1000 | ThermoFisher | Rockford, IL/USA |
| ALEXA Fluor 555 nm | A-31572 | polyclonal | Anti-Rabbit | 1:1000 | ThermoFisher | Rockford, IL/USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinowski, D.; Bogus-Nowakowska, K.; Kozłowska, A.; Równiak, M. The Co-Expression Pattern of Calcium-Binding Proteins with γ-Aminobutyric Acid and Glutamate Transporters in the Amygdala of the Guinea Pig: Evidence for Glutamatergic Subpopulations. Int. J. Mol. Sci. 2023, 24, 15025. https://doi.org/10.3390/ijms241915025
Kalinowski D, Bogus-Nowakowska K, Kozłowska A, Równiak M. The Co-Expression Pattern of Calcium-Binding Proteins with γ-Aminobutyric Acid and Glutamate Transporters in the Amygdala of the Guinea Pig: Evidence for Glutamatergic Subpopulations. International Journal of Molecular Sciences. 2023; 24(19):15025. https://doi.org/10.3390/ijms241915025
Chicago/Turabian StyleKalinowski, Daniel, Krystyna Bogus-Nowakowska, Anna Kozłowska, and Maciej Równiak. 2023. "The Co-Expression Pattern of Calcium-Binding Proteins with γ-Aminobutyric Acid and Glutamate Transporters in the Amygdala of the Guinea Pig: Evidence for Glutamatergic Subpopulations" International Journal of Molecular Sciences 24, no. 19: 15025. https://doi.org/10.3390/ijms241915025





