Abstract
Antiphospholipid syndrome (APS) is an acquired highly prothrombotic disorder in which thrombo-inflammatory antiphospholipid antibodies (aPL) cause thrombosis via multiple mechanisms, including endothelial damage and activation. Obstetric complications in APS are caused by placental thrombosis, inflammation and complement activation. Anticoagulation is poorly effective in some patients especially those with triple positive aPL who are at ~30% risk of thrombosis recurrence within 10 years. Increasing therapeutic anticoagulation intensity may be beneficial but leads to excess bleeding with serious complications, such as intracerebral haemorrhage. Nonetheless, anticoagulation is still the mainstay of treatment despite the autoimmune nature of APS. The antimalarial immunomodulatory drug hydroxychloroquine (HCQ) has been used for many years for the treatment of inflammatory rheumatic diseases. HCQ has complex pleiotropic mechanisms of action upon multiple cell types. The proposed biological processes that HCQ regulates support the hypothesis that it may be a successful adjunctive treatment in the prevention of recurrent thrombosis and pregnancy complications.
1. Introduction
Antiphospholipid syndrome (APS) is the paradigm example of an autoantibody-mediated prothrombotic disorder, resulting in a high frequency of thrombosis and pregnancy complications. Unlike autoimmune vasculitis where inflammation is the hallmark of disease progression, inflammation is not the predominant feature of APS. Instead, the presence of inflammation may act as a “second hit” in the process of developing thrombosis or pregnancy complications in an individual with antiphospholipid antibodies (aPL) [1]. Thrombosis in APS can be venous, arterial, microvascular or a combination of events occurring in any organ or tissue. Deep vein thrombosis is the most common venous thrombotic event, although thrombosis at unusual sites including cerebral venous sinus thrombosis, portal vein thrombosis and retinal vein thrombosis is a presenting feature. Stroke is the most common arterial thrombotic event and around one-third of strokes <50 years of age may be due to APS [1]. Pregnancy complications include recurrent miscarriages and late pregnancy complications such as foetal loss due to placental insufficiency. The diagnosis of APS requires the presence of clinical events (either thrombosis, pregnancy complication or both) with persistently positive aPL (detection on ≥two consecutive occasions at least 12 weeks apart) [2]. Laboratory diagnostic criteria aPL tests measure lupus anticoagulant (LA), IgG/IgM anticardiolipin (aCL) and IgG/IgM anti-β2-glycoprotein I (anti-β2GPI) antibodies.
In addition to the standardised clinical criteria for APS diagnosis (Sydney criteria) [2], several other clinical features are frequently associated with APS, collectively known as non-criteria features. These include heart valve disease, livedo reticularis, thrombocytopenia, nephropathy and alveolar haemorrhage which may present an association with thrombosis and/or pregnancy morbidity or as isolated features with persistently positive aPL. APS is a relatively rare disease with an estimated UK incidence of 1 in 2000 [3] but it has major clinical significance. APS can present in isolation (primary APS) or in association with other autoimmune diseases, mainly systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) (secondary APS). A study of 1000 patients with SLE over 10 years demonstrated that, of the 7% overall mortality, 26.5% of those deaths were due to thrombotic APS [4]. Patients with triple-positive aPL (i.e., presence of LA, aCL and anti-β2GPI) are at the highest risk of developing first and recurrent events of thrombosis [5].
Besides aCL and anti-β2GPI, autoantibodies against various other targets are present in APS patients, which may variably contribute to the pathogenesis of the disorder. Of these, anti-phosphatidylserine/prothrombin antibodies (anti-PS/PT) that target the complex of anionic phospholipid phosphatidylserine (PS) and the procoagulant plasma protein prothrombin (PT) have been shown to be involved in pathogenesis and thrombotic risk prediction [6,7]. This target is analogous to the complex of cardiolipin with β2GPI. Recent evidence suggests that LA positivity, determined by functional clotting assays, is likely due to the presence of anti-PS/PT rather than aCL/anti-β2GPI [7]. Several studies have shown an enhanced performance of anti-PS/PT compared with antibodies against prothrombin alone (aPT) in APS [8,9].
aPL target multiple cell types and particularly endothelial cells (EC) to cause thrombosis [1]. The endothelium, forming the internal wall of blood vessels, naturally presents an antithrombotic surface to the flowing blood, expressing and secreting factors that modulate vascular contractility, coagulation, fibrinolysis and platelet adhesion. Inflammation reverses this phenotype, leading to the upregulation of procoagulant/antifibrinolytic factors such as the tissue factor (TF), von Willebrand factor (VWF) and plasminogen activator inhibitor-I (PAI-I) and downregulation of anticoagulant/fibrinolytic factors such as thrombomodulin (TM), the tissue factor pathway inhibitor (TFPI), endothelial protein C receptor (EPCR), tissue–plasminogen activator (t-PA) and nitric oxide (NO), creating an environment favouring thrombosis [10]. Additionally, inflammation upregulates cell adhesion molecules (e.g., VCAM, ICAM and selectins), promoting endothelial interaction with blood cells, including platelets and leukocytes. Largely via effects on the vasculature, inflammation plays a critical role in the pathogenesis of thrombosis in APS [11]. The binding of aPL to EC upregulates TF expression and promotes leukocyte adhesion, cytokine secretion and PGE2 synthesis. aPL interfere with naturally occurring anticoagulant pathways, including protein C and TFPI pathways, either by producing antibodies against these anticoagulants or indirectly, for example, via the TM pathway [12]. aPL also inhibits fibrinolysis through various mechanisms, including the downregulation of tPA via antibodies against tPA, disruption of the TM pathway or upregulation of PAI-1. In addition, aPL binds platelets through phospholipid-binding proteins leading to platelet aggregation. All these mechanisms contribute to a prothrombotic state, regarded as the first hit in developing thrombosis. Notably, thrombotic events occur only intermittently and not all individuals with aPL developing thrombosis, underlining the importance of a ‘second hit’ or ‘trigger’ to precipitate thrombosis [1]. A second hit such as inflammation, infection or genetic susceptibility for developing thrombosis tips the balance, and combined with activation of the complement, leads to thrombosis [12].
