Genome-Wide Identification and Characterization of Aldo-Keto Reductase (AKR) Gene Family in Response to Abiotic Stresses in Solanum lycopersicum
Abstract
1. Introduction
2. Results
2.1. Identification and Characteristics of SlAKR in S. lycopersicum
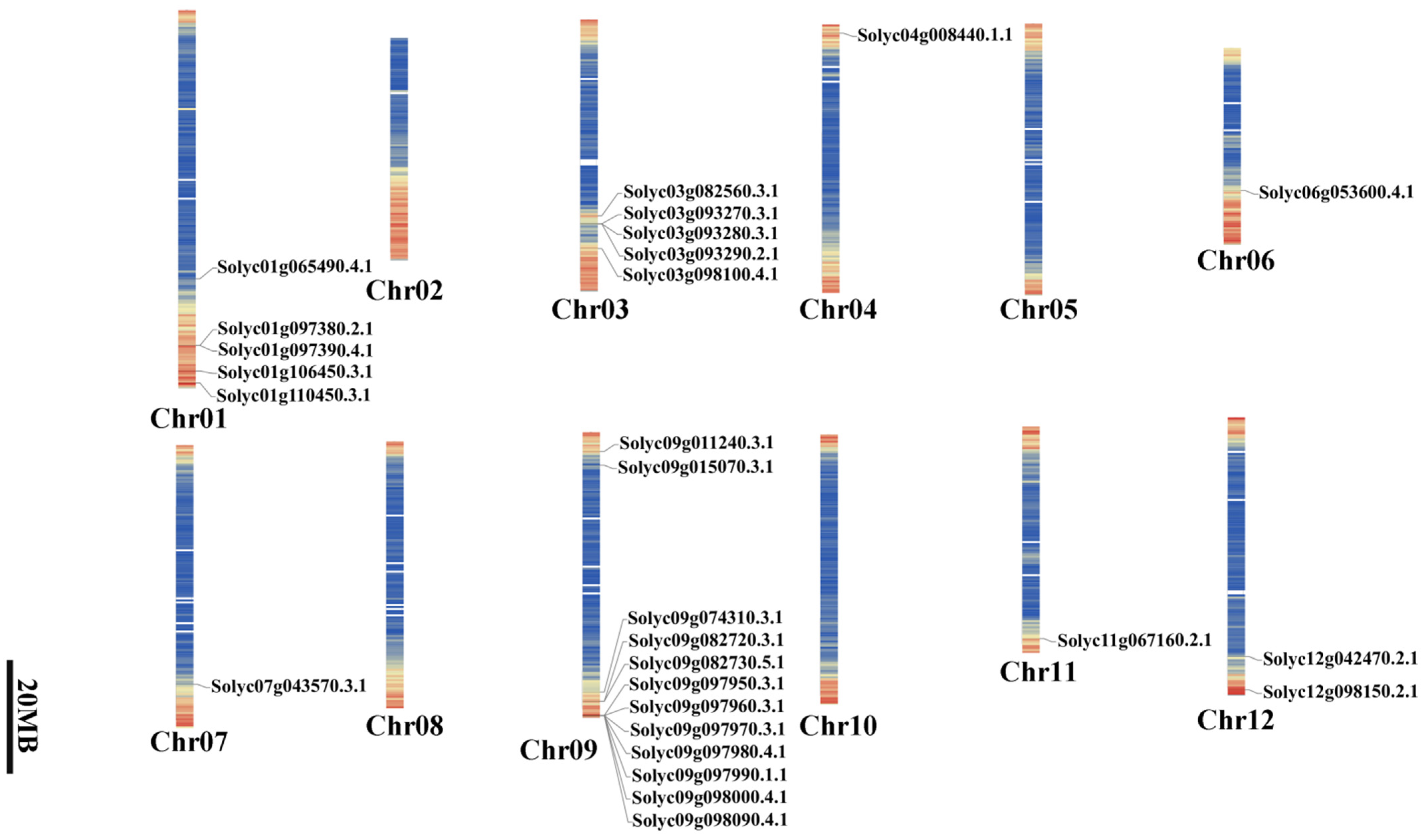
2.2. Locations of SlAKR on the S. lycopersicum Genome
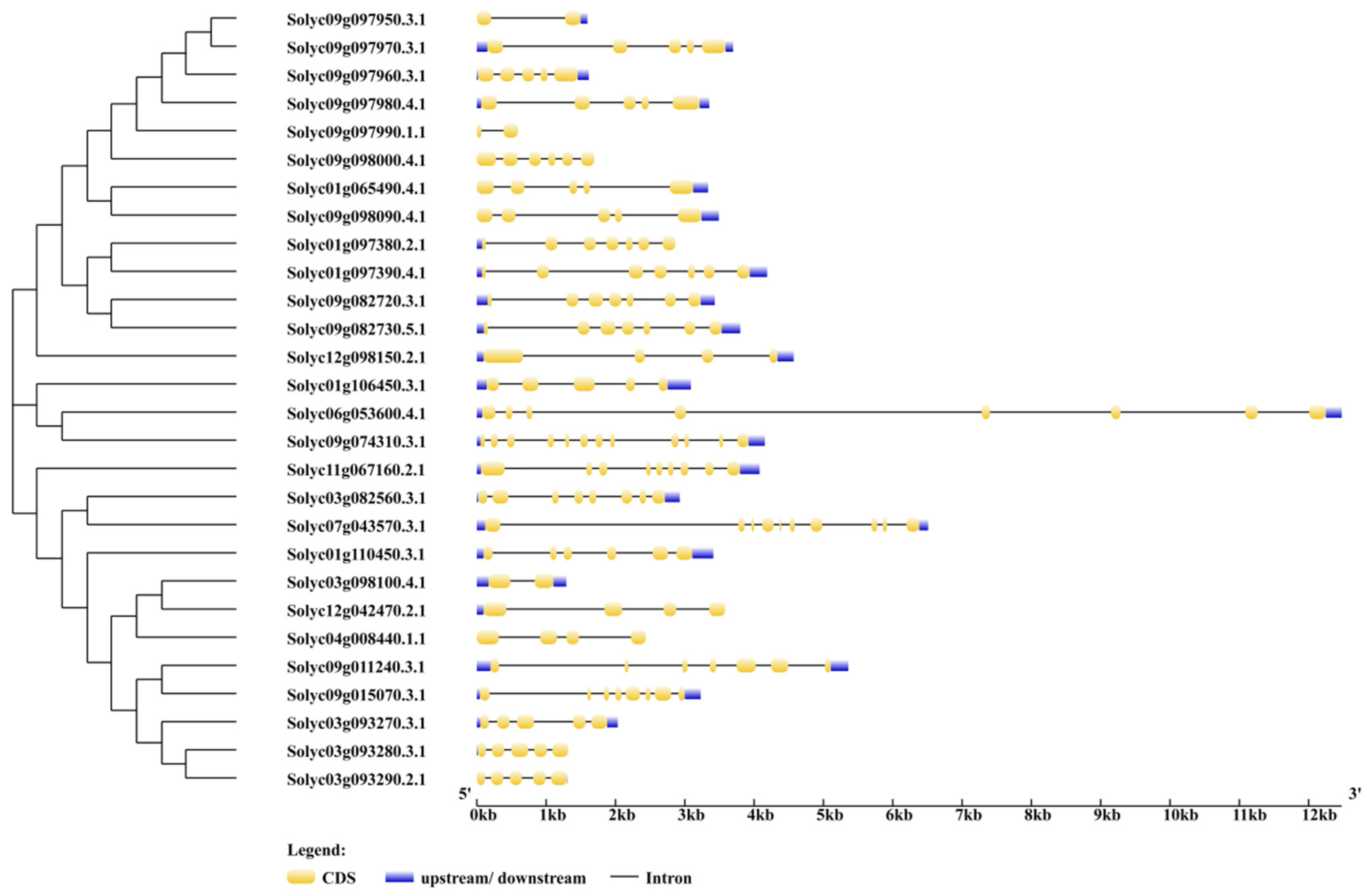
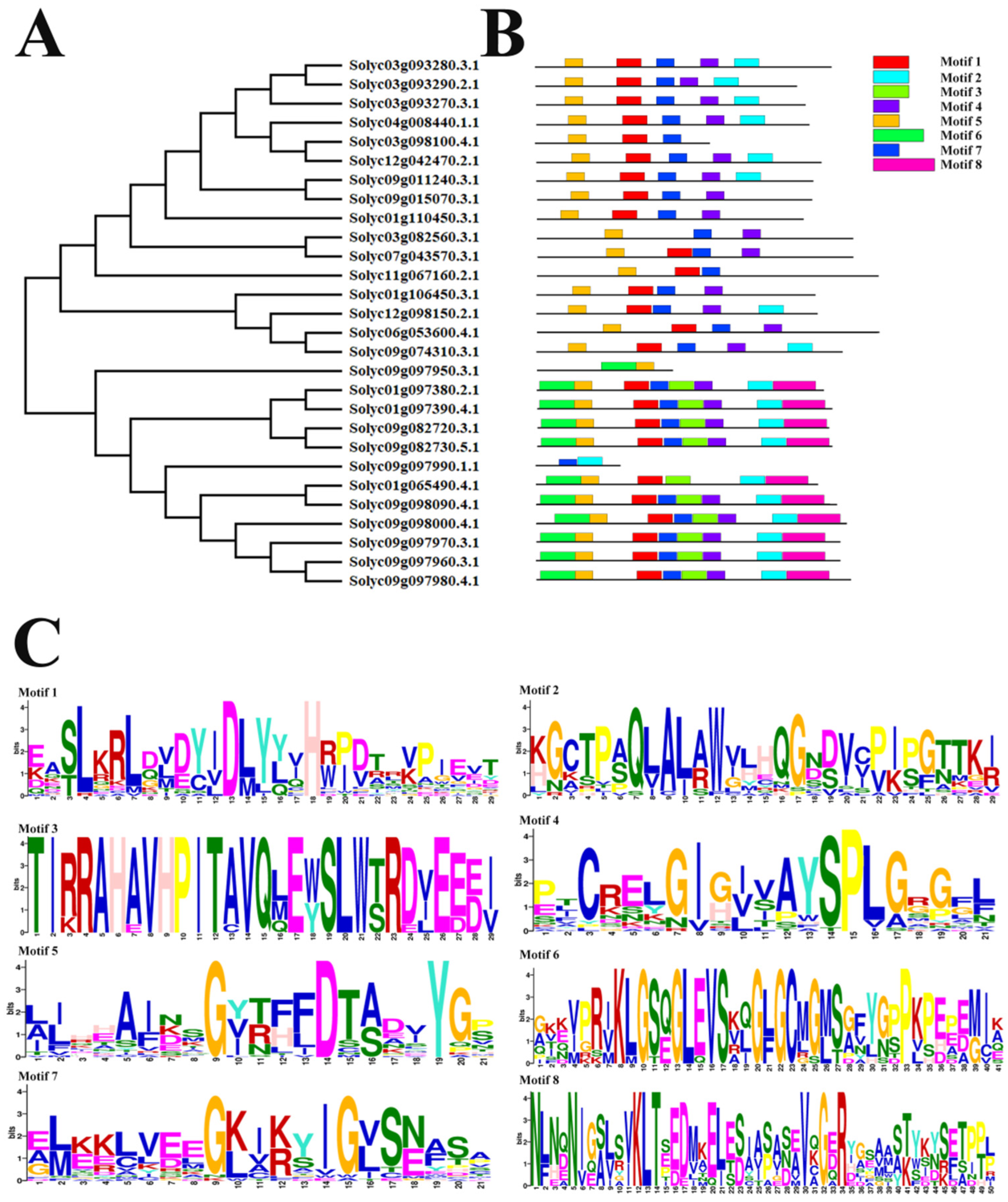
2.3. Phylogenic Relationship and Gene Structures of AKR Genes in S. lycopersicum
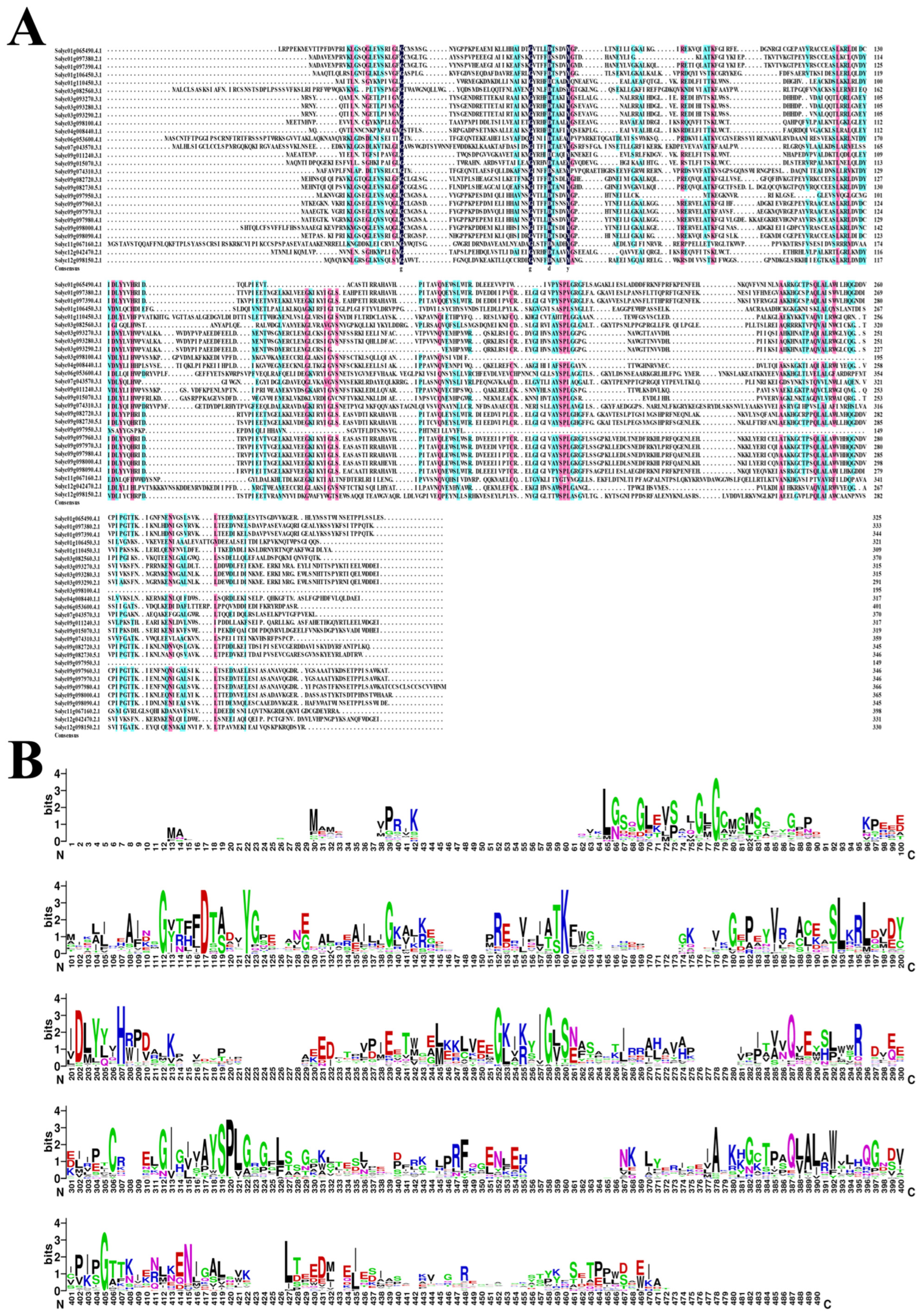
2.4. Alignment and Phylogenic Analysis of the AKR Protein in S. lycopersicum
2.5. Phylogenic Relationships of AKR Protein in S. lycopersicum, A. thaliana, and Oryza sativa
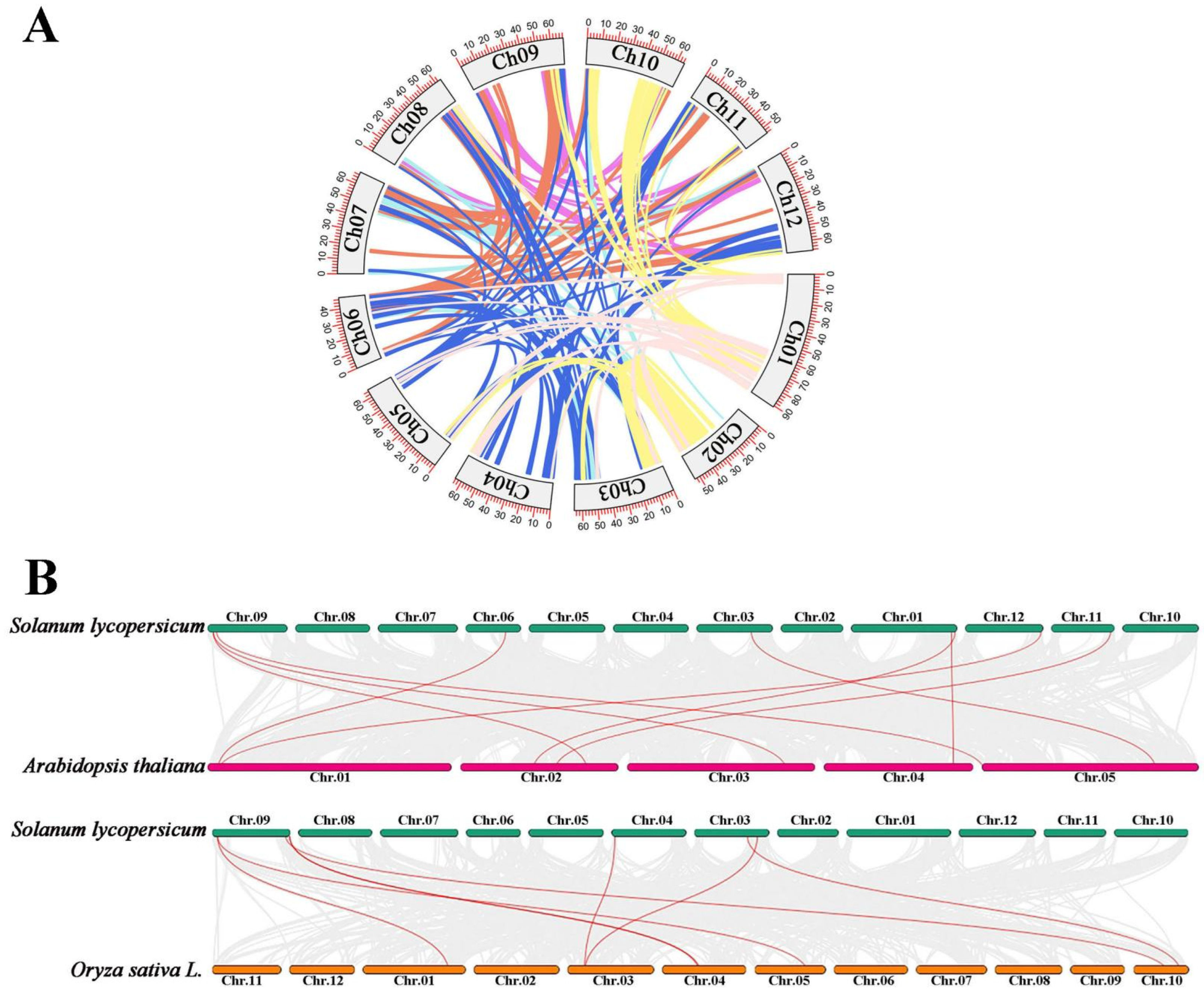
2.6. Collinearity Relationship of AKR Genes in S. lycopersicum, A. thaliana, and O. sativa
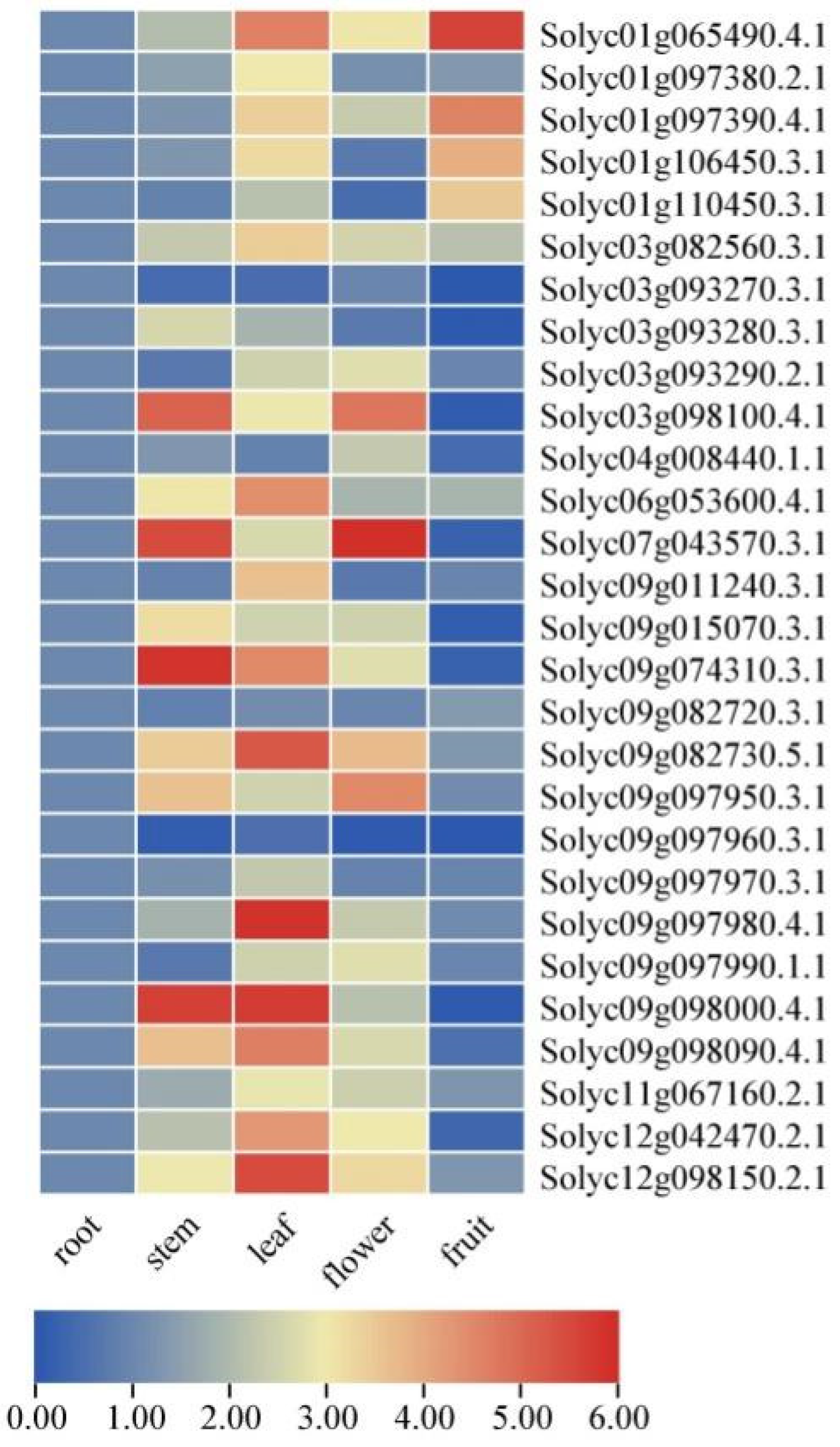
2.7. Expression Levels of SlAKR Family Genes in Various Tissues
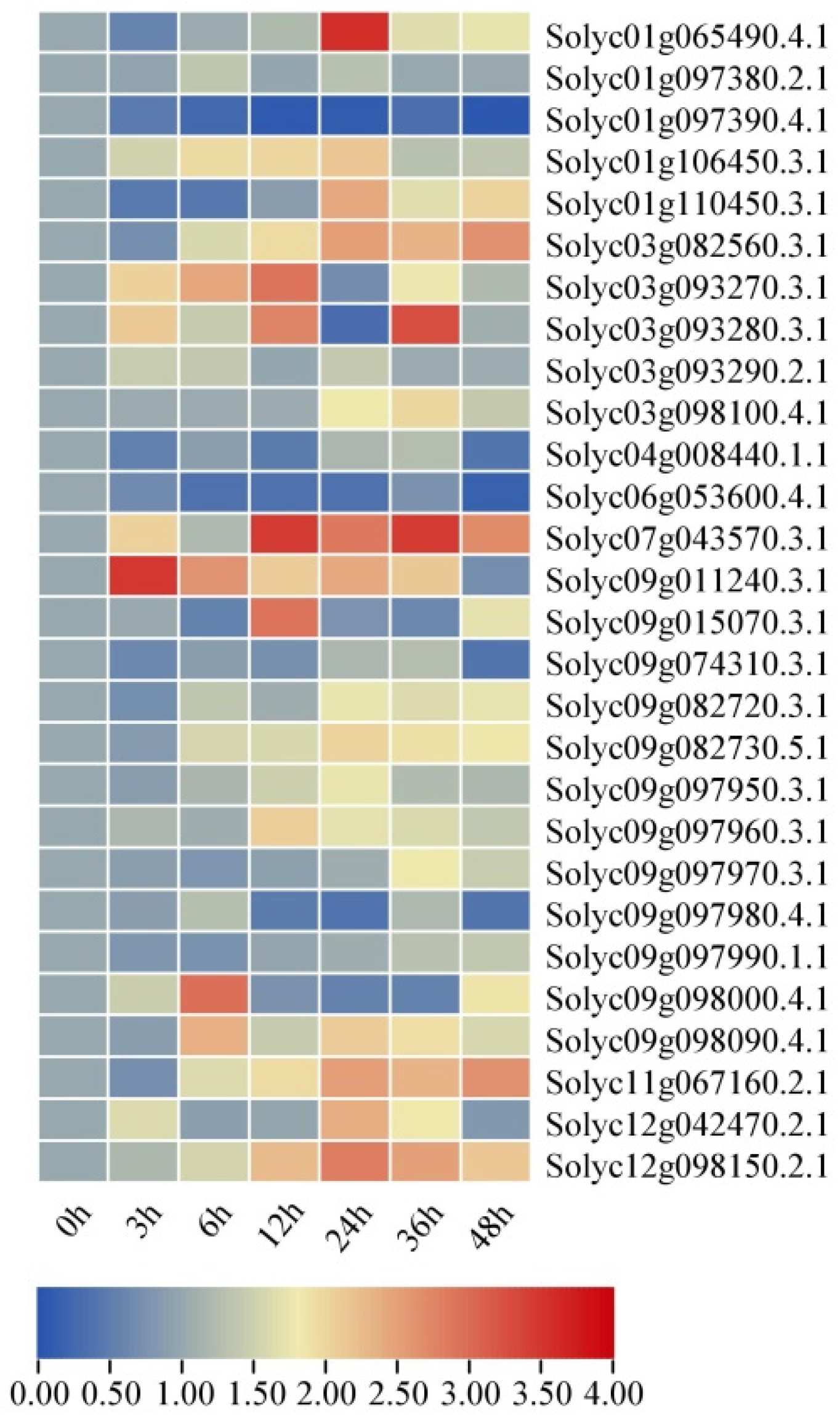
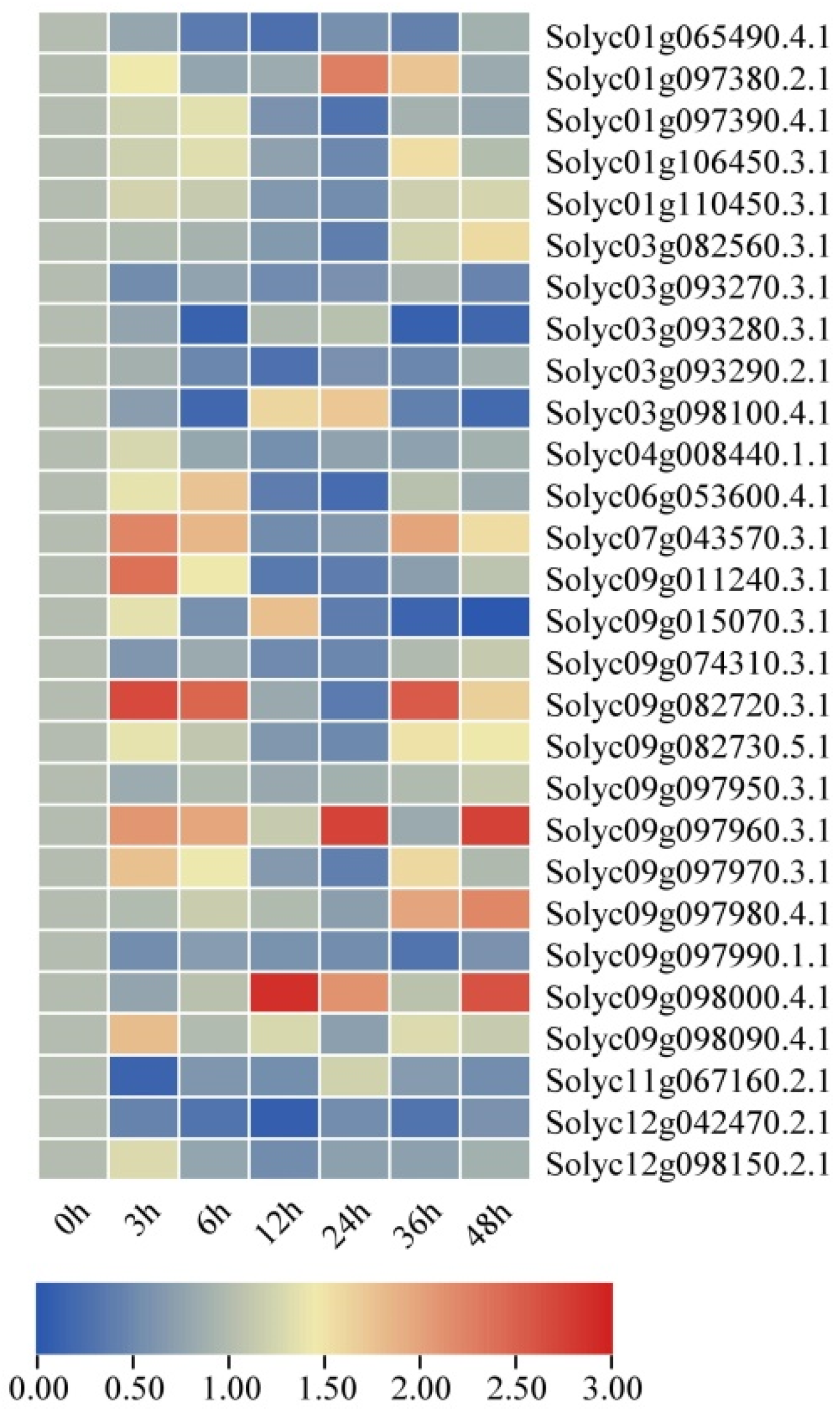
2.8. Expression Profiles of SlAKR Family Genes under Drought and Salt Stresses in AC Lines
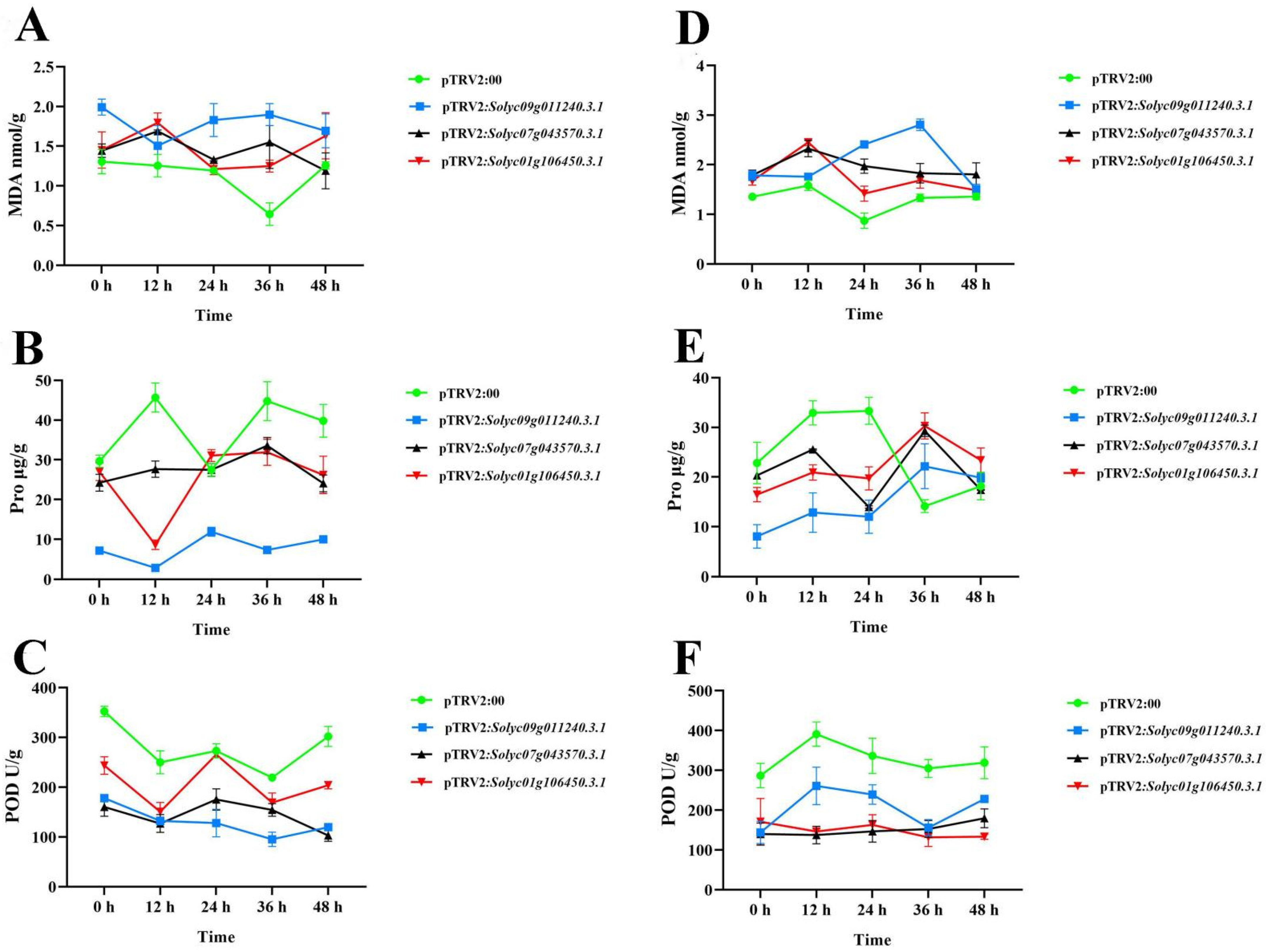
2.9. Functional Analysis of Silencing Solyc09g0112403.1, Solyc07g043570.3.1, and Solyc01g106450.3.1 in S. lycopersicum
3. Discussion
3.1. Analysis of SlAKRs Characterization and Phylogenetic Relationships
3.2. Expression Profiles and Candidate Genes of SlAKRs
4. Materials and Methods
4.1. Plant Materials
4.2. RNA Extraction and Real-Time PCR
4.3. Isolation of AKR Family Members
4.4. Location and Structures of ARK Members
4.5. Phylogenetic Analysis
4.6. Synteny
