Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans
Abstract
:1. Introduction
2. Results
2.1. Minimum Inhibitory Concentration (MIC)
2.2. Synergistic Activity of Capsaicin with Fluconazole
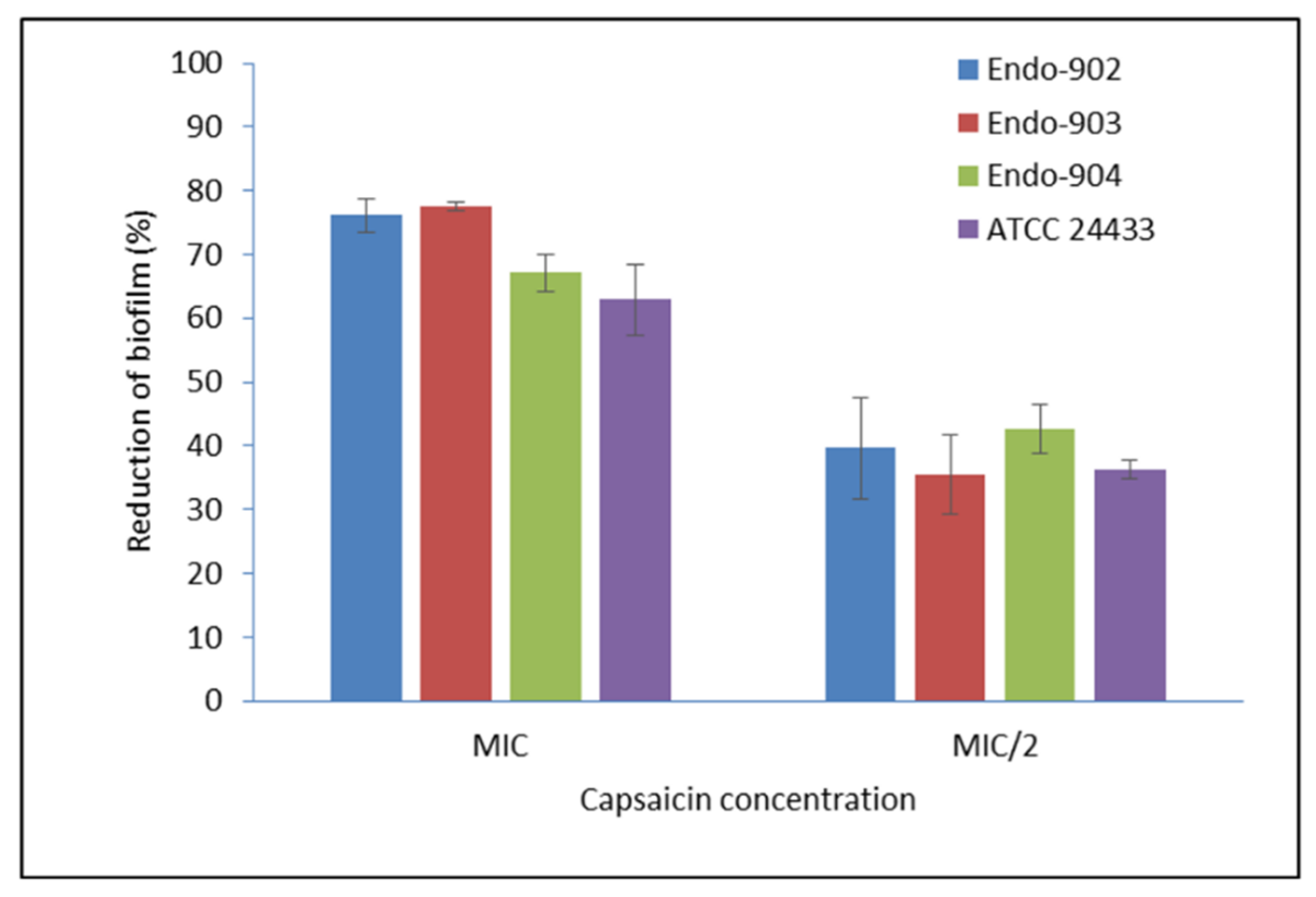
2.3. Biofilm Inhibition
2.4. Inhibitory Activity on Hyphae Formation
2.5. Inhibition Activity of Ergosterol Synthesis
2.6. Cell Membrane Permeability
2.7. Change of Cellular Morphology
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Sample Collection
4.3. Minimum Inhibitory Concentration
4.4. Synergistic Activity of Capsaicin and Fluconazole
4.5. Biofilm Inhibition Assay
4.6. Study of Hyphae Formation and Cells Morphology
4.7. Ergosterol Extraction and Estimation
4.8. Confocal Scanning Laser Microscopy (CSLM)
4.9. Effect of Capsaicin on the Biofilm of Dentine Substrate
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aminzadeh, A.; Sabeti Sanat, A.; Nik Akhtar, S. Frequency of Candidiasis and Colonization of Candida albicans in Relation to Oral Contraceptive Pills. Iran. Red Crescent Med. J. 2016, 18, e38909. [Google Scholar] [CrossRef] [Green Version]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Ohshima, T.; Ikawa, S.; Kitano, K.; Maeda, N. A Proposal of Remedies for Oral Diseases Caused by Candida: A Mini Review. Front. Microbiol. 2018, 9, 1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behbehani, J.; Shreaz, S.; Irshad, M.; Karched, M. The natural compound magnolol affects growth, biofilm formation, and ultrastructure of oral Candida isolates. Microb. Pathog. 2017, 113, 209–217. [Google Scholar] [CrossRef]
- Reynaud, A.H.; Nygaard-Østby, B.; Bøygard, G.K.; Eribe, E.R.; Olsen, I.; Gjermo, P. Yeasts in periodontal pockets. J. Clin. Periodontol. 2001, 28, 860–864. [Google Scholar] [CrossRef]
- Shah, B.; Harshe, D.G.; Shah, H.; Shetty, N.; Shenoy, A.; Ramakrishnan, A.; Cholera, R.; Kale, S. Perceived hassles and uplifts and their impact on perceived cognitive performance during pregnancy: A pilot study. Endodontology 2016, 28, 109–113. [Google Scholar]
- Kumar, J.; Sharma, R.; Sharma, M.; Prabhavathi, V.; Paul, J.; Chowdary, C.D. Presence of Candida albicans in Root Canals of Teeth with Apical Periodontitis and Evaluation of their Possible Role in Failure of Endodontic Treatment. J. Int. Oral Health 2015, 7, 42–45. [Google Scholar] [PubMed]
- Mohammadi, Z. Sodium hypochlorite in endodontics: An update review. Int. Dent. J. 2008, 58, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Candida spp. in the USA: Species distribution, clinical features and antifungal susceptibilities. Int. J. Antimicrob. Agents 2014, 43, 78–81. [Google Scholar] [CrossRef]
- Jensen, R.H.; Astvad, K.M.T.; Silva, L.V.; Sanglard, D.; Jørgensen, R.; Nielsen, K.F.; Mathiasen, E.G.; Doroudian, G.; Perlin, D.S.; Arendrup, M.C. Stepwise emergence of azole, echinocandin and amphotericin B multidrug resistance in vivo in Candida albicans orchestrated by multiple genetic alterations. J. Antimicrob. Chemother. 2015, 70, 2551–2555. [Google Scholar] [CrossRef] [Green Version]
- Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal Activity of Natural Compounds vs. Candida spp.: A Mixture of Cinnamaldehyde and Eugenol Shows Promising In Vitro Results. Antibiotics 2022, 11, 73. [Google Scholar] [PubMed]
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Le Pape, P.; Ganesan, A. A Decade of Antifungal Leads from Natural Products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soumya, S.; Nair, B.R. Antifungal efficacy of Capsicum frutescens L. extracts against some prevalent fungal strains associated with groundnut storage. J. Agric. Technol. 2012, 8, 739–750. [Google Scholar]
- Dorantes, L.; Colmenero, R.; Hernández, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solano, C. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. Int. J. Food Microbiol. 2000, 57, 125–128. [Google Scholar] [CrossRef]
- Menezes, R.D.P.; Bessa, M.A.D.S.; Siqueira, C.D.P.; Teixeira, S.C.; Ferro, E.A.V.; Martins, M.M.; Cunha, L.C.S.; Martins, C.H.G. Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics 2022, 11, 1154. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Velez-Haro, J.M.; Buitimea-Cantúa, N.E.; Molina-Torres, J.; Rosas-Burgos, E.C. GC-EIMS analysis, antifungal and anti-aflatoxigenic activity of Capsicum chinense and Aspergillus parasiticus fruits and their bioactive compounds capsaicin and piperine upon Aspergillus parasiticus. Nat. Prod. Res. 2020, 34, 1452–1455. [Google Scholar] [CrossRef]
- Gerber, W.; Steyn, D.; Kotzé, A.; Svitina, H.; Weldon, C.; Hamman, J. Capsaicin and Piperine as Functional Excipients for Improved Drug Delivery across Nasal Epithelial Models. Planta Med. 2019, 85, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Hayman, M.; Kam, P.C.A. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Saito, A.; Yamamoto, M. Acute oral toxicity of Capsaicin in mice and rats. J. Toxicol. Sci. 1996, 21, 195–200. [Google Scholar] [CrossRef]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and Anti-Virulence Activity of Capsaicin against Erythromycin-Resistant, Cell-Invasive Group A Streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Guan, X.; Zhu, W.; Liu, Z.; Wang, X.; Yu, H.; Wang, H. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mathé, L.; Van Dijck, P. Recent insights into Candida albicans biofilm resistance mechanisms. Curr. Genet. 2013, 59, 251–264. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Wang, D. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Hu, C.; Yu, J.H. Lipid Biosynthesis as an Antifungal Target. J. Fungi 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behbehani, J.M.; Irshad, M.; Shreaz, S.; Karched, M. Synergistic effects of tea polyphenol epigallocatechin 3-O-gallate and azole drugs against oral Candida isolates. J. Mycol. Med. 2019, 29, 158–167. [Google Scholar] [CrossRef]
- Liao, R.S.; Rennie, R.P.; Talbot, J.A. Sublethal injury and resuscitation of Candida albicans after amphotericin B treatment. Antimicrob. Agents Chemother. 2003, 47, 1200–1206. [Google Scholar] [CrossRef] [Green Version]
