The Beneficial Effects of Pine Nuts and Its Major Fatty Acid, Pinolenic Acid, on Inflammation and Metabolic Perturbations in Inflammatory Disorders
Abstract
:1. Introduction
2. Composition of PNLA and Pine Nuts Oils
3. PNLA and Its Metabolism
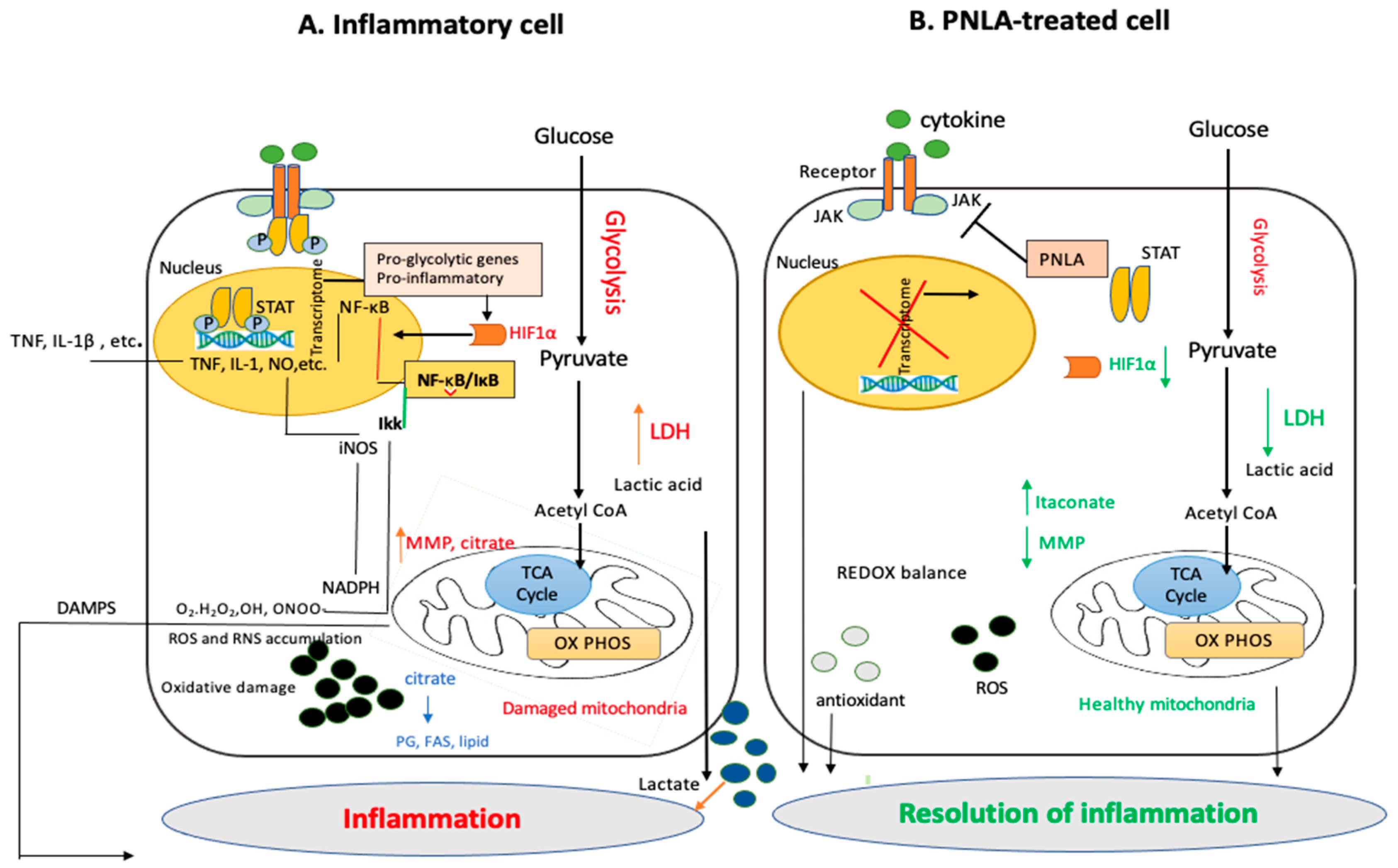
4. PNO and PNLA Inhibit the Inflammatory Response
4.1. Effects on Cell Culture
4.2. Effects on Animal Models
4.3. Effects on Healthy Individuals and Patients with Chronic Inflammatory Diseases
5. PNO and PNLA Inhibit Oxidative Reactions
6. PNLA and PNO protect against Hyperlipidaemia and Atherosclerosis and Regulate the Lipid and Metabolic State Based on Cell Line, Animal, and Human Studies
7. Novel Potential Metabolic, Anti-Inflammatory, and Immune-Regulatory Effects Discovered by Transcriptomic and Bioinformatic Analyses
8. PNLA and Modulation of miRNAs
9. Discussion and Conclusions
10. Prospects and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Pasman, W.J.; Heimerikx, J.; Rubingh, C.M.; van den Berg, R.; O’Shea, M.; Gambelli, L.; Hendriks, H.F.; Einerhand, A.W.; Scott, C.; Keizer, H.G.; et al. The effect of Korean pine nut oil on in vitro CCK release, on appetite sensations and on gut hormones in post-menopausal overweight women. Lipids Health Dis. 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, K.; Miles, E.A.; Calder, P.C. A review of the potential health benefits of pine nut oil and its characteristic fatty acid pinolenic acid. J. Funct. Foods 2016, 23, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Le, N.H.; Shin, S.; Tu, T.H.; Kim, C.-S.; Kang, J.-H.; Tsuyoshi, G.; Teruo, K.; Han, S.N.; Yu, R. Diet enriched with Korean pine nut oil improves mitochondrial oxidative metabolism in skeletal muscle and brown adipose tissue in diet-induced obesity. J. Agric. Food Chem. 2012, 60, 11935–11941. [Google Scholar] [CrossRef]
- Takala, R.; Ramji, D.P.; Andrews, R.; Zhou, Y.; Burston, J.; Choy, E. Anti-inflammatory and immunoregulatory effects of pinolenic acid in rheumatoid arthritis. Rheumatology 2021, 61, 992–1004. [Google Scholar] [CrossRef]
- Takala, R.; Ramji, D.P.; Andrews, R.; Zhou, Y.; Farhat, M.; Elmajee, M.; Rundle, S.; Choy, E. Pinolenic acid exhibits anti-inflammatory and anti-atherogenic effects in peripheral blood-derived monocytes from patients with rheumatoid arthritis. Sci. Rep. 2022, 12, 8807. [Google Scholar] [CrossRef]
- Amr, A.R.; Abeer, E.E.K. Hypolipideimic and hypocholestermic effect of pinenuts in rats fed high fat, cholesterol-diet. World Appl. Sci. J. 2011, 15, 1677. [Google Scholar]
- Lee, A.R.; Han, S.N. Pinolenic acid downregulates lipid anabolic pathway in HepG2 Cells. Lipids 2016, 51, 847–855. [Google Scholar] [CrossRef]
- Destaillats, F.; Cruz-Hernandez, C.; Giuffrida, F.; Dionisi, F. Identification of the botanical origin of pine nuts found in food products by gas−liquid chromatography analysis of fatty acid profile. J. Agric. Food Chem. 2010, 58, 2082–2087. [Google Scholar] [CrossRef]
- Kang, Y.H.; Kim, K.K.; Kim, T.W.; Choe, M. Anti-atherosclerosis effect of pine nut oil in high-cholesterol and high-fat diet fed rats and its mechanism studies in human umbilical vein endothelial cells. Food Sci. Biotechnol. 2015, 24, 323–332. [Google Scholar] [CrossRef]
- Park, Y.S.; Chung, M.S. Cholesterol-lowering effect of pine nut in plasma of rats. Korean J. Food Sci. Technol. 2005, 37, 702–708. [Google Scholar]
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.; O’Brien, N.M. Fatty acid profile, tocopherol, squalene and phytosterol content of brazil, pecan, pine, pistachio and cashew nuts. Int. J. Food Sci. Nutr. 2006, 57, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Zadernowski, R.; Naczk, M.; Czaplicki, S. Chemical composition of Pinus sibirica nut oils. Eur. J. Lipid Sci. Technol. 2009, 111, 698–704. [Google Scholar] [CrossRef]
