Functionalized Magnetite Nanoparticles: Characterization, Bioeffects, and Role of Reactive Oxygen Species in Unicellular and Enzymatic Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Microstructure and Magnetic Properties of Samples
2.1.1. Local Environment of Fe Atom/Ions
2.1.2. Magnetic Parameters
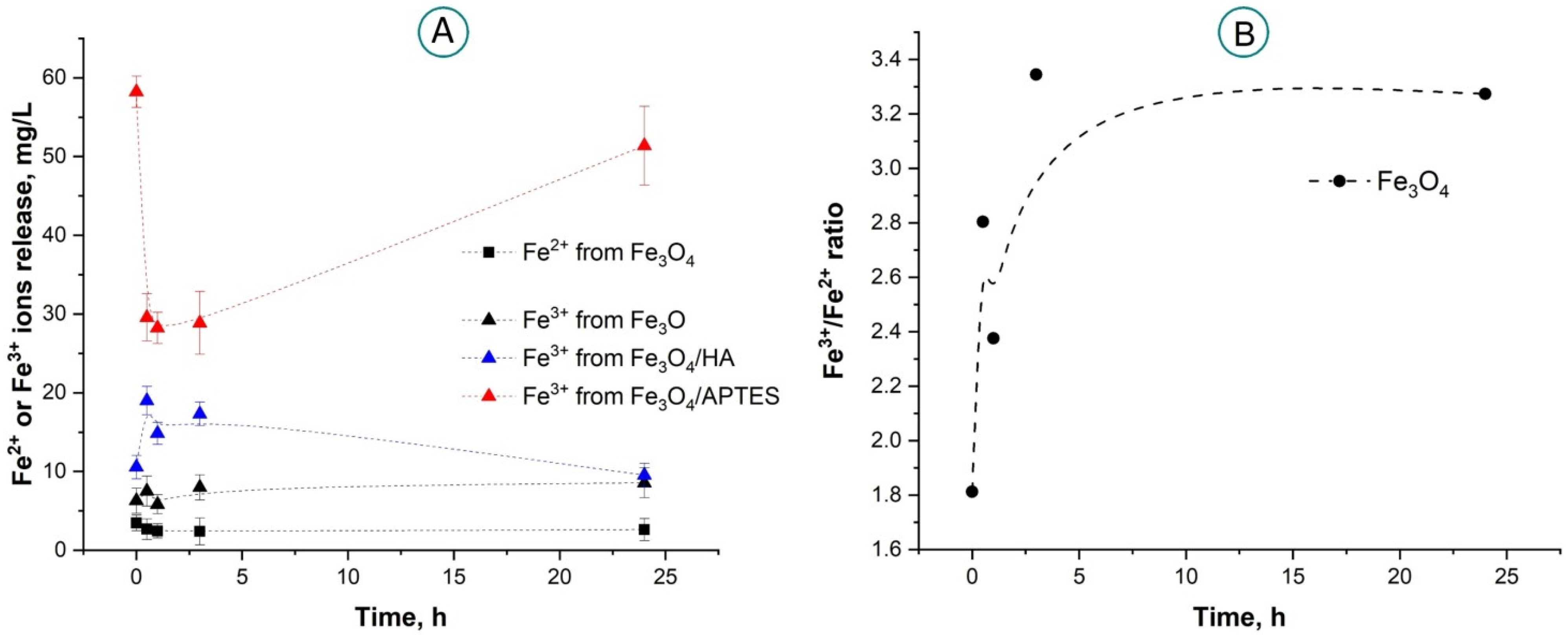
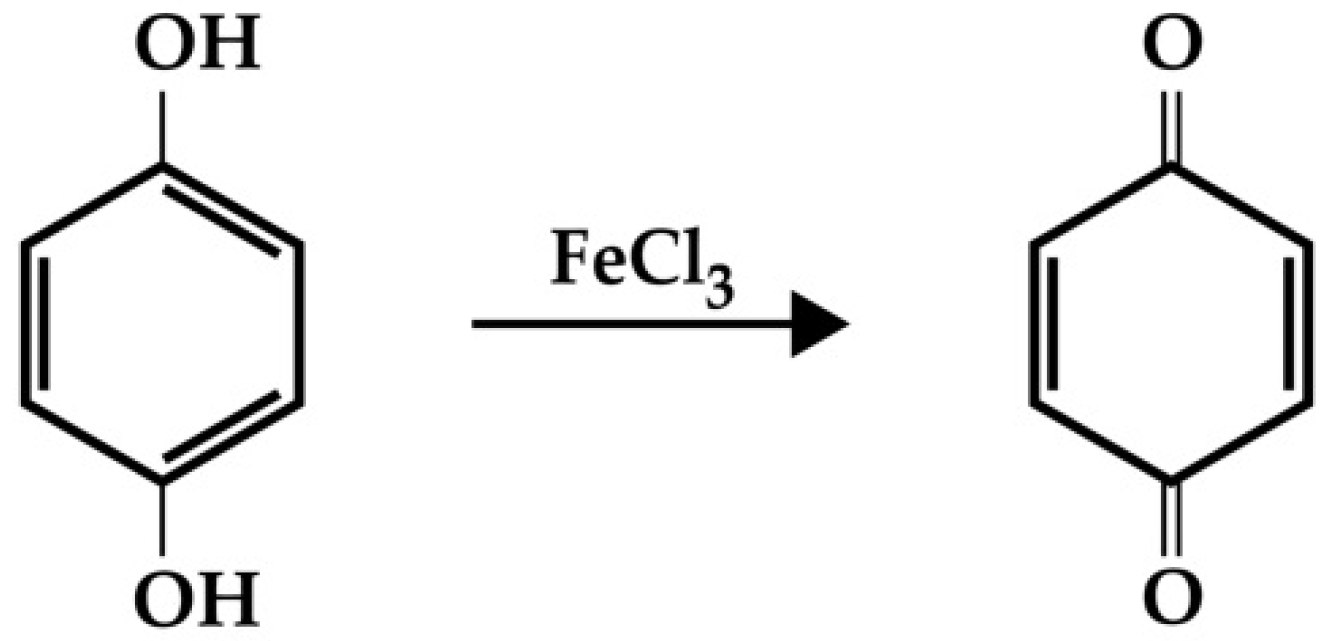
2.1.3. Iron Ion Release
2.2. Effects of MNPs on Bacterial Cells
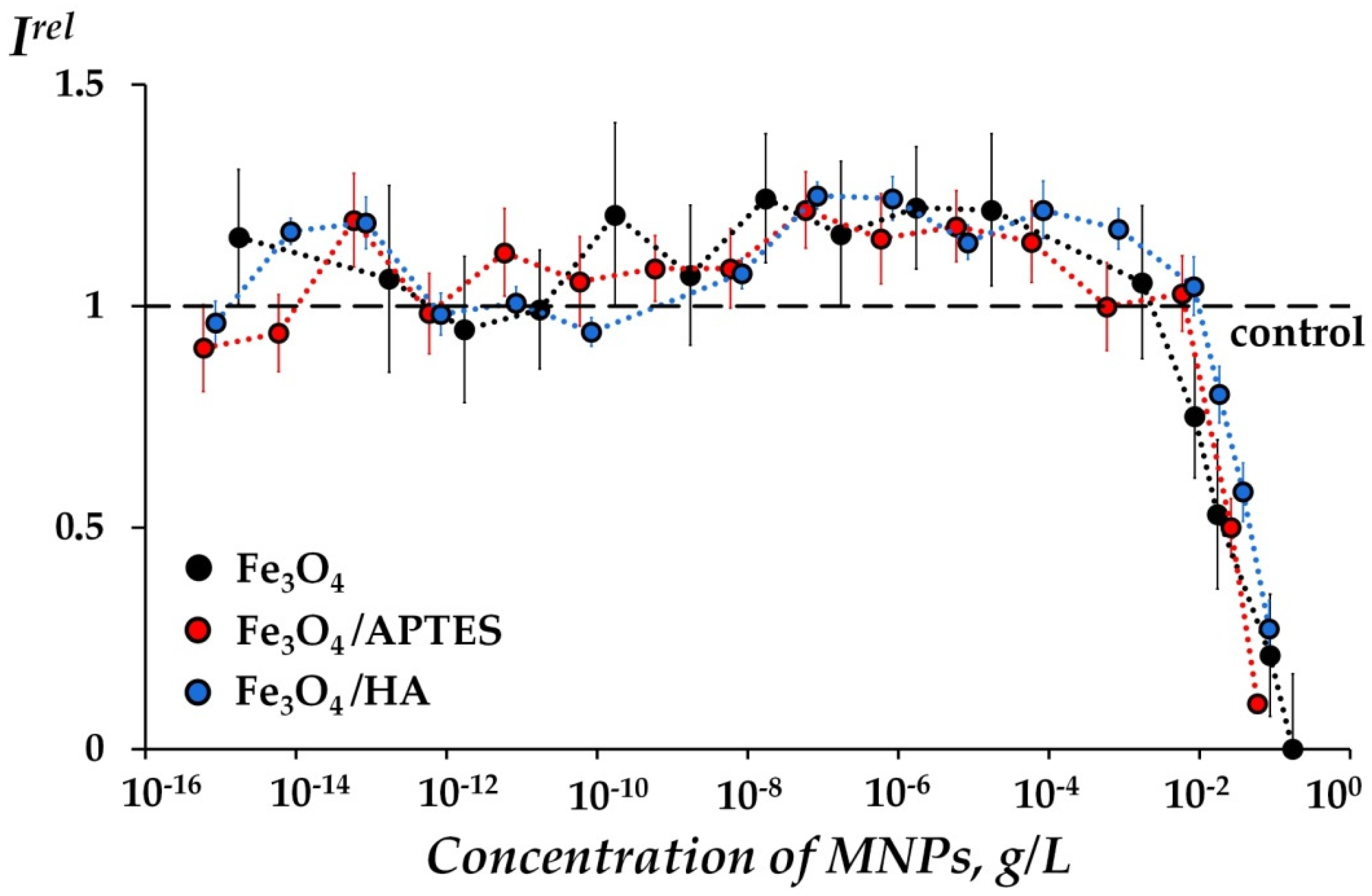
2.2.1. Toxic Effects of MNPs in Bacterial Suspension
- (a)
- Effects of MNPs on Bacterial Bioluminescence Intensity
- (b)
- Effects of MNPs on ROS Content in Bacterial Suspension
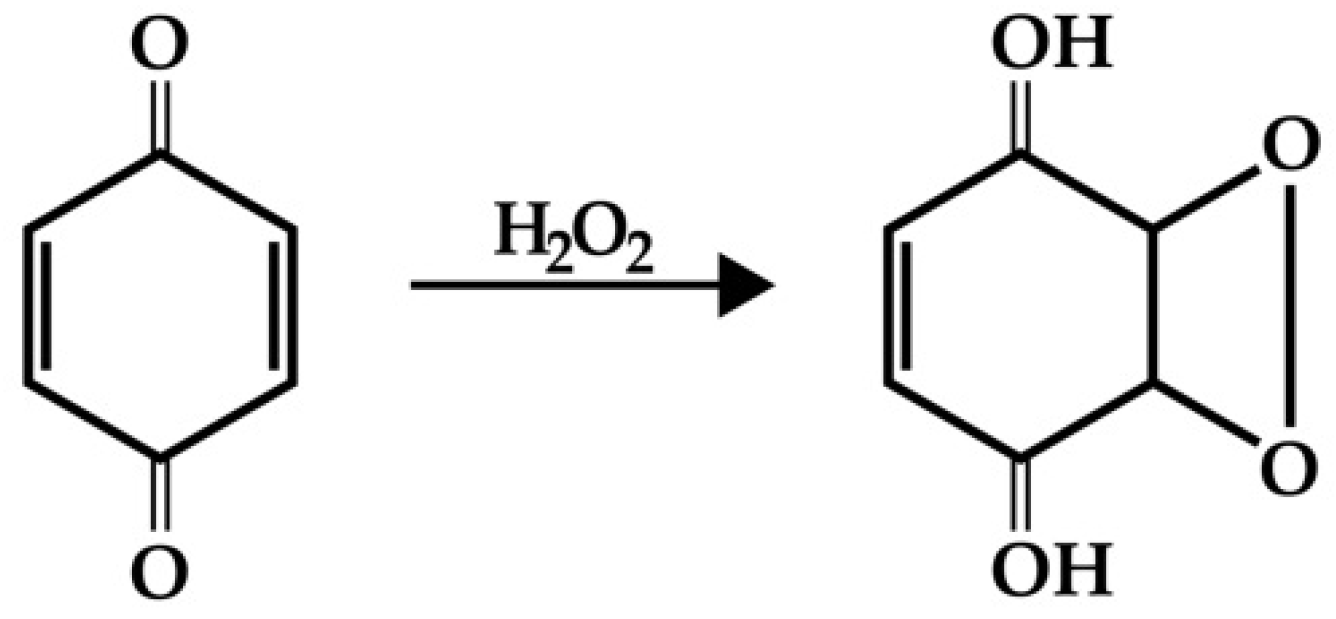
2.2.2. Effects of MNPs in Bacterial Suspension under Model Oxidative Stress
- (a)
- Effects of MNPs on Bacterial Bioluminescence Intensity under Model Oxidative Stress
- (b)
- Effects of MNPs on ROS Content in Bacterial Suspension under Model Oxidative Stress
2.3. Effects of MNPs on Enzymatic Reactions
2.3.1. Effects of MNPs without Oxidative Stress
- (a)
- Effects of MNPs on Bioluminescence Intensity of Enzyme Reactions
- (b)
- Effects of MNPs on ROS Content in Enzyme System
2.3.2. Effects of MNPs in Enzyme System under Model Oxidative Stress
3. Materials and Methods
3.1. Preparations of Fe3O4 MNPs and Humic Acids- and Amino-Silica Functionalized Fe3O4 MNPs
3.2. Characterization of the MNPs
3.3. Bioluminescence Assay Systems and Luminol Chemiluminescence Assay
3.3.1. Bioluminescence Assay Systems
Bioluminescence Cellular Assay
Bioluminescence Enzymatic Assay
3.3.2. Experimental Data Processing
- The toxic (for bacterial assay) and inhibitory (for enzymatic assay) effects of MNPs on the bioluminescent systems were characterized by the relative bioluminescence intensity, Irel:
- 2.