4.7. Construction of Recombinant Plasmids and Transformation of Tomato
4.8. Measurement of Physiological Indices
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vemanna, R.S.; Babitha, K.C.; Solanki, J.K.; Reddy, V.A.; Sarangi, S.K.; Udayakumar, M. Aldo-keto reductase-1 (AKR1) protect cellular enzymes from salt stress by detoxifying reactive cytotoxic compounds. Plant Physiol. Biochem. 2017, 113, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. The aldo-keto reductases (AKRs): Overview. Chem. Biol. Interact 2015, 234, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.X.; Du, B.; Sun, S.F.; He, S.Z.; Zhao, N.; Liu, Q.C.; Zhai, H. A novel aldo-keto reductase gene IbAKR from sweet potato confers higher tolerance to cadmium stress in tobacco. Front. Agric. Sci. Eng. 2018, 5, 206–213. [Google Scholar]
- Jez, J.M.; Bennett, M.J.; Schlegel, B.P.; Lewis, M.; Penning, T.M. Comparative anatomy of the aldo-keto reductase superfamily. Biochem. J. 1997, 326, 625–636. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Pothiraj, R.; Suthanthiram, B.; Somasundaram, S.M.; Subbaraya, U. Phylogenomic classification and synteny network analyses deciphered the evolutionary landscape of aldo-keto reductase (AKR) gene superfamily in the plant kingdom. Gene 2022, 816, 146169. [Google Scholar] [CrossRef]
- Rajib, S.A.; Sharif Siam, M.K. Characterization and Analysis of Mammalian AKR7A Gene Promoters: Implications for Transcriptional Regulation. Biochem. Genet. 2020, 58, 171–188. [Google Scholar] [CrossRef]
- Morita, H.; Mizuuchi, Y.; Abe, T.; Kohno, T.; Noguchi, H.; Abe, I. Cloning and Functional Analysis of a Novel Aldo-Keto Reductase from Aloe arborescens. Biol. Pharm. Bull. 2007, 30, 2262–2267. [Google Scholar] [CrossRef]