- Matthäus, B.; Li, P.; Ma, F.; Zhou, H.; Jiang, J.; Özcan, M.M. Is the profile of fatty acids, tocopherols, and amino acids suitable to differentiate Pinus armandii suspicious to be responsible for the pine nut syndrome from other pinus species? Chem. Biodivers. 2018, 15, e1700323. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takimoto, T.; Morishige, J.-I.; Kikuta, Y.; Sugiura, T.; Satouchi, K. Non-methylene-interrupted polyunsaturated fatty acids: Effective substitute for arachidonate of phosphatidylinositol. Biochem. Biophys. Res. Commun. 1999, 264, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.-T.; Tsai, P.-J.; Lee, C.-L.; Huang, Y.-S. Uptake and Incorporation of pinolenic acid reduces n-6 polyunsaturated fatty acid and downstream prostaglandin formation in murine macrophage. Lipids 2009, 44, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Chuang, L.-T.; Liao, J.-S.; Huang, W.-C.; Lin, H.-H. Phospholipid incorporation of non-methylene-interrupted fatty acids (NMIFA) in murine microglial BV-2 cells reduces pro-inflammatory mediator production. Inflammation 2015, 38, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hattori, T.; Kouchi, M.; Hirano, K.; Satouchi, K. Methylene-interrupted double bond in polyunsaturated fatty acid is an essential structure for metabolism by the fatty acid chain elongation system of rat liver. Biochim. Biophys. Acta 1998, 1393, 299–306. [Google Scholar] [CrossRef]
- Chen, S.J.; Huang, W.C.; Shen, H.J.; Chen, R.Y.; Chang, H.; Ho, Y.S.; Tsai, P.J.; Chuang, L.T. Investigation of modulatory effect of pinolenic acid (PNA) on inflammatory responses in human THP-1 macrophage-like cell and mouse models. Inflammation 2020, 43, 518–531. [Google Scholar] [CrossRef]
- Baker, E.J.; Valenzuela, C.A.; De Souza, C.O.; Yaqoob, P.; Miles, E.; Calder, P.C. Comparative anti-inflammatory effects of plant- and marine-derived omega-3 fatty acids explored in an endothelial cell line. Biochim. Biophys. Acta 2020, 1865, 158662. [Google Scholar] [CrossRef]
- Baker, E.J.; Yusof, M.H.; Yaqoob, P.; Miles, E.A.; Calder, P.C. Omega-3 fatty acids and leukocyte-endothelium adhesion: Novel anti-atherosclerotic actions. Mol. Asp. Med. 2018, 64, 169–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-J.; Hsu, C.-P.; Li, C.-W.; Lu, J.-H.; Chuang, L.-T. Pinolenic acid inhibits human breast cancer MDA-MB-231 cell metastasis in vitro. Food Chem. 2011, 126, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Morishige, J.I.; Iwawaki, D.; Fukuhara, T.; Hamamura, N.; Hirano, K.; Osumi, T.; Satouchi, K. Metabolic pathway that produces essential fatty acids from polymethylene-interrupted polyunsaturated fatty acids in animal cells. FEBS J. 2007, 274, 2728–2737. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Makarova, M.N. Anti-inflammatory effect of Pinus sibirica oil extract in animal models. J. Nat. Med. 2008, 62, 436–440. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.D.; Wang, P.; Guo, N.; Wang, W.; Yao, L.P.; Yang, Q.; Efferth, T.; Jiao, J.; Fu, Y.J. Pinolenic acid ameliorates oleic acid-induced lipogenesis and oxidative stress via AMPK/SIRT1 signaling pathway in HepG2 cells. Eur. J. Pharm. 2019, 861, 172618. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.J.; Miles, E.A.; Calder, P.C. A review of the functional effects of pine nut oil, pinolenic acid and its derivative eicosatrienoic acid and their potential health benefits. Prog. Lipid Res. 2021, 82, 101097. [Google Scholar] [CrossRef]
- Calder, P.C. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis, and plaque stability. Mol. Nutr. Food Res. 2012, 56, 1073–1080. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, P.J.; Huang, Y.L.; Chen, S.N.; Chuang, L.T. PGE2 production is suppressed by chemically synthesized Delta7-eicosatrienoic acid in macrophages through the competitive inhibition of COX-2. Food Chem. Toxicol. 2014, 66, 122–133. [Google Scholar] [CrossRef]
- Matsuo, N.; Osada, K.; Kodama, T.; Lim, B.O.; Nakao, A.; Yamada, K. Effects of gamma-linolenic acid and its positional isomer pinolenic acid on immune parameters of brown-Norway rats. Prostagland Leukot. Essent. Fat. Acids 1996, 55, 223–229. [Google Scholar] [CrossRef]