- Li, W.R.; Shi, Q.S.; Dai, H.Q.; Liang, Q.; Xie, X.B.; Huang, X.M.; Zhao, G.Z.; Zhang, L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, Y. Antifungal Activity of Salvia miltiorrhiza against Candida albicans Is Associated with the Alteration of Membrane Permeability and (1,3)-β-D-Glucan Synthase Activity. J. Microbiol. Biotechnol. 2016, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L.P.; MacFarlane, T.W.; Lamey, P.J.; Ferguson, M.M. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphylococcus aureus carriage in the oral cavity. J. Oral Pathol. 1986, 15, 386–388. [Google Scholar] [CrossRef]
- Wayne, P. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Breivik, O.N.; Owades, J.L. Yeast Analysis, Spectrophotometric Semimicrodetermination of Ergosterol in Yeast. J. Agric. Food Chem. 1957, 5, 360–363. [Google Scholar] [CrossRef]
- Irshad, M.; Ahmad, A.; Zafaryab, M.; Ahmad, F.; Manzoor, N.; Singh, M.; Rizvi, M.M.A. Composition of Cassia fistula oil and its antifungal activity by disrupting ergosterol biosynthesis. Nat. Prod. Commun. 2013, 8, 261–264. [Google Scholar] [CrossRef] [PubMed]




| C. albicans (Strain/Isolates) | MIC (µg/mL) | ||
|---|---|---|---|
| Capsaicin | Fluconazole | Ketoconazole | |
| ATCC-24433 | 25 | 64 | 16 |
| Isolates | |||
| Endo-902 | 25 | 64 | 16 |
| Endo-903 | 25 | 64 | 16 |
| Endo-904 | 25 | 128 | 8 |
| Endo-905 | 25 | 64 | 16 |
| Endo-906 | 25 | 128 | 16 |
| Endo-908 | 25 | 32 | 8 |
| Endo-910 | 25 | 16 | 8 |
| Endo-911 | 25 | 128 | 16 |
| Oral isolates# | 12.5–50 | 18–128 | -- |
| C. albicans (ATCC/Isolates#) | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| Capsaicin | Fluconazole | Σ FICI | |||
| Alone/Combo | FICI | Alone/Combo | FICI | ||
| ATCC-24433 | 25/6.25 | 0.25 | 64/4 | 0.063 | 0.313 |
| Endo-902 | 25/6.25 | 0.25 | 64/16 | 0.250 | 0.500 |
| Endo-903 | 25/6.25 | 0.25 | 64/8 | 0.125 | 0.375 |
| Endo-904 | 25/12.5 | 0.50 | 128/16 | 0.125 | 0.625 * |
| Endo-905 | 25/6.25 | 0.25 | 64/4 | 0.063 | 0.313 |
| Endo-906 | 25/6.25 | 0.25 | 128/16 | 0.125 | 0.375 |
| Endo-908 | 25/6.25 | 0.25 | 32/8 | 0.250 | 0.500 |
| Endo-910 | 25/12.5 | 0.50 | 16/8 | 0.500 | 1.000 * |
| Endo-911 | 25/12.5 | 0.50 | 128/16 | 0.125 | 0.625 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behbehani, J.M.; Irshad, M.; Shreaz, S.; Karched, M. Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans. Int. J. Mol. Sci. 2023, 24, 1046. https://doi.org/10.3390/ijms24021046
Behbehani JM, Irshad M, Shreaz S, Karched M. Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans. International Journal of Molecular Sciences. 2023; 24(2):1046. https://doi.org/10.3390/ijms24021046
Chicago/Turabian StyleBehbehani, Jawad M., Mohammad Irshad, Sheikh Shreaz, and Maribasappa Karched. 2023. "Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans" International Journal of Molecular Sciences 24, no. 2: 1046. https://doi.org/10.3390/ijms24021046
APA StyleBehbehani, J. M., Irshad, M., Shreaz, S., & Karched, M. (2023). Anticandidal Activity of Capsaicin and Its Effect on Ergosterol Biosynthesis and Membrane Integrity of Candida albicans. International Journal of Molecular Sciences, 24(2), 1046. https://doi.org/10.3390/ijms24021046