- −
- Values of IrelBq > 1 revealed a decrease in the toxicity under the exposure to MNPs, i.e., the antioxidant activity of MNPs in the Bq solutions.
- −
- Values of IrelBq ≈ 1 revealed the absence of the MNP effects.
- −
- Values of IrelBq < 1 revealed an increase in toxicity under exposure to MNPs, i.e., the pro-oxidant activity of MNPs in the Bq solutions.
3.3.3. Luminol Chemiluminescence Assay
3.3.4. Preparation of the MNP Suspensions for the Bioluminescence Analyses
3.3.5. Equipment
3.3.6. Statistical Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APTES | 3-Aminopropyltrietoxysilane |
| Bq | 1,4-benzoquinone |
| FMN | Flavin mononucleotide |
| HA | Humic acids |
| MNPs | Magnetite nanoparticles |
| NADH | Nicotinamide adenine dinucleotide disodium salt-reduced |
| ROS | Reactive oxygen species |
References
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macêdo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Mylkie, K.; Nowak, P.; Rybczynski, P.; Ziegler-Borowska, M. Polymer-Coated Magnetite Nanoparticles for Protein Immobilization. Materials 2021, 14, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Kadam, U.S.; Irudayaraj, J. One-stop genomic DNA extraction by salicylic acid-coated magnetic nanoparticles. Anal. Biochem. 2013, 442, 249–252. [Google Scholar] [CrossRef]
- Halavaara, J.; Tervahartiala, R.; Isoniemi, H.; Hockerstedt, K. Efficacy of sequential use of superparamagnetic iron oxide and gadolinium in liver MR imaging. Acta Radiol. 2002, 43, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A. Green synthesis of Fe3O4 nanoparticles and its application in preparation of Fe3O4/cellulose magnetic nanocomposite: A suitable proposal for drug delivery system. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3552–3561. [Google Scholar] [CrossRef]
- Hafeli, U.; Schutt, W.; Teller, J.; Zborowski, M. Scientific and Clinical Applications of Magnetic Carriers, 1st ed.; Springer: New York, NY, USA, 1997; 628p. [Google Scholar] [CrossRef]
- Chauhan, N.; Narang, J.; Jain, U. Amperometric acetylcholinesterase biosensor for pesticides monitoring utilising iron oxide nanoparticles and poly(indole-5-carboxylic acid). J. Exper. Nanosci. 2016, 11, 111–122. [Google Scholar] [CrossRef]
- Ali, A.; AlSalhi, M.S.; Atif, M.; Ansari, A.A.; Israr, M.Q.; Sadaf, J.R.; Ahmed, E.; Nur, O.; Willander, M. Potentiometric urea biosensor utilizing nanobiocomposite of chitosan-iron oxide magnetic nanoparticles. J. Phys. 2013, 414, 012024. [Google Scholar] [CrossRef]
- Lubbe, A.S.; Alexiou, C.; Bergemann, C. Clinical applications of magnetic drug targeting. J. Surg. Res. 2001, 95, 200–206. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Shen, M. Hydrothermal synthesis and functionalization of iron oxide nanoparticles for MR imaging applications. Part. Part. Syst. Charact. 2014, 31, 1223–1237. [Google Scholar] [CrossRef]
- Bilici, K.; Muti, A.; Sennaroğlu, A.; Acar, H.Y. Indocyanine green loaded APTMS coated SPIONs for dual phototherapy of cancer. J. Photochem. Photobiol. B 2019, 201, 111648. [Google Scholar] [CrossRef]
- Daoush, W.M. Co-precipitation and magnetic properties of magnetite nanoparticles for potential biomedical applications. J. Nanomed. Res. 2017, 5, 00118. [Google Scholar] [CrossRef]
- Shan, J.; Wang, L.; Yu, H.; Ji, J.; Amer, W.A.; Chen, Y.; Jing, G.; Khalid, H.; Akram, M.; Abbasi, N.M. Recent progress in Fe3O4 based magnetic nanoparticles: From synthesis to application. Mater. Sci. Technol. 2016, 32, 602–614. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Yang, Y.; Teranishi, T.C.; Thi, M.; Cao, Y.; Nogami, M. Biomedical applications of advanced multifunctional magnetic nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Nassar, H.N. Biosynthesized magnetite nanoparticles as an environmental opulence and sustainable wastewater treatment. Sci. Total Environ. 2021, 774, 145610. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Anuchina, M.M. Nature-inspired synthesis of magnetic non-stoichiometric Fe3O4 nanoparticles by oxidative in situ method in a humic medium. Mater. Chem. Phys. 2019, 231, 216–224. [Google Scholar] [CrossRef]
- Anuchina, M.; Pankratov, D.; Abroskin, D. Estimating the Toxicity and Biological Availability for Interaction Products of Metallic Iron and Humic Substances. Moscow Univ. Soil Sci. Bull. 2019, 74, 193–198. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Stockwell, B.R. Ferroptosis: An irondependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Shams, M.; Owczarczak, B.; Manderscheid-Kern, P.; Bellnier, D.A.; Gollnick, S.O. Development of photodynamic therapy regimens that control primary tumor growth and inhibit secondary disease. Cancer Immunol. Immunother. 2014, 64, 287–297. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Dan, Q.; Hu, D.; Ge, Y.; Zhang, S.; Li, S.; Gao, D.; Sheng, Z. Ultrasmall theranostic nanozymes to modulate tumor hypoxia for augmenting photodynamic therapy and radiotherapy. Biomater. Sci. 2020, 107, 2411–2502. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, H.-W. Iron ions-releasing mesoporous bioactive glass ultrasmall nanoparticles designed as ferroptosis-based bone cancer nanotherapeutics: Ultrasonic-coupled sol–gel synthesis, properties and iron ions release. Mater. Lett. 2021, 294, 129759. [Google Scholar] [CrossRef]