- Park, S.; Lim, Y.; Shin, S.; Han, S.N. Impact of Korean pine nut oil on weight gain and immune responses in high-fat diet-induced obese mice. Nutr. Res. Pract. 2013, 7, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-Y.; Wang, W.-H.; Chen, S.-H.; Chang, Y.-W.; Hung, L.-C.; Chen, C.-Y.; Chen, Y.-H. Lipopolysaccharide-induced nitric oxide, prostaglandin E2, and cytokine production of mouse and human macrophages are suppressed by pheophytin-b. Int. J. Mol. Sci. 2017, 18, 2637. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxid. Med. Cell 2012, 2012, 480895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhang, Y.; Wang, Z.; Zu, Y. In vivo antioxidant activity of Pinus koraiensis nut oil obtained by optimised supercritical carbon dioxide extraction. Nat. Prod. Res. 2011, 25, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, S.; Sheng, Z.; Li, X.; Chang, Y.; Dai, W.; Chang, S.K.; Liu, J.; Yang, Y. Effect of pinolenic acid on oxidative stress injury in HepG2 cells induced by H2O2. Food Sci. Nutr. 2021, 9, 5689–5697. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme oxygenase-1 in kidney health and disease. Antioxid. Redox Signal 2016, 25, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Long, X.; Song, J.; Zhao, X.; Zhang, Y.; Wang, H.; Liu, X.; Suo, H. Silkworm pupa oil attenuates acetaminophen-induced acute liver injury by inhibiting oxidative stress-mediated NF-κB signaling. Food Sci. Nutr. 2020, 8, 237–245. [Google Scholar] [CrossRef]
- Yuemin, W.; Yanlei, X.; Aiping, Z.; Nannan, Z.; Jiashen, Z.; Dongmei, Z.; Zhenhai, Y.; Ning, X.; Yancun, Y.; Xiying, L.; et al. Oligosaccharide attenuates ageing-related liver dysfunction by activating Nrf2 antioxidant signaling. Food Sci. Nutr. 2020, 8, 3872–3881. [Google Scholar]
- Li, R.L.; He, L.Y.; Zhang, Q.; Liu, J.; Lu, F.; Duan, H.X.Y.; Fan, L.H.; Peng, W.; Huang, Y.L.; Wu, C.J. HIF-1α is a potential molecular target for herbal medicine to treat diseases. Drug Des. Dev. Ther. 2020, 14, 4915. [Google Scholar] [CrossRef]
- Marcelin, G.; Silveira, A.L.M.; Martins, L.B.; Ferreira, A.V.; Clément, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Invest. 2019, 129, 4032–4040. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Pérez-Calahorra, S.; Marco-Benedí, V.; Plana, N.; Sánchez, R.; Ros, E.; Ascaso, J.F.; Puzo, J.; Almagro, F.; Lahoz, C.; et al. Effect of LDL cholesterol, statins and presence of mutations on the prevalence of type 2 diabetes in heterozygous familial hypercholesterolemia. Sci. Rep. 2017, 7, 5596. [Google Scholar] [CrossRef] [Green Version]
- Defesche, J.C.; Gidding, S.S.; Harada-Shiba, M.; Hegele, R.A.; Santos, R.D.; Wierzbicki, A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Primers 2017, 3, 17093. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Ikeda, I.; Wakamatsu, K.; Oka, T. Influence of Korean pine (Pinus koraiensis)-seed oil containing cis-5, cis-9, cis-12-octadecatrienoic acid on polyunsaturated fatty acid metabolism, eicosanoid production and blood pressure. Br. J. Nutr. 1994, 72, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Asset, G.; Bauge, E.; Wolff, R.L.; Fruchart, J.-C.; Dallongeville, J. Effects of dietary maritime pine seed oil on lipoprotein metabolism and atherosclerosis development in mice expressing human apolipoprotein B. Eur. J. Nutr. 2001, 40, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.C.; Kim, M.H.; Nam, Y.K.; Yang, W.M. Fat regulatory mechanisms of pine nut oil based on protein interaction network analysis. Phytomedicine 2021, 86, 153557. [Google Scholar] [CrossRef]
- No, D.S.; Kim, I.-H. Pinolenic acid as a new source of phyto-polyunsaturated fatty acid. Lipid Technol. 2013, 25, 135–138. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, K.W.; Lee, S.W.; Kim, I.H.; Rhee, C. Selective increase in pinolenic acid (all-cis-5,9,12-18:3) in Korean pine nut oil by crystallization and its effect on LDL-receptor activity. Lipids 2004, 39, 383–387. [Google Scholar] [CrossRef]
- Park, S.; Shin, S.; Lim, Y.; Shin, J.H.; Seong, J.K.; Han, S.N. Korean pine nut oil attenuated hepatic triacylglycerol accumulation in high-fat diet-induced obese mice. Nutrients 2016, 8, 59. [Google Scholar] [CrossRef] [Green Version]
- Ferramosca, A.; Savy, V.; Einerhand, A.; Zara, V. Pinus koraiensis seed oil (PinnoThinTM) supplementation reduces body weight gain and lipid concentration in liver and plasma of mice. J. Anim. Feed Sci. 2008, 17, 621–630. [Google Scholar] [CrossRef]
