Pretreated Mesenchymal Stem Cells and Their Secretome: Enhanced Immunotherapeutic Strategies
Abstract
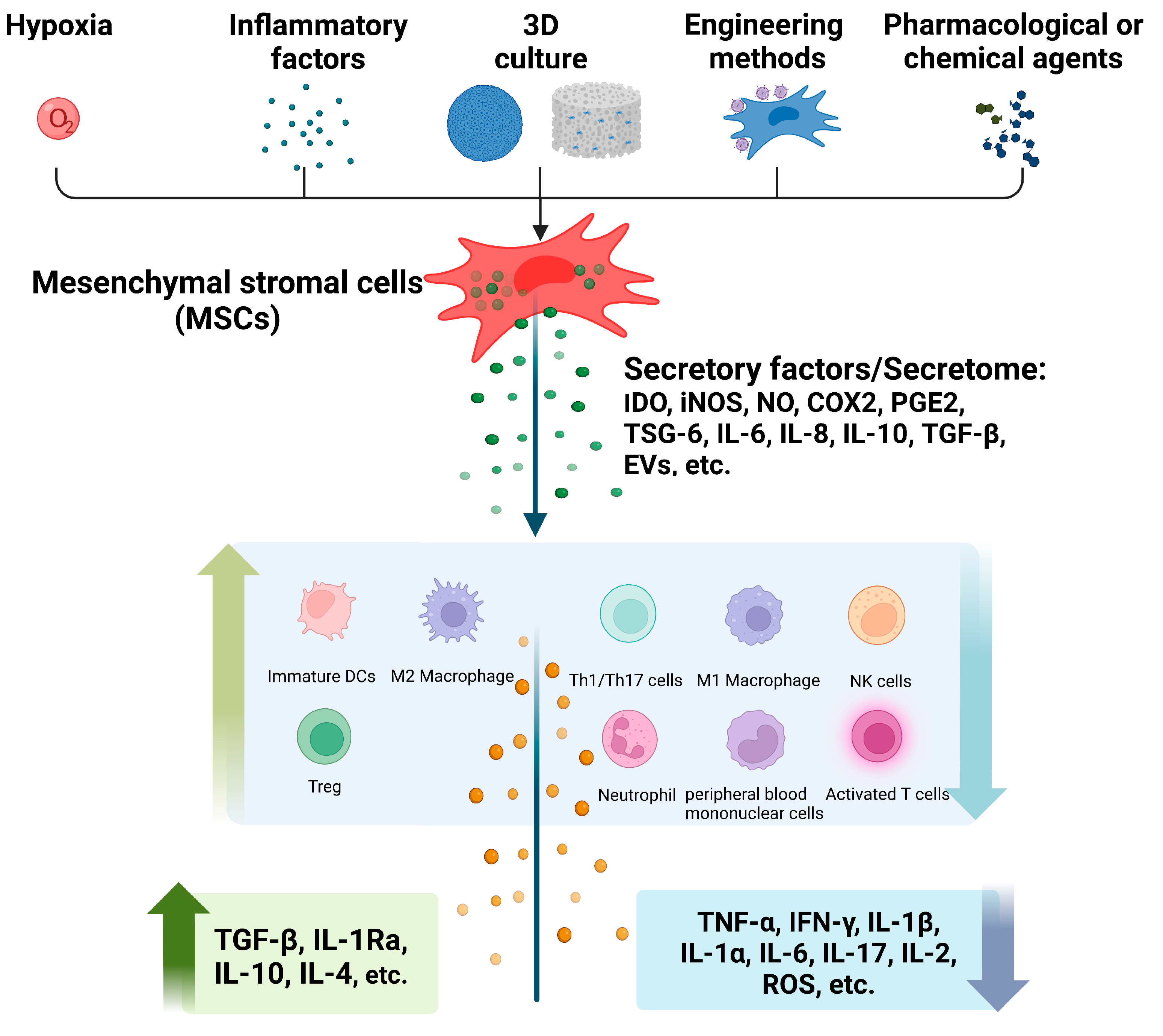
:1. Introduction
1.1. Immunoregulatory Characteristics of MSCs
1.2. MSC-Derived Secretome
2. The Immunomodulatory Effects of Pretreated MSCs and Their Secretome
2.1. Hypoxia
2.2. Inflammatory Factors
2.2.1. IFN-γ
2.2.2. TNF-α
2.2.3. IL-1β
2.2.4. Lipopolysaccharide (LPS)
2.2.5. Polyinosinic–Polycytidylic Acid (poly(I:C))
2.2.6. Combined Pretreatment of Inflammatory Factors
2.3. D Cell Culture
2.4. Engineered Pretreatment
2.5. Pharmacological or Chemical Agents
3. Immunomodulatory Mechanisms of Pretreated MSCs and Their Secretome
3.1. IDO/iNOS
3.2. COX2/PGE2
3.3. TSG-6
3.4. MSC-Derived EVs
3.5. IL-6
4. Problems and Prospects
- Selection of pretreatment methods: it is essential to understand how each pretreatment method affects MSCs’ paracrine behavior. Some studies suggest that the combination of different pretreatment methods is superior to one single method, but further studies are still required to confirm this. Meanwhile, pretreatment strategies can be categorized as selective or non-selective: selective approaches, such as engineered methods, target a single pathway or a small number of related pathways to achieve a desired secretome, whereas non-selective strategies, such as hypoxia or inflammatory factors, activate multiple signaling pathways that collectively increase expression of downstream signal factors or receptors.
- Heterogeneity: it is extremely difficult to understand the mechanism underlying the reported effects on pretreatment of MSCs due to their tissue origin, the health and age of the donors, cells separation and culture techniques and the animal models.
- Purification: the current method for collecting and purifying secretome is centrifugation to remove the cell debris in CM [112], and some studies concentrated the secretome after centrifugation [108,143]. However, additional culture media components may be collected simultaneously and may influence the purity of secretome. Currently, there is no standard purification method of secretome as there is for exosomes.
- Components selection: the comparative effect of the specific components (soluble factors or EVs) and the overall application of secretome remains to be verified.
- Standardization and optimization: this is problematic because secretome is a combination of various molecules and deserves further study to evaluate its potency and determine a safe dosage.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, M.; Ma, L.; Yuan, Y.; Ye, X.; Montagne, A.; He, J.; Ho, T.V.; Wu, Y.; Zhao, Z.; Sta Maria, N.; et al. Cranial Suture Regeneration Mitigates Skull and Neurocognitive Defects in Craniosynostosis. Cell 2021, 184, 243–256. [Google Scholar] [CrossRef]
- Mrahleh, M.A.; Matar, S.; Jafar, H.; Wehaibi, S.; Aslam, N.; Awidi, A. Human Wharton’s Jelly-Derived Mesenchymal Stromal Cells Primed by Tumor Necrosis Factor-α and Interferon-γ Modulate the Innate and Adaptive Immune Cells of Type 1 Diabetic Patients. Front. Immunol. 2021, 12, 732549. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Bátiz, L.F.; Contreras, R.A.; Ortúzar, M.I.; Kurte, M.; Elizondo-Vega, R.; et al. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020, 21, e48052. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, J.; Liu, Y.; Qin, Y.; Luo, Q.; Wang, Q.; Duan, H. TLR4 plays a crucial role in MSC-induced inhibition of NK cell function. Biochem. Biophys. Res. Commun. 2015, 464, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Planella, L.; Monguió-Tortajada, M.; Borràs, F.E.; Franquesa, M. Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Front. Immunol. 2019, 10, 1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrou, P.; Kassis, I.; Levin, N.; Paul, F.; Backner, Y.; Benoliel, T.; Oertel, F.C.; Scheel, M.; Hallimi, M.; Yaghmour, N.; et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 2020, 143, 3574–3588. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, L.; Wang, X.; Zhou, X.; Zhang, X.; Li, L.; Wu, J.; Kou, M.; Cai, C.; Lian, Q.; et al. Topical and intravenous administration of human umbilical cord mesenchymal stem cells in patients with diabetic foot ulcer and peripheral arterial disease: A phase I pilot study with a 3-year follow-up. Stem Cell Res. Ther. 2022, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yu, J.; Zhang, N.; Li, W.; Wang, J.; Cai, G.; Chen, Y.; Yang, Y.; Liu, Z. Bone marrow mesenchymal stem cells transfer in patients with ST-segment elevation myocardial infarction: Single-blind, multicenter, randomized controlled trial. Stem Cell Res. Ther. 2021, 12, 33. [Google Scholar] [CrossRef]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar] [CrossRef]
- Garcia-Olmo, D.; Gilaberte, I.; Binek, M.; AJL, D.H.; Lindner, D.; Selvaggi, F.; Spinelli, A.; Panés, J. Follow-up Study to Evaluate the Long-term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn’s Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis. Colon Rectum 2022, 65, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. BioFactors 2017, 43, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Veenendaal, T.; Wiendels, M.; Ruiz-Zapata, A.M.; van Laar, J.; Kyranas, R.; Enting, H.; van Cranenbroek, B.; Koenen, H.; Mihaila, S.M.; et al. Synthetic Extracellular Matrices as a Toolbox to Tune Stem Cell Secretome. ACS Appl. Mater. Interfaces 2020, 12, 56723–56730. [Google Scholar] [CrossRef] [PubMed]
- Roemeling-van Rhijn, M.; Mensah, F.K.; Korevaar, S.S.; Leijs, M.J.; van Osch, G.J.; Ijzermans, J.N.; Betjes, M.G.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Effects of Hypoxia on the Immunomodulatory Properties of Adipose Tissue-Derived Mesenchymal Stem cells. Front. Immunol. 2013, 4, 203. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amodeo, G.; Niada, S.; Moschetti, G.; Franchi, S.; Savadori, P.; Brini, A.T.; Sacerdote, P. Secretome of human adipose-derived mesenchymal stem cell relieves pain and neuroinflammation independently of the route of administration in experimental osteoarthritis. Brain. Behav. Immun. 2021, 94, 29–40. [Google Scholar] [CrossRef]
- Isildar, B.; Ozkan, S.; Ercin, M.; Gezginci-Oktayoglu, S.; Oncul, M.; Koyuturk, M. 2D and 3D cultured human umbilical cord-derived mesenchymal stem cell-conditioned medium has a dual effect in type 1 diabetes model in rats: Immunomodulation and beta-cell regeneration. Inflamm. Regen. 2022, 42, 55. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef]
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S.; Cheng, K. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv. Healthc. Mater. 2019, 8, e1900411. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Alam, P.; Pacelli, S.; Chakravarti, A.R.; Ahmed, R.P.H.; Paul, A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 2018, 69, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, C.; Ceresa, D.; Lesage, R.; Villa, F.; Reverberi, D.; Balbi, C.; Santamaria, S.; Cortese, K.; Malatesta, P.; Geris, L.; et al. Dissecting the effects of preconditioning with inflammatory cytokines and hypoxia on the angiogenic potential of mesenchymal stromal cell (MSC)-derived soluble proteins and extracellular vesicles (EVs). Biomaterials 2021, 269, 120633. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Raggi, F.; Pelassa, S.; Pierobon, D.; Penco, F.; Gattorno, M.; Novelli, F.; Eva, A.; Varesio, L.; Giovarelli, M.; Bosco, M.C. Regulation of Human Macrophage M1-M2 Polarization Balance by Hypoxia and the Triggering Receptor Expressed on Myeloid Cells-1. Front. Immunol. 2017, 8, 1097. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.M.; Liu, J.; Zhao, J.Y.; Xiao, L.; An, S.; Gou, Y.C.; Quan, H.X.; Cheng, Q.; Zhang, Y.L.; He, W.; et al. Effects of hypoxia on the immunomodulatory properties of human gingiva-derived mesenchymal stem cells. J. Dent. Res. 2015, 94, 69–77. [Google Scholar] [CrossRef]
- Kim, Y.; Jin, H.J.; Heo, J.; Ju, H.; Lee, H.Y.; Kim, S.; Lee, S.; Lim, J.; Jeong, S.Y.; Kwon, J.; et al. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia 2018, 32, 2672–2684. [Google Scholar] [CrossRef]
- Kadle, R.L.; Abdou, S.A.; Villarreal-Ponce, A.P.; Soares, M.A.; Sultan, D.L.; David, J.A.; Massie, J.; Rifkin, W.J.; Rabbani, P.; Ceradini, D.J. Microenvironmental cues enhance mesenchymal stem cell-mediated immunomodulation and regulatory T-cell expansion. PloS ONE 2018, 13, e0193178. [Google Scholar] [CrossRef]
- Ying, J.; You, Q.; Wang, Z.; Hu, Z. Hypoxic preconditioning promotes the immunosuppressive effects of mesenchymal stem cells in mice with colitis. Res. Vet. Sci. 2022, 144, 157–163. [Google Scholar] [CrossRef]
- Wobma, H.M.; Kanai, M.; Ma, S.P.; Shih, Y.; Li, H.W.; Duran-Struuck, R.; Winchester, R.; Goeta, S.; Brown, L.M.; Vunjak-Novakovic, G. Dual IFN-γ/hypoxia priming enhances immunosuppression of mesenchymal stromal cells through regulatory proteins and metabolic mechanisms. J. Immunol. Regen. Med. 2018, 1, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Zhao, J.; Zhang, Z.; Yang, R.; Xie, J.; Liu, X.; Qi, S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PloS ONE 2014, 9, e96161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paquet, J.; Deschepper, M.; Moya, A.; Logeart-Avramoglou, D.; Boisson-Vidal, C.; Petite, H. Oxygen Tension Regulates Human Mesenchymal Stem Cell Paracrine Functions. Stem Cells Transl. Med. 2015, 4, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Diao, Y.F.; Wang, J.; Liang, J.; Xu, H.H.; Zhao, M.L.; Zheng, B.; Luan, Z.; Wang, J.J.; Yang, X.P.; et al. Intravenously Infusing the Secretome of Adipose-Derived Mesenchymal Stem Cells Ameliorates Neuroinflammation and Neurological Functioning After Traumatic Brain Injury. Stem Cells Dev. 2020, 29, 222–234. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Lin, L.; Fan, Y.; Huselstein, C.; De Isla, N.; He, X.; Chen, Y.; Li, Y. Secretome of Mesenchymal Stem Cells from Consecutive Hypoxic Cultures Promotes Resolution of Lung Inflammation by Reprogramming Anti-Inflammatory Macrophages. Int. J. Mol. Sci. 2022, 23, 4333. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Y.; Zheng, T.; Pu, Y.; Ma, Y.; Qi, X.; Zhang, W.; Xue, F.; Shan, Z.; Liu, J.; et al. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell Res. Ther. 2021, 12, 4. [Google Scholar] [CrossRef]