- Li, J.; Xiong, Z.; Yu, Y.; Wang, X.; Zhou, H.; Huang, B.; Lai, B. Efficient degradation of carbamazepine by electro-Fenton system without any extra oxidant in the presence of molybdate: The role of slow release of iron ions. Appl. Catal. B 2021, 298, 120506. [Google Scholar] [CrossRef]
- Yang, Y.; Zuo, S.; Li, L.; Kuang, X.; Li, J.; Sun, B.; Sun, J. Iron-doxorubicin prodrug loaded liposome nanogenerator programs multimodal ferroptosis for efficient cancer therapy. Asian J. Pharm. Sci. 2021, 16, 784–793. [Google Scholar] [CrossRef]
- Shen, Z.; Song, J.; Yung, B.C.; Zhou, Z.; Wu, A.; Chen, X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 2018, 30, 1704007. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Deng, J.; Liu, F.; Guo, T.; Liu, M.; Dai, P.; Zhao, Y. Triggered all-active metal organic framework: Ferroptosis machinery contributes to the apoptotic photodynamic antitumor therapy. Nano Lett. 2019, 19, 7866–7876. [Google Scholar] [CrossRef]
- Shan, X.; Li, S.; Sun, B.; Chen, Q.; Sun, J.; He, Z.; Luo, C. Ferroptosis-driven nanotherapeutics for cancer treatment. J. Control. Release 2020, 319, 322–332. [Google Scholar] [CrossRef]
- An, Q.; Sun, C.; Li, D.; Xu, K.; Guo, J.; Wang, C. Peroxidase-like activity of Fe3O4@carbon nanoparticles enhances ascorbic acidinduced oxidative stress and selective damage to PC-3 prostate cancer cells. ACS Appl. Mater. Interfaces 2013, 5, 13248–13257. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Shi, J. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew. Chem. Int. Ed. 2016, 55, 2101–2106. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, L.; Peng, C.; Zhang, S.; Chen, S.; Qian, X.; Zhao, B. Tumor microenvironments self-activated nanoscale metalorganic frameworks for ferroptosis based cancer chemodynamic/photothermal/chemo therapy. Acta Pharm. Sin. B 2021, 11, 3231–3243. [Google Scholar] [CrossRef]
- Huang, G.; Chen, H.; Dong, Y.; Luo, X.; Yu, H.; Moore, Z.; Gao, J. Superparamagnetic Iron Oxide Nanoparticles: Amplifying ROS Stress to Improve Anticancer Drug Efficacy. Theranostics 2013, 3, 116–126. [Google Scholar] [CrossRef]
- Touyz, R.M. Molecular and cellular mechanisms in vascular injury in hypertension: Role of angiotensin II- editorial review. Curr. Opin. Nephrol. Hypertens. 2005, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.F.; Laude, K.; McNally, J.S.; Harrison, D.G. Redox mechanisms in blood vessels. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Schipper, H.M. Principles of Free Radical Biomedicine; Nova Science Publisher: New York, NY, USA, 2011; 670p. [Google Scholar]
- Paravicini, T.M.; Touyz, R.M. NADPH oxidases, reactive oxygen species, and hypertension clinical implications and therapeutic possibilities. Diabetes Care 2008, 31, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Wu, H.; Yin, J.J.; Wamer, W.G.; Zeng, M.; Lo, M.Y. Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides. J. Food Drug Anal. 2014, 22, 86–94. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Kadam, U.S.; Hong, J.C. Selenoprotein: Potential Player in Redox Regulation in Chlamydomonas reinhardtii. Antioxidants 2022, 11, 1630. [Google Scholar] [CrossRef]
- Huang, C.C.; Liao, Z.X.; Lu, H.M.; Pan, W.Y.; Wan, W.L.; Chen, C.C.; Sung, H.W. Cellular organelle-dependent cytotoxicity of iron oxide nanoparticles and its implications for cancer diagnosis and treatment: A mechanistic investigation. Chem. Mater. 2016, 28, 9017–9025. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D.C. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2022, 199, 16–27. [Google Scholar] [CrossRef]
- Illes, E.; Tombacz, E. The role of variable surface charge and surface complexation in the adsorption of humic acid on magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Rashid, M.; Price, N.T.; Pinilla, M.Á.G.; O’Shea, K.E. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Res. 2017, 123, 353–360. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Anuchina, M.M.; Spiridonov, F.M. Fe3−δO4 Nanoparticles Synthesized in the Presence of Natural Polyelectrolytes. Crystallogr. Rep. 2020, 65, 393–397. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Irzhak, V.I.; Bratskaya, S.Y.; Mayorov, V.Y.; Privar, Y.O.; Kasymova, E.D.; Kulyabko (Bondarenko), L.S.; Zhorobekova, S.; Kydralieva, K.A. Stabilization of magnetite nanoparticles in a medium of humic acids and study of their sorption properties. Colloid J. 2020, 82, 1–7. [Google Scholar] [CrossRef]