- Ferramosca, A.; Savy, V.; Conte, L.; Zara, V. Dietary combination of conjugated linoleic acid (CLA) and pine nut oil prevents CLA-induced fatty liver in mice. J. Agric. Food Chem. 2008, 56, 8148–8158. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ting, A.T.; Seed, B. PPAR-γ agonists inhibit the production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Su, C.G.; Wen, X.; Bailey, S.T.; Jiang, W.; Rangwala, S.M.; Keilbaugh, S.A.; Flanigan, A.; Murthy, S.; Lazar, M.A.; Wu, G.D. A novel therapy for colitis utilizing PPAR-γ ligands to inhibit the epithelial inflammatory response. J. Clin. Invest. 1999, 104, 383–389. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Sliwinski, T.; Zajdel, R. Anti-inflammatory activity of extracts and pure compounds derived from plants via modulation of signalling pathways, especially PI3K/AKT in macrophages. Int. J. Mol. Sci. 2020, 21, 9605. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Orekhov, A.N. Peroxisome proliferator-activated receptor (PPAR) gamma agonists as therapeutic agents for cardiovascular disorders: Focus on atherosclerosis. Curr. Pharm. Des. 2017, 23, 1119–1124. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.-D. PPARs and angiogenesis-implications in pathology. Int. J. Mol. Sci. 2020, 21, 5723. [Google Scholar] [CrossRef]
- Rigamonti, E.; Chinetti-Gbaguidi, G.; Staels, B. Regulation of macrophage functions by PPAR-α, PPAR-γ, and LXRs in mice and men. Arterscler. Thromb Vasc. Biol. 2008, 28, 1050–1059. [Google Scholar] [CrossRef] [Green Version]
- Li, A.C.; Brown, K.K.; Silvestre, M.J.; Willson, T.M.; Palinski, W.; Glass, C.K. Peroxisome proliferator-activated receptor γ ligands inhibit the development of atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 2000, 106, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Young, J.M.; Shand, B.I.; McGregor, P.M.; Scott, R.S.; Frampton, C.M. Comparative effects of and vitamin C supplementation versus vitamin C alone on endothelial function and biochemical markers of oxidative stress and inflammation in chronic smokers. Free. Radic. 2006, 40, 85–94. [Google Scholar] [CrossRef]
- Shand, B.; Strey, C.; Scott, R.; Morrison, Z.; Gieseg, S. Pilot study on the clinical effects of dietary supplementation with Enzogenol, a flavonoid extract of pine bark and vitamin C. Phytother. Res. 2003, 17, 490–494. [Google Scholar] [CrossRef]
- Christiansen, E.; Watterson, K.R.; Stocker, C.J.; Sokol, E.; Jenkins, L.; Simon, K.; Ulven, T. Activity of dietary fatty acids on FFA1 and FFA4 and characterisation of pinolenic acid as a dual FFA1/FFA4 agonist with potential effect against metabolic diseases. Br. J. Nutr. 2015, 113, 1677–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Fujino, M. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar]
- Watterson, K.R.; Hudson, B.D.; Ulven, T.; Milligan, G. Treatment of type 2 diabetes by free fatty acid receptor agonists. Front. Endocrinol. 2014, 5, 137. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Park, S.; Lim, Y.; Shin, S.; Han, S.N. Korean pine nut oil replacement decreases intestinal lipid uptake while improves hepatic lipid metabolism in mice. Nutr. Res. Pract. 2016, 10, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Hughes, G.M.; Boyland, E.J.; Williams, N.J.; Mennen, L.; Scott, C.; Kirkham, T.C.; Harrold, J.A.; Keizer, H.G.; Halford, J.C. The effect of Korean pine nut oil (PinnoThin™) on food intake, feeding behaviour and appetite: A double-blind placebo-controlled trial. Lipids Health Dis. 2008, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Frantisek, Z.; Meuselbach, K. Effect of Pinus koraiensis seed oil on satiety hormones CCK and GLP-1 and appetite suppression. Transl Biomed. 2010, 1, 5. [Google Scholar]
- Chung, M.; Kim, J.; Choi, H.; Choi, H.; Noh, S.K.; Kim, B.H. A structured pine nut oil has hypocholesterolemic activity by increasing LDLR gene expression in the livers of obese mice. Eur. J. Lipid Sci. Technol. 2019, 121, 1900049. [Google Scholar] [CrossRef]
- Liu, B.; Xu, L.; Yu, X.; Li, W.; Sun, X.; Xiao, S.; Wang, H. Protective effect of KLF15 on vascular endothelial dysfunction induced by TNF α. Mol. Med. Rep. 2018, 18, 1987–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Zhang, L.; Liao, X.; Sangwung, P.; Prosdocimo, D.A.; Zhou, G.; Votruba, A.R.; Brian, L.; Han, Y.J.; Gao, H.; et al. Kruppel-like factor 15 is critical for vascular inflammation. J. Clin. Investig. 2013, 123, 4232–4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furer, V.; Greenberg, J.D.; Attur, M.; Abramson, S.; Pillinger, M.H. The role of microRNA in rheumatoid arthritis and other autoimmune diseases. Clin Immunol. 2010, 136, 1–15. [Google Scholar] [CrossRef]