- Éva, C.; Tóth, G.; Oszvald, M.; Tamás, L. Overproduction of an Arabidopsis aldo–keto reductase increases barley tolerance to oxidative and cadmium stress by an in vivo reactive aldehyde detoxification. Plant Growth Regul. 2014, 74, 55–63. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Simpson, P.J.; Tantitadapitak, C.; Reed, A.M.; Mather, O.C.; Bunce, C.M.; White, S.A.; Ride, J.P. Characterization of Two Novel Aldo–Keto Reductases from Arabidopsis: Expression Patterns, Broad Substrate Specificity, and an Open Active-Site Structure Suggest a Role in Toxicant Metabolism Following Stress. J. Mol. Biol. 2009, 392, 465–480. [Google Scholar] [CrossRef]
- Turoczy, Z.; Kis, P.; Torok, K.; Cserhati, M.; Lendvai, A.; Dudits, D.; Horvath, G.V. Overproduction of a rice aldo-keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol. Biol. 2011, 75, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Bartels, D.; Engelhardt, K.; Roncarati, R.; Schneider, K.; Rotter, M.; Salamini, F. An ABA and GA modulated gene expressed in the barley embryo encodes an aldose reductase related protein. EMBO J. 1991, 10, 1037–1043. [Google Scholar] [CrossRef]
- Yu, J.; Sun, H.; Zhang, J.; Hou, Y.; Zhang, T.; Kang, J.; Wang, Z.; Yang, Q.; Long, R. Analysis of Aldo–Keto Reductase Gene Family and Their Responses to Salt, Drought, and Abscisic Acid Stresses in Medicago truncatula. Int. J. Mol. Sci. 2020, 21, 754. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.Z.; Liu, H.B.; Ni, Y.; Dong, J.; Zhong, C.F.; Sun, R.; Li, S.T.; Xiong, R.; Wang, G.X.; Sun, J.; et al. FaAKR23 Modulates Ascorbic Acid and Anthocyanin Accumulation in Strawberry (Fragaria x ananassa) Fruits. Antioxidants 2022, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Suekawa, M.; Fujikawa, Y.; Inada, S.; Murano, A.; Esaka, M. Gene expression and promoter analysis of a novel tomato aldo-keto reductase in response to environmental stresses. J. Plant Physiol. 2016, 200, 35–44. [Google Scholar] [CrossRef]
- Kanayama, Y.; Mizutani, R.; Yaguchi, S.; Hojo, A.; Ikeda, H.; Nishiyama, M.; Kanahama, K. Characterization of an uncharacterized aldo-keto reductase gene from peach and its role in abiotic stress tolerance. Phytochemistry 2014, 104, 30–36. [Google Scholar] [CrossRef]
- Auiyawong, B.; Narawongsanont, R.; Tantitadapitak, C. Characterization of AKR4C15, a Novel Member of Aldo–Keto Reductase, in Comparison with Other Rice AKR(s). Protein J. 2017, 36, 257–269. [Google Scholar] [CrossRef]
- Gavidia, I.; Perez-Bermudez, P.; Seitz, H.U. Cloning and expression of two novel aldo-keto reductases from Digitalis purpurea leaves. Eur. J. Biochem. 2002, 269, 2842–2850. [Google Scholar] [CrossRef]
- Liu, H.B.; Wei, L.Z.; Ni, Y.; Chang, L.L.; Dong, J.; Zhong, C.F.; Sun, R.; Li, S.T.; Xiong, R.; Wang, G.X.; et al. Genome-Wide Analysis of Ascorbic Acid Metabolism Related Genes in Fragaria x ananassa and Its Expression Pattern Analysis in Strawberry Fruits. Front. Plant Sci. 2022, 13, 954505. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Ansari, W.A.; Soumia, P.S.; Yadav, A.; Jaiswal, D.K.; Kumar, S.; Singh, A.K.; Singh, M.; Verma, J.P. Biotechnological Interventions in Tomato (Solanum lycopersicum) for Drought Stress Tolerance: Achievements and Future Prospects. BioTech 2022, 11, 48. [Google Scholar] [CrossRef]
