Repair of Long Peripheral Nerve Defects in Sheep: A Translational Model for Nerve Regeneration
Abstract
1. Introduction
2. Results
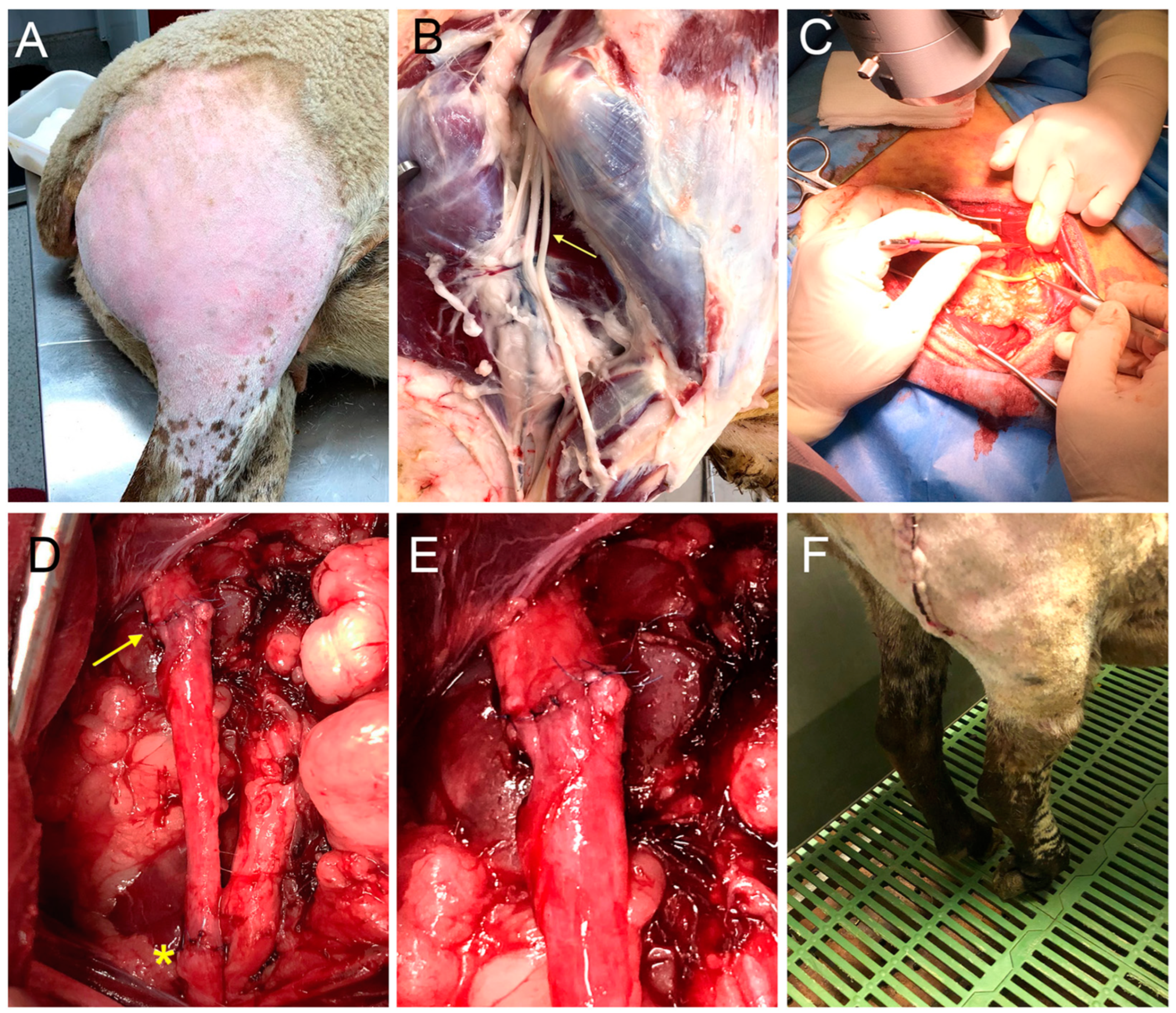
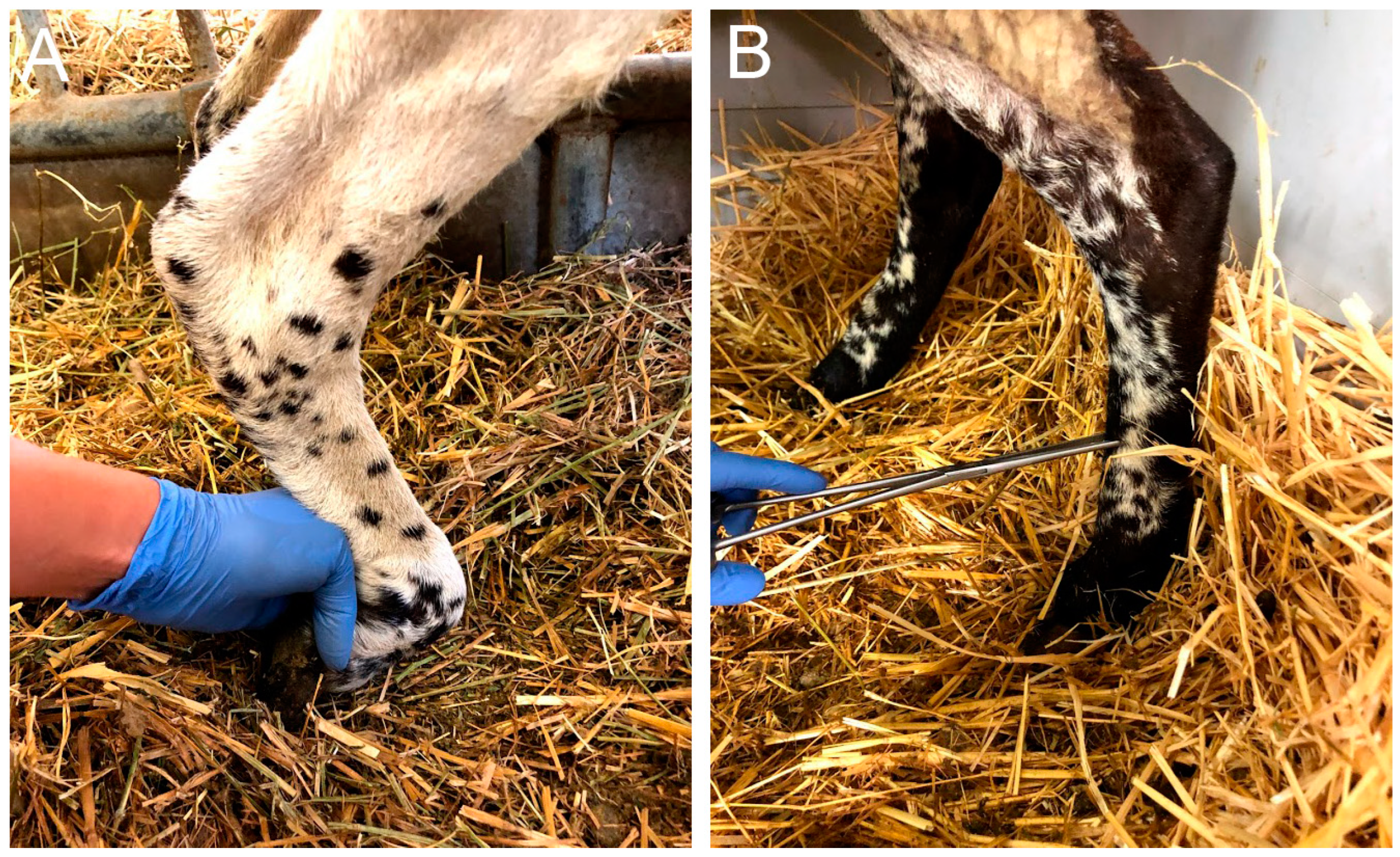
2.1. Clinical Observations
2.2. Functional Evaluation
2.3. Electrophysiological Results
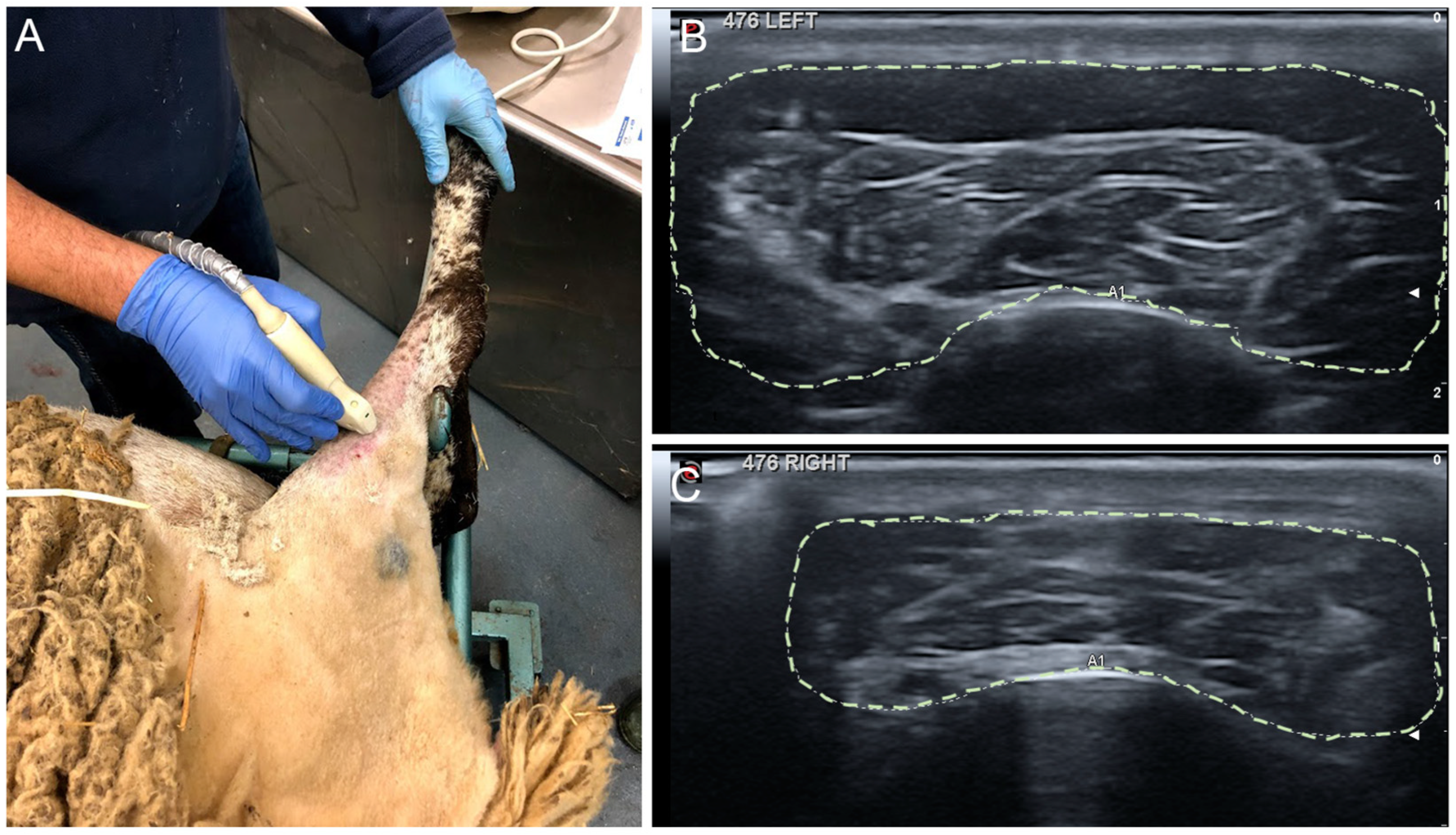
2.4. Echographic Evaluation of TA Muscle
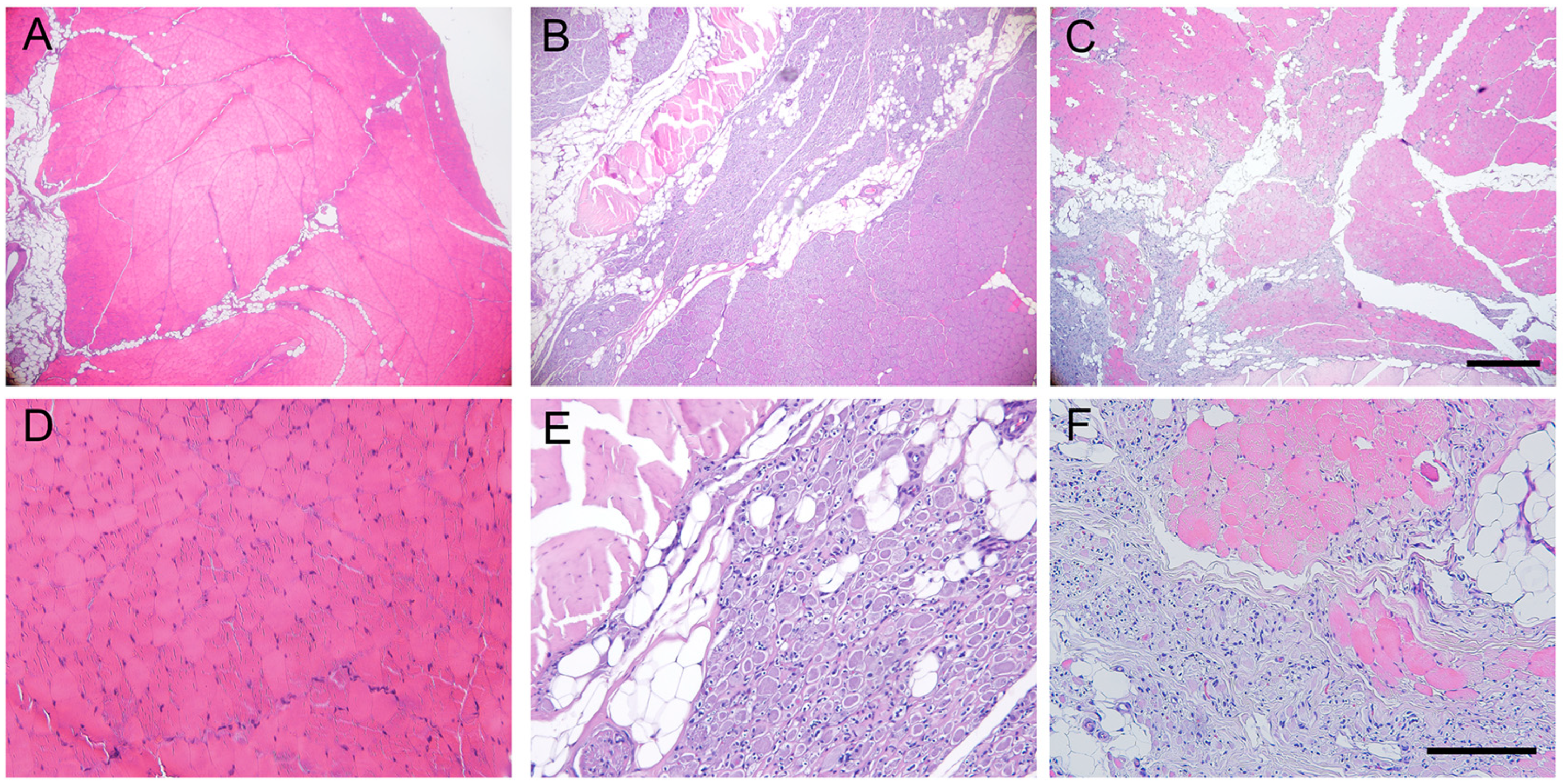
2.5. Histological Results of the Grafted Nerve
2.6. Histological Evaluation of Reinnervated Targets
3. Discussion
4. Materials and Methods
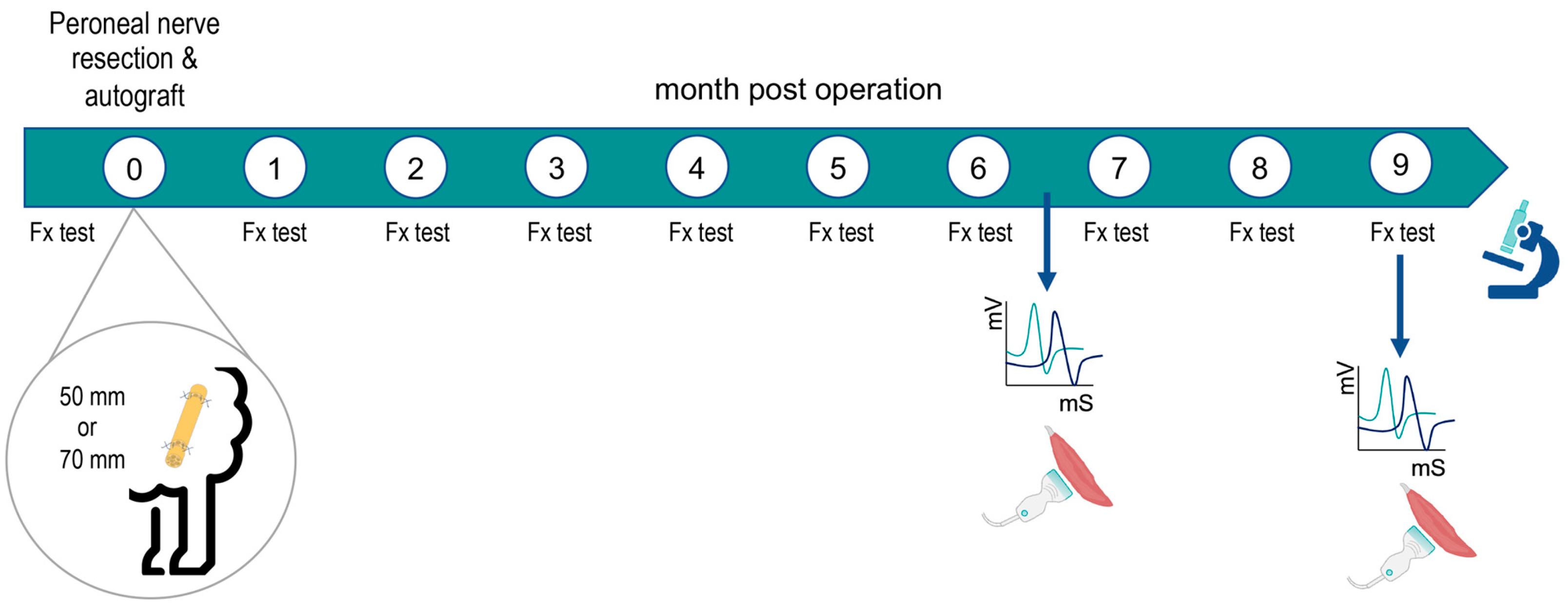
4.1. Animals and Study Design
4.2. Surgical Procedure
4.3. Functional Testing
4.4. Electrophysiological Tests
4.5. Ultrasound Test
4.6. Histological Evaluation
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navarro, X.; Vivó, M.; Valero-Cabré, A. Neural Plasticity after Peripheral Nerve Injury and Regeneration. Prog. Neurobiol. 2007, 82, 163–201. [Google Scholar] [CrossRef]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of Peripheral Nerve Regeneration: Interactions at the Axon Level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Keynes, R.J. Peripheral nerve regeneration. Annu Rev Neurosci. 1990, 13, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.; Coleman, M.P. Lessons from Injury: How Nerve Injury Studies Reveal Basic Biological Mechanisms and Therapeutic Opportunities for Peripheral Nerve Diseases. Neurotherapeutics 2021, 18, 2200–2221. [Google Scholar] [CrossRef]
- Kline, D.G.; Hudson, A.R. Nerve Injuries. In Operative Results for Major Nerve Injuries, Entrapments, and Tumors, 2nd ed.; Saunders: Philadelphia, PA, USA, 1995. [Google Scholar]
- Lundborg, G. A 25-Year Perspective of Peripheral Nerve Surgery: Evolving Neuroscientific Concepts and Clinical Significance. J. Hand Surg. Am. 2000, 25, 391–414. [Google Scholar] [CrossRef]
- Ray, W.Z.; Mackinnon, S.E. Management of Nerve Gaps: Autografts, Allografts, Nerve Transfers, and End-to-Side Neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve Grafting for Peripheral Nerve Injuries with Extended Defect Sizes. Wien Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G.; Dahlin, L.B.; Danielsen, N.; Gelberman, R.H.; Longo, F.M.; Powell, H.C.; Varon, S. Nerve Regeneration in Silicone Chambers: Influence of Gap Length and of Distal Stump Components. Exp. Neurol. 1982, 76, 361–375. [Google Scholar] [CrossRef]
- Butí, M.; Verdú, E.; Labrador, R.O.; Vilches, J.J.; Forés, J.; Navarro, X. Influence of Physical Parameters of Nerve Chambers on Peripheral Nerve Regeneration and Reinnervation. Exp. Neurol. 1996, 137, 26–33. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Dellon, A.L. A study of nerve regeneration across synthetic (Maxon) and biologic (collagen) nerve conduits for nerve gaps up to 5 cm in the primate. J. Reconstr. Microsurg. 1990, 6, 117–121. [Google Scholar] [CrossRef]