- Chen, K.-C.; Juo, S.-H.H. MicroRNAs in atherosclerosis. Kaohsiung J. Med. Sci. 2010, 28, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, E.; Wang, B.; McClelland, A.; Mohan, M.; Marai, M.; Beuscart, O.; Derouiche, S.; Gray, S.; Pickering, R.; Tikellis, C.; et al. Protective effect of let-7miRNA family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes 2017, 66, 2266–2277. [Google Scholar] [CrossRef] [PubMed]
- Pastore, N.; Brady, O.A.; Diab, H.I.; Martina, J.A.; Sun, L.; Huynh, T.; Lim, J.A.; Zare, H.; Raben, N.; Ballabio, A.; et al. TFEB and TFE3 cooperate in the regulation of the innate immune response in activated macrophages. Autophagy 2016, 12, 1240–1258. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Chae, H.J.; Thomas, M.; Miyazaki, T.; Monosov, A.; Monosov, E.; Krajewska, M.; Krajewski, S.; Reed, J.C. Mammalian dap3 is an essential gene required for mitochondrial homeostasis in vivo and contributes to the extrinsic pathway for apoptosis. FASEB J. 2007, 21, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Busso, N.; Peclat, V.; So, A.; Sappino, A.-P. Plasminogen activation in synovial tissues: Differences between normal, osteoarthritis, and rheumatoid arthritis joints. Ann. Rheum. Dis. 1997, 56, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Raghu, H.; Jone, A.; Cruz, C.; Rewerts, C.L.; Frederick, M.D.; Thornton, S.; Degen, J.L.; Flick, M.J. Plasminogen is a joint-specific positive or negative determinant of arthritis pathogenesis in mice. Arthritis Rheumatol. 2014, 66, 1504–1516. [Google Scholar] [CrossRef] [Green Version]
- Veras, F.P.; Peres, R.S.; Saraiva, A.L.; Pinto, L.G.; Louzada-Junior, P.; Cunha, T.M.; Alves-Filho, J.C. Fructose 1, 6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Sci. Rep. 2015, 5, 15171. [Google Scholar] [CrossRef] [Green Version]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2011, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, B.; Mazidi, M.; Penson, P.; Gluba-Brzózka, A.; Rysz, J.; Banach, M. The sirtuin family members SIRT1, SIRT3 and SIRT6: Their role in vascular biology and atherogenesis. Atherosclerosis 2017, 265, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, Y.; Wang, Y.; Zhao, K.; Chi, Y.; Wang, B. Pyrroloquinoline quinine protects HK-2 cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signalling pathway. Biochem. Biophys. Res. Commun. 2019, 508, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, S.; Yeung, P.; Fang, D. The class III histone deacetylase sirtuin 1 in immune suppression and its therapeutic potential in rheumatoid arthritis. J. Genet. Genom. 2013, 40, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, X.; Liu, Y.; Tan, S.; Li, Y. The role of sirtuin-1 in immune response and systemic lupus erythematosus. Front. Immunol. 2021, 2021, 632383. [Google Scholar] [CrossRef]
- Niederer, F.; Ospelt, C.; Brentano, F.; Hottiger, M.O.; Gay, R.E.; Gay, S.; Detmar, M.; Kyburz, D. SIRT1 overexpression in the rheumatoid arthritis synovium contributes to proinflammatory cytokine production and apoptosis resistance. Ann. Rheum. Dis. 2011, 70, 1866. [Google Scholar] [CrossRef]
- Dlamini, Z.; Ntlabati, P.; Mbita, Z.; Shoba-Zikhali, L. Pyruvate dehydrogenase kinase 4 (PDK4) could be involved in a regulatory role in apoptosis and a link between apoptosis and insulin resistance. Exp. Mol. Pathol. 2015, 98, 574–584. [Google Scholar] [CrossRef]
- Drexler, S.K.; Kong, P.; Inglis, J.; Williams, R.O.; Garlanda, C.; Mantovani, A.; Yazdi, A.S.; Brennan, F.; Feldmann, M.; Foxwell, B.M. SIGIRR/TIR-8 is an inhibitor of toll-like receptor signalling in primary human cells and regulates inflammation in models of rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2249–2261. [Google Scholar] [CrossRef]
- Al-Ahmadi, W.; Webberley, T.S.; Joseph, A.; Harris, F.; Chan, Y.; Alotibi, R.; Williams, J.O.; Alahmadi, A.; Decker, T.; Hughes, T.R.; et al. Pro-atherogenic actions of signal transducer and activator of transcription 1 serine 727 phosphorylation in LDL receptor deficient mice via modulation of plaque inflammation. FASEB J. 2021, 35, e21892. [Google Scholar] [CrossRef]
- O’Morain, V.L.; Chan, Y.; Williams, J.O.; Alotibi, R.; Alahmadi, A.; Rodrigues, N.P.; Plummer, S.F.; Hughes, T.R.; Michael, D.R.; Ramji, D.P. The Lab4P consortium of probiotics attenuates atherosclerosis in LDL receptor deficient mice fed a high fat diet and causes plaque stabilization by inhibiting inflammation and several pro-atherogenic processes. Mol. Nutr. Food Res. 2021, 65, e2100214. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.E.; Williams, J.O.; Al-Ahmadi, W.; O’Morain, V.; Chan, Y.H.; Hughes, T.R.; Menendez-Gonzalez, J.B.; Almotiri, A.; Plummer, S.F.; Rodrigues, N.P.; et al. Protective effects of a unique combination of nutritionally active ingredients on risk factors and gene expression associated with atherosclerosis in C57BL/6J mice fed a high fat diet. Food Funct. 2021, 12, 3657–3671. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, M.; Kawano, S.; DuBois, R.N. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc. Natl. Acad. Sci. USA 1997, 94, 3336–3340. [Google Scholar] [CrossRef] [PubMed]


| Model | PNO or PNLA | Experimental Design | Results and Outcome of PNO and PNLA Treatment | Reference(s) |
|---|---|---|---|---|
| Murine RAW264.7 macrophages | PNLA | 10, 25, 50 or 100 μM PNLA for 24 h followed by lipopolysaccharide (LPS) stimulation (100 ng/mL) for 16 h. 50 μM PNLA for 24 h followed by LPS stimulation (100 ng/mL) for 16 h. | Release of PGE1 by RAW264.7 cells is reduced by 10, 25, 50 and 100 μM PNLA by 33, 49, 73, and 84%, respectively. COX-2 is not affected by 50 μM PNLA. | [16] |
| Human breast cancer MDA- MB-231 cells | PNLA | 50 or 100 μM PNLA for 24 h followed by 12-O-tetradecanoylphorbol-13-acetate (TPA) stimulation (100 ng/mL) for 12 h. 100 μM PNLA for 24 h followed by TPA stimulation (0.1 μg/mL) for 12 h. | Release of PGE2 is reduced by 50 and 75% by 50 and 100 μM PNLA, respectively. COX-2 mRNA and protein is both reduced about 55% by 100 μM PNLA. | [22] |
| Murine macrophage RAW264.7 cells and rat primary peritoneal macrophages | PNLA | 25, 50 and 100 μM PNLA for 24 h followed by LPS stimulation (100 ng/mL) for 16 h. 50 and 100 μM PNLA for 24 h followed by LPS stimulation (0.1 μg/mL) for 8 h. 50 and 100 uM PNLA for 24 h followed by LPS stimulation (100 ng/mL) for 30 min. 50 and 100 μM PNLA for 24 h followed by LPS stimulation (100 ng/mL) for 15 min. | Release of PGE2 by RAW264.7 cells at 50 and 100 μM PNLA is reduced by 67% and 80%, respectively. Release of PGE2 by perineal macrophages is reduced by 13% by 50 μM PNLA but unaffected by 25 μM PNLA. COX-2 in RAW264.7 cells is reduced by 50 and 100 μM PNLA by 20 and 40%, respectively. NF-κB/p65 protein ratio in RAW264.7 cells treated with 50 and 100 μM PNLA is reduced by 40 and 50%, respectively. | [29] |
| Murine microglial BV-2 cells and rat primary peritoneal macrophages | PNLA | 50 μM PNLA for 24 h followed by LPS stimulation (100 ng/mL) for 16 h. | Release of IL-6, TNF-α, NO and PGE2 by BV-2 cells is reduced by 71, 27, 41 and 89%, respectively. In BV-2 cells, iNOS and COX-2 proteins expression are reduced by 53 and 10%, respectively. Production of NO and PGE2 by peritoneal macrophages is reduced by 31 and 35%, respectively. | [17] |
| THP-1 macrophages | PNLA | 10, 25, 50 and 100 μM PNLA for 24 h followed by LPS stimulation (200 ng/mL) for 16 h. | Release of TNF-α is reduced by 9 and 18%, respectively by 50 and 100 μM PNLA. IL-6 is reduced by 9, 24, 33 and 48% by 10, 25, 50 and 100 μM PNLA, respectively. PGE2 is reduced by 55, 67, 78, and 83%, respectively by 10, 25, 50 and 100 μM PNLA. Protein expression of COX-2 is reduced by 50 and 100 μM PNLA by 20 and 25%, respectively. | [19] |
| Hep G2 cells | PNLA | 25 μM PNLA for 12 h followed by 0.5 mM oleic acid for 24h. | Synthesis of NO by HepG2 cells is reduced by 60% with 25 μM PNLA. | [25] |
| EA. hy296 cells | PNLA | 10, 25 and 50 μM PNLA for 48 h followed by TNF-α stimulation (1 ng/mL) for 24 h. 50 μM PNLA for 48 h followed by TNF-α stimulation (1 ng/mL) for 1 h. 25 and 50 μM PNLA for 48 h followed by TNF-α stimulation (1 ng/mL) for 6 h. | Levels of soluble intercellular adhesion molecule (ICAM)-1 by 10, 25, and 50 μM PNLA is reduced by 15, 23 and 24%, respectively. Release of monocyte chemotactic protein (MCP)-1 is reduced with 50 μM PNLA by 25%. Production of regulated on activation, normal T cell expressed and secreted (RANTES) is reduced by 46% with 50 μM PNLA. Phosphorylated-NFκB/NFκB protein ratio is reduced by 50%. Adherence of THP-1 cells to EA.hy296 cell monolayers is reduced by 25% with 50 μM PNLA. | [20,27] |
| THP-1 monocytes, PMA-differentiated THP-1 macrophages and human monocyte-derived macrophages (HMDMs). | PNLA | 25, 50, 75 and 100 μM PNLA for 24 h followed by 20 ng/mL MCP-1 stimulation for 3 h in monocyte migration, and Lucifer Yellow (LY) and oxidized LDL (ox-LDL) incubation for 24 h for macropinocytosis and Dil-ox-LDL uptake, respectively for both THP-1 macrophages and HMDMs. | The mean decrease in MCP-1-mediated THP-1 monocyte migration across all PNLA concentrations was 55%. Macropinocytosis and DiI- ox-LDL uptake was reduced by 50% and 40% in THP-1 macrophages and by 40% and 25% in HMDM, respectively. | [5] |
| Peripheral blood mononuclear cells (PBMCs) from healthy controls (HCs) and rheumatoid arthritis (RA) patients. | PNLA | 25 and 50 μM PNLA for 24 h followed by LPS stimulation (100 ng /mL) for 16 h. | TNF-α and IL-6 levels in cell free supernatants were reduced by 60% from RA patients and in HCs were reduced by 50 and 35%, respectively by 25 and 50 μM PNLA. PGE2 was reduced by 50% from both HCs and RA patients by 50 μM PNLA. | [5] |
| CD14 monocytes purified from RA patients with active disease. | PNLA | 25 and 50 μM PNLA for 24 h followed by LPS stimulation (0.1 μg /mL) for 9 h. The proportions of CD14 monocytes, or CD14 monocytes expressing TNF-α, IL-6, IL-1β, and IL-8 in purified monocytes were assessed by flow cytometry. | The percentage of monocytes expressing TNF-α, IL-6, and IL-1 was decreased by 23%, 25%, and 23%, respectively, by PNLA. Percentages of CD14+ monocytes were reduced by 20% following 25 or 50 μM PNLA. | [6] |
| Male Wistar rats | PNO (P. sibirica) | 300 mg/kg bodyweight PNO for 2 days and 4 h prior to carrageenan injection into right feet and exposing the feet to a heat of 55 °C. | Swelling (paw volume) at 3-, 12- and 24 h following carrageenan injection was reduced by 24, 36 and 45%, respectively. Fever-reducing effect (surface temperature of adjuvant-inflamed paw) was reduced by 5%, 10% and 10%, respectively following 3, 12 and 24 h after carrageenan injection. Analgesic effect (response time to 55 °C thermal-induced hot-plate) was induced by more than 100% following 3 and 12 h while unaffected following 24 h. | [24] |
| Male ICR mice | PNLA | PNLA (3 g) was injected intradermally into the ears for 18 hours, followed by TPA (5 g) for 6 or 24 h. Topical application of PNLA (3 μg) to dorsal skin followed by TPA injection (5 μg) for 2 h. | Ear swelling, thickness and COX-2 protein expression in mouse ear tissue homogenates was reduced by 29, 15, and 53%, respectively. Infiltration of leukocytes (CD45+), neutrophils (Ly6G+CD45+) and macrophages (F4/80+CD45+) was reduced by 63, 50 and 70%, respectively. IL-1, IL-6, TNF-α, and PGE2 levels in the dorsal skin cell-free supernatant was reduced by 79, 68, 42 and 51%, respectively. Phosphorylated p38 expression was dorsal skin tissue is reduced by 55%. | [19] |
| Gene Name | Gene Biotype | Change in Expression |
|---|---|---|
| PDK4 | protein coding | increase |
| TMEM52B | protein coding | increase |
| AC092118.1 | lncRNA | increase |
| CPT1A | protein coding | increase |
| SERPINE1 | protein coding | increase |
| AC087289.4 | lncRNA | increase |
| IGSF6 | Protein coding | increase |
| AKR1C1 | Protein coding | increase |
| AC138207.5 | lncRNA | increase |
| TSPAN10 | Protein coding | increase |
| Gene Name | Gene Biotype | Change in Expression |
|---|---|---|
| FBP1 | protein coding | increase |
| PCAT7 | lncRNA | increase |
| NDRG2 | protein coding | increase |
| PDK4 | protein coding | increase |
| AC015660.2 | lncRNA | increase |
| LINC02244 | lncRNA | increase |
| LRRC32 | protein coding | increase |
| LOXL2 | protein coding | increase |
| CD163L1 | protein coding | decrease |
| DIXDC1 | protein coding | increase |
| Upregulated Genes | Downregulated Genes |
|---|---|
| LY6G5B | SPCS1 |
| PDK4 * | RHNO1 |
| BRF1 | MRPL9 |
| ACAA2 | MT-ND1 |
| ZBTB34 | HSPA1L |