- Bai, Y.; Kissoudis, C.; Yan, Z.; Visser, R.G.F.; van der Linden, G. Plant behaviour under combined stress: Tomato responses to combined salinity and pathogen stress. Plant J. 2018, 93, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, D.; Bauman, D.R.; Heredia, V.V.; Penning, T.M. The aldo-keto reductase superfamily homepage. Chem.-Biol. Interact 2003, 143, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, D.; Naik, D.; Reddy, A.R. Plant aldo-keto reductases (AKRs) as multi-tasking soldiers involved in diverse plant metabolic processes and stress defense: A structure-function update. J. Plant Physiol. 2015, 179, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Landherr, L.L.; Frohlich, M.W.; Leebens-Mack, J.; Ma, H.; dePamphilis, C.W. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: Evidence for multiple mechanisms of rapid gene birth. Plant J. 2007, 50, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Nagy, T.; Oberschall, A.; Dudits, D.; Vass, I. Detoxification function of aldose/aldehyde reductase during drought and ultraviolet-B (280-320 nm) stresses. Plant Cell Environ. 2003, 26, 513–522. [Google Scholar] [CrossRef]
- Colrat, S.G.N.; Latché, A.; Guis, M.; Pech, J.-C.; Bouzayen, M.; Fallot, J.; Roustan, J.-P. Purification and Characterization of a NADPH-Dependent Aldehyde Reductase from Mung Bean That Detoxifies Eutypine, a Toxin from Eutypa lata1. Plant Physiol. 1999, 119, 621–626. [Google Scholar] [CrossRef]
- Guillen, P.; Guis, M.; Martinez-Reina, G.; Colrat, S.; Dalmayrac, S.; Deswarte, C.; Bouzayen, M.; Roustan, J.P.; Fallot, J.; Pech, J.C.; et al. A novel NADPH-dependent aldehyde reductase gene from Vigna radiata confers resistance to the grapevine fungal toxin eutypine. Plant J. 1998, 16, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Oberschall, A.; Deak, M.; Torok, K.; Sass, L.; Vass, I.; Kovacs, I.; Feher, A.; Dudits, D.; Horvath, G.V. A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses. Plant J. 2000, 24, 437–446. [Google Scholar] [CrossRef]
- Éva, C.; Zelenyánszki, H.; Tömösközi-Farkas, R.; Tamás, L. Transgenic barley expressing the Arabidopsis AKR4C9 aldo-keto reductase enzyme exhibits enhanced freezing tolerance and regenerative capacity. S. Afr. J. Bot. 2014, 93, 179–184. [Google Scholar] [CrossRef]
- Somyong, S.; Poopear, S.; Jomchai, N.; Uthaipaisanwong, P.; Ruang-areerate, P.; Sangsrakru, D.; Sonthirod, C.; Ukoskit, K.; Tragoonrung, S.; Tangphatsornruang, S. The AKR gene family and modifying sex ratios in palms through abiotic stress responsiveness. Funct. Integr. Genom. 2015, 15, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, X.Y.; Liu, S.N.; Yang, M.; Ren, J.H.; Guo, M.; Huang, Z.H.; Zhang, Y.W. Genome-Wide Identification and Analysis of TCP Transcription Factors Involved in the Formation of Leafy Head in Chinese Cabbage. Int. J. Mol. Sci. 2018, 19, 847. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]

| Gene ID | Location | Exon | CDS (bp) | Protein (aa) | MW (da) | PI |
|---|---|---|---|---|---|---|
| Solyc01g065490.4.1 | Ch01:64503126..64507794 | 5 | 961 | 319 | 35,408.56 | 5.46 |
| Solyc01g097380.2.1 | Ch01:80521659..80524521 | 7 | 1002 | 333 | 36,601.78 | 5.47 |