- Archibald, S.J.; Shefner, J.; Krarup, C.; Madison, R.D. Monkey Median Nerve Repaired by Nerve Graft or Collagen Nerve Guide Tube. J. Neurosci. 1995, 15, 4109–4123. [Google Scholar] [CrossRef]
- Kaplan, H.M.; Mishra, P.; Kohn, J. The Overwhelming Use of Rat Models in Nerve Regeneration Research May Compromise Designs of Nerve Guidance Conduits for Humans. J. Mater. Sci. Mater. Med. 2015, 26, 226. [Google Scholar] [CrossRef] [PubMed]
- Tos, P.; Ronchi, G.; Papalia, I.; Sallen, V.; Legagneux, J.; Geuna, S.; Giacobini-Robecchi, M.G. Chapter 4 Methods and Protocols in Peripheral Nerve Regeneration Experimental Research: Part I-Experimental Models. Int. Rev. Neurobiol. 2009, 87, 47–79. [Google Scholar] [PubMed]
- Siemionow, M.; Brzezicki, G. Chapter 8 Current Techniques and Concepts in Peripheral Nerve Repair. Int. Rev. Neurobiol. 2009, 87, 141–172. [Google Scholar]
- Angius, D.; Wang, H.; Spinner, R.J.; Gutierrez-Cotto, Y.; Yaszemski, M.J.; Windebank, A.J. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials 2012, 33, 8034–8039. [Google Scholar] [CrossRef]