- Bondarenko, L.; Terekhova, V.; Kahru, A.; Dzhardimalieva, G.; Kelbysheva, E.; Tropskaya, N.; Kydralieva, K. Sample preparation considerations for surface and crystalline properties and ecotoxicity of bare and silicacoated magnetite nanoparticles. RSC Adv. 2021, 11, 32227–32235. [Google Scholar] [CrossRef]
- Bondarenko (Kulyabko), L.; Illés, E.; Tombácz, E.; Dzhardimalieva, G.; Golubeva, N.; Tushavina, O.; Adachi, Y.; Kydralieva, K. Fabrication, Microstructure and Colloidal Stability of Humic Acids Loaded Fe3O4/APTES Nanosorbents for Environmental Applications. Nanomaterials 2021, 11, 1418. [Google Scholar] [CrossRef]
- Koesnarpadi, S.; Santosa, S.J.; Siswanta, D.; Rusdiarso, B. Synthesis and Characterizatation of Magnetite Nanoparticle Coated Humic Acid (Fe3O4/HA). Procedia Environ. Sci. 2015, 30, 103–108. [Google Scholar] [CrossRef]
- Carlos, L.; Cipollone, M.; Soria, D.B.; Moreno, M.S.; Ogilby, P.R.; Einschlag, F.S.G.; Mártire, D. The effect of humic acid binding to magnetite nanoparticles on the photogeneration of reactive oxygen species. Sep. Purif. Technol. 2012, 91, 23–29. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Q.; Xu, W.; Yang, M.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Cr(VI) Adsorption and Reduction by Humic Acid Coated on Magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef]
- Yang, S.; Zong, P.; Ren, X.; Wang, Q.; Wang, X. Rapid and Highly Efficient Preconcentration of Eu(III) by Core–Shell Structured Fe3O4@Humic Acid Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 6891–6900. [Google Scholar] [CrossRef]
- Peng, L.; Qin, P.F.; Lei, M.; Zeng, Q.R.; Song, H.J.; Yang, J.; Gu, J.D. Modifying Fe3O4 nanoparticles with humic acid for removal of rhodamine B in water. J. Hazard. Mater. 2012, 209, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Xiao, Y.; Wang, G.; Fan, J.; Wan, K.; He, Q.; Gao, M.; Miao, Z. Adsorption of heavy metals in water by modifying Fe3O4 nanoparticles with oxidized humic acid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126333. [Google Scholar] [CrossRef]
- Bayrakci, M.; Gezici, O.S.; Bas, Z.; Ozmen, M.; Maltas, E. Novel humic acid-bonded magnetite nanoparticles for protein immobilization. Mater. Sci. Eng. C 2014, 42, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.M.; Andrade, A.L.; Fabris, J.D.; Valério, P.; Góes, A.M.; Leite, M.F.; Domingues, R.Z. Synthesis and in vitro evaluation of toxicity of silica-coated magnetite nanoparticles. J. Non-Cryst. Solids 2008, 354, 4894–4897. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef]
- Bulich, A.A.; Isenberg, D.L. Use of the luminescent bacterial system for rapid assessment of aquatic toxicity. ISA Trans. 1981, 20, 29–33. [Google Scholar]
- Girotti, S.; Ferri, E.N.; Fumo, M.G.; Maiolini, E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal. Chim. Acta 2008, 608, 2–29. [Google Scholar] [CrossRef]
- Roda, A.; Pasini, P.; Mirasoni, M.; Michchelini, E.; Guardigli, M. Biotechnological application of bioluminescence and chemiluminescence. Trends Biotechnol. 2004, 22, 295–303. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Ismailov, A.D.; Aleskerova, L.E. Photobiosensors containing luminescent bacteria. Biochemistry 2015, 80, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Kurvet, I.; Ivask, A.; Bondarenko, O.; Sihtmäe, M.; Kahru, A. LuxCDABE—Transformed constitutively bioluminescent Escherichia coli for toxicity screening: Comparison with naturally luminous Vibrio fischeri. Sensors 2011, 11, 7865–7878. [Google Scholar] [CrossRef] [PubMed]
- Kratasyuk, V.A.; Esimbekova, E.N. Applications of luminous bacteria enzymes in toxicology. Comb. Chem. High Throughput Screen. 2015, 18, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Kovel, E.S.; Sachkova, A.S.; Vnukova, N.G.; Churilov, G.N.; Knyazeva, E.M.; Kudryasheva, N.S. Antioxidant activity and toxicity of fullerenols via bioluminescence signaling: Role of oxygen substituents. Int. J. Mol. Sci. 2019, 20, 2324. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Kudryasheva, N.S.; Kratasyuk, V.A. Redox compounds influence on the NAD(P)H:FMN-oxidoreductase-luciferase bioluminescent system. Photochem. Photobiol. Sci. 2007, 6, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Esimbekova, E.N.; Torgashina, I.G.; Kalyabina, V.P.; Kratasyuk, V.A. Enzymatic biotesting: Scientific basis and application. Contem. Probl. Ecol. 2021, 14, 290–304. [Google Scholar] [CrossRef]
- Kudryasheva, N.; Vetrova, E.; Kuznetsov, A.; Kratasyuk, V.; Stom, D. Bioluminescent Assays: Effects of Quinones and Phenols. Ecotoxicol. Environ. Saf. 2002, 53, 221–225. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Effect of humic substances on toxicity of inorganic oxidizer bioluminescent monitoring. Environ. Toxicol. Chem. 2011, 30, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Kudryasheva, N.S.; Tarasova, A.S. Pollutant toxicity and detoxification by humic substances: Mechanisms and quantitative assessment via luminescent biomonitoring. Environ. Sci. Pollut. Res. 2015, 22, 155–167. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Kislan, S.L.; Fedorova, E.S.; Kuznetsov, A.M.; Mogilnaya, O.A.; Stom, D.I.; Kudryasheva, N.S. Bioluminescence as a tool for studying detoxification processes in metal salt solutions involving humic substances. J. Photochem. Photobiol. B 2012, 117, 164–170. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Antioxidant activity of humic substances via bioluminescent monitoring in vitro. Environ. Monit. Assess. 2015, 187, 89. [Google Scholar] [CrossRef] [PubMed]
- Selivanova, M.A.; Mogilnaya, O.A.; Badun, G.A.; Vydryakova, G.A.; Kuznetsov, A.M.; Kudryasheva, N.S. Effect of tritium on luminous marine bacteria and enzyme reactions. J. Environ. Radioact. 2013, 120, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kudryasheva, N.S.; Kovel, E.S.; Sachkova, A.S.; Vorobeva, A.A.; Isakova, V.G.; Churilov, G.N. Bioluminescent enzymatic assay as a tool for studying antioxidant activity and toxicity of bioactive compounds. Photochem. Photobiol. 2017, 93, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Sachkova, A.S.; Kovel, E.S.; Churilov, G.N.; Guseynov, O.A.; Bondar, A.A.; Dubinina, I.A.; Kudryasheva, N.S. On mechanism of antioxidant effect of fullerenols. Biochem. Biophys. Rep. 2017, 9, 1–8. [Google Scholar] [CrossRef]
- Sachkova, A.S.; Kovel, E.S.; Churilov, G.N.; Stom, D.I.; Kudryasheva, N.S. Biological activity of carbonic nanostructures—Comparison via enzymatic bioassay. J. Soils Sediments 2019, 19, 2689–2696. [Google Scholar] [CrossRef]
- Kovel, E.S.; Kicheeva, A.G.; Vnukova, N.G.; Churilov, G.N.; Stepin, E.A.; Kudryasheva, N.S. Toxicity and Antioxidant Activity of Fullerenol C60,70 with Low Number of Oxygen Substituents. Int. J. Mol. Sci. 2021, 22, 6382. [Google Scholar] [CrossRef]
- Sushko, E.S.; Vnukova, N.G.; Churilov, G.N.; Kudryasheva, N.S. Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems. Int. J. Mol. Sci. 2022, 23, 5152. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, L.S.; Kovel, E.S.; Kydralieva, K.A.; Dzhardimalieva, G.I.; Illés, E.; Tombácz, E.; Kicheeva, A.G.; Kudryasheva, N.S. Effects of Modified Magnetite Nanoparticles on Bacterial Cells and Enzyme Reactions. Nanomaterials 2020, 10, 1499. [Google Scholar] [CrossRef]
- Klygach, D.S.; Vakhitov, M.G.; Pankratov, D.A.; Zherebtsov, D.A.; Tolstoguzov, D.S.; Raddaoui, Z.; Kossi, S.E.; Dhahri, J.; Vinnik, D.A.; Trukhanov, A.V. MCC: Specific of preparation, correlation of the phase composition and electrodynamic properties. J. Magn. Magn. Mater. 2021, 526, 167694. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Kazantsev, R.V.; Pankina, G.V.; Pankratov, D.A.; Maksimov, S.V.; Eliseev, O.L. Unusual Effect of Support Carbonization on the Structure and Performance of Fe/Mgal2O4 Fischer–Tropsch Catalyst. Energy Technol. 2021, 9, 2000877. [Google Scholar] [CrossRef]
- Bondarenko, L.S.; Pankratov, D.A.; Dzeranov, A.A.; Dzhardimalieva, G.I.; Streltsova, A.N.; Zarrelli, M.; Kydralieva, K.A. A simple method for quantification of nonstoichiometric magnetite nanoparticles using conventional X-ray diffraction technique. Mendeleev Commun. 2022, 32, 642–644. [Google Scholar] [CrossRef]
- Pankratov, D.A. Mössbauer study of oxo derivatives of iron in the Fe2O3-Na2O2 system. Inorg. Mater. 2014, 50, 82–89. [Google Scholar] [CrossRef]
- Sajja, H.K.; East, M.P.; Mao, H.; Wang, Y.A.; Nie, S.; Yang, L. Development of multifunctional nanoparticles for targeted drug delivery and noninvasive imaging of therapeutic effect. Curr. Drug Discov. Technol. 2009, 6, 43–51. [Google Scholar] [CrossRef]
- Thorek, D.L.; Tsourkas, A. Size, charge and concentration dependent uptake of iron oxide particles by non-phagocytic cells. Biomaterials 2008, 29, 3583–3590. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- He, Y.-J.; Liu, X.-Y.; Xing, L.; Wan, X.; Chang, X.; Jiang, H.L. Fenton reaction-independent ferroptosis therapy via Glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials 2020, 241, 119911. [Google Scholar] [CrossRef]
- Dong, Z.; Feng, L.; Chao, Y.; Hao, Y.; Chen, M.; Gong, F.; Han, X.; Zhang, R.; Cheng, L.; Liu, Z. Amplification of Tumor Oxidative Stresses with Liposomal Fenton Catalyst and Glutathione Inhibitor for Enhanced Cancer Chemotherapy and Radiotherapy. Nano Lett. 2019, 19, 805–815. [Google Scholar] [CrossRef]
- Huo, M.; Wang, L.; Chen, Y.; Shi, J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yin, J.-J.; Zhou, X.; Kurash, I.; Chai, Z.; Zhao, Y.; Feng, W. Physicochemical origin for free radical generation of iron oxide nanoparticles in biomicroenvironment: Catalytic activities mediated by surface chemical states. J. Phys. Chem. C 2012, 117, 383–392. [Google Scholar] [CrossRef]
- Roden, D.M. Drug-induced prolongation of the QT interval. N. Engl. J. Med. 2004, 350, 1013–1022. [Google Scholar] [CrossRef]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef]
- Ersoy, B.; Tosun, I.; Günay, A.; Dikmen, S. Turbidity Removal from Wastewaters of Natural Stone Processing by Coagulation/Flocculation Methods. CLEAN–Soil Air Water 2009, 37, 225–232. [Google Scholar] [CrossRef]
- Tipping, E.; Rey-Castro, C.; Bryan, S.E.; Hamilton-Taylor, J. Al(III) and Fe(III) binding by humic substances in freshwaters, and implications for trace metal speciation. Geochim. Cosmochim. Acta 2002, 66, 3211–3224. [Google Scholar] [CrossRef]
- Oliveira, R.J.; Conto, J.F.; Oliveira, M.R.; Egues, S.M.S.; Borges, G.R.; Dariva, C.; Franceschi, E. CO2/CH4 adsorption at high pressure using silica-APTES aerogel as adsorbent and near infrared as a monitoring technique. J. CO2 Util. 2019, 32, 232–240. [Google Scholar] [CrossRef]
- Otalvaro, J.O.; Avena, M.; Brigante, M. Adsorption of organic pollutants by amine functionalized mesoporous silica in aqueous solution. Effects of pH, ionic strength and some consequences of APTES stability. J. Environ. Chem. Eng. 2019, 7, 103325. [Google Scholar] [CrossRef]
- Minotti, G.; Aust, S.D. The role of iron in the initiation of lipid peroxidation. Chem. Phys. Lipids 1987, 44, 191–208. [Google Scholar] [CrossRef]
- Vasquez-Medrano, R.; Prato-Garcia, D.; Vedrenne, M. Ferrioxalate-Mediated Processes. In Advanced Oxidation Processes for Waste Water Treatment, 1st ed.; Ameta, S.C., Ameta, R., Eds.; Academic Press: New York, NY, USA, 2018; pp. 89–113. [Google Scholar] [CrossRef]
- Masomboon, N.; Ratanatamskul, C.; Lu, M.C. Chemical oxidation of 2,6-dimethylaniline in the fenton process. Environ. Sci. Technol. 2009, 43, 8629–8634. [Google Scholar] [CrossRef]
- Fedorova, E.; Kudryasheva, N.; Kuznetsov, A.; Mogil’naya, O.; Stom, D. Bioluminescent monitoring of detoxification processes: Activity of humic substances in quinone solutions. J. Photochem. Photobiol. B 2007, 88, 131–136. [Google Scholar] [CrossRef]
- Calabrese, E. Hormesis: Path and Progression to Significance. Int. J. Mol. Sci. 2018, 19, 2871. [Google Scholar] [CrossRef]
- Jargin, S.V. Hormesis and Radiation Safety Norms: Comments for an Update. Hum. Exp. Toxicol. 2018, 37, 1233–1243. [Google Scholar] [CrossRef]
- Shibamoto, Y.; Nakamura, H. Overview of Biological, Epidemiological, and Clinical Evidence of Radiation Hormesis. Int. J. Mol. Sci. 2018, 19, 2387. [Google Scholar] [CrossRef]
- Ge, H.; Zhou, M.; Lv, D.; Wang, M.; Xie, D.; Yang, X.; Dong, C.; Li, S.; Lin, P. Novel Segmented Concentration Addition Method to Predict Mixture Hormesis of Chlortetracycline Hydrochloride and Oxytetracycline Hydrochloride to Aliivibrio fischeri. Int. J. Mol. Sci. 2020, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J. Hormesis: Sipping from a poisoned chalice Science. Science 2003, 302, 376–379. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Grebowski, J.; Kazmierska, P.; Krokosz, A. Fullerenols as a new therapeutic approach in nanomedicine. Biomed. Res. Int. 2013, 2013, 751913. [Google Scholar] [CrossRef]
- Mirkov, S.M.; Djordjevic, A.N.; Andric, N.L.; Andric, S.A.; Kostic, T.S.; Bogdanovic, G.M.; Vojinovic-Miloradov, M.B.; Kovacevic, R.Z. Nitric oxide-scavenging activity of polyhydroxylated fullerenol, C60(OH)24. Nitric Oxide 2004, 11, 201–207. [Google Scholar] [CrossRef]
- Injac, R.; Prijatelj, M.; Strukelj, B. Fullerenol nanoparticles: Toxicity and antioxidant activity. Methods Mol. Biol. 2013, 1028, 75–100. [Google Scholar] [CrossRef]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of synthesis and antioxidant potential of fullerenol nanoparticles. J. Nanomater. 2015, 2015, 567073. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Lu, Z.; Gao, X. Syntheses, structures and antioxidant activities of fullerenols: Knowledge learned at the atomistic level. J. Clust. Sci. 2015, 26, 375–388. [Google Scholar] [CrossRef]
- Djordjevic, A.; Canadanovic-Brunet, J.M.; Vojinovic-Miloradov, M.; Bogdanovic, G. Antioxidant properties and hypothetic radical mechanism of fullerenol C60(OH)24. Oxid. Commun. 2005, 27, 806–812. [Google Scholar]
- Nemtseva, E.V.; Kudryasheva, N. The mechanism of electronic excitation in bacterial bioluminescent reaction. Uspekhi Khimii 2007, 76, 101–112. [Google Scholar] [CrossRef]
- Kuznetsov, A.M.; Rodicheva, E.K.; Shilova, E.V. Bioassay based on lyophilized bacteria. Biotekhnologiya 1996, 9, 57–61. [Google Scholar]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-Based Chemiluminescent Signals: Clinical and Non-Clinical Application and Future Uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef]
- Vasil’ev, R.F.; Veprintsev, T.L.; Dolmatova, L.S.; Naumov, V.V.; Trofimov, A.V.; Tsaplev, Y.B. Kinetics of Ethylbenzene Oxy-Chemiluminescence in the Presence of Antioxidants from Tissues of the Marine Invertebrate Eupentacta Fraudatrix: Estimaing the Concentration and Reactivity of the Natural Antioxidants. Kinet. Catal. 2014, 55, 148–153. [Google Scholar] [CrossRef]
- Gmurman, V.E. Fundamentals of Probability Theory and Mathematical Statistics; Berenblut, I.I., Ed.; Iliffe Book Ltd.: London, UK, 1968; p. 249p. [Google Scholar]
- Rostovshchikova, T.N.; Korobov, M.S.; Pankratov, D.A. Catalytic conversions of chloroolefins over iron oxide nanoparticles 2. Isomerization of dichlorobutenes over iron oxide nanoparticles stabilized on the surface of ultradispersed poly(tetrafluoroethylene). Russ. Chem. Bull. 2005, 54, 1425–1432. [Google Scholar] [CrossRef]
- Jones, D.H.; Srivastava, K.K.P. Many-state relaxation model for the Mössbauer spectra of superparamagnets. Phys. Rev. B 1998, 34, 7542–7548. [Google Scholar] [CrossRef]
- Nadeem, K.; Krenn, H.; Traussnig, T.; Würschum, R.; Szabó, D.V.; Letofsky-Papst, I. Effect of dipolar and exchange interactions on magnetic blocking of maghemite nanoparticles. J. Magn. Magn. Mater. 2011, 323, 1998–2004. [Google Scholar] [CrossRef]
- Adolphi, N.L.; Huber, D.L.; Bryant, H.C.; Monson, T.C.; Fegan, D.L.; Lim, J.; Trujillo, J.E.; Tessier, T.E.; Lovato, D.M.; Butler, K.S.; et al. Characterization of single-core magnetite nanoparticles for magnetic imaging by SQUID relaxometry. Phys. Med. Biol. 2010, 55, 5985–6003. [Google Scholar] [CrossRef] [PubMed]
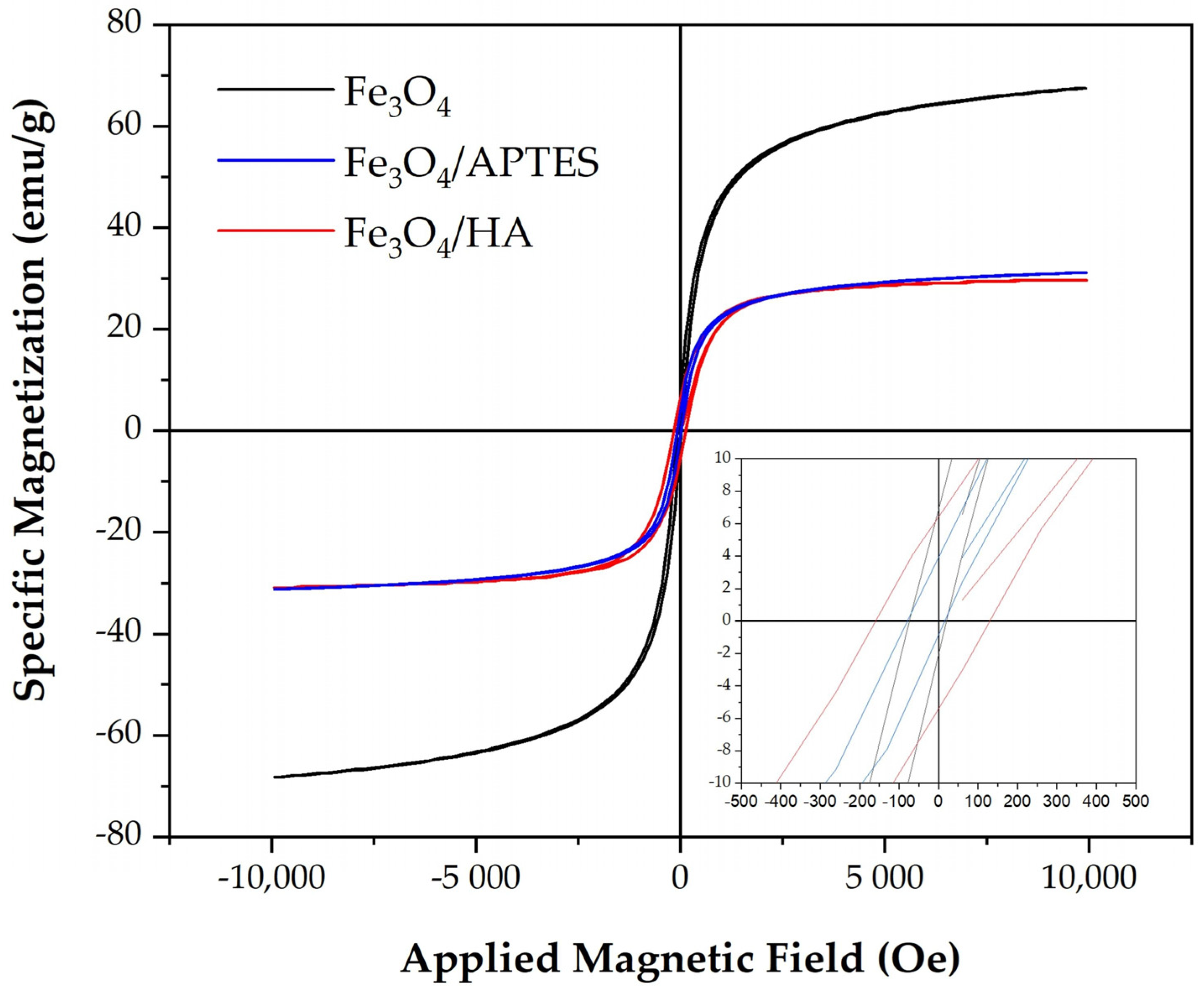



| Sample | Composition Fe3−δO4 * | D, nm | Saturation Magnetization Ms, emu/g | Remanent Magnetization Mr, emu/g | Coercive Force Hc, Oe |
|---|---|---|---|---|---|
| Fe3O4 | Fe2.718O4 | 15.64 ± 0.03 | 68.2 | 6.88 | 74.1 |
| Fe3O4-APTES | Fe2.734O4 | 15.73 ± 0.02 | 31.2 | 3.93 | 79.0 |
| Fe3O4-HA | Fe2.682O4 | 12.61 ± 0.06 | 30.9 | 6.40 | 160.0 |
| MNPs | EC50, g/L |
|---|---|
| Fe3O4 | 0.05 |
| Fe3O4/APTES | 0.07 |
| Fe3O4/HA | 0.12 |
| MNPs | MNP Concentration Range, g/L | r |
|---|---|---|
| Fe3O4 | (10−9–10−1) | −0.21 |
| Fe3O4/APTES | (10−9–10−1) | −0.88 |
| Fe3O4/HA | (10−9–10−1) | −0.79 |
| MNPs | MNP Concentration Range, g/L | r |
|---|---|---|
| Fe3O4 | (10−7–10−3) | 0.92 |
| Fe3O4/APTES | (10−7–10−3) | 0.91 |
| Fe3O4/HA | (10−7–10−3) | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicheeva, A.G.; Sushko, E.S.; Bondarenko, L.S.; Kydralieva, K.A.; Pankratov, D.A.; Tropskaya, N.S.; Dzeranov, A.A.; Dzhardimalieva, G.I.; Zarrelli, M.; Kudryasheva, N.S. Functionalized Magnetite Nanoparticles: Characterization, Bioeffects, and Role of Reactive Oxygen Species in Unicellular and Enzymatic Systems. Int. J. Mol. Sci. 2023, 24, 1133. https://doi.org/10.3390/ijms24021133
Kicheeva AG, Sushko ES, Bondarenko LS, Kydralieva KA, Pankratov DA, Tropskaya NS, Dzeranov AA, Dzhardimalieva GI, Zarrelli M, Kudryasheva NS. Functionalized Magnetite Nanoparticles: Characterization, Bioeffects, and Role of Reactive Oxygen Species in Unicellular and Enzymatic Systems. International Journal of Molecular Sciences. 2023; 24(2):1133. https://doi.org/10.3390/ijms24021133
Chicago/Turabian StyleKicheeva, Arina G., Ekaterina S. Sushko, Lyubov S. Bondarenko, Kamila A. Kydralieva, Denis A. Pankratov, Nataliya S. Tropskaya, Artur A. Dzeranov, Gulzhian I. Dzhardimalieva, Mauro Zarrelli, and Nadezhda S. Kudryasheva. 2023. "Functionalized Magnetite Nanoparticles: Characterization, Bioeffects, and Role of Reactive Oxygen Species in Unicellular and Enzymatic Systems" International Journal of Molecular Sciences 24, no. 2: 1133. https://doi.org/10.3390/ijms24021133
APA StyleKicheeva, A. G., Sushko, E. S., Bondarenko, L. S., Kydralieva, K. A., Pankratov, D. A., Tropskaya, N. S., Dzeranov, A. A., Dzhardimalieva, G. I., Zarrelli, M., & Kudryasheva, N. S. (2023). Functionalized Magnetite Nanoparticles: Characterization, Bioeffects, and Role of Reactive Oxygen Species in Unicellular and Enzymatic Systems. International Journal of Molecular Sciences, 24(2), 1133. https://doi.org/10.3390/ijms24021133