| ACADVL * | MT-CO2 |
| AC007375.2 | CHCHD4 |
| SPINK4 | NIT1 |
| AC090227.2 | OTUB1 |
| CPT1A * | PAIP1 |
| GRIK1 | MEN1 |
| HSD17B8 | OTUB1 |
| NPEPL1 | MEN1 |
| ZNF48 | MT-ATP6 |
| ANKRD23 | ENOX2 |
| CCER2 | LSM1 |
| SLC25A42 | ARL2BP |
| GHRL | MT-ND5 |
| ALG13 | MT-ND4 |
| CRABP2 | SLC10A3 |
| MTRNR2L8 | DEDD |
| ROM1 | DSTN |
| ST14 | DCTN2 |
| SIGIRR | TRAPPC2L |
| ETFA | EDARADD |
| JMJD7-PLA2G4B | NDUFA7 |
| SLC25A20 * | GZF1 |
| Downregulated miRNA | Upregulated miRNA |
|---|---|
| mIR637 | mIR8066 |
| mIR4326 | mIR1276 |
| mIR6886 | mIR3173 |
| mIR1909 | mIR664B |
| mIR671 | mIR6773 |
| mIR7111 | mIR6778 |
| mIR374C | |
| mIR374B | |
| mIR3161 | |
| mIR219B | |
| mIR3922 | |
| mIR219A2 | |
| mIR1914 | |
| mIR505 | |
| mIR3140 | |
| mIR941-3 | |
| mIR324 | |
| mIR4722 | |
| mIR4755 | |
| mIR3176 | |
| mIR3978 | |
| mIR4435-2 | |
| mIR4440 | |
| mIR1470 | |
| mIR570 | |
| mIR6719 |
| miRNA ID | Change in Expression | mRNA Target | Effect | Pathway(s) |
|---|---|---|---|---|
| miR-3173 | increase | CRABP2 | Increase | Acute phase response, signalling for apoptosis mediated by retinoic acid, and regulation of cellular processes by glucocorticoids. |
| miR1260B | increase | JMJD7-PLA2G4B | Increase | VEGF family ligand-receptor interactions, ERK/MAPK signalling, glucocorticoid receptor signalling, MIF regulation of innate immunity, MIF-mediated glucocorticoid regulation, p38 MAPK signalling, and phospholipase C signalling. |
| miR-646 | increase | |||
| miR-1909 | decrease | FZD2 | Decrease | Pathways for adipogenesis, osteoarthritis, and the control of macrophages, fibroblasts, and endothelial cells in rheumatoid arthritis, as well as osteoblasts, osteoclasts, and chondrocytes. |
| miR-1909 | decrease | SIGIRR | Increase | Signalling from the NF-κB transcription factor, TLRs, and TREM1. Signalling by IL-6, TNF-α, and IL-1. Both anti-atherogenic and anti-inflammatory pathways. |
| miR-7150 | increase | |||
| miR-6868-5P | increase | |||
| miR-2861 | decrease | LSM1 | Decrease | Systemic lupus erythematosus signalling. |
| miR-374B | increase | ETFA | Increase | NAD signalling pathway involved in energy production from fats and proteins. |
| miR-4440 | increase | ATMIN | Decrease | Control of the cell cycle checkpoint by CHK proteins. Role in development of the immune system. |
| miR-4440 | increase | GHRL | Increase | Leptin signalling in obesity, appetite and growth hormone regulation. |
| miR-548L | increase | |||
| miR-626 | increase | PDK4 | Increase | Reelin signalling, glucocorticoid receptor signalling, and senescence pathways. TNF-α and NF-κB checkpoint. Lipid and glucose metabolism regulation. Associated with mitochondrial function and cellular energy regulation. Improves VSMCs oxidative stress resistance. |
| miR-3173 | increase | |||
| miR-2861 | decrease | |||
| miR-28 | increase | |||
| miR-7150 | increase | |||
| miR-3188 | increase | |||
| miR-637 | Decrease | SPCS1 | Decrease | Insulin secretion signalling pathways. |
| miR-671 | Decrease | MT-ATP6 | Decrease | Glucocorticoid receptor signalling, mitochondrial dysfunction, oxidative phosphorylation, sirtuin signalling pathway. |
| miR-Let-7 | increase | PDE12 RRP1B TARBP-2 GZF1 NRAS | All decrease | Anti-inflammatory and anti-atherogenic actions. Suppression of immune-modulatory cytokines IL-6 and IL-10. TLR4 signalling. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takala, R.; Ramji, D.P.; Choy, E. The Beneficial Effects of Pine Nuts and Its Major Fatty Acid, Pinolenic Acid, on Inflammation and Metabolic Perturbations in Inflammatory Disorders. Int. J. Mol. Sci. 2023, 24, 1171. https://doi.org/10.3390/ijms24021171
Takala R, Ramji DP, Choy E. The Beneficial Effects of Pine Nuts and Its Major Fatty Acid, Pinolenic Acid, on Inflammation and Metabolic Perturbations in Inflammatory Disorders. International Journal of Molecular Sciences. 2023; 24(2):1171. https://doi.org/10.3390/ijms24021171
Chicago/Turabian StyleTakala, Rabaa, Dipak P. Ramji, and Ernest Choy. 2023. "The Beneficial Effects of Pine Nuts and Its Major Fatty Acid, Pinolenic Acid, on Inflammation and Metabolic Perturbations in Inflammatory Disorders" International Journal of Molecular Sciences 24, no. 2: 1171. https://doi.org/10.3390/ijms24021171