- Tian, J.; Chen, W.; Xiong, Y.; Li, Q.; Kong, S.; Li, M.; Pang, C.; Qiu, Y.; Xu, Z.; Gong, Q.; et al. Small extracellular vesicles derived from hypoxic preconditioned dental pulp stem cells ameliorate inflammatory osteolysis by modulating macrophage polarization and osteoclastogenesis. Bioact. Mater. 2023, 22, 326–342. [Google Scholar] [CrossRef]
- Braga, C.L.; da Silva, L.R.; Santos, R.T.; de Carvalho, L.R.P.; Mandacaru, S.C.; de Oliveira Trugilho, M.R.; Rocco, P.R.M.; Cruz, F.F.; Silva, P.L. Proteomics profile of mesenchymal stromal cells and extracellular vesicles in normoxic and hypoxic conditions. Cytotherapy 2022, 24, 1211–1224. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Noone, C.; Kihm, A.; English, K.; O’Dea, S.; Mahon, B.P. IFN-γ stimulated human umbilical-tissue-derived cells potently suppress NK activation and resist NK-mediated cytotoxicity in vitro. Stem Cells Dev. 2013, 22, 3003–3014. [Google Scholar] [CrossRef]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced Immunosuppressive Properties of Human Mesenchymal Stem Cells Primed by Interferon-γ. EBioMedicine 2018, 28, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duijvestein, M.; Wildenberg, M.E.; Welling, M.M.; Hennink, S.; Molendijk, I.; van Zuylen, V.L.; Bosse, T.; Vos, A.C.; de Jonge-Muller, E.S.; Roelofs, H.; et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011, 29, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Copland, I.B.; Patel, S.R.; Galipeau, J. IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J. Immunol. 2014, 192, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Huang, H.; Cui, S.; Zhou, Y.; Zhang, T.; Zhou, Y. IFN-γ promoted exosomes from mesenchymal stem cells to attenuate colitis via miR-125a and miR-125b. Cell Death Dis. 2020, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Choi, D.; Lora, M.; Shum-Tim, D.; Rak, J.; Colmegna, I. Human multipotent mesenchymal stromal cells cytokine priming promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo. Stem Cell Res. Ther. 2020, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Li, Q.; Bu, H.; Lin, F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010, 19, 1803–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.; Wan, Q.; Huang, J.; Han, L.; Chen, X.; Chen, G.; Olsen, N.; Zheng, S.G.; Liang, D. Culture medium from TNF-α-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J. Allergy Clin. Immunol. 2015, 136, 423–432.e428. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, L.; Hu, H.; Wang, H.; Wang, X.; Jiang, J.; Ma, Y.; Yang, J.; Hou, Y.; Xie, D.; et al. Pretreatment of exosomes derived from hUCMSCs with TNF-α ameliorates acute liver failure by inhibiting the activation of NLRP3 in macrophage. Life Sci. 2020, 246, 117401. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhao, G.; Liu, L.; Liu, F.; Gong, W.; Liu, X.; Yang, L.; Wang, J.; Hou, Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell. Mol. Immunol. 2012, 9, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, M.; Cui, B.; Zhang, W.; Ma, W.; Zhao, G.; Xing, L. Exosomal miR-21 secreted by IL-1β-primed-mesenchymal stem cells induces macrophage M2 polarization and ameliorates sepsis. Life Sci. 2021, 264, 118658. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cai, G.L.; Zhuang, Z.; Pei, S.Y.; Xu, S.N.; Wang, Y.N.; Wang, H.; Wang, X.; Cui, C.; Sun, M.C.; et al. Interleukin-1β-Treated Mesenchymal Stem Cells Inhibit Inflammation in Hippocampal Astrocytes Through Exosome-Activated Nrf-2 Signaling. Int. J. Nanomed. 2021, 16, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, D.I.; Choi, B.H.; Min, B.H. Exosomes from IL-1β-Primed Mesenchymal Stem Cells Inhibited IL-1β- and TNF-α-Mediated Inflammatory Responses in Osteoarthritic SW982 Cells. Tissue Eng. Regen. Med. 2021, 18, 525–536. [Google Scholar] [CrossRef]
- Magne, B.; Dedier, M.; Nivet, M.; Coulomb, B.; Banzet, S.; Lataillade, J.J.; Trouillas, M. IL-1β-Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing via Matrix Metalloproteinases and Transforming Growth Factor-β1. J. Investig. Dermatol. 2020, 140, 688–698.e621. [Google Scholar] [CrossRef]
- Redondo-Castro, E.; Cunningham, C.; Miller, J.; Martuscelli, L.; Aoulad-Ali, S.; Rothwell, N.J.; Kielty, C.M.; Allan, S.M.; Pinteaux, E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res. Ther. 2017, 8, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, J.R.; Teixeira, G.Q.; Neto, E.; Ribeiro-Machado, C.; Silva, A.M.; Caldeira, J.; Leite Pereira, C.; Bidarra, S.; Maia, A.F.; Lamghari, M.; et al. IL-1β-pre-conditioned mesenchymal stem/stromal cells’ secretome modulates the inflammatory response and aggrecan deposition in intervertebral disc. Eur. Cell. Mater. 2021, 41, 431–453. [Google Scholar] [CrossRef]
- Tang, J.; Wu, T.; Xiong, J.; Su, Y.; Zhang, C.; Wang, S.; Tang, Z.; Liu, Y. Porphyromonas gingivalis lipopolysaccharides regulate functions of bone marrow mesenchymal stem cells. Cell Prolif. 2015, 48, 239–248. [Google Scholar] [CrossRef]
- Liu, G.Y.; Liu, Y.; Lu, Y.; Qin, Y.R.; Di, G.H.; Lei, Y.H.; Liu, H.X.; Li, Y.Q.; Wu, C.; Hu, X.W.; et al. Short-term memory of danger signals or environmental stimuli in mesenchymal stem cells: Implications for therapeutic potential. Cell. Mol. Immunol. 2016, 13, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Bahroudi, M.; Bakhshi, B.; Soudi, S.; Najar-Peerayeh, S. Immunomodulatory effects of mesenchymal stem cell-conditioned media on lipopolysaccharide of Vibrio cholerae as a vaccine candidate. Stem Cell Res. Ther. 2021, 12, 564. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Zhang, F.; Chai, R.; Zhou, W.; Hu, M.; Liu, B.; Chen, X.; Liu, M.; Xu, Q.; Liu, N.; et al. Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J. Cell. Mol. Med. 2019, 23, 7617–7631. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Liu, Q.; Liu, L.; Huo, F.; Guo, S.; Tian, W. Lipopolysaccharide-Preconditioned Dental Follicle Stem Cells Derived Small Extracellular Vesicles Treating Periodontitis via Reactive Oxygen Species/Mitogen-Activated Protein Kinase Signaling-Mediated Antioxidant Effect. Int. J. Nanomed. 2022, 17, 799–819. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Jakob, M.; Bruderek, K.; Bootz, F.; Giebel, B.; Radtke, S.; Mauel, K.; Jäger, M.; Flohé, S.B.; Lang, S. Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PloS ONE 2014, 9, e106903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Letter, A.M.; Kurte, M.; Fernández-O’Ryan, C.; Gauthier-Abeliuk, M.; Fuenzalida, P.; Moya-Uribe, I.; Altamirano, C.; Figueroa, F.; Irarrázabal, C.; Carrión, F. Differential TLR activation of murine mesenchymal stem cells generates distinct immunomodulatory effects in EAE. Stem Cell Res. Ther. 2016, 7, 150. [Google Scholar] [CrossRef] [Green Version]
- Fuenzalida, P.; Kurte, M.; Fernández-O’ryan, C.; Ibañez, C.; Gauthier-Abeliuk, M.; Vega-Letter, A.M.; Gonzalez, P.; Irarrázabal, C.; Quezada, N.; Figueroa, F.; et al. Toll-like receptor 3 pre-conditioning increases the therapeutic efficacy of umbilical cord mesenchymal stromal cells in a dextran sulfate sodium-induced colitis model. Cytotherapy 2016, 18, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; Sun, L.; Sun, Y.; Zhao, F.; Liu, P.; Peng, X.; Xuan, X.; Li, Y.; Wang, P.; et al. Exosomes derived from LPS-stimulated human thymic mesenchymal stromal cells enhance inflammation via thrombospondin-1. Biosci. Rep. 2021, 41, BSR20203573. [Google Scholar] [CrossRef] [PubMed]
- Kurte, M.; Vega-Letter, A.M.; Luz-Crawford, P.; Djouad, F.; Noël, D.; Khoury, M.; Carrión, F. Time-dependent LPS exposure commands MSC immunoplasticity through TLR4 activation leading to opposite therapeutic outcome in EAE. Stem Cell Res. Ther. 2020, 11, 416. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, W.H.; Lee, M.W.; Park, H.J.; Jang, I.K.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Involvement of TLR3-Dependent PGES Expression in Immunosuppression by Human Bone Marrow Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2018, 14, 286–293. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, J.; Mao, R.; Chao, K.; Chen, B.L.; He, Y.; Zeng, Z.R.; Zhang, S.H.; Chen, M.H. TLR3 preconditioning enhances the therapeutic efficacy of umbilical cord mesenchymal stem cells in TNBS-induced colitis via the TLR3-Jagged-1-Notch-1 pathway. Mucosal Immunol. 2017, 10, 727–742. [Google Scholar] [CrossRef]
- Contreras-Lopez, R.; Elizondo-Vega, R.; Paredes, M.J.; Luque-Campos, N.; Torres, M.J.; Tejedor, G.; Vega-Letter, A.M.; Figueroa-Valdés, A.; Pradenas, C.; Oyarce, K.; et al. HIF1α-dependent metabolic reprogramming governs mesenchymal stem/stromal cell immunoregulatory functions. FASEB J. 2020, 34, 8250–8264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, L.; Burand, A.J.; Brown, A.J.; Boyt, D.; Lira, V.A.; Ankrum, J.A. IFN-γ and TNF-α Pre-licensing Protects Mesenchymal Stromal Cells from the Pro-inflammatory Effects of Palmitate. Mol. Ther. 2018, 26, 860–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- François, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, Y.; Du, P.; Yang, F.; Guo, P.; Tang, X.; Diao, L.; Lu, G. Mesenchymal stem cells pretreated with proinflammatory cytokines accelerate skin wound healing by promoting macrophages migration and M2 polarization. Regen. Ther. 2022, 21, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Y.; Lu, Y.; Du, P.; Li, X.; Wang, C.; Guo, P.; Diao, L.; Lu, G. Mesenchymal stromal cells pretreated with proinflammatory cytokines enhance skin wound healing via IL-6-dependent M2 polarization. Stem Cell Res. Ther. 2022, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Feng, C.; Huang, P.; Li, Y.; Liu, R.; Liu, C.; Han, Y.; Chen, L.; Ding, Y.; Shao, C.; et al. TNFα and IFNγ rapidly activate PI3K-AKT signaling to drive glycolysis that confers mesenchymal stem cells enhanced anti-inflammatory property. Stem Cell Res. Ther. 2022, 13, 491. [Google Scholar] [CrossRef]
- Jin, P.; Zhao, Y.; Liu, H.; Chen, J.; Ren, J.; Jin, J.; Bedognetti, D.; Liu, S.; Wang, E.; Marincola, F.; et al. Interferon-γ and Tumor Necrosis Factor-α Polarize Bone Marrow Stromal Cells Uniformly to a Th1 Phenotype. Sci. Rep. 2016, 6, 26345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, J.; Gu, Y.; Xue, M.; Qian, F.; Wang, B.; Yang, W.; Yu, H.; Wang, Q.; Guo, X.; et al. Inflammation-induced inhibition of chaperone-mediated autophagy maintains the immunosuppressive function of murine mesenchymal stromal cells. Cell. Mol. Immunol. 2021, 18, 1476–1488. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Ren, G.; Cao, G.; Chen, Q.; Shou, P.; Zheng, C.; Du, L.; Han, X.; Jiang, M.; Yang, Q.; et al. miR-155 regulates immune modulatory properties of mesenchymal stem cells by targeting TAK1-binding protein 2. J. Biol. Chem. 2013, 288, 11074–11079. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wang, J.; Wang, Q.; He, Z.; Sun, T.; Yao, Y.; Wang, W.; Shen, P. Pretreatment of umbilical cord derived MSCs with IFN-γ and TNF-α enhances the tumor-suppressive effect on acute myeloid leukemia. Biochem. Pharmacol. 2022, 199, 115007. [Google Scholar] [CrossRef]
- Yu, Y.; Yoo, S.M.; Park, H.H.; Baek, S.Y.; Kim, Y.J.; Lee, S.; Kim, Y.L.; Seo, K.W.; Kang, K.S. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J. Tissue Eng. Regen. Med. 2019, 13, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Philipp, D.; Suhr, L.; Wahlers, T.; Choi, Y.H.; Paunel-Görgülü, A. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res. Ther. 2018, 9, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Yang, Q.; Lin, L.; Xu, C.; Zheng, C.; Chen, X.; Han, Y.; Li, M.; Cao, W.; Cao, K.; et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014, 21, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, G.; Zhang, L.; Li, F.; Liu, K.; Wang, Y.; Shi, Y.; Cao, K. Interleukin-17 promotes nitric oxide-dependent expression of PD-L1 in mesenchymal stem cells. Cell Biosci. 2020, 10, 73. [Google Scholar] [CrossRef]
- Lin, T.; Pajarinen, J.; Nabeshima, A.; Lu, L.; Nathan, K.; Jämsen, E.; Yao, Z.; Goodman, S.B. Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res. Ther. 2017, 8, 277. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Y.; Kim, B.S.; Ryu, D.B.; Kim, T.W.; Park, G.; Min, C.K. The therapeutic efficacy of mesenchymal stromal cells on experimental colitis was improved by the IFN-γ and poly(I:C) priming through promoting the expression of indoleamine 2,3-dioxygenase. Stem Cell Res. Ther. 2021, 12, 37. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, L.; Liang, Y.; Guo, Z.; Wang, L.; Ma, C.; Wang, H. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J. Cell. Physiol. 2018, 233, 6832–6840. [Google Scholar] [CrossRef]
- Vinci, P.; Bastone, A.; Schiarea, S.; Cappuzzello, C.; Del Prete, A.; Dander, E.; Biondi, A.; D’Amico, G. Mesenchymal stromal cell-secreted chemerin is a novel immunomodulatory molecule driving the migration of ChemR23-expressing cells. Cytotherapy 2017, 19, 200–210. [Google Scholar] [CrossRef]
- Yao, M.; Chen, Z.; He, X.; Long, J.; Xia, X.; Li, Z.; Yang, Y.; Ao, L.; Xing, W.; Lian, Q.; et al. Cross talk between glucose metabolism and immunosuppression in IFN-γ-primed mesenchymal stem cells. Life Sci. Alliance 2022, 5, e202201493. [Google Scholar] [CrossRef]
- Chae, B.S. Pretreatment of Low-Dose and Super-Low-Dose LPS on the Production of In Vitro LPS-Induced Inflammatory Mediators. Toxicol. Res. 2018, 34, 65–73. [Google Scholar] [CrossRef]
- Saether, E.E.; Chamberlain, C.S.; Aktas, E.; Leiferman, E.M.; Brickson, S.L.; Vanderby, R. Primed Mesenchymal Stem Cells Alter and Improve Rat Medial Collateral Ligament Healing. Stem Cell Rev. Rep. 2016, 12, 42–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PloS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, T.; Pajarinen, J.; Kohno, Y.; Huang, J.F.; Maruyama, M.; Romero-Lopez, M.; Nathan, K.; Yao, Z.; Goodman, S.B. Trained murine mesenchymal stem cells have anti-inflammatory effect on macrophages, but defective regulation on T-cell proliferation. FASEB J. 2019, 33, 4203–4211. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Mayshar, Y.; Benvenisty, N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 2011, 9, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Xu, G.; Wang, Q.; Yang, L.; Zheng, L.; Zhao, J.; Zhang, X. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017, 8, e2851. [Google Scholar] [CrossRef] [Green Version]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.K.; Wang, J.Y.; Chen, C.F.; Chao, K.Y.; Chang, M.C.; Chen, W.M.; Hung, S.C. Early Passage Mesenchymal Stem Cells Display Decreased Radiosensitivity and Increased DNA Repair Activity. Stem Cell. Transl. Med. 2017, 6, 1504–1514. [Google Scholar] [CrossRef]
- Rao, V.V.; Vu, M.K.; Ma, H.; Killaars, A.R.; Anseth, K.S. Rescuing mesenchymal stem cell regenerative properties on hydrogel substrates post serial expansion. Bioeng. Transl. Med. 2019, 4, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Gwon, Y.; Park, S.; Kim, H.; Kim, J. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact. Mater. 2023, 19, 50–74. [Google Scholar] [CrossRef]
- Burand, A.J., Jr.; Di, L.; Boland, L.K.; Boyt, D.T.; Schrodt, M.V.; Santillan, D.A.; Ankrum, J.A. Aggregation of Human Mesenchymal Stromal Cells Eliminates Their Ability to Suppress Human T Cells. Front. Immunol. 2020, 11, 143. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, T.; Xu, A.; Zhang, L. 3D spheroid culture enhances survival and therapeutic capacities of MSCs injected into ischemic kidney. J. Cell. Mol. Med. 2016, 20, 1203–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Guo, G.; Li, L.; Chen, F.; Bao, J.; Shi, Y.J.; Bu, H. Three-dimensional spheroid culture of human umbilical cord mesenchymal stem cells promotes cell yield and stemness maintenance. Cell Tissue Res. 2015, 360, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.A.; McDevitt, T.C. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy 2014, 16, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosh, T.J.; Ylöstalo, J.H.; Bazhanov, N.; Kuhlman, J.; Prockop, D.J. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1). Stem Cells 2013, 31, 2443–2456. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.M.; Camões, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simões, S.; Gaspar, M.; Mosqueira, D.; et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res. Ther. 2015, 6, 90. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.P.; Camões, S.P.; Gaspar, M.M.; Rodrigues, J.S.; Carvalheiro, M.; Bárcia, R.N.; Cruz, P.; Cruz, H.; Simões, S.; Santos, J.M. The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front. Immunol. 2019, 10, 18. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Khodadadi Yazdi, M.; Baradaran Ghavami, S.; Farokhimanesh, S.; Mohammadi Amirabad, L.; Zarrintaj, P.; Saeb, M.R.; Hamblin, M.R.; Zare, M.; Mozafari, M. Mesenchymal Stem Cell Spheroids Embedded in an Injectable Thermosensitive Hydrogel: An In Situ Drug Formation Platform for Accelerated Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5096–5109. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Fu, H.; Kuang, S.; He, F.; Zhang, M.; Shen, Z.; Qin, W.; Lin, Z.; Huang, S. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int. J. Oral. Sci. 2021, 13, 43. [Google Scholar] [CrossRef]
- Costa, M.H.G.; Serra, J.; McDevitt, T.C.; Cabral, J.M.S.; da Silva, C.L.; Ferreira, F.C. Dimethyloxalylglycine, a small molecule, synergistically increases the homing and angiogenic properties of human mesenchymal stromal cells when cultured as 3D spheroids. Biotechnol. J. 2021, 16, e2000389. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.; Lee, H.J.; Na, K.S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicer, M.; Cottrell, G.S.; Widera, D. Impact of 3D cell culture on bone regeneration potential of mesenchymal stromal cells. Stem Cell Res. Ther. 2021, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.E.; Doron, G.; Levy, M.J.; Temenoff, J.S. Hydrogel Culture Surface Stiffness Modulates Mesenchymal Stromal Cell Secretome and Alters Senescence. Tissue Eng Part A 2020, 26, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Rao, V.V.; Borelli, A.N.; Anseth, K.S. Engineering the MSC Secretome: A Hydrogel Focused Approach. Adv. Healthc. Mater. 2021, 10, e2001948. [Google Scholar] [CrossRef]
- Song, S.Y.; Hong, J.; Go, S.; Lim, S.; Sohn, H.S.; Kang, M.; Jung, G.J.; Yoon, J.K.; Kang, M.L.; Im, G.I.; et al. Interleukin-4 Gene Transfection and Spheroid Formation Potentiate Therapeutic Efficacy of Mesenchymal Stem Cells for Osteoarthritis. Adv. Healthc. Mater. 2020, 9, e1901612. [Google Scholar] [CrossRef]