- Geuna, S. The Sciatic Nerve Injury Model in Pre-Clinical Research. J. Neurosci. Methods 2015, 243, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Borschel, G.H. The Use of the Rat as a Model for Studying Peripheral Nerve Regeneration and Sprouting after Complete and Partial Nerve Injuries. Exp. Neurol. 2017, 287, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Diogo, C.C.; Camassa, J.A.; Pereira, J.E.; da Costa, L.M.; Filipe, V.; Couto, P.A.; Geuna, S.; Maurício, A.C.; Varejão, A.S. The Use of Sheep as a Model for Studying Peripheral Nerve Regeneration Following Nerve Injury: Review of the Literature. Neurol. Res. 2017, 39, 926–939. [Google Scholar] [CrossRef]
- Glasby, M.A.; Gilmour, J.A.; Gschmeissner, S.E.; Hems, T.E.; Myles, L.M. The Repair of Large Peripheral Nerves Using Skeletal Muscle Autografts: A Comparison with Cable Grafts in the Sheep Femoral Nerve. Br. J. Plast. Surg. 1990, 43, 169–178. [Google Scholar] [CrossRef]
- Kettle, S.J.A.; Starritt, N.E.; Glasby, M.A.; Hems, T.E.J. End-to-Side Nerve Repair in a Large Animal Model: How Does It Compare with Conventional Methods of Nerve Repair? J. Hand Surg. Eur. Vol. 2013, 38, 192–202. [Google Scholar] [CrossRef]
- Atchabahian, A.; Genden, E.M.; Mackinnon, S.E.; Doolabh, V.B.; Hunter, D.A. Regeneration through long nerve grafts in the swine model. Microsurgery 1998, 18, 379–382. [Google Scholar] [CrossRef]
- Forden, J.; Xu, Q.G.; Khu, K.J.; Midha, R. A Long Peripheral Nerve Autograft Model in the Sheep Forelimb. Neurosurgery 2011, 68, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Zen, F.; Maurina, M.; Raimondo, S.; Mendonça, C.; Atayde, L.; Geuna, S.; Varejão, A.S.P.; et al. Establishment of a Sheep Model for Hind Limb Peripheral Nerve Injury: Common Peroneal Nerve. Int. J. Mol. Sci. 2021, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Al Abri, R.; Kolethekkat, A.A.; Kelleher, M.O.; Myles, L.M.; Glasby, M.A. Effect of Locally Administered Ciliary Neurotrophic Factor on the Survival of Transected and Repaired Adult Sheep Facial Nerve. Oman Med. J. 2014, 29, 208–213. [Google Scholar] [CrossRef]
- Glasby, M.A.; Mountain, R.E.; Murray, J.A.M. Repair of the Facial Nerve Using Freeze-Thawed Muscle Autografts A Surgical Model in the Sheep. Arch. Otolaryngol. Head Neck Surg. 1993, 119, 461–465. [Google Scholar] [CrossRef]
- Hems, T.E.J.; Glasby, M.A. Repair of cervical nerve roots proximal to the root ganglia an experimental study in sheep. J. Bone Jt. Surg. Br. 1992, 74, 918–922. [Google Scholar] [CrossRef]
- Turner, A.S. Experiences with Sheep as an Animal Model for Shoulder Surgery: Strengths and Shortcomings. J. Shoulder Elb. Surg. 2007, 16, S158–S163. [Google Scholar] [CrossRef]
- Ozturk, C.; Uygur, S.; Lukaszuk, M. Sheep as a Large Animal Model for Nerve Regeneration Studies. In Plastic and Reconstructive Surgery: Experimental Models and Research Designs; Springer: London, UK, 2015; pp. 507–511. ISBN 9781447163350. [Google Scholar]
- Costa, D.; Diogo, C.C.; da Costa, L.M.; Pereira, J.E.; Filipe, V.; Couto, P.A.; Geuna, S.; Armada-Da-Silva, P.A.; Maurício, A.C.; Varejão, A.S.P. Kinematic Patterns for Hindlimb Obstacle Avoidance during Sheep Locomotion. Neurol. Res. 2018, 40, 963–971. [Google Scholar] [CrossRef]
- Strasberg, S.R.; Mackinnon, S.E.; Genden, E.M.; Bain, J.R.; Purcell, C.M.; Hunter, D.A.; Hay, J.B. Long-segment nerve allograft regeneration in the sheep model experimental study and review of the literature. J. Reconstr. Microsurg. 1996, 12, 529–537. [Google Scholar] [CrossRef]
- Lawson, G.M.; Glasby, M.A. A comparison of immediate and delayed nerve repair using autologous freeze-thawed muscle grafts in a large animal model. The simple injury. J. Hand Surg. Br. 1995, 20, 663–700. [Google Scholar] [CrossRef] [PubMed]
- Jeans, L.A.; Gilchrist, T.; Healy, D. Peripheral Nerve Repair by Means of a Flexible Biodegradable Glass Fibre Wrap: A Comparison with Microsurgical Epineurial Repair. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kline, D.G. Management and results of peroneal nerve lesions. Neurosurgery 1996, 39, 319–320. [Google Scholar] [CrossRef]
- Nath, R.K.; Lyons, A.B.; Paizi, M. Successful Management of Foot Drop by Nerve Transfers to the Deep Peroneal Nerve. J. Reconstr. Microsurg. 2008, 24, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Roballo, K.C.S.; Burns, D.T.; Ghnenis, A.B.; Osimanjiang, W.; Bushman, J.S. Long-Term Neural Regeneration Following Injury to the Peroneal Branch of the Sciatic Nerve in Sheep. Eur. J. Neurosci. 2020, 52, 4385–4394. [Google Scholar] [CrossRef]
- Lezak, B.; Massel, D.H.; Varacallo, M. Peroneal Nerve Injury. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549859/ (accessed on 4 September 2022).
- Marciniak, C. Fibular (Peroneal) Neuropathy. Electrodiagnostic Features and Clinical Correlates. Phys. Med. Rehabil. Clin. N. Am. 2013, 24, 121–137. [Google Scholar] [CrossRef]
- Baima, J.; Krivickas, L. Evaluation and Treatment of Peroneal Neuropathy. Curr. Rev. Musculoskelet. Med. 2008, 1, 147–153. [Google Scholar] [CrossRef]
- Matsuyama, T.; Midha, R.; Mackinnon, S.E.; Munro, C.A.; Wong, P.-Y.; Ang, L.C. Long nerve allografts in sheep with cyclosporin a immunosuppression. J. Reconstr. Miscrosurg. 2000, 16, 219–225. [Google Scholar] [CrossRef]
- Siemionow, M.; Cwykiel, J.; Uygur, S.; Kwiecien, G.; Oztürk, C.; Szopinski, J.; Madajka, M. Application of Epineural Sheath Conduit for Restoration of 6-Cm Long Nerve Defects in a Sheep Median Nerve Model. Microsurgery 2019, 39, 332–339. [Google Scholar] [CrossRef]
- Gilchrist, T.; