The Effect of Mesenchymal Stem Cells on Dry Eye in Sjogren Syndrome Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Clinical DE Parameters and Goblet Cell Counts Are Improved in hMSCs-Treated Mice
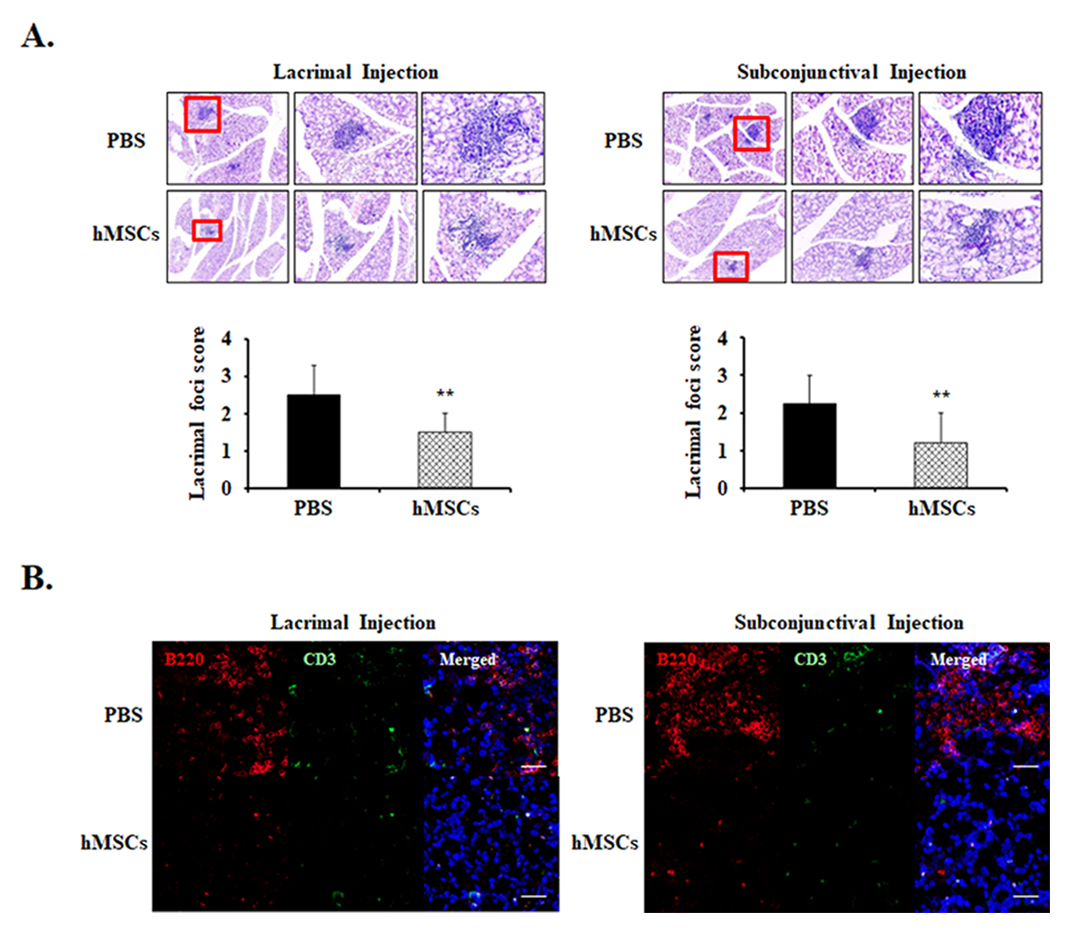
2.2. Lacrimal Gland Inflammatory Foci Decreased in hMSCs-Treated Mice
2.3. hMSCs-Treated Mice Demonstrated Autophagy Marker Attenuations in the Lacrimal Glands
2.4. Expression of Proinflammatory Cytokines Decreased in hMSCs-Treated Mice
3. Discussion
4. Methods and Materials
4.1. Mouse Model of SS
4.2. hMSCs Treatment
4.3. Phenol Red Thread Test
4.4. Corneal Surface Staining
4.5. Periodic Acid Schiff (PAS) Staining for Conjunctival Goblet Cell Histology
4.6. Histologic Analysis of Lacrimal Glands
4.7. RNA Isolation and Real-Time PCR
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, R.I. Sjögren’s syndrome. Lancet 2005, 366, 321–331. [Google Scholar] [CrossRef]
- Mielle, J.; Tison, A.; Cornec, D.; Le Pottier, L.; Daien, C.; Pers, J.O. B cells in Sjögren’s syndrome: From pathophysiology to therapeutic target. Rheumatology (Oxford) 2021, 60, 2545–2560. [Google Scholar] [CrossRef] [PubMed]
- Maehara, T.; Moriyama, M.; Hayashida, J.N.; Tanaka, A.; Shinozaki, S.; Kubo, Y.; Matsumura, K.; Nakamura, S. Selective localization of T helper subsets in labial salivary glands from primary Sjögren’s syndrome patients. Clin. Exp. Immunol. 2012, 169, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflugfelder, S.C.; Huang, A.J.; Feuer, W.; Chuchovski, P.T.; Pereira, I.C.; Tseng, S.C. Conjunctival cytologic features of primary Sjögren’s syndrome. Ophthalmology 1990, 97, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Chihaby, N.; Orliaguet, M.; Le Pottier, L.; Pers, J.O.; Boisramé, S. Treatment of Sjögren’s Syndrome with Mesenchymal Stem Cells: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10474. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal 2011, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Cagliani, J.; Grande, D.; Molmenti, E.P.; Miller, E.J.; Rilo, H.L.R. Immunomodulation by Mesenchymal Stromal Cells and Their Clinical Applications. J. Stem Cell Regen. Biol. 2017, 3, 126–139. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.; Song, S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef]
- Maria, A.T.; Maumus, M.; Le Quellec, A.; Jorgensen, C.; Noël, D.; Guilpain, P. Adipose-Derived Mesenchymal Stem Cells in Autoimmune Disorders: State of the Art and Perspectives for Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2017, 52, 234–259. [Google Scholar] [CrossRef]
- Nasri, F.; Mohtasebi, M.S.; Hashemi, E.; Zarrabi, M.; Gholijani, N.; Sarvestani, E.K. Therapeutic Efficacy of Mesenchymal Stem Cells and Mesenchymal Stem Cells-derived Neural Progenitors in Experimental Autoimmune Encephalomyelitis. Int. J. Stem Cells 2018, 11, 68–77. [Google Scholar] [CrossRef]
- Genc, B.; Bozan, H.R.; Genc, S.; Genc, K. Stem Cell Therapy for Multiple Sclerosis. Tissue Eng. Regen. Med. 2018, 1084, 145–174. [Google Scholar]
- Lu, X.; Wang, X.; Nian, H.; Yang, D.; Wei, R. Mesenchymal stem cells for treating autoimmune dacryoadenitis. Stem Cell Res. Ther. 2017, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aluri, H.S.; Samizadeh, M.; Edman, M.C.; Hawley, D.R.; Armaos, H.L.; Janga, S.R.; Meng, Z.; Sendra, V.G.; Hamrah, P.; Kublin, C.L.; et al. Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Tear Production in a Mouse Model of Sjögren’s Syndrome. Stem Cells Int. 2017, 2017, 3134543. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, D.; Liu, D.; Fan, Z.; Zhang, H.; Liu, O.; Ding, G.; Gao, R.; Zhang, C.; Ding, Y.; et al. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood 2012, 120, 3142–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Ko, A.Y.; Ko, J.H.; Lee, H.J.; Kim, M.K.; Wee, W.R.; Khwarg, S.I.; Oh, J.Y. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 2015, 23, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Yang, C.; Shen, M.; Yang, M.; Jin, Z.; Ding, L.; Jiang, W.; Yang, J.; Chen, H.; Cao, F.; et al. Autophagy mediates the beneficial effect of hypoxic preconditioning on bone marrow mesenchymal stem cells for the therapy of myocardial infarction. Stem Cell Res. Ther. 2017, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Byun, Y.S.; Lee, H.J.; Shin, S.; Chung, S.H. Elevation of autophagy markers in Sjögren syndrome dry eye. Sci. Rep. 2017, 7, 17280. [Google Scholar] [CrossRef] [Green Version]
- Byun, Y.S.; Lee, H.J.; Shin, S.; Choi, M.Y.; Kim, H.S.; Chung, S.H. Tear ATG5 as a Potential Novel Biomarker in the Diagnosis of Sjögren Syndrome. Diagnostics 2021, 11, 71. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, S.; Yoon, S.G.; Cheon, E.J.; Chung, S.H. The Effect of Chloroquine on the Development of Dry Eye in Sjögren Syndrome Animal Model. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3708–3716. [Google Scholar] [CrossRef] [Green Version]
- Yao, G.; Qi, J.; Liang, J.; Shi, B.; Chen, W.; Li, W.; Tang, X.; Wang, D.; Lu, L.; Chen, W.; et al. Mesenchymal stem cell transplantation alleviates experimental Sjögren’s syndrome through IFN-β/IL-27 signaling axis. Theranostics 2019, 9, 8253–8265. [Google Scholar] [CrossRef]
- Beyazyıldız, E.; Pınarlı, F.A.; Beyazyıldız, O.; Hekimoğlu, E.R.; Acar, U.; Demir, M.N.; Albayrak, A.; Kaymaz, F.; Sobacı, G.; Delibaşı, T. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. 2014, 2014, 250230. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.Y.; Karunanithi, P.; Loo, W.C.; Naveen, S.; Chen, H.; Hussin, P.; Chan, L.; Kamarul, T. The effects of staged intra-articular injection of cultured autologous mesenchymal stromal cells on the repair of damaged cartilage: A pilot study in caprine model. Arthritis Res. Ther. 2013, 15, R129. [Google Scholar] [CrossRef] [Green Version]
- Tessier, L.; Bienzle, D.; Williams, L.B.; Koch, T.G. Phenotypic and immunomodulatory properties of equine cord blood-derived mesenchymal stromal cells. PLoS ONE 2015, 10, e0122954. [Google Scholar] [CrossRef]
- Gebler, A.; Zabel, O.; Seliger, B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol. Med. 2012, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Liu, Y.; Kornete, M.; Roescher, N.; Kodama, S.; Peterson, A.; Piccirillo, C.A.; Tran, S.D. Mesenchymal stromal cells improve salivary function and reduce lymphocytic infiltrates in mice with Sjögren’s-like disease. PLoS ONE 2012, 7, e38615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abughanam, G.; Elkashty, O.A.; Liu, Y.; Bakkar, M.O.; Tran, S.D. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren’s Syndrome-Like Disease. Int. J. Mol. Sci. 2019, 20, 4750. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.Q.; Cha, S.R.; Peck, A.B. Sjögren’s syndrome (SjS)-like disease of mice: The importance of B lymphocytes and autoantibodies. Front. Biosci. 2007, 12, 1767–1789. [Google Scholar] [CrossRef] [Green Version]
- Umazume, T.; Thomas, W.M.; Campbell, S.; Aluri, H.; Thotakura, S.; Zoukhri, D.; Makarenkova, H.P. Lacrimal Gland Inflammation Deregulates Extracellular Matrix Remodeling and Alters Molecular Signature of Epithelial Stem/Progenitor Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8392–8402. [Google Scholar] [CrossRef] [Green Version]
- Schenke-Layland, K.; Xie, J.; Angelis, E.; Starcher, B.; Wu, K.; Riemann, I.; MacLellan, W.R.; Hamm-Alvarez, S.F. Increased degradation of extracellular matrix structures of lacrimal glands implicated in the pathogenesis of Sjögren’s syndrome. Matrix Biol. 2008, 27, 53–66. [Google Scholar] [CrossRef] [Green Version]
- Schenke-Layland, K.; Xie, J.; Magnusson, M.; Angelis, E.; Li, X.; Wu, K.; Reinhardt, D.P.; Maclellan, W.R.; Hamm-Alvarez, S.F. Lymphocytic infiltration leads to degradation of lacrimal gland extracellular matrix structures in NOD mice exhibiting a Sjögren’s syndrome-like exocrinopathy. Exp. Eye Res. 2010, 90, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Katsiougiannis, S.; Tenta, R.; Skopouli, F.N. Endoplasmic reticulum stress causes autophagy and apoptosis leading to cellular redistribution of the autoantigens Ro/Sjögren’s syndrome-related antigen A (SSA) and La/SSB in salivary gland epithelial cells. Clin. Exp. Immunol. 2015, 181, 244–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan-Bathke, M.; Lin, H.H.; Chibly, A.M.; Zhang, W.; Sun, X.; Chen, C.H.; Flodby, P.; Borok, Z.; Wu, R.; Arnett, D.; et al. Deletion of ATG5 shows a role of autophagy in salivary homeostatic control. J. Dent. Res. 2013, 92, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakovljevic, J.; Harrell, C.R.; Fellabaum, C.; Arsenijevic, A.; Jovicic, N.; Volarevic, V. Modulation of autophagy as new approach in mesenchymal stem cell-based therapy. Biomed. Pharmacother. 2018, 104, 404–410. [Google Scholar] [CrossRef]
- Beeken, L.J.; Ting, D.S.J.; Sidney, L.E. Potential of mesenchymal stem cells as topical immunomodulatory cell therapies for ocular surface inflammatory disorders. Stem Cells Transl. Med. 2021, 10, 39–49. [Google Scholar] [CrossRef]
- Omoto, M.; Katikireddy, K.R.; Rezazadeh, A.; Dohlman, T.H.; Chauhan, S.K. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6631–6638. [Google Scholar] [CrossRef] [Green Version]
- Orozco Morales, M.L.; Marsit, N.M.; McIntosh, O.D.; Hopkinson, A.; Sidney, L.E. Anti-inflammatory potential of human corneal stroma-derived stem cells determined by a novel in vitro corneal epithelial injury model. World J. Stem Cells 2019, 11, 84–99. [Google Scholar] [CrossRef]
- Ruan, G.F.; Zheng, L.; Huang, J.S.; Huang, W.X.; Gong, B.D.; Fang, X.X.; Zhang, X.Y.; Tang, J.P. Effect of mesenchymal stem cells on Sjögren-like mice and the microRNA expression profiles of splenic CD4+ T cells. Exp. Ther. Med. 2017, 13, 2828–2838. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Li, Z.R.; Su, W.R.; Li, Y.P.; Lin, M.L.; Zhang, W.X.; Liu, Y.; Wan, Q.; Liang, D. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS ONE 2012, 7, e30842. [Google Scholar] [CrossRef] [Green Version]
- Xuan, J.; Shen, L.; Malyavantham, K.; Pankewycz, O.; Ambrus, J.L., Jr.; Suresh, L. Temporal histological changes in lacrimal and major salivary glands in mouse models of Sjogren’s syndrome. BMC Oral Health 2013, 13, 51. [Google Scholar] [CrossRef] [Green Version]
- Greenspan, J.S.; Daniels, T.E.; Talal, N.; Sylvester, R.A. The histopathology of Sjögren’s syndrome in labial salivary gland biopsies. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 217–229. [Google Scholar] [CrossRef] [PubMed]



| Gene | Forward Primer Sequence (5’-3’) | Reverse Primer Sequence (5’-3’) |
|---|---|---|
| GAPDH | GGT CCT CAG TGT AGC CCA AG | AAT GTG TCC GTC GTG GAT CT |
| IL-6 | AGT TGC CTT CTT GGG ACT GA | TTG GGA GTG GTA TCC TCT GTG |
| IL-1β | CAG GCA GGC AGT ATC ACT CA | AGG TGC TCA TGT CCT CAT CC |
| TNF-α | CAC CTG GCC TCT CTA CCT TG | TGG TCA CCA AAT CAG CGT TA |
| IFN-γ | GCT TTA ACA GCA GGC CAG AC | GGA AGC ACC AGG TGT CAA GT |
| IL-17A | TTC AGG GTC GAG AAG ATG CT | AAA CGT GGG GGT TTC TTA GG |
| MMP9 | AGG TGG ACC ATG AGG TGA AC | CGG TTG AAG CAA AGA AGG AG |
| HIF-1α | GTC GGA CAG CCT CAC CAA ACA G | TAG GTA GTG AGC CAC CAG TGT CC |
| mTOR | CTC AAG CGA TCC AGT TGT CA | AGA AGG TGG GGA CAC TGA TG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.; Yoon, S.-G.; Kim, M.; Cheon, E.J.; Jeon, Y.; Lee, H.J.; Chung, S.-H. The Effect of Mesenchymal Stem Cells on Dry Eye in Sjogren Syndrome Mouse Model. Int. J. Mol. Sci. 2023, 24, 1039. https://doi.org/10.3390/ijms24021039
Shin S, Yoon S-G, Kim M, Cheon EJ, Jeon Y, Lee HJ, Chung S-H. The Effect of Mesenchymal Stem Cells on Dry Eye in Sjogren Syndrome Mouse Model. International Journal of Molecular Sciences. 2023; 24(2):1039. https://doi.org/10.3390/ijms24021039
Chicago/Turabian StyleShin, Soojung, Seul-Gi Yoon, Miso Kim, Eun Jeong Cheon, Youngseo Jeon, Hyun Jung Lee, and So-Hyang Chung. 2023. "The Effect of Mesenchymal Stem Cells on Dry Eye in Sjogren Syndrome Mouse Model" International Journal of Molecular Sciences 24, no. 2: 1039. https://doi.org/10.3390/ijms24021039
APA StyleShin, S., Yoon, S. -G., Kim, M., Cheon, E. J., Jeon, Y., Lee, H. J., & Chung, S. -H. (2023). The Effect of Mesenchymal Stem Cells on Dry Eye in Sjogren Syndrome Mouse Model. International Journal of Molecular Sciences, 24(2), 1039. https://doi.org/10.3390/ijms24021039