- Niu, J.; Yue, W.; Song, Y.; Zhang, Y.; Qi, X.; Wang, Z.; Liu, B.; Shen, H.; Hu, X. Prevention of acute liver allograft rejection by IL-10-engineered mesenchymal stem cells. Clin. Exp. Immunol. 2014, 176, 473–484. [Google Scholar] [CrossRef]
- Lu, X.; Ru, Y.; Chu, C.; Lv, Y.; Gao, Y.; Jia, Z.; Huang, Y.; Zhang, Y.; Zhao, S. Lentivirus-mediated IL-10-expressing Bone Marrow Mesenchymal Stem Cells promote corneal allograft survival via upregulating lncRNA 003946 in a rat model of corneal allograft rejection. Theranostics 2020, 10, 8446–8467. [Google Scholar] [CrossRef]
- Tang, J.; Yang, R.; Lv, L.; Yao, A.; Pu, L.; Yin, A.; Li, X.; Yu, Y.; Nyberg, S.L.; Wang, X. Transforming growth factor-β-Expressing Mesenchymal Stem Cells Induce Local Tolerance in a Rat Liver Transplantation Model of Acute Rejection. Stem Cells 2016, 34, 2681–2692. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, N.; Li, B.; Gao, H.; Yan, Y.; Guo, H. Inhibition of cardiac allograft rejection in mice using interleukin-35-modified mesenchymal stem cells. Scand. J. Immunol. 2019, 89, e12750. [Google Scholar] [CrossRef]
- He, Y.; Zhou, S.; Liu, H.; Shen, B.; Zhao, H.; Peng, K.; Wu, X. Indoleamine 2, 3-Dioxgenase Transfected Mesenchymal Stem Cells Induce Kidney Allograft Tolerance by Increasing the Production and Function of Regulatory T Cells. Transplantation 2015, 99, 1829–1838. [Google Scholar] [CrossRef]
- He, J.G.; Li, B.B.; Zhou, L.; Yan, D.; Xie, Q.L.; Zhao, W. Indoleamine 2,3-dioxgenase-transfected mesenchymal stem cells suppress heart allograft rejection by increasing the production and activity of dendritic cells and regulatory T cells. J. Investig. Med. 2020, 68, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, H.L.; Zhang, W.; Wu, B.J.; Fu, N.N.; Dong, C.; Shen, Z.Y. Heme oxygenase-1-transduced bone marrow mesenchymal stem cells in reducing acute rejection and improving small bowel transplantation outcomes in rats. Stem Cell Res. Ther. 2016, 7, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Song, H.L.; Yang, Y.; Yin, M.L.; Zhang, B.Y.; Cao, Y.; Dong, C.; Shen, Z.Y. Improvement of Liver Transplantation Outcome by Heme Oxygenase-1-Transduced Bone Marrow Mesenchymal Stem Cells in Rats. Stem Cells Int. 2016, 2016, 9235073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.Y.; Wu, B.; Liu, T.; Yang, Y.; Yin, M.L.; Zheng, W.P.; Zhang, B.Y.; Song, H.L. Immunomodulatory effects of bone marrow mesenchymal stem cells overexpressing heme oxygenase-1: Protective effects on acute rejection following reduced-size liver transplantation in a rat model. Cell. Immunol. 2017, 313, 10–24. [Google Scholar] [CrossRef]
- Ou, Q.; Dou, X.; Tang, J.; Wu, P.; Pan, D. Small extracellular vesicles derived from PD-L1-modified mesenchymal stem cell promote Tregs differentiation and prolong allograft survival. Cell Tissue Res. 2022, 389, 465–481. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; Yuan, P.; Guo, W.; Hu, X.; Xing, W.; Ao, L.; Tan, Y.; Wu, X.; Ao, X.; et al. Antibacterial Fusion Protein BPI21/LL-37 Modification Enhances the Therapeutic Efficacy of hUC-MSCs in Sepsis. Mol. Ther. J. Am. Soc. Gene Ther. 2020, 28, 1806–1817. [Google Scholar] [CrossRef]
- Martinez, V.G.; Ontoria-Oviedo, I.; Ricardo, C.P.; Harding, S.E.; Sacedon, R.; Varas, A.; Zapata, A.; Sepulveda, P.; Vicente, A. Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ferrer, M.; Amaro-Prellezo, E.; Dorronsoro, A.; Sánchez-Sánchez, R.; Vicente, Á.; Cosín-Roger, J.; Barrachina, M.D.; Baquero, M.C.; Valencia, J.; Sepúlveda, P. HIF-Overexpression and Pro-Inflammatory Priming in Human Mesenchymal Stromal Cells Improves the Healing Properties of Extracellular Vesicles in Experimental Crohn’s Disease. Int. J. Mol. Sci. 2021, 22, 11269. [Google Scholar] [CrossRef]
- Gómez-Ferrer, M.; Villanueva-Badenas, E.; Sánchez-Sánchez, R.; Sánchez-López, C.M.; Baquero, M.C.; Sepúlveda, P.; Dorronsoro, A. HIF-1α and Pro-Inflammatory Signaling Improves the Immunomodulatory Activity of MSC-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 3416. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, X.; Lu, J.; Li, Z.; Jia, H.; Chen, M.; Chang, Y.; Liu, Y.; Li, P.; Zhang, B.; et al. Mesenchymal stem cells transfected with sFgl2 inhibit the acute rejection of heart transplantation in mice by regulating macrophage activation. Stem Cell Res. Ther. 2020, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Wei, F.; Zhou, Y.; Hu, L.; Ge, Z.; Jin, J.; Wang, H.; Wu, C.T. Overexpression of Hepatocyte Growth Factor in Dental Pulp Stem Cells Ameliorates the Severity of Psoriasis by Reducing Inflammatory Responses. Stem Cells Dev. 2021, 30, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.A.; Hettiaratchi, M.H.; McDevitt, T.C. Enhanced Immunosuppression of T Cells by Sustained Presentation of Bioactive Interferon-γ Within Three-Dimensional Mesenchymal Stem Cell Constructs. Stem Cells Transl. Med. 2017, 6, 223–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankrum, J.A.; Dastidar, R.G.; Ong, J.F.; Levy, O.; Karp, J.M. Performance-enhanced mesenchymal stem cells via intracellular delivery of steroids. Sci. Rep. 2014, 4, 4645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Yang, Z.; Concannon, J.; Ng, K.S.; Seyb, K.; Mortensen, L.J.; Ranganath, S.; Gu, F.; Levy, O.; Tong, Z.; Martyn, K.; et al. Tetrandrine identified in a small molecule screen to activate mesenchymal stem cells for enhanced immunomodulation. Sci. Rep. 2016, 6, 30263. [Google Scholar] [CrossRef] [Green Version]
- Rawat, S.; Dadhwal, V.; Mohanty, S. Dexamethasone priming enhances stemness and immunomodulatory property of tissue-specific human mesenchymal stem cells. BMC Dev. Biol. 2021, 21, 16. [Google Scholar] [CrossRef]
- Rossi, F.; Tortora, C.; Palumbo, G.; Punzo, F.; Argenziano, M.; Casale, M.; Di Paola, A.; Locatelli, F.; Perrotta, S. CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. Int. J. Mol. Sci. 2019, 20, 1049. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Heo, J.S.; Lim, J.Y.; Yoon, D.W.; Pyo, S.; Kim, J. Exosome and Melatonin Additively Attenuates Inflammation by Transferring miR-34a, miR-124, and miR-135b. Biomed. Res. Int. 2020, 2020, 1621394. [Google Scholar] [CrossRef]
- Jahandideh, S.; Maghsood, F.; Ghahhari, N.M.; Lotfinia, M.; Mohammadi, M.; Johari, B.; Kadivar, M. The effect of Trimetazidine and Diazoxide on immunomodulatory activity of human embryonic stem cell-derived mesenchymal stem cell secretome. Tissue Cell 2017, 49, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, S.; Khatami, S.; Eslami Far, A.; Kadivar, M. Anti-inflammatory effects of human embryonic stem cell-derived mesenchymal stem cells secretome preconditioned with diazoxide, trimetazidine and MG-132 on LPS-induced systemic inflammation mouse model. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1178–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Li, H.; Su, X.; Zhang, Y.; Xu, H.; Fan, L.; Fan, J.; Han, Q.; Bai, X.; Zhao, R.C. Chlorzoxazone, a small molecule drug, augments immunosuppressive capacity of mesenchymal stem cells via modulation of FOXO3 phosphorylation. Cell Death Dis. 2020, 11, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.G.; Lee, J.; Hong, S.M.; Kwok, S.K.; Cho, M.L.; Park, S.H. Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology 2020, 59, 1426–1438. [Google Scholar] [CrossRef]
- Jin, Y.; Hong, H.S.; Son, Y. Substance P enhances mesenchymal stem cells-mediated immune modulation. Cytokine 2015, 71, 145–153. [Google Scholar] [CrossRef]
- Gao, L.; Cen, S.; Wang, P.; Xie, Z.; Liu, Z.; Deng, W.; Su, H.; Wu, X.; Wang, S.; Li, J.; et al. Autophagy Improves the Immunosuppression of CD4+ T Cells by Mesenchymal Stem Cells Through Transforming Growth Factor-β1. Stem Cells Transl. Med. 2016, 5, 1496–1505. [Google Scholar] [CrossRef] [Green Version]
- Cen, S.; Wang, P.; Xie, Z.; Yang, R.; Li, J.; Liu, Z.; Wang, S.; Wu, X.; Liu, W.; Li, M.; et al. Autophagy enhances mesenchymal stem cell-mediated CD4(+) T cell migration and differentiation through CXCL8 and TGF-β1. Stem Cell Res. Ther. 2019, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Li, H.; He, L.; Huang, Y.; Cai, J.; Chen, L.; Zhou, C.; Fu, H.; Lu, T.; Zhang, Y.; et al. Preconditioning of umbilical cord-derived mesenchymal stem cells by rapamycin increases cell migration and ameliorates liver ischaemia/reperfusion injury in mice via the CXCR4/CXCL12 axis. Cell Prolif. 2019, 52, e12546. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; Aimaletdinov, A.M.; Bondar, O.V.; Starostina, I.G.; Gorshkova, N.V.; Neustroeva, O.A.; Kletukhina, S.K.; Kurbangaleeva, S.V.; Vorobev, V.V.; Garanina, E.E.; et al. Immunosuppressive properties of cytochalasin B-induced membrane vesicles of mesenchymal stem cells: Comparing with extracellular vesicles derived from mesenchymal stem cells. Sci. Rep. 2020, 10, 10740. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; Kletukhina, S.K.; Kurbangaleeva, S.V.; Neustroeva, O.A.; Vasileva, O.S.; Garanina, E.E.; Khaiboullina, S.F.; Rizvanov, A.A. Mesenchymal Stem Cell Derived Biocompatible Membrane Vesicles Demonstrate Immunomodulatory Activity Inhibiting Activation and proliferation of Human Mononuclear Cells. Pharmaceutics 2020, 12, 577. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, K.; Liu, K.; Xue, Y.; Roberts, A.I.; Li, F.; Han, Y.; Rabson, A.B.; Wang, Y.; Shi, Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018, 25, 1209–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, G.G.; Hastreiter, A.A.; Sartori, T.; Borelli, P.; Fock, R.A. L-Glutamine in vitro Modulates some Immunomodulatory Properties of Bone Marrow Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2017, 13, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Tang, R.; Xu, J.; Jiang, W.; Gong, Z.; Zhang, L.; Ning, Y.; Huang, P.; Xu, J.; Chen, G.; et al. Tongxinluo-pretreated mesenchymal stem cells facilitate cardiac repair via exosomal transfer of miR-146a-5p targeting IRAK1/NF-κB p65 pathway. Stem Cell Res. Ther. 2022, 13, 289. [Google Scholar] [CrossRef]
- da Silva Lima, F.; da Rocha Romero, A.B.; Hastreiter, A.; Nogueira-Pedro, A.; Makiyama, E.; Colli, C.; Fock, R.A. An insight into the role of magnesium in the immunomodulatory properties of mesenchymal stem cells. J. Nutr. Biochem. 2018, 55, 200–208. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhou, W.; Du, Q.; Yang, M.; Ding, Y.; Hu, R. Blockade of IDO-Kynurenine-AhR Axis Ameliorated Colitis-Associated Colon Cancer via Inhibiting Immune Tolerance. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1179–1199. [Google Scholar] [CrossRef]
- Zhai, L.; Spranger, S.; Binder, D.C.; Gritsina, G.; Lauing, K.L.; Giles, F.J.; Wainwright, D.A. Molecular Pathways: Targeting IDO1 and Other Tryptophan Dioxygenases for Cancer Immunotherapy. Clin. Cancer Res. 2015, 21, 5427–5433. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; Bell, A.; Ladomersky, E.; Lauing, K.L.; Bollu, L.; Sosman, J.A.; Zhang, B.; Wu, J.D.; Miller, S.D.; Meeks, J.J.; et al. Immunosuppressive IDO in Cancer: Mechanisms of Action, Animal Models, and Targeting Strategies. Front. Immunol. 2020, 11, 1185. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; Wainwright, D.A. IDO1 in cancer: A Gemini of immune checkpoints. Cell. Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Serrador, J.M. Nitric Oxide Signaling in T Cell-Mediated Immunity. Trends Mol. Med. 2018, 24, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Luo, Y.; Yuan, C.; Han, L.; Wu, Q.; Xu, L.; Gao, Y.; Sun, Y.; Ma, S.; Tang, G.; et al. Downregulation of Nitric Oxide Collaborated with Radiotherapy to Promote Anti-Tumor Immune Response via Inducing CD8+ T Cell Infiltration. Int. J. Biol. Sci. 2020, 16, 1563–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Ozaki, K.; Oh, I.; Meguro, A.; Hatanaka, K.; Nagai, T.; Muroi, K.; Ozawa, K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood 2007, 109, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, W.; Besnard, A.G.; Jiang, H.R.; Alves-Filho, J.C.; Fukada, S.Y.; Nascimento, D.; Mitani, A.; Pushparaj, P.; Alqahtani, M.H.; Liew, F.Y. Nitric oxide-induced regulatory T cells inhibit Th17 but not Th1 cell differentiation and function. J. Immunol. 2013, 191, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.H.; Hong, H.M.; Lin, C.L.; Jhang, A.Z.; Tsai, J.H.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T.; Chen, W.J. Se-methylselenocysteine inhibits lipopolysaccharide-induced NF-κB activation and iNOS induction in RAW 264.7 murine macrophages. Mol. Nutr. Food Res. 2011, 55, 723–732. [Google Scholar] [CrossRef]
- Gazdic, M.; Simovic Markovic, B.; Vucicevic, L.; Nikolic, T.; Djonov, V.; Arsenijevic, N.; Trajkovic, V.; Lukic, M.L.; Volarevic, V. Mesenchymal stem cells protect from acute liver injury by attenuating hepatotoxicity of liver natural killer T cells in an inducible nitric oxide synthase- and indoleamine 2,3-dioxygenase-dependent manner. J. Tissue Eng. Regen. Med. 2018, 12, e1173–e1185. [Google Scholar] [CrossRef] [Green Version]
- Simovic Markovic, B.; Gazdic, M.; Arsenijevic, A.; Jovicic, N.; Jeremic, J.; Djonov, V.; Arsenijevic, N.; Lukic, M.L.; Volarevic, V. Mesenchymal Stem Cells Attenuate Cisplatin-Induced Nephrotoxicity in iNOS-Dependent Manner. Stem Cells Int. 2017, 2017, 1315378. [Google Scholar] [CrossRef] [Green Version]
- Laing, A.G.; Fanelli, G.; Ramirez-Valdez, A.; Lechler, R.I.; Lombardi, G.; Sharpe, P.T. Mesenchymal stem cells inhibit T-cell function through conserved induction of cellular stress. PloS ONE 2019, 14, e0213170. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Jia, J. Natural COX-2 Inhibitors as Promising Anti-inflammatory Agents: An Update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar] [CrossRef]
- Linard, C.; Strup-Perrot, C.; Lacave-Lapalun, J.V.; Benderitter, M. Flagellin preconditioning enhances the efficacy of mesenchymal stem cells in an irradiation-induced proctitis model. J. Leukoc. Biol. 2016, 100, 569–580. [Google Scholar] [CrossRef]
- Vasandan, A.B.; Jahnavi, S.; Shashank, C.; Prasad, P.; Kumar, A.; Prasanna, S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE(2)-dependent mechanism. Sci. Rep. 2016, 6, 38308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Huang, S.; Wu, S.; Qi, J.; Li, W.; Liu, S.; Cong, Y.; Chen, H.; Lu, L.; Shi, S.; et al. Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. EBioMedicine 2019, 45, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Shin, T.H.; Lee, B.C.; Yu, K.R.; Seo, Y.; Lee, S.; Seo, M.S.; Hong, I.S.; Choi, S.W.; Seo, K.W.; et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology 2013, 145, 1392–1403. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, L.; Fu, B.; Bai, J.; Zhang, Y.; Cai, G.; Bai, X.; Feng, Z.; Sun, S.; Chen, X. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 2018, 93, 814–825. [Google Scholar] [CrossRef]
- Ortiz-Virumbrales, M.; Menta, R.; Pérez, L.M.; Lucchesi, O.; Mancheño-Corvo, P.; Avivar-Valderas, Á.; Palacios, I.; Herrero-Mendez, A.; Dalemans, W.; de la Rosa, O.; et al. Human adipose mesenchymal stem cells modulate myeloid cells toward an anti-inflammatory and reparative phenotype: Role of IL-6 and PGE2. Stem Cell Res. Ther. 2020, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Yang, H.; Wu, L.; Deng, H.; Chen, Y.; Zhou, H.; Liu, M.; Wang, S.; Zheng, L.; Zhu, L.; Lv, X. Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-κB signaling pathway in spinal microglia. J. Neuroinflamm. 2020, 17, 154. [Google Scholar] [CrossRef]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.; Day, A.J.; Milner, C.M. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef] [Green Version]
- Kota, D.J.; Wiggins, L.L.; Yoon, N.; Lee, R.H. TSG-6 produced by hMSCs delays the onset of autoimmune diabetes by suppressing Th1 development and enhancing tolerogenicity. Diabetes 2013, 62, 2048–2058. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Rose-John, S. The Soluble Interleukin 6 Receptor: Advanced Therapeutic Options in Inflammation. Clin. Pharmacol. Ther. 2017, 102, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, D.P.; Suresh, S.; Newsome, P.N.; Frampton, J.; Kalia, N. Pretreatment of Mesenchymal Stem Cells Manipulates Their Vasculoprotective Potential While Not Altering Their Homing Within the Injured Gut. Stem Cells 2015, 33, 2785–2797. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Wu, F.; Zhou, Y.; Bao, Z.; Li, H.; Zheng, P.; Zhao, S. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019, 10, 918. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Hao, H.; Tong, C.; Cheng, Y.; Liu, J.; Pang, Y.; Si, Y.; Guo, Y.; Zang, L.; Mu, Y.; et al. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells 2016, 34, 627–639. [Google Scholar] [CrossRef]
- Stumhofer, J.S.; Silver, J.S.; Laurence, A.; Porrett, P.M.; Harris, T.H.; Turka, L.A.; Ernst, M.; Saris, C.J.; O’Shea, J.J.; Hunter, C.A. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007, 8, 1363–1371. [Google Scholar] [CrossRef]
- Shu, Y.; Ma, M.; Pan, X.; Shafiq, M.; Yu, H.; Chen, H. Cobalt protoporphyrin-induced nano-self-assembly for CT imaging, magnetic-guidance, and antioxidative protection of stem cells in pulmonary fibrosis treatment. Bioact. Mater. 2023, 21, 129–141. [Google Scholar] [CrossRef]
- Danisovic, L.; Oravcova, L.; Krajciova, L.; Varchulova Novakova, Z.; Bohac, M.; Varga, I.; Vojtassak, J. Effect of long-term culture on the biological and morphological characteristics of human adipose tissue-derived stem Cells. J. Physiol. Pharmacol. 2017, 68, 149–158. [Google Scholar]
- Yan, K.; Zhang, J.; Yin, W.; Harding, J.N.; Ma, F.; Wu, D.; Deng, H.; Han, P.; Li, R.; Peng, H.; et al. Transcriptomic heterogeneity of cultured ADSCs corresponds to embolic risk in the host. iScience 2022, 25, 104822. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; de Castro, L.F.; Jin, P.; Civini, S.; Ren, J.; Reems, J.A.; Cancelas, J.; Nayak, R.; Shaw, G.; O’Brien, T.; et al. Manufacturing Differences Affect Human Bone Marrow Stromal Cell Characteristics and Function: Comparison of Production Methods and Products from Multiple Centers. Sci. Rep. 2017, 7, 46731. [Google Scholar] [CrossRef] [Green Version]
- Oliver-Vila, I.; Coca, M.I.; Grau-Vorster, M.; Pujals-Fonts, N.; Caminal, M.; Casamayor-Genescà, A.; Ortega, I.; Reales, L.; Pla, A.; Blanco, M.; et al. Evaluation of a cell-banking strategy for the production of clinical grade mesenchymal stromal cells from Wharton’s jelly. Cytotherapy 2016, 18, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Sipp, D.; Robey, P.G.; Turner, L. Clear up this stem-cell mess. Nature 2018, 561, 455–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.S.; Xu, X.P.; Chang, W.; Lu, Z.H.; Huang, L.L.; Xu, J.Y.; Liu, L.; Qiu, H.B.; Yang, Y.; Guo, F.M. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res. Ther. 2018, 9, 280. [Google Scholar] [CrossRef] [PubMed]

| Treatment Conditions | MSCs Sources | Secreted Factors or Expressed Genes | Research Scenarios | Immunomodulatory Effects (Signaling Pathway) | Reference |
|---|---|---|---|---|---|
| 2% O2 24 h | GT-MSCs | FasL, IL-10↑ TNF-α↓ | PBMCs proliferation in vitro skin wound model of mice | PBMCs apoptosis↑ inflammatory cells↓, skin wound closure ↑ | [27] |
| 3% O2 + calcium (1.8 mmol/L) | UC- MSCs | PLK1↑ | T-cell proliferation in vitro GVHD model of mice | T-cell proliferation↓ survival↑, weight loss↓ and histopathologic injuries↓ in GVHD target organs in vivo (MCP-1 and p53/p21) | [28] |
| 5% O2 | BM-MSCs | IDO↑ | T-cell differentiation in vitro | Treg proliferation↑ | [29] |
| 1% O2 48 h | BM-MSCs | iNOS, NO↑ | inflammatory bowel disease model of mice | CD8+ T-cell activation↓ body weight loss, colon shortening and colon inflammation↓ | [30] |
| 1% O2 + IFN-γ (50 ng/mL) + TNF-α (20 ng/mL) 6, 24, 72 h | AT-MSCs | IDO, CXCL10↑ | T-cell proliferation in vitro | inhibition of CD4+ and CD8+ T-cell↑ in vitro | [15] |
| 1% O2 + IFN-γ (100 ng/mL) 48 h | AT-MSCs | IDO, HLA-G↑ | mixed lymphocyte reactions in vitro | T-cell inhibition↑ | [31] |
| 2% O2 | BM-MSCs | FGF, VEGF, IL-6 and IL-8↑ | monocytes migration in vitro skin wound model of mice | CD14+ monocyte migration↑ macrophages recruitment↑ | [32] |
| 0.1% O2 7 d | BM-MSCs | IL-8, VEGF, MCP-1, RANTES↑ IL-6, IL-1β, IL-15, IL-1Ra↓ | CM implanting subcutaneously in mice | inflammatory effect↓ after subcutaneous transplantation in vivo | [33] |
| 5% O2 24 h | AT-MSCs | CM | traumatic brain injury model of rats | neurological impairment and cognitive deficiency↓, neuroinflammatory edema and nerve fiber damage↓, M1 macrophages↓ and M2 macrophages↑, IL-6, TNF-α↓ and TSG-6, TGF-β↑ | [34] |
| 1% O2 48 h | UC-MSCs | IGF1, IL-10, TSG-6, TGF-β and PGE2↑ IL-6, IL-8↓ | lung injury model of mice | anti-inflammatory polarization and efferocytosis of macrophages↑ | [35] |
| 5% O2 24 h | UC-MSCs | EVs (miR-146a-5p) ↑ | allergic asthma model of mice | eosinophils↓and IL-4, IL-13↓ | [36] |
| 1% O2 48 h | DP-MSCs | EVs (miR-210-3p) ↑ | calvarial osteolysis model of mice osteoclast differentiation and macrophages polarization in vitro | inflammatory bone loss↓ in vivo M2 polarization↑ and osteoclastogenesis↓ in vivo and in vitro (NF-κB1) | [37] |
| 1% O2 48 h | BM-MSCs | exosomal miR-216a-5p↑ | contusive spinal cord injury model of mouse microglia/macrophages polarization in vitro | functional behavioral recovery after spinal cord injury↑ in vivo M1 to M2 microglia/macrophages polarization↑ in vivo and in vitro (TLR4/NF-κB/PI3K/AKT) | [25] |
| Treatment Conditions | MSCs Sources | Secreted Factors or Expressed Genes | Research Scenarios | Immunomodulatory Effects (Signaling Pathway) | Reference |
|---|---|---|---|---|---|
| IFN-γ (2 ng/mL) | BM-MSCs | IDO↑ | T, NK cells proliferation in vitro | proliferation of activated T or NK cells↓ | [39] |
| IFN-γ (50 ng/m) 48 h | BM-MSCs | IDO, PGE2↑ | NK cells activation in vitro | NK cells activation↓ | [40] |
| IFN-γ (200 IU/mL) 24 h | BM-MSCs UC-MSCs AT-MSCs WJ-MSCs | IDO↑ | PBMCs proliferation in vitro GVHD model of mice | PBMCs proliferation↓ immune cells infiltration in skin and small intestine↓ and survival rate↑ of GVHD mice (JAK/STAT1) | [41] |
| IFN-γ (500 U/mL) | BM-MSCs | IDO, iNOS↑ | PBMCs proliferation in vitro colitis model of mice | PBMCs proliferation↓ serum amyloid A protein levels and local proinflammatory cytokine levels↓ in colonic tissues | [42] |
| IFN-γ (50 ng/mL) 72 h | BM-MSCs | IDO (independent)↑, B7H1, B7DC↑ | T-cell proliferation and cytokines production in vitro | T-cell proliferation↓ Th1 cytokine (IFN-γ, TNF-α and IL-2) ↓ T-cell degranulation↓ (B7H1 and B7DC/PD1) | [43] |
| IFN-γ (50 ng/mL) | BM-MSCs | Exosomal miR-125a and miR-125b↑ | T-cell differentiation in vitro colitis model of mice | differentiation of Treg↑ and Th17 cells ↓in vitro body weight loss, disease activity index, colon shortening, impaired intestinal structure, TNF-α, IFN-γ, IL-6 and Th 17 cells↓ and Treg↑ in vivo (Stat3) | [44] |
| IFN-γ (10 ng/mL)/ TNF-α (15 ng/mL) 72 h | AT-MSCs | EVs (A20 and TSG-6) ↑ RAB27B | T-cell proliferation in vitro | activated CD4+ T-cell↓ | [45] |
| IFN-γ (10 and 100 ng/mL) 12, 24, 48 h/ TNF-α (100 ng/mL) 12, 24, 48 h | MSCs | H factor↑ | modified sheep erythrocytes hemolytic assay in vitro | complement activation↓ | [46] |
| TNF-α (10 ng/mL) 48 h | BM-MSCs | COX2, PGE2↑ | experimental allergic conjunctivitis model of mice | TNF-a, IL-4, IL-5, IL-1β↓and TGF-β↑in the conjunctiva B-cell IgE release↓ activation of mast cells↓ histamine release↓ (COX2/PGE2) | [47] |
| TNF-α (100 ng/mL) 48 h | GT-MSCs | exosomal miR-1260b, exosomal CD73↑ | macrophages polarization in vitro skin wound model of mice periodontitis model of mice | M2 macrophages polarization↑ in vitro and in vivo wound healing↑ TRAP+ osteoclasts and bone resorption↓ (Wnt5a/RANKL) | [48] |
| TNF-α (1 ng/mL) 72 h | UC-MSCs | exsomal miR-299-3p↑ | LPS-activated Kupffer cells cytokines production in vitro acute liver failure model of mice | secretion of IL-1β, IL-18 and IL-6 in Kupffer cells↓ the damage of liver tissue↓, IL-6, IL-1β, IL-18, aspartate aminotransferase and alanine aminotransferase in peripheral blood serum↓ of acute liver failure mice (NLRP3) | [49] |
| IL-1β (10 ng/mL) 48 h | UC-MSCs | COX2, IL-6 and IL-8↑ | colitis model of mice | M1 macrophages↓ in the peritoneal cavity of mice Th1, Th17↓ and Treg, Th2↑ in spleen and lymph nodes | [50] |
| IL-1β (10 ng/mL) 12 h | UC-MSCs | exosomal miR-146a↑ | macrophages polarization in vitro sepsis model of mice | M2 macrophages polarization↑ in vitro and in vivo survival rate↑, TNF-α, IL-6↓ and IL-10↑ in serum of septic mice | [51]. |
| IL-1β (10 ng/mL) 12 h | MSCs | exosomal miR-21↑ | macrophages polarization in vitro sepsis model of mice | M2 macrophages polarization↑ in vitro and in vivo survival rate↑, TNF-α↓ and IL-10↑ in serum of septic mice | [52] |
| IL-1β (10 ng/mL) 24 h | BM-MSCs | exosome | LPS-activated astrogliosis in vitro status epilepticus model of mice | astrogliosis↓ C3, CD81 and Ki67↓, BDNF, IL-1Ra, VEGF, IL-10 and NGF↑ in vitro and in vivo (Nrf-2) | [53] |
| IL-1β (25 ng/mL) 24 h | BM-MSCs | exosomal miR-147b | IL-1β-induced inflammatory SW982 cells cytokines production in vitro | IL-1β, IL-6, and MCP-1↓ in inflammatory SW982 cells (NF-κB) | [54] |
| IL-1β (1 ng/mL) 24 h | GT-MSCs | TGF-β1, MMP-1, MMP-9↑ | LPS-activated THP-1 cytokines production in vitro skin wound model of mice | TNF-α↓ and IL-1Ra↑ in THP-1 skin wound healing↑ | [55] |
| IL-1β (10 ng/mL) 24 h | BM-MSCs | G-CSF↑ | LPS-treated BV2 microglial cells cytokines production in vitro | IL-6, TNF-α↓ and IL-10↑ of BV2 microglial cells | [56] |
| IL-1β (10 ng/mL) + 6 % O2 48 h | BM-MSCs | IL-6, RANTES, IL-8, MCP-1 and PGE2↑ | degenerative intervertebral disc model of bovina in vitro | IL-6, IL-8↓, aggrecan↑ in intervertebral disc | [57] |
| LPS (0.1 μg/mL) 12 h | BM-MSCs | NO↑ | activated T-cell apoptosis in vitro | apoptosis of T-cell↑ | [58] |
| LPS (100 ng/Ml) 24 h | AT-MSCs | IL-6 and IL-8↑ | skin flap model of diabetic rats | skin flap survival↑ in diabetic rats | [59] |
| LPS (5 µg/mL) 72 h | MSCs | CM | immunized model of mice | serum IL-4, IL-5, BAFF, TGF-β↑ and TNF-α↓ in humoral immunity CD4+ T-cell↑ serum IgG, saliva IgA↑ in immunized mice serum IL-6, TNF-α, IL-10↑ in immunized mice vibriocidal activity↑ | [60] |
| LPS (100 ng/mL) 48 h | UC-MSCs | exosomal miRlet-7b↑ | macrophages polarization in vitro cutaneous wound model of diabetic rats | M2 macrophages polarization↑ in vitro and in vivo M1 macrophages and inflammation↓ in diabetic wounds (TLR4/NF-κB/STAT3/AKT) | [61] |
| LPS (100 ng/mL) 24 h | BM-MSCs | exosomes | macrophages polarization and cytokines production in vitro myocardial infarction model of mice | M2↑ and M1↓ in vitro and in vivo IL-6, TNF-α, IL 1β↓ and IL-10↑of macrophages post-infarction inflammation↓ (NF-κB/AKT1/AKT2) | [62] |
| LPS (250 ng/mL) 24 h | DF-MSCs | EVs | periodontitis model of beagle dogs | ROS and RANKL/OPG ratio↓ of LPS pretreated PDLSCs (ROS/JNK) M2 macrophages polarization↑ (ROS/ERK) periodontal tissue regeneration↑ of periodontitis dog | [63] |
| LPS (10 ng/mL) 24 h | BM-MSCs PG-MSCs | IL-6, IL-8, MIF↑ | anti-microbial PMNs activity in vitro | migration of PMNs↑ CCL4 secretion of PMNs↑ (NF-κB) anti-microbial activity of PMNs↑ | [64] |
| LPS (500 ng/mL) 1 h | BM-MSCs | NO↓ IL-6, IL-8↑ | T-cell proliferation and differentiation in vitro EAE model of mice | proliferation of CD3+ T-cell, Th1 and Th17↑ percentages of Th1 and Th17↑ in EAE mice reverse the therapeutic immunosuppressive effect of MSCs | [65] |
| LPS (10 ng/mL) 1 h | UC-MSCs | IL-6, IL-8↑ | T-cell proliferation in vitro colitis model of mice | T-cell proliferation↑ clinical signs and severity of intestinal inflammation↑ in vivo | [66] |
| LPS (1 μg/mL), 72 h | T-MSCs | thrombospondin-1↑ exosomes | macrophages polarization, cytokines production and T-cell differentiation in vitro | M1 macrophages, IL-6, TNF-α↑ differentiation of CD4+ T-cell to Th17 cells↑ | [67] |
| LPS (500 ng/mL) 48 h | BM-MSCs | IL6, iNOS↑ | T-cell proliferation in vitro EAE model of mice | T-cell proliferation↓ clinical score↓, Th17↓ and Treg↑ in vivo | [68] |
| Poly (I:C)) (10 μg/mL) 1 h | BM-MSCs | NO↑ IL-6↓ | T-cell proliferation and differentiation in vitro EAE model of mice | proliferation of CD3+ T-cell, Th1 and Th17↓ percentages of Th1 and Th17↓ in EAE mice clinical signs and the severity↓ of EAE | [65] |
| Poly (I:C)) (1 μg/mL) 1 h | UC-MSCs | IDO, IL-6 and IL-8↑ | T-cell proliferation in vitro colitis model of mice | T-cell proliferation↓ clinical signs and severity of intestinal inflammation↓ in the acute murine model of colitis | [66] |
| Poly (I:C)) (100 μg/mL) 24 h | BM-MSCs | COX2, PGES↑ | GVHD model of mice | (TLR3/PGES/COX2/PGE2) | [69] |
| Poly (I:C)) (1 μg/mL) | UC-MSCs | PGE2↑ | colitis model of mice | IFN-γ, IL-17A, IL-21, IL-23↓ and IL-10↑ in colitis tissues proliferation of activated mesenteric lymphnode cells↓ Th1/17 cells↓ and Treg↑ in the colon proliferation of mononuclear cells↓ clinical and pathological manifestations in colitic mice↓ (TLR3/Jagged-1/Notch-1) | [70] |