| Solyc01g097390.4.1 | Ch01:80526571..80530758 | 7 | 1036 | 344 | 38,093.9 | 7.14 |
| Solyc01g106450.3.1 | Ch01:86648540..86651626 | 5 | 967 | 321 | 34,659.69 | 5.4 |
| Solyc01g110450.3.1 | Ch01:89495104..89498514 | 6 | 931 | 309 | 34,691.84 | 6.15 |
| Solyc03g082560.3.1 | Ch03:47065999..47068924 | 8 | 1113 | 370 | 44,692.65 | 9.28 |
| Solyc03g093270.3.1 | Ch03:49011703..49013733 | 5 | 947 | 315 | 35,907.79 | 5.12 |
| Solyc03g093280.3.1 | Ch03:49018029..49019346 | 5 | 948 | 315 | 36,067.09 | 5.75 |
| Solyc03g093290.2.1 | Ch03:49025805..49027630 | 5 | 876 | 291 | 33,388.95 | 5.75 |
| Solyc03g098100.4.1 | Ch03:54950796..54952085 | 2 | 589 | 195 | 21,617.42 | 5.81 |
| Solyc04g008440.1.1 | Ch04:2097095..2100505 | 4 | 954 | 317 | 35,736.61 | 7.11 |
| Solyc06g053600.4.1 | Ch06:34195838..34208314 | 8 | 1207 | 401 | 45,400.67 | 7.59 |
| Solyc07g043570.3.1 | Ch07:57339225..57345735 | 10 | 1069 | 355 | 39,163.51 | 7.65 |
| Solyc09g011240.3.1 | Ch09:4605903..4611260 | 7 | 955 | 317 | 35,568.88 | 6.27 |
| Solyc09g015070.3.1 | Ch09:7921034..7924262 | 8 | 961 | 319 | 36,210.64 | 5.97 |
| Solyc09g074310.3.1 | Ch09:62302505..62306658 | 12 | 1080 | 359 | 40,259.85 | 6.20 |
| Solyc09g082720.3.1 | Ch09:64521009..64524440 | 7 | 1039 | 345 | 38,306.89 | 5.91 |
| Solyc09g082730.5.1 | Ch09:64525521..64529319 | 7 | 1042 | 346 | 38,202.57 | 5.43 |
| Solyc09g097950.3.1 | Ch09:67972899..67974512 | 5 | 450 | 149 | 16,033.51 | 6.03 |
| Solyc09g097960.3.1 | Ch09:67972899..67974512 | 5 | 1042 | 349 | 38,212.53 | 5.87 |
| Solyc09g097970.3.1 | Ch09:67974976..67978670 | 5 | 1042 | 346 | 37,766.09 | 5.9 |
| Solyc09g097980.4.1 | Ch09:67978934..67982284 | 5 | 1057 | 351 | 38,574.17 | 6.24 |