Although APS is an autoantibody-mediated disease, anticoagulation with vitamin K antagonists (VKA, primarily warfarin) rather than immunomodulation remains the mainstay of management. A major concern in APS is arterial thrombosis, which, unlike atherosclerosis, mainly affects young patients [13] and is driven by pathogenic mechanisms unrelated to conventional vascular risk factors and plaque rupture. For primary and secondary prevention of ischaemic stroke, combinations of anticoagulants and antiplatelet agents are utilised. However, despite adequate anticoagulation, APS patients may suffer recurrent life-threatening thrombotic events. A 10-year prospective study of 1000 patients with APS reported that 25% suffered from further thromboses despite antithrombotic therapy and were the most common cause of death (36.5% of total deaths over the 10-year period, 9.3% of the entire cohort) [14]. Importantly, warfarin can be poorly effective in triple aPL-positive patients and is associated with a ~30% risk of recurrence within 10 years despite otherwise adequate anticoagulation [5]. Direct oral anticoagulants such as rivaroxaban or apixaban are not as effective as warfarin, especially in patients with triple aPL positivity [15]. The management of recurrence by increasing the intensity of anticoagulation is limited by the risk of major bleeding including life-threatening or life-changing intracerebral haemorrhage (ICH).
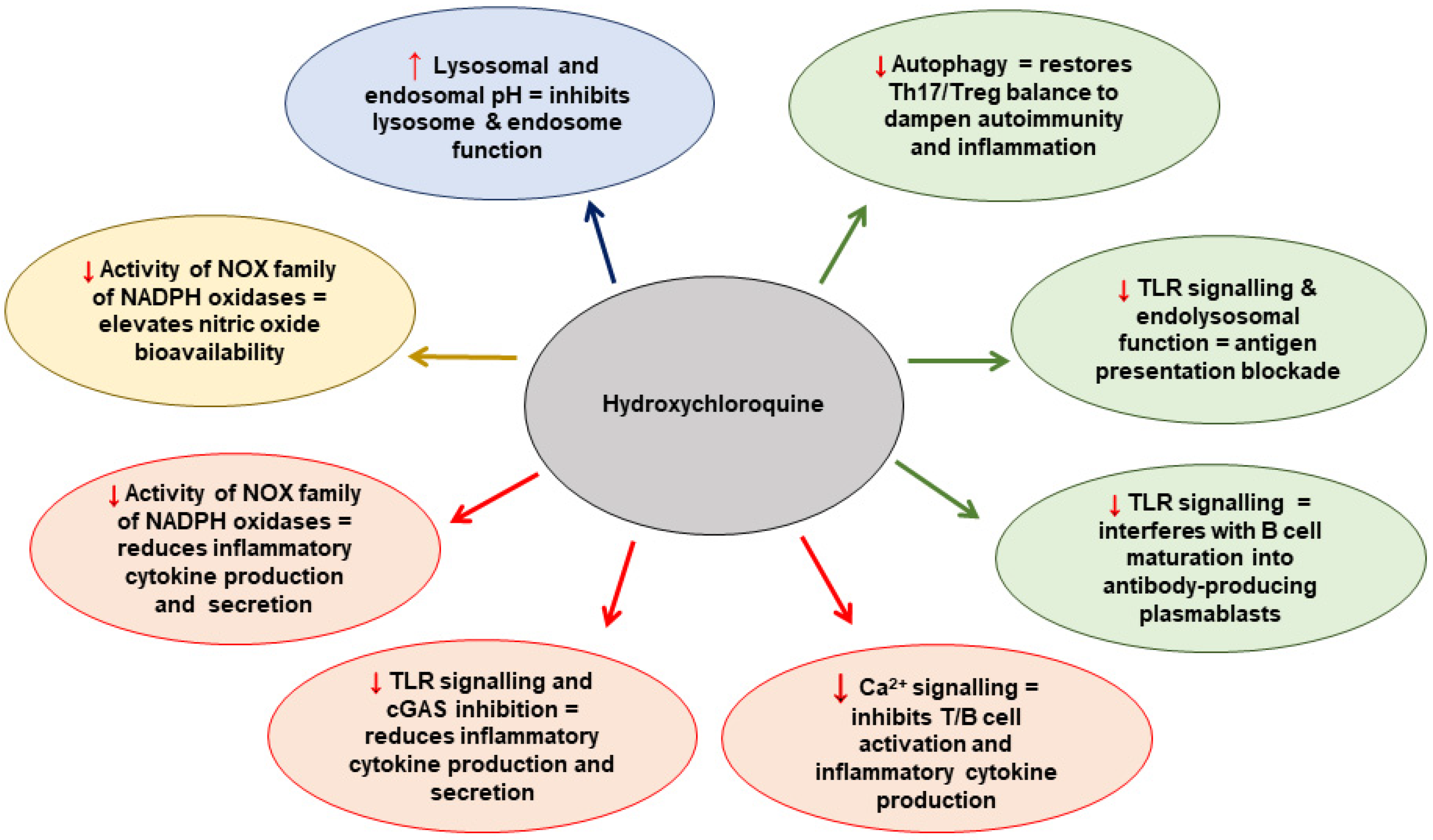
The antimalarial drug hydroxychloroquine (HCQ) has been used for many years for the treatment of inflammatory rheumatic diseases, especially SLE and RA. HCQ is cheap and easily available. It has complex pleiotropic mechanisms of action [16] upon multiple cell types, collectively supporting the hypothesis that HCQ may act as a successful adjunctive treatment in the prevention of recurrent thrombosis or pregnancy complications. The proposed mechanisms that HCQ modulates at the cellular level are summarised in Figure 1 [17]. In this review, we discuss the role of HCQ as an immunomodulatory, antithrombotic and antiplatelet agent and its potential benefits for patients with thrombotic and obstetric APS.
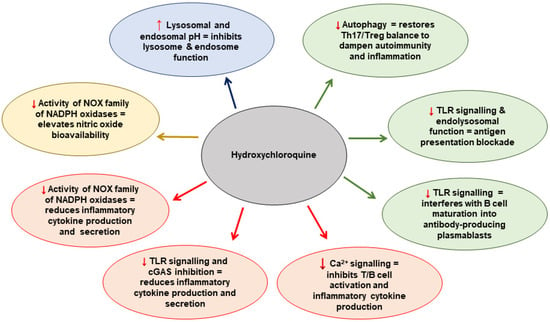
Figure 1.
The proposed mechanisms that HCQ modulates at the cellular level. Adapted from Nirk et al. 2020 [17]. NADPH = nicotinamide adenine dinucleotide phosphate hydrogen; NOX = NADPH oxidase; TLR = Toll-like receptor; cGAS = cyclic GMP–AMP synthase; Th17 = T-helper cell 17.
2. Hydroxychloroquine in Thrombotic APS
The antithrombotic and immunomodulatory properties of HCQ were demonstrated many years ago in a thrombotic APS mouse model. Edwards et al. showed that HCQ significantly diminished both the thrombus size and duration of thrombus formation in mice injected with aPL [18]. More recently, Miranda et al. showed that HCQ reduced TF expression in arterial tissue isolated from thrombotic APS mice, as well as reduced the plasma levels of soluble cell adhesion molecules E-selectin and vascular cell adhesion molecule (VCAM-1). HCQ also improved endothelial nitric oxide synthase (eNOS) mRNA and protein expression, suggesting that it may stimulate the posttranscriptional expression of eNOS in the mouse model [19]. eNOS regulates NO production which, in turn, supports the endothelial antithrombotic function by inhibiting platelet activation and promoting vasodilation [20]. Interestingly, in a separate in vivo model, anti-β2GPI were reported to promote thrombosis by downregulation of eNOS and reducing generation of NO. These results were replicated in an in vitro system using cultured human EC [21].In a separate thrombotic APS mouse model, Urbanski et al. demonstrated that a single injection of monoclonal anti-β2GPIinduced arterial resistance that lasted up to three weeks; HCQ improved endothelium-dependent arterial dilation, which was mediated by an increase in eNOS [22].
Numerous other APS-related in vitro studies reported beneficial effects of HCQ upon the endothelium and other cell types. For example, HCQ reduced endothelial and monocyte TF expression [23], upregulated endothelial TM expression [19] and protected from aPL-mediated disruption of the endothelial annexin V anticoagulant shield [24]. In patients, HCQ treatment reduced soluble plasma TF [25]. Furthermore, Dong et al. showed that HCQ can reverse aPL-inhibited angiogenesis and endothelial migration [26]. In SLE, several lines of in vitro and in vivo evidence suggest that a significant benefit of HCQ may also arise from its effects on the endothelium, as seen in murine SLE models where early treatment with HCQ prevented the development of endothelial dysfunction [27]. HCQ has been shown to affect the antigen–antibody interaction (formation of immunocomplexes), an essential biological step that facilitates aPL-induced pathology. In a study using ellipsometry and atomic force microscopy (AFM), Rand et al. demonstrated HCQ at ≥1 μg/mL directly affected the formation of IgG aPL–β2GPI complexes on phospholipid bilayers. The incubation of IgG aPL–β2GPI with HCQ significantly reduced the binding of complexes to phospholipids compared with complexes incubated with the control buffer (no HCQ). This effect was completely reversed when the HCQ–protein solutions were dialyzed against the buffer (0.01 M HEPES, 0.14 M NaCl 0.05%) [28].
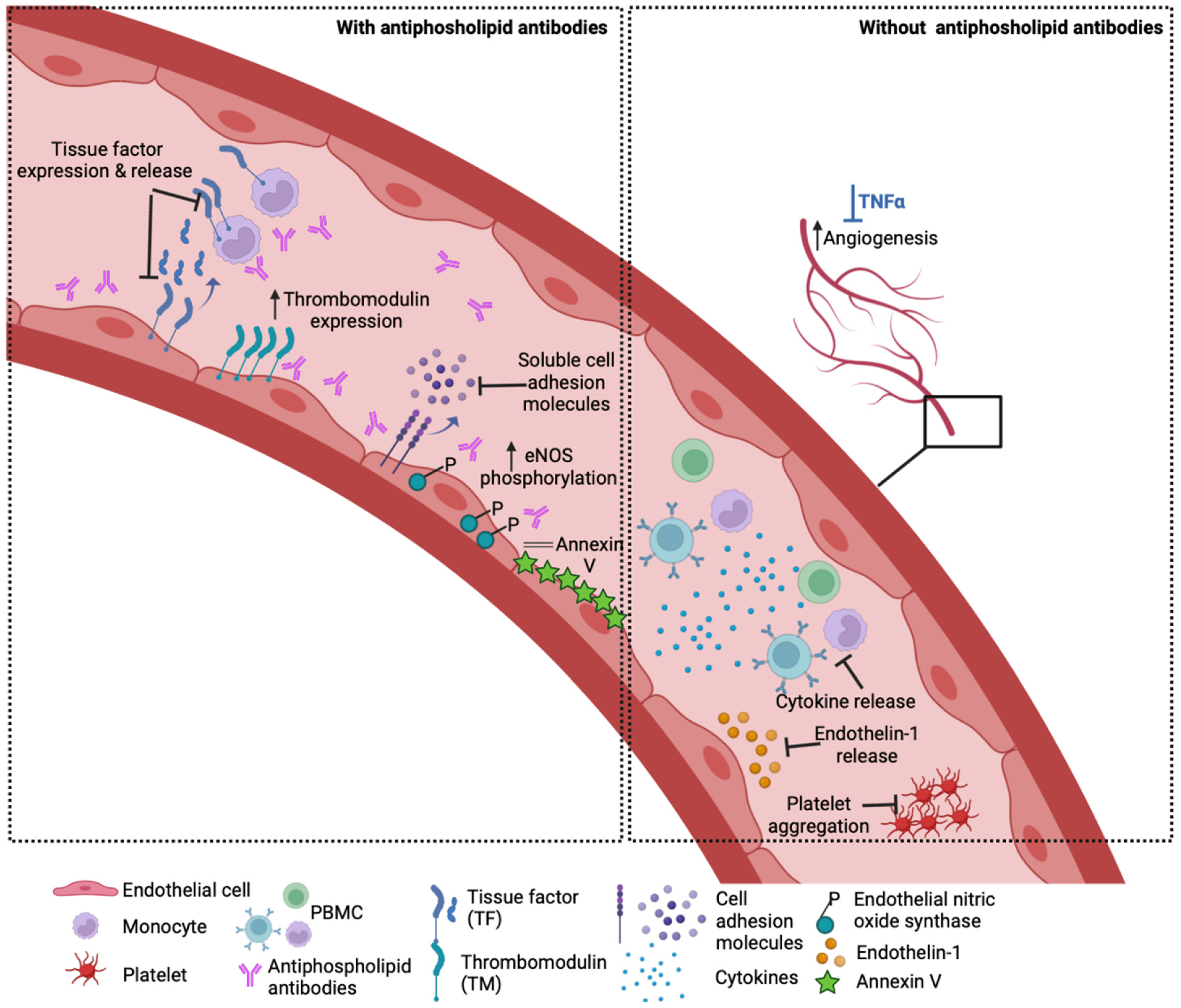
Outside the context of APS, HCQ was shown to block the release of the potent vasoconstrictor endothelin-1 and restore impaired angiogenesis in vitro in TNF-α-treated EC [29]. HCQ reduced the production of inflammatory cytokines such as TNF-α, IFN-γ and IL-6 from peripheral blood mononuclear cells [30,31]; upregulated IL-10 [32] and reduced platelet aggregation [33]. HCQ also targets lysosomal activity, autophagy, membrane stability and alters signalling pathways and transcriptional activity [16,34]. However, its exact mechanism of action and its cellular binding site are still not well understood, and its effects may vary according to the type of vascular endothelium examined (venous, arterial or microvascular). Experimental HUVEC models of inflammation showed that TNF-α-induced endothelial–leukocyte adhesion and the expression of endothelial ICAM-1 and VCAM-1 were profoundly reduced by HCQ treatment [29,35]. In an ex vivo vasomotion model, HCQ induced the relaxation of rat aortic rings in a concentration-dependent manner that was mediated by endothelium-derived NO [36]. In patients with chronic renal failure, HCQ caused a significant reduction in vascular endothelial dysfunction and improvement in vascular elasticity and flow with preservation of the blood vessel wall thickness [37]. The potential effects of HCQ on the vasculature are summarised in Figure 2.
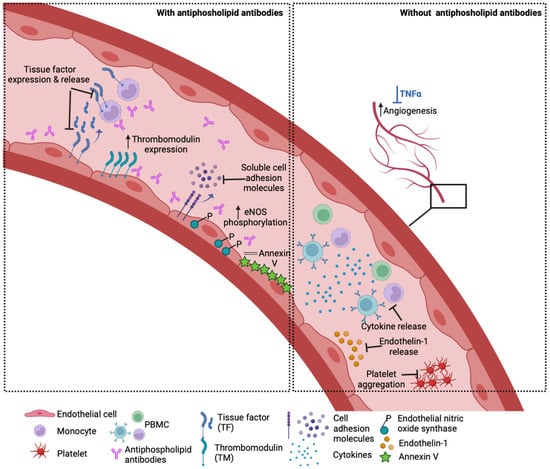
Figure 2.
How HCQ may benefit the vasculature. Left panel: In the presence of antiphospholipid antibodies, HCQ has been shown to reduce endothelial and monocyte tissue factor expression in vitro and lower soluble TF levels in patients. Other in vitro and in vivo studies demonstrate that HCQ reverses the aPL-induced increase in soluble cell adhesion molecules, reduction in thrombomodulin expression and eNOS phosphorylation, as well as disruption of the anticoagulant annexin V shield. Right panel: HCQ ameliorates TNF-a induced endothelin-1 release by endothelial cells and prevents impaired angiogenesis mediated by TNF-a in vitro. HCQ also lowers the cytokine production from stimulated healthy peripheral blood mononuclear cells (PBMC) and agonist-stimulated platelet aggregation.
The European Medicines Agency has licenced the use of HCQ for the treatment of APS as an orphan medicinal product in patients with refractory or recurrent thrombosis despite adequate anticoagulation [38]. Importantly, the risk of bleeding associated with HCQ is extremely low. HCQ therefore appears an excellent candidate as an adjunct to anticoagulation. In a small pilot open label randomised prospective study, 50 patients with primary APS were allocated in a 1:1 ratio to receive HCQ and anticoagulation with or without antiplatelet treatment vs. anticoagulation with or without antiplatelet treatment (no HCQ) [39]. In this study, at a 2.6-year follow-up, patients who received HCQ had lower rates of recurrent thrombosis (0.001 vs. 0.007, log-rank p = 0.048). However, following the adjustment for age, gender, vascular risk factors, presence of triple-positive aPL, previous history of recurrent thrombosis and time in therapeutic range of anticoagulation with VKAs, there was no effect of HCQ towards reducing the risk of recurrent thrombosis. This could be due to a lack of statistical power due to the small number of patients. Additionally, no adverse events were reported related to HCQ. Interestingly, HCQ reduced IgG aCL and both IgG and IgM anti-β2GPItitres [39]. Prior to the above study, a small prospective nonrandomised study included 40 patients allocated to receive HCQ (400 mg daily) with VKA (target INR 2.5) or VKA alone (n = 20 patients per treatment group) [40]. Over a follow-up period of 6–36 months, six patients developed recurrent thrombosis in the VKA alone group, whilst none had recurrence in the HCQ group. In contrast to the aforementioned randomised pilot study, HCQ had no effect on the aPL level. Importantly, there was again no difference in the bleeding events in the two groups [40]. Another study showed that treatment with HCQ reduced the aPL levels in patients with primary APS [41]. Evidence supporting the vasculoprotective effects of HCQ was also reported in a large case–control study of 10,268 cases and 29,969 controls that evaluated HCQ use and the occurrence of cardiovascular events among patients with SLE or RA. The study found a lower risk of overall cardiovascular events, lower risk of venous thromboembolism and a trend towards lower risks for myocardial infarction and ischemic stroke associated with the current HCQ use [42]. Additionally, a systematic review and meta-analysis of HCQ in rheumatic disease showed a significant reduction in the risk of cardiovascular events (pooled risk ratio, RR, 0.72, 95% CI 0.56–0.94, p = 0.013) [43].
3. Hydroxychloroquine in Obstetric APS
Placental thrombosis, inflammation and complement activation all play major roles in the pathogenesis of obstetric APS [44]. Heparin and low-dose aspirin (LDA) are the treatment of choice for obstetric complications. This treatment regime is based on several clinical studies and meta-analyses on the use of heparin and LDA in obstetric APS [45]. Low molecular weight heparin (LMWH) is the preferred heparin and has been the standard of care due to its lower risk of heparin-induced thrombocytopenia (HIT) and osteopenia and the relatively easy subcutaneous administration compared to unfractionated heparin (UFH). With standard treatment, approximately 70% of pregnant women with APS have successful pregnancy outcomes [46]. However, for the remaining 20–30% of women with obstetric APS, there are limited therapeutic options and no guidelines for those who do not respond to heparin and LDA [46,47,48]. Several adjunctive treatments have been used in women with refractory obstetric APS with variable success [49]. These include steroids, intravenous immunoglobulin, plasma exchange and HCQ [49]. Retrospective studies reported better outcomes with patients with obstetric APS treated with HCQ in addition to the standard treatment [50]. Sciascia et al. reported that HCQ treatment was associated with a higher rate of live births (67% vs. 57%; p = 0.05) and a lower prevalence of aPL-related pregnancy morbidity (47% vs. 63%; p = 0.004) [51]. Gerde et al. reported on a retrospective study assessing the effects of HCQ in achieving successful pregnancy outcomes [52]. In this study, pregnancy outcomes (live birth rates as the primary outcome; early and late pregnancy loss and placental mediated complications as secondary outcomes) in 87 women with refractory primary obstetric APS and no other autoimmune disease who received 400 mg HCQ with 60 mg LMWH and LDA were compared with women who received 60 mg LMWH and LDA. Both the primary and secondary outcomes were significantly better in women treated with HCQ (97.1% (67/69) vs. 62.5% (20/32); p < 0.001). Overall, the pregnancy complications were lower in women treated with HCQ compared to the standard treatment alone (8.7% (6/69) vs. 37.5% (12/32); p < 0.001) [52]. Currently, three prospective clinical trials (HYPATIA: A prospective randomised controlled trial of HYdroxychloroquine to improve Pregnancy outcome in women with AnTIphospholipid Antibodies; HIBISCUS: Hydroxychloroquine for the secondary prevention of thrombotic and obstetrical events in primary antiphospholipid syndrome; HYDROSAPL: HYDROxychloroquine in Syndrome Primary AntiPhospholipid) are assessing the safety and efficacy of HCQ in obstetric APS in combination with the standard treatment. These studies will provide us with more definitive evidence of the use of HCQ in the prevention of obstetric complications.
Similarly, the clinical utility of HCQ has been demonstrated in in vivo models of obstetric APS, preventing foetal death and placental metabolic changes [53]. HCQ protected not only from aPL-mediated placental insufficiency but also abnormal foetal brain development [53]. In the same study, the authors showed that HCQ prevented complement activation both in vivo and in vitro. APS mice had lower C5a levels following treatment with HCQ, and this was observed in patients with APS as well [53]. In cultured human extravillous trophoblast cells, Liu et al. demonstrated that aPL reduced the ability of normal trophoblast cells to invade, migrate and form tubules, which was partially reversed by HCQ [54]. Furthermore, in an obstetric APS mouse model, HCQ was able to reduce pregnancy complications and prevent foetal death [54]. Wu et al. showed that HCQ reversed the effects of aPL on primary syncytiotrophoblasts generated from cytotrophoblasts in vitro by significantly reducing IgG binding and restoring annexin A5 expression [55]. Using the human placental BeWo cell line as a model for trophoblast fusion and differentiation, Marchetti et al. showed that anti-β2GPI decrease trophoblastic differentiation via Toll-like receptor 4 (TLR4). HCQ reversed this phenotype and reduced TLR4 expression levels [56]. Finally, in a human first-trimester trophoblast cell line (SVneo-transformed HTR8 cells), HCQ was able to partially reverse the aPL-mediated inhibition of cell migration despite the observation that HCQ treatment increased the secretion of promigratory IL-6 by trophoblasts [57].
4. Hydroxychloroquine for Asymptomatic aPL Carriers
Individuals with aPL but no history of thrombosis (aPL carriers), although usually asymptomatic at the time of detection, are also at increased risk of developing thrombosis and thereby join the APS group. A multicentre prospective observational study of individuals with a high-risk aPL profile (triple-positive) demonstrated a thromboembolism rate of 5.3% per year, with a cumulative incidence of 37.1% after 10 years (95% CI, 19.9–54.3%) [58]. Another study of asymptomatic carriers reported the annual first thrombosis rate of 2.3% per patient/year during a median of 13 years and noted the risk to be three-fold higher in triple aPL-positive carriers (OR 3.38 (95% CI: 1.24–9.22)) [58]. The identification of this high-risk group raises the question of whether they should be offered primary prophylaxis. However, anticoagulation carries a high risk of bleeding and no evidence to support its implementation in this setting. A recent Preventive Services Task Force (USPSTF) report suggests that primary prophylaxis with aspirin should only rarely be used for the prevention of myocardial infarction (MI) in the general population [59]. Thus, an anti-inflammatory approach may be preferable for this group. In a cross-sectional study of aPL-positive patients with connective tissue disease and APS, a logistic regression analysis demonstrated the reduced risk of a thrombotic event by taking LDA and/or HCQ [60]. Another pilot randomised prospective study of 65 patients investigated the impact of HCQ on thrombosis development and aPL titres in both APS patients and aPL carriers. The results of this trial showed that the use of HCQ plus the standard care was associated with a lower incidence of thrombosis during a 2.6-year follow-up [60]. In a small, randomised control study with 20 aPL carriers where 9/20 were randomised to HCQ, this study was prematurely terminated due to logistic reasons. However, over a 1.7-year follow-up period, no patients developed thrombosis or a serious adverse event [61].
5. Conclusions
HCQ has pleotropic effects on multiple cell types. The drug’s effects on EC, immune cells and platelets in particular, modulate inflammation and the thrombotic risk. The potential benefits of HCQ in reducing the risk of thrombosis has been demonstrated in thrombotic APS and asymptomatic carriers of aPL from in vivo, in vitro and clinical studies. Importantly, HCQ is not associated with an increased risk of bleeding when used in combination with an anticoagulation or in isolation. HCQ’s biological effects may also contribute to protection in obstetric APS given the central role of the vasculature during foetal/placental development. However, there remains a need for more robust evidence on HCQ’s mechanisms of action in reducing thrombosis and obstetric complications to definitively demonstrate the clinical benefits and promote the establishment of multicentre clinical trials assessing the development of APS and the risk of thrombotic or obstetric recurrence.
Funding
This article was not funded by any external sources. D.J.A. was funded by MRC UK (MR/V037633/1). C.P. was funded by Versus Arthritis (21223) and the Imperial College-Wellcome Trust Institutional Strategic Support Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
J.A. received funding from Bayer plc to set up the multicentre database of the study as investigator-initiated funding and received a research grant from Leo Pharma. M.L. received consultation and speaker fees from Leo-Pharma, Takeda and Pfizer. C.P. has no conflicts of interest to declare.
Abbreviations
| anti-β2GPI | anti-β2-glycoprotein I |
| aCL | Anticardiolipin antibodies |
| Anti-PS/PT | anti-phosphatidylserine/prothrombin antibodies |
| APS | Antiphospholipid syndrome |
| aPL | Antiphospholipid antibodies |
| EC | Endothelial cells |
| EPCR | endothelial protein C receptor |
| HCQ | hydroxychloroquine |
| ICH | intracerebral haemorrhage |
| LA | lupus anticoagulant |
| LDA | low dose aspirin |
| LMWH | Low molecular weight heparin (LMWH |
| NO | Nitric oxide |
| eNOS | Nitric oxide synthase |
| PAI-I | Plasminogen activator inhibitor I |
| RA | Rheumatoid arthritis |
| SLE | systemic lupus erythematosus |
| TM | Thrombomodulin |
| TF | Tissue factor |
| TFPI | Tissue factor pathway inhibitor |
| tPA | Tissue-plasminogen activator |
| TLR4 | Toll like receptor 4 |
| UFH | unfractionated heparin |
| USPSTF | US Preventive Services Task Force |
| VKA | vitamin K antagonists |
| VWF | von Willebrand factor |
References
- Arachchillage, D.R.; Laffan, M. Pathogenesis and management of antiphospholipid syndrome. Br. J. Haematol. 2017, 178, 181–195. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Duarte-García, A.; Pham, M.M.; Crowson, C.S.; Amin, S.; Moder, K.G.; Pruthi, R.K.; Warrington, K.; Matteson, E.L. The Epidemiology of Antiphospholipid Syndrome: A Population-Based Study. Arthritis Rheumatol. 2019, 71, 1545–1552. [Google Scholar] [CrossRef]
- Cervera, R.; Khamashta, M.A.; Font, J.; Sebastiani, G.D.; Gil, A.; Lavilla, P.; Mejia, J.C.; Aydintug, A.O.; Chwalinska-Sadowska, H.; de Ramon, E.; et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: A comparison of early and late manifestations in a cohort of 1000 patients. Medicine 2003, 82, 299–308. [Google Scholar] [CrossRef]
- Pengo, V.; Ruffatti, A.; Legnani, C.; Testa, S.; Fierro, T.; Marongiu, F.; De Micheli, V.; Gresele, P.; Tonello, M.; Ghirarduzzi, A.; et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: A multicenter prospective study. Blood 2011, 118, 4714–4718. [Google Scholar] [CrossRef] [PubMed]
- Pontara, E.; Cattini, M.G.; Cheng, C.; Bison, E.; Denas, G.; Pengo, V. Insight into the hypercoagulable state of high-risk thrombotic APS patients: Contribution of aβ2GPI and aPS/PT antibodies. J. Thromb. Haemost. 2021, 19, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Cattini, M.G.; Bison, E.; Pontara, E.; Cheng, C.; Denas, G.; Pengo, V. Tetra positive thrombotic antiphospholipid syndrome: Major contribution of anti-phosphatidyl-serine/prothrombin antibodies to lupus anticoagulant activity. J. Thromb. Haemost. 2020, 18, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Sciascia, S.; Sanna, G.; Murru, V.; Roccatello, D.; Khamashta, M.A.; Bertolaccini, M.L. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome. Thromb. Haemost. 2014, 111, 354–364. [Google Scholar] [CrossRef]
- Jaskowski, T.D.; Wilson, A.R.; Hill, H.R.; Branch, W.D.; Tebo, A.E. Autoantibodies against phosphatidylserine, prothrombin and phosphatidylserine-prothrombin complex: Identical or distinct diagnostic tools for antiphospholipid syndrome? Clin. Chim. Acta 2009, 410, 19–24. [Google Scholar] [CrossRef]
- Libby, P.; Simon, D.I. Inflammation and thrombosis: The clot thickens. Circulation 2001, 103, 1718–1720. [Google Scholar] [CrossRef]
- Foley, J.; Conway, E.M. Cross Talk Pathways between Coagulation and Inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef] [PubMed]
- Meroni, P.L.; Borghi, M.O.; Raschi, E.; Tedesco, F. Pathogenesis of antiphospholipid syndrome: Understanding the antibodies. Nat. Rev. Rheumatol. 2011, 7, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Cheng, G.-Y.; Denas, G.; Pengo, V. Arterial thrombosis in antiphospholipid syndrome (APS): Clinical approach and treatment. A systematic review. Blood Rev. 2021, 48, 100788. [Google Scholar] [CrossRef] [PubMed]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; De Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef]
- Arachchillage, D.R.J.; Gomez, K.; Alikhan, R.; Anderson, J.A.M.; Lester, W.; Laffan, M. British Society for Haematology, T. Thrombosis, Addendum to British Society for Haematology Guidelines on Investigation and Management of Antiphospholipid syndrome, 2012 (Br. J. Haematol. 2012; 157: 47–58): Use of direct acting oral anticoagulants. Br. J. Haematol. 2020, 189, 212–215. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Nirk, E.L.; Reggiori, F.; Mauthe, M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol. Med. 2020, 12, e12476. [Google Scholar] [CrossRef]
- Edwards, M.H.; Pierangeli, S.; Liu, X.; Barker, J.H.; Anderson, G.; Harris, E.N. Hydroxychloroquine Reverses Thrombogenic Properties of Antiphospholipid Antibodies in Mice. Circulation 1997, 96, 4380–4384. [Google Scholar] [CrossRef]
- Miranda, S.; Billoir, P.; Damian, L.; Thiebaut, P.A.; Schapman, D.; Le Besnerais, M.; Jouen, F.; Galas, L.; Levesque, H.; Le Cam-Duchez, V.; et al. Hydroxychloroquine reverses the prothrombotic state in a mouse model of antiphospholipid syndrome: Role of reduced inflammation and endothelial dysfunction. PLoS ONE 2019, 14, e0212614. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Ramesh, S.; Morrell, C.N.; Tarango, C.; Thomas, G.D.; Yuhanna, I.S.; Girardi, G.; Herz, J.; Urbanus, R.T.; De Groot, P.G.; Thorpe, P.E.; et al. Antiphospholipid antibodies promote leukocyte–endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2. J. Clin. Investig. 2011, 121, 120–131. [Google Scholar] [CrossRef]
- Urbanski, G.; Caillon, A.; Poli, C.; Kauffenstein, G.; Begorre, M.-A.; Loufrani, L.; Henrion, D.; Belizna, C. Hydroxychloroquine partially prevents endothelial dysfunction induced by anti-beta-2-GPI antibodies in an in vivo mouse model of antiphospholipid syndrome. PLoS ONE 2018, 13, e0206814. [Google Scholar] [CrossRef]
- Saraiva-Mangolin, S.; Vaz, C.D.O.; Ruiz, T.; Mazetto, B.M.; Orsi, F.A. Use of hydroxychloroquine to control immune response and hypercoagulability in patients with primary antiphospholipid syndrome. Eur. J. Intern. Med. 2021, 90, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.H.; Wu, X.-X.; Quinn, A.S.; Ashton, A.W.; Chen, P.P.; Hathcock, J.J.; Andree, H.A.M.; Taatjes, D.J. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: Evidence for a novel effect for an old antimalarial drug. Blood 2010, 115, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.; Breen, K.; Parmar, K.; Rand, J.H.; Wu, X.-X.; Hunt, B.J. The effect of hydroxychloroquine on haemostasis, complement, inflammation and angiogenesis in patients with antiphospholipid antibodies. Rheumatology 2018, 57, 120–124. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Xia, Y.; Wang, X. Effect of hydroxychloroquine on antiphospholipid antibodies-inhibited endometrial angiogenesis. J. Matern. Neonatal Med. 2022, 35, 7084–7092. [Google Scholar] [CrossRef]
- Virdis, A.; Tani, C.; Duranti, E.; Vagnani, S.; Carli, L.; Kühl, A.A.; Solini, A.; Baldini, C.; Talarico, R.; Bombardieri, S.; et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Thromb. Haemost. 2015, 17, 277. [Google Scholar] [CrossRef]
- Rand, J.H.; Wu, X.X.; Quinn, A.S.; Chen, P.P.; Hathcock, J.J.; Taatjes, D.J. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-beta2-glycoprotein I complexes to phospholipid bilayers. Blood 2008, 112, 1687–1695. [Google Scholar] [CrossRef]
- Rahman, R.; Murthi, P.; Singh, H.; Gurusinghe, S.; Mockler, J.C.; Lim, R.; Wallace, E.M. The effects of hydroxychloroquine on endothelial dysfunction. Pregnancy Hypertens. 2016, 6, 259–262. [Google Scholar] [CrossRef]
- van den Borne, B.E.; Dijkmans, B.A.; de Rooij, H.H.; le Cessie, S.; Verweij, C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J. Rheumatol. 1997, 24, 55–60. [Google Scholar] [PubMed]
- Tishler, M.; Yaron, I.; Shirazi, I.; Yaron, M. Hydroxychloroquine treatment for primary Sjögren’s syndrome: Its effect on salivary and serum inflammatory markers. Ann. Rheum. Dis. 1999, 58, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Ghasemnejad-Berenji, H.; Ghaffari Novin, M.; Hajshafiha, M.; Nazarian, H.; Hashemi, S.M.; Ilkhanizadeh, B.; Ghasemnejad, T.; Sadeghpour, S.; Ghasemnejad-Berenji, M. Immunomodulatory effects of hydroxychloroquine on Th1/Th2 balance in women with repeated implantation failure. Biomed. Pharmacother. 2018, 107, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Achuthan, S.; Ahluwalia, J.; Shafiq, N.; Bhalla, A.; Pareek, A.; Chandurkar, N.; Malhotra, S. Hydroxychloroquine’s Efficacy as an Antiplatelet Agent Study in Healthy Volunteers: A Proof of Concept Study. J. Cardiovasc. Pharmacol. Ther. 2015, 20, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Shippey, E.A.; Wagler, V.D.; Collamer, A.N. Hydroxychloroquine: An old drug with new relevance. Cleve Clin. J. Med. 2018, 85, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, H.; Ye, Y.; Xiao, Y.; Xu, S.; Wang, J.; Wang, C.; Zou, Y.; Shi, M.; Liang, L.; et al. Attenuation of antimalarial agent hydroxychloroquine on TNF-α-induced endothelial inflammation. Int. Immunopharmacol. 2018, 63, 261–269. [Google Scholar] [CrossRef]
- Arslan, S.O.; Doğan, M.F.; Çam, S.A.; Omar, I.A.; Uysal, F.; Parlar, A.; Andaç, A.C.; Yıldız, O. Hydroxychloroquine induces endothelium-dependent and endothelium-independent relaxation of rat aorta. Turk. J. Med. Sci. 2022, 52, 848–857. [Google Scholar] [CrossRef]
- Shukla, A.M.; Bose, C.; Karaduta, O.; Apostolov, E.O.; Kaushal, G.P.; Fahmi, T.; Segal, M.S.; Shah, S.V. Impact of Hydroxychloroquine on Atherosclerosis and Vascular Stiffness in the Presence of Chronic Kidney Disease. PLoS ONE 2015, 10, e0139226. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3161820 (accessed on 16 October 2022).
- Kravvariti, E.; Koutsogianni, A.; Samoli, E.; Sfikakis, P.P.; Tektonidou, M.G. The effect of hydroxychloroquine on thrombosis prevention and antiphospholipid antibody levels in primary antiphospholipid syndrome: A pilot open label randomised prospective study. Autoimmun. Rev. 2020, 19, 102491. [Google Scholar] [CrossRef]
- Schmidt-Tanguy, A.; Voswinkel, J.; Henrion, D.; Subra, J.F.; Loufrani, L.; Rohmer, V.; Ifrah, N.; Belizna, C. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J. Thromb. Haemost. 2013, 11, 1927–1929. [Google Scholar] [CrossRef]
- Nuri, E.; Taraborelli, M.; Andreoli, L.; Tonello, M.; Gerosa, M.; Calligaro, A.; Argolini, L.M.; Kumar, R.; Pengo, V.; Meroni, P.L.; et al. Long-term use of hydroxychloroquine reduces antiphospholipid antibodies levels in patients with primary antiphospholipid syndrome. Immunol. Res. 2017, 65, 17–24. [Google Scholar] [CrossRef]
- Jorge, A.; Lu, N.; Choi, H.; Esdaile, J.M.; Lacaille, D.; Avina-Zubieta, J.A. Hydroxychloroquine Use and Cardiovascular Events among Patients With Systemic Lupus Erythematosus and Rheumatoid Arthritis. Arthritis Care Res. (Hoboken) 2021. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.; Zhang, Y.; Kwong, J.S.-W.; Li, L.; Zhang, Y.; Xu, C.; Li, Q.; Sun, X.; Tian, H.; et al. Chloroquine and hydroxychloroquine are associated with reduced cardiovascular risk: A systematic review and meta-analysis. Drug Des. Dev. Ther. 2018, 12, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Anunciación-Llunell, A.; Marques-Soares, J.; Pardos-Gea, J.; Miró-Mur, F. Pathogenesis, Diagnosis and Management of Obstetric Antiphospholipid Syndrome: A Comprehensive Review. J. Clin. Med. 2022, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Ziakas, P.D.; Pavlou, M.; Voulgarelis, M. Heparin treatment in antiphospholipid syndrome with recurrent pregnancy loss: A systematic review and meta-analysis. Obstet. Gynecol. 2010, 115, 1256–1262. [Google Scholar] [CrossRef]
- Bramham, K.; Hunt, B.J.; Germain, S.; Calatayud, I.; Khamashta, M.; Bewley, S.; Nelson-Piercy, C. Pregnancy outcome in different clinical phenotypes of antiphospholipid syndrome. Lupus 2010, 19, 58–64. [Google Scholar] [CrossRef]
- Mak, A.; Cheung, M.; Cheak, A.A.-C.; Ho, R.C.-M. Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: A meta-analysis of randomised controlled trials and meta-regression. Rheumatology 2009, 49, 281–288. [Google Scholar] [CrossRef]
- Bouvier, S.; Cochery-Nouvellon, E.; Lavigne-Lissalde, G.; Mercier, E.; Marchetti, T.; Balducchi, J.-P.; Marès, P.; Gris, J.-C. Comparative incidence of pregnancy outcomes in treated obstetric antiphospholipid syndrome: The NOH-APS observational study. Blood 2014, 123, 404–413. [Google Scholar] [CrossRef]
- Ruffatti, A.; Favaro, M.; Calligaro, A.; Zambon, A.; Del Ross, T. Management of pregnant women with antiphospholipid antibodies. Expert Rev. Clin. Immunol. 2019, 15, 347–358. [Google Scholar] [CrossRef]
- Mekinian, A.; Lazzaroni, M.G.; Kuzenko, A.; Alijotas-Reig, J.; Ruffatti, A.; Levy, P.; Canti, V.; Bremme, K.; Bezanahary, H.; Bertero, T.; et al. The efficacy of hydroxychloroquine for obstetrical outcome in anti-phospholipid syndrome: Data from a European multicenter retrospective study. Autoimmun. Rev. 2015, 14, 498–502. [Google Scholar] [CrossRef]
- Sciascia, S.; Branch, D.W.; Levy, R.A.; Middeldorp, S.; Pavord, S.; Roccatello, D.; Ruiz-Irastorza, G.; Tincani, A.; Khamashta, M.; Schreiber, K.; et al. The efficacy of hydroxychloroquine in altering pregnancy outcome in women with antiphospholipid antibodies. Evid. Clin. Judgm. Thromb. Haemost. 2016, 115, 285–290. [Google Scholar] [CrossRef]
- Gerde, M.; Ibarra, E.; Mac Kenzie, R.; Suarez, C.F.; Heer, C.; Alvarez, R.; Iglesias, M.; Balparda, J.; Beruti, E.; Rubinstein, F. The impact of hydroxychloroquine on obstetric outcomes in refractory obstetric antiphospholipid syndrome. Thromb. Res. 2021, 206, 104–110. [Google Scholar] [CrossRef]
- Bertolaccini, M.L.; Contento, G.; Lennen, R.; Sanna, G.; Blower, P.J.; Ma, M.T.; Sunassee, K.; Girardi, G. Complement inhibition by hydroxychloroquine prevents placental and fetal brain abnormalities in antiphospholipid syndrome. J. Autoimmun. 2016, 75, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Tian, Y.; Wan, S.; Hu, M.; Song, S.; Zhang, M.; Zhou, Q.; Xia, Y.; Wang, X. Protection by hydroxychloroquine prevents placental injury in obstetric antiphospholipid syndrome. J. Cell. Mol. Med. 2022, 26, 4357–4370. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-X.; Guller, S.; Rand, J.H. Hydroxychloroquine reduces binding of antiphospholipid antibodies to syncytiotrophoblasts and restores annexin A5 expression. Am. J. Obstet. Gynecol. 2011, 205, 576.e7–576.e14. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, T.; Ruffatti, A.; Wuillemin, C.; de Moerloose, P.; Cohen, M. Hydroxychloroquine restores trophoblast fusion affected by antiphospholipid antibodies. J. Thromb. Haemost. 2014, 12, 910–920. [Google Scholar] [CrossRef]
- Albert, C.R.; Schlesinger, W.J.; Viall, C.A.; Mulla, M.J.; Brosens, J.J.; Chamley, L.W.; Abrahams, V.M. Effect of Hydroxychloroquine on Antiphospholipid Antibody-Induced Changes in First Trimester Trophoblast Function. Am. J. Reprod. Immunol. 2014, 71, 154–164. [Google Scholar] [CrossRef]
- Yelnik, C.M.; Urbanski, G.; Drumez, E.; Sobanski, V.; Maillard, H.; Lanteri, A.; Morell-Dubois, S.; Caron, C.; Dubucquoi, S.; Launay, D.; et al. Persistent triple antiphospholipid antibody positivity as a strong risk factor of first thrombosis, in a long-term follow-up study of patients without history of thrombosis or obstetrical morbidity. Lupus 2017, 26, 163–169. [Google Scholar] [CrossRef]
- Available online: https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2022/04/25/21/07/uspstf-report-on-aspirin (accessed on 20 October 2022).
- Erkan, D.; Yazici, Y.; Peterson, M.G.; Sammaritano, L.; Lockshin, M.D. A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology 2002, 41, 924–929. [Google Scholar] [CrossRef]
- Erkan, D.; Unlu, O.; Sciascia, S.; Belmont, H.; Branch, D.W.; Cuadrado, M.J.; Gonzalez, E.; Knight, J.S.; Uthman, I.; Willis, R.; et al. Hydroxychloroquine in the primary thrombosis prophylaxis of antiphospholipid antibody positive patients without systemic autoimmune disease. Lupus 2018, 27, 399–406. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).