| TNF-α + IFN-γ 24 h | BM-MSCs | ROS, HIF1α↑ | delayed-type hypersensitivity model of mice | oxidative phosphorylation metabolism↓ Th1, Th17↓in vitro and in vivo | [71] |
| TNF-α (1 ng/mL) + IFN-γ (10 ng/mL) 24 h | MSCs | IDO↑ PTGS2 and IL-6↓ in the presence of palmitate | PBMCs proliferation and cytokines production in vitro | PBMCs proliferation and production of IFN-γ, TNF-α↓ in the presence of palmitate (IFN-γ/JAK1/2) | [72] |
| TNF-α (3 ng/mL) + IFN-γ (10 ng/mL) 24 h | BM-MSCs | IDO↑ | macrophages polerization and T-cell proliferation in vitro | M2 macrophages↑ T-cell proliferation↓ | [73] |
| TNF-α (20 ng/mL) + IFN-γ (20 ng/mL) 24 h | UC-MSCs | CM | macrophages migration, polarization and cytokines production in vitro skin wound model of mice | migration, M2 polarization, and phagocytic ability of macrophages↑ secretion of VEGF, IL-10, IL-13 and IL-4↑ and TNF-α↓ of macrophages proangiogenic ability↑ wound closure↑ (IL-6/IL-4Rα/STAT6/PPARγ and IL-6/STAT3) | [74,75] |
| TNF-α (10 ng/mL) + IFN-γ (10 ng/mL) 24 h | UC-MSCs | IDO, TSG-6↑ CXCL9, CXCL10 and CXCL11↑ Hexokinase II↑ | inflammatory bowel disease model of mice | inflammatory parameters in inflammatory bowel diseases mice↓ (PI3K/AKT) | [76] |
| TNF-α (1.5 ng/15 ng) + IFN-γ (6.5 ng/65 ng) 48 h | BM-MSCs | CCL5, CXCL9, CXCL10 and CXCL11↑ IDO, PD-L1 and HLA-G↑ | / | / | [77] |
| TNF-α (10 ng/mL) + IFN-γ (10 ng/mL) 24 h | BM-MSCs | CMA, LAMP-2A↓ | T-cell proliferation and recruitment t in vitro inflammatory liver injury model of mice | T-cell↓ in vitro and in vivo T-cell recruitment to MSCs↑ (NF-κB and STAT1/AKT) | [78] |
| TNF-α (10 ng/mL) + IFN-γ (10 ng/mL) 24 h | BM-MSCs | miR-155↑ | T-cell proliferation in vitro | T-cell proliferation↓ iNOS and NO expression of MSCs↓ (TAB2) | [79] |
| TNF-α (20 ng/mL) + IFN-γ (50 ng/mL) 24 h | UC-MSCs | tumor necrosis factor-α-related apoptosis-inducing ligand and IDO↑ | leukemia cells cycle, apoptosis in vitro acute myeloid leukemia model of mice | leukemia cells apoptosis↑ survival↑, leukemia burden in peripheral blood and bone marrow↓ of acute myeloid leukemia mice | [80] |
| IL-1β (5 ng/mL) + IFN-γ (20 ng/mL) 24 h | UC-MSCs | PGE2, IDO↑ | PBMC proliferation, T-cell differentiation and migration in vitro colitis model of mice | PBMCs proliferation↓ Th1 differentiation↓ and Treg differentiation↑ CD4 + T-cell migration↑ body weight, colon structure improvement↑ | [81] |
| IL-1β (3 ng/mL) + IFN-γ (30ng/mL) 24 h | BM-MSCs | NO, IL-6 and PGE2↑ | macrophages polarization in vitro | M1 macrophages↓ in inflammation M2b macrophages↓ in anti- inflammation (IL-6) | [82] |
| IL-17 (10 ng/mL) + IFN-γ (10 ng/mL) + TNF-α (10 ng/mL) 12 h | BM-MSCs | iNOS↑ | T-cell proliferation in vitro hepatitis model of mice | T-cell proliferation↓ mononuclear cells and T-cell infiltration↓ in liver injury mouse model | [83] |
| IL-17 (10 ng/mL) + IFN-γ (10 ng/mL) + TNF-α (10 ng/mL) 24 h | BM-MSCs | iNOS, NO↑ | tumor model of mice | tumor growth↑ (NO/STAT3) | [84] |
| LPS (20 μg/mL) + TNF-α (20 ng/mL) 72 h | BM-MSCs | PGE2 and Arginase-1↑ | macrophages polarization in vitro | M2 macrophages↑ M1 macrophages↓ | [85] |
| IFN-γ (100 ng/mL) + poly(I:C) (10 μg/mL) | BM-MSCs | IDO1↑ | colitis model of mice | body weight loss, colon shortening and colon epithelial loss, crypt destruction, inflammatory cells infiltration↓, intestinal stem cells proliferation, enterocyte differentiation, and epithelial regeneration↑ in inflammatory bowel diseases mice IL-1β, TNF-α,, IL-6↓, IL-10↑ in colon tissue Treg↑ in lymphoid organs and in vitro | [86] |
| TGF-β (10 ng/mL) + IFN-γ (1000 IU/mL) 72 h | UC-MSCs | exosomes (IL-10, IFN-γ, IDO) ↑ | PBMCs proliferation and differentiation in vitro | PBMCs proliferation↓ proportion of Treg↑ | [87] |
| IL-1β (50 ng/mL) + IL-6 (40 ng/mL) + TNF-α (100 ng/mL) + IFN-γ (200 ng/mL) 72 h | BM-MSCs | chemerin↑ | L1.2-ChemR23 cells migration in vitro | L1.2-ChemR23 cells migration↑ | [88] |
| Treatment Conditions | MSCs Sources | Secreted Factors or Expressed Genes | Research Scenarios | Immunomodulatory Effects (Signaling Pathway) | Reference |
|---|---|---|---|---|---|
| spheroids | BM-MSCs UC-MSCs | COX2, PGE2↑ CD73, Kynurenine and free phosphate↓ | PBMC proliferation in vitro | T-cell suppressive abilities↓ | [100] |
| AT-MSCs | TSG-6↑ | acute kidney injury model of rats | therapeutic effects of AKI↑ | [101] | |
| BM-MSCs | TSG-6, stanniocalcin-1↑ | LPS-activated macrophages cytokines production in vitro Peritonitis model of mice | secretion of TNF-α↓of macrophages neutrophil activity, TNF-α, IL-1β, CXCL2/MIP-2, PGE2↓in inflammatory exudates and plasmin activity↓ in serum | [104] | |
| BM-MSCs | PGE2↑ | LPS-activated macrophages macrophages polarization and cytokines production in vitro | TNF-α, IL-6, IL-12P40, IL-23, CXCL2↓ and IL-1Ra, IL-10↑ in macrophages polarization of M1 to M2↑ (COX2/PGE2/EP4) (caspases/NF-κB) | [105] | |
| BM-MSCs | IL-1α, IL-1β, COX2, TSG-6, stanniocalcin-1 and PGE2↑ | macrophages polarization in vitro | polarization of M1 to M2↑ (caspases/NF-κB/IL-1/PGE2 and Notch/PGE2) | [106] | |
| UC-MSCs | MMP-2, MMP-9, TGF-β1, IL-6, G-CSF↑ | skin wound model of rats | wound healing↑ in skin wound | [107] | |
| UC-MSCs | IFN-γ, IL-6 ↑ TNF-α↓ | acute liver failure model of mice | necrosis↓, regeneration↑ and liver repair↑ | [102] | |
| BM-MSCs | PGE2, TGF-β, IDO and IL-6↑ | lipopolysaccharide and IFN-γ activated macrophages in vitro | TNF-a secretion↓ of macrophages | [103] | |
| DP-MSCs | exosomes exosomal miR-1246 | periodontitis and colitis model of mice | restored Th17 cells/Treg balance in both the inflamed periodontium and colon alveolar bone loss↓, inflammatory cells↓ and osteoclasts↓ in experimental periodontitis colon length↑, inflammatory cells↓, IL-1β, IL-6, TNF-α↓ in colitis (miR-1246/Nfat5) | [110] | |
| UC-MSCs | IL-10, LIF↑ | arthritis model of rats | local and systemic arthritic manifestations↓ | [108] | |
| spheroids +microbeads+ hydrogel | AT-MSCs | IL-10, TGF-β↑ | ear full thickness wound model of rabbits | infiltration of lymphocytes↓ in wound ear full-thickness wound healing↑ | [109] |
| spheroids + 2% O2/ dimethyloxalylglycine | BM-MSCs | TSG-6, MMP-2 and VEGF↑ | tube formation assay in vitro | tube formation of HUVECs↑ | [111] |
| polycaprolactone and gelatin electrospun fiber scaffolds | BM-MSCs | eotaxin, IL-6, LIF, MCP-1 and MCP-3↑ | corneal wound model of rabbits in vitro | corneal wound healing↑ | [112] |
| polyisocyanide hydrogel | AT-MSCs | IL-10↑ | wound healing assay in vitro | wound healing↑ | [14] |
| Treatment Conditions | MSCs Sources | Secreted Factors or Expressed Genes | Research Scenarios | Immunomodulatory Effects (Signaling Pathway) | Reference |
|---|---|---|---|---|---|
| IL-4 gene transfection +spheroids | AT-MSCs | IL-4↑ | IL-1β-treated chondrocytes apoptosis and cytokines production in vitro osteoarthritis model of rats | chondrocytes apoptosis↓, NO, iNOS MMP-13)↓and cartilage ECM (Col2) ↑ in chondrocytes production of NO↓, chondrocyte apoptosis↓, expression of the pain mediators↓ in osteoarthritis | [117] |
| IL-10 gene transfection | MSCs | IL-10↑ | orthotopic liver transplantation model of rats | liver allograft survival↑ acute rejection↓ Th17↓ and Treg↑ IL-17, IL-23, IL-6, IFN-γ, TNF-α↓ and IL-10, TGF-β1↑ in T-cell of liver and in serum | [118] |
| BM-MSCs | IL-10↑ | T-cell proliferation in vitro corneal allograft model of rats | proliferation of T-cell↓ corneal allograft survival time↑ infiltration of CD4+, CD68+ T-cell↓ in the corneal grafts CD4+, CD68+ T-cell↓ and Treg↑ in the draining lymph nodes lncRNA 003946 expression↑ in CD68+ infiltrating cells | [119] | |
| TGF-β1 gene transfection | BM-MSCs | TGF-β1↑ | T-cell proliferation, cytokines production and differentiation in vitro liver allograft model of rats | T-cell proliferation and IFN-γ secretion↓ and Treg↑ acute rejection↓ and survival↑ after liver transplant T-cell, Th17 cells, IL-1β, IL-6, IFN-γ↓, Treg, IL-10↑ in vivo | [120] |
| IL-35 gene transfection | AT-MSCs | IL-35↑ | cardiac allograft model of mice | acute cellular rejection↓ allograft survival↑ Th17, Th1/Th2↓ and Treg↑ in spleen IL-17↓ in graft | [121] |
| IDO gene transfection | BM-MSCs | IDO↑ | T-cell proliferation, differentiation and cytokines production in vitro orthotopic renal transplantation model of rabbits | T-cell proliferation↓, Treg↑, CTLA-4, IL-10 and TGF-β1 expression of Treg↑ in vitro renal graft survival and tolerance↑ | [122] |
| BM-MSCs | IDO↑ | T-cell and DCs differentiation in vitro heterotopic heart transplantation model of rats | DCs markers↓ and Treg↑ in vitro and in vivo IL-10, TGF-β↑ and IL-2, IFN-γ↓ in serum infiltration of inflammatory cells, hemorrhage, edema, and myocardial damage↓ in the transplantation mode | [123] | |
| HO-1 | BM-MSC | HO-1↑ | small bowel allograft model of rats | recipient survival rates↑ clinical manifestation and weight loss↓ grading of acute rejection↓ in small bowel graft apoptotic↓ cells in small intestine mucosa↓ NK cells activity↓ in graft IFN-γ. IL-2, IL-17, IL-6, IL-23, TNF-α↓ and IL-10, TGF-β↑ in serum Treg↑ in the spleen | [124] |
| BM-MSC | HO-1↑ | orthotopic liver transplantation model of rats | recipient survival rates and liver function↑ clinical manifestations↓ grading of acute rejection↓ in hepatic grafts apoptotic cells↓ in hepatic tissue IFN-γ. IL-2, IL-17, IL-6, IL-23, TNF-α↓ and IL-10, TGF-β↑ in serum Treg↑ in the spleen | [125] | |
| BM-MSC | HO-1↑ | lymphocytes proliferation and differentiation in vitro reduced-size liver transplantation model of rats | T-cell, NK cells activation↓ and Treg↑ in vitro recipient survival rates↑ clinical manifestations↓ grading of acute rejection↓ in hepatic graft Treg↑ in the spleen TNF-α, IL-2, IL-17, IL-23↓ and IL-10, TGF-β↑ in serum NK cells activation↓ in hepatic graft | [126] | |
| PD-L1 gene transfection | BM-MSC | EVs (PD-L1) | T-cell proliferation, differentiation and cytokines production in vitro GVHD model | Treg↑, T-cell proliferation↓ IL-10, IL-2, TGF-β, IFN-γ↑ of CD4+ T-cell host versus graft rejection↓ graft survival↑ Treg↑ and Th17, Th1 cells↓ in vivo | [127] |
| BPI21/LL-37 gene transfection | UC-MSCs | BPI21/LL-37↑ | antibacterial activity and endotoxin neutralization assay LPS-activated macrophages in vitro sepsis model of mice | antibacterial and endotoxin-neutralizing activity↑ IL-1β, TNF-α, IL-6↓ and IL-10↑ both in macrophages and in serum bacterial clearance and endotoxin-neutralizing↑ in septic mice | [128] |
| HIF1α gene transfection | DP-MSCs | HIF1α, CCL2/MCP-1, galectin 1, IL-6↑ | T-cell proliferation, DCs differentiation, monocytes recruitment and differentiation, and NK cells-mediated lysis in vitro | T-cell proferization↓ DCs differentiation↓ recruitment of monocytes and differentiation into suppressor macrophages↑ degranulation and IFN-γ production of NK cells↓ | [129] |
| HIF1α gene transfection/+ IFN-γ (50 ng/mL) + TNF-α (10 ng/mL) + IL-1β (10 ng/mL) | DP-MSCs | EVs IL-6, IDO↑ | macrophages polarization, T-cell proliferation, PBMCs adhesion in vitro delayed type hypersensitivity model of mice colitis model of mice | M1 repolarizes to M2↑ efferocytic and immunosuppressive capacity of M1↑ fibrosis Induced by TGF-β expression of VCAM and P-selectin of HUVECs↓ PBMCs adhesion on activated endothelium↓ proliferation of CD4+, CD8+ T-cell↓ leucocyte infiltration↓ ear-swelling response↓ hyperplasia↓ and CD45+ cells infiltration↓, M1↓and M2↑ in a DTH mice inflammatory cells infiltration↓, TNF-α, IL-1β, IL-6↓, M1↓and M2↑ in colitis mouse model (PD-L1/PD-1/NF-κB) | [130,131] |
| sFgl2 gene transfection | AT-MSCs | sFgl2↑ | IFN-γ and LPS-activated macrophages polarization, migration and phagocytosis in vitro heart transplantation model of mice | M1↓, M2↑ in vitro and in vivo (JAK/STAT and NF-κB) phagocytosis and migration of macrophages↑ myocyte necrosis, vasculitis, lymphocytes infiltration↓ in the heart grafts Treg↑ in spleen IFN-γ, IL-12, TNF-α, IL-6, and IL-1β↓, TGF-β1, IL-4, IL-10↑ in the serum acute rejection after heart transplantation↓ | [132] |
| hepatocyte growth factor gene transfection | DP-MSCs | hepatocyte growth factor↑ | T-cell differentiation in vitro psoriasis model of mice | Th1, Th17 cells↓, Treg↑ expression of cytokeratin 6 and cytokeratin 17↓ in the psoriatic skin lesions. IFN-γ, IL-17A, TNF-α↓ in the serums T-box transcription factor 21, IFN-γ, retinoic acid-related orphan receptor-γt, IL-17A, IL-17F, IL-23↓ and Foxp3, IL-10↑ in the psoriatic skin lesions. | [133] |
| heparin microparticle loaded with IFN-γ (20 µg/mg microparticle) + spheroids | BM-MSCs | IDO↑ | T-cell proliferation, activation and cytokines production in vitro | T-cell activation and proliferation↓ secretion of TNF-α↓, IL-10↑ of PBMCs | [134] |
| internalization of PLGA microparticle loaded with budesonide (loading 7.05%) | MSCs | IDO↑ | PBMCs proliferation and cytokines production in vitro | proliferation and IFN-γ production of PBMCs↓ (STAT1/ FOXO3) | [135] |
| Treatment Conditions | MSCs Sources | Secreted Factors or Expressed Genes | Research Scenarios | Immunomodulatory Effects (Signaling Pathway) | Reference |
|---|---|---|---|---|---|
| tetrandrine (5 μM and 10 μM) 24 h | BM-MSCs | PGE2↑ | LPS-activated macrophages cytokine production in vitro ear skin inflammation model of mice | TNF-α secretion of LPS-activated macrophages↓ TNF-α↓ in ear skin inflammation sites (NF-κB/COX2) | [137] |
| budesonide (1 μM) 24 h + IFN-γ (100ng/mL) 48 h | MSCs | IDO↑ | PBMCs proliferation and cytokines production in vitro | proliferation and IFN-γ production of PBMCs↓ (STAT1/ FOXO3) | [135] |
| dexamethasone (1000 ng/mL, 2000 ng/mL, 3000 ng/mL) 24 h and 48 h | UC-MSCs DP-MSCs AT-MSCs BM-MSCs | PGE2, IDO, HLA-G↑ | PBMCs proliferationt in vitro | proliferation of PBMCs↓ | [138] |
| JWH-133 (2.5µM) / + dexamethasone (100 nM) 24 h | ITP-MSCs | IL-6↓ IL-4, Bcl2↑ | T lymphocytes proliferation and cytokines production in vitro | T lymphocytes proliferation↓ TNF-α↓ in LPS-treated T-cell (Bcl2) | [139] |
| melatonin (1 μM) 48 h | BM-MSCs | exosomes exosomal miR-34a, miR-124 and miR-135b↑ | monocytes polarization and cytokines production in vitro air pouch model of mice diabetic wound healing of rats | ratio of M2 to M1↑ in vitro and in vivo IL-1β, TNF-α, iNOS↓, Arginase-1, IL-10↑ of macrophages in vitro gene expression of TGF-β1, Il-10 and TSG-6↑ of activated THP-1 cells angiogenesis and collagen synthesis↑ in diabetic wound (PTEN/AKT) | [140] |
| melatonin (10 μM) 72 h | BM-MSCs | exosomal miR-34a, miR-124 and miR-135b↑ | monocytes polarization and cytokines production in vitro | M2 polarization and gene expression of TGF-β1, Il-10 and TSG-6↑of activated THP-1 cells | [141] |
| trimetazidine (50 μM) 6 h /diazoxide (100 μM) 0.5 h | ESC-MSCs | secretome | LPS-activated PBMCS cytokines production in vitro endotoxemia model of mice | IL-10, TNF-α and IL-1β↑ secreted by PBMCs CXCL13, IL-12, CCL2, TNFR1, IL↓ and IL-3, IL-10, KC, CXCL2α, XCL1, CCL5↑ in serum of LPS injected mice necroinflammatory score↓ in kidney and liver of LPS injected mice alveolar space↑ and inflammatory infiltration↓ in lung of LPS injected mice | [142,143] |
| chlorzoxazone (10 μM) 24 h | UC-MSCs | IDO, COX2, IL-4, TSG-6, CCL5, CXCL9 and CXCL10↑ IL-6↓ | T-cell proliferation acute nephritis model of mice | T-cell activation and proliferation↓ inflammatory infiltration and tissue damage in AKI rat model↓ (FOXO3) | [144] |
| metformin (0.1, 1 and 5 mM) 72 h | AT-MSCs | IDO, IL-10 and TGF-β↑ | T-cell proliferation in vitro lupus model of mice | CD4+ T-cell proliferation↓ cervical lymph node and kidney weight, proteinuria, serum anti-dsDNA IgG and renal pathology↓ in lupus nephritis mice regulatory effect on peripheral blood and splenic cellular subsets in lupus nephritis mice Th17/Treg ratio↓ of spleen and kidney in lupus nephritis mice | [145] |
| SP (100 nM) 48 h | BM-MSCs | TGF-β1↑ | T-cell proliferation and cytokines production in vitro | activity and IL-2/ IFN-γ secretion of T-cell↓ | [146] |
| rapamycin (3 μM) 24 h | UC-MSCs | TGF-β1↑ | T-cell proliferation in vitro | CD4+ T-cell proliferation↓ | [147] |
| BM-MSCs | TGF-β1, CXCL8↑ | T-cell migration and differentiation in vitro | migration and Treg differentiation↑ Th1 cells differentiation and IL-17A, IFN-γ, IL-2 production of CD3/CD28+ T-cell↓ | [148] | |
| UC-MSCs | IL-10, TGF-β1, IDO↑ | liver ischemia/reperfusion injury model of mice | neutrophils infiltration and ROS↓ in liver tissues IL-1β, IL-6, TNF-α gene↓ in liver tissues | [149] | |
| CB (10 µg/mL) 0.5 h | AT-MSCs | microvesicles | PBMCs proliferation and cytokine production, T-cytotoxic lymphocytes, Th cells, and B cells proliferation in vitro transplantation sheep red blood cells immunization model of mice allogeneic and xnogeneic microvesicles in mice | anti-sheep red blood cells antibody↓ in serum proliferation of PBMCs↓, activation of Th cells. B cells and T-cytotoxic lymphocytes↓ fractalkine↓, G-CSF, GM-CSF, MCP-3, MDC, IL-12p70, IL-1β, MCP-1 of PBMCs↑ | [150,151] |
| kynurenic acid (200 μM) 48 h | UC-MSCs | TSG-6↑ | acute lung injury model of mice | neutrophil infiltration in ALI↓ (AhR) | [152] |
| glutamine (2 mM and 10 mM) 14 d | BM-MSCs | IL-1β, IL-6↓ IL-10, TGF-β↑ | lymphocytes and macrophages proliferation and cytokine production in vitro | lymphocytes and macrophages proliferation↓ IL-10 production↑ of lymphocytes and macrophages IFN-γ production↓ of lymphocytes (NF-κB/STAT3) | [153] |
| Tongxinluo (400 μg/mL) 24 h | BM-MSCs | exosomal miR-146a-5p | acute myocardial infarction model of rats | cardiomyocyte apoptosis↓ apoptotic cardiomyocytes, Bax, cleaved-Caspase 3, IL-6, TNF-α, infarct size and cardiac fibrosis↓ and angiogenesis↑ in the infarct region (IRAK1/NF-κB p65) | [154] |
| Magnesium (5 mM) 24 h + LPS (1.25 μg/mL) /TNF-α (10 ng/mL) 2 h | C3H/10T1/2 MSCs | IL-1β, IL-6↓ IL-10, PGE2↑ | LPS-activated macrophages proliferation and cytokine production, lymphocytes proliferation and cytokine production in vitro | Proliferation production of TNF-α, IL-β, IL-6 ↓ and IL-10↑ of macrophages IL-10 production↑ of lymphocytes (NF-κB/STAT3) | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Xu, C.; Cheng, W.; Zhao, Y.; Sui, L.; Zhao, Y. Pretreated Mesenchymal Stem Cells and Their Secretome: Enhanced Immunotherapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 1277. https://doi.org/10.3390/ijms24021277
Su Y, Xu C, Cheng W, Zhao Y, Sui L, Zhao Y. Pretreated Mesenchymal Stem Cells and Their Secretome: Enhanced Immunotherapeutic Strategies. International Journal of Molecular Sciences. 2023; 24(2):1277. https://doi.org/10.3390/ijms24021277
Chicago/Turabian StyleSu, Yuran, Chenyu Xu, Wen Cheng, Yanmei Zhao, Lei Sui, and Yanhong Zhao. 2023. "Pretreated Mesenchymal Stem Cells and Their Secretome: Enhanced Immunotherapeutic Strategies" International Journal of Molecular Sciences 24, no. 2: 1277. https://doi.org/10.3390/ijms24021277
APA StyleSu, Y., Xu, C., Cheng, W., Zhao, Y., Sui, L., & Zhao, Y. (2023). Pretreated Mesenchymal Stem Cells and Their Secretome: Enhanced Immunotherapeutic Strategies. International Journal of Molecular Sciences, 24(2), 1277. https://doi.org/10.3390/ijms24021277