| Solyc09g097990.1.1 | Ch09:67983109..67983935 | 2 | 273 | 90 | 10,277.02 | 8.48 |
| Solyc09g098000.4.1 | Ch09:67985560..67987253 | 6 | 1051 | 349 | 38,675.3 | 5.92 |
| Solyc09g098090.4.1 | Ch09:68042656..68046145 | 5 | 1038 | 345 | 38,729.75 | 5.61 |
| Solyc11g067160.2.1 | Ch11:50922853..50926930 | 9 | 1197 | 398 | 44,437.18 | 8.85 |
| Solyc12g042470.2.1 | Ch12:57401753..57405333 | 4 | 996 | 331 | 37,500.62 | 6.47 |
| Solyc12g098150.2.1 | Ch12:65298986..65303554 | 4 | 993 | 330 | 36,956.2 | 7.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Yu, L.; Wang, A. Genome-Wide Identification and Characterization of Aldo-Keto Reductase (AKR) Gene Family in Response to Abiotic Stresses in Solanum lycopersicum. Int. J. Mol. Sci. 2023, 24, 1272. https://doi.org/10.3390/ijms24021272
Guan X, Yu L, Wang A. Genome-Wide Identification and Characterization of Aldo-Keto Reductase (AKR) Gene Family in Response to Abiotic Stresses in Solanum lycopersicum. International Journal of Molecular Sciences. 2023; 24(2):1272. https://doi.org/10.3390/ijms24021272
Chicago/Turabian StyleGuan, Xiaoyu, Lei Yu, and Aoxue Wang. 2023. "Genome-Wide Identification and Characterization of Aldo-Keto Reductase (AKR) Gene Family in Response to Abiotic Stresses in Solanum lycopersicum" International Journal of Molecular Sciences 24, no. 2: 1272. https://doi.org/10.3390/ijms24021272
APA StyleGuan, X., Yu, L., & Wang, A. (2023). Genome-Wide Identification and Characterization of Aldo-Keto Reductase (AKR) Gene Family in Response to Abiotic Stresses in Solanum lycopersicum. International Journal of Molecular Sciences, 24(2), 1272. https://doi.org/10.3390/ijms24021272






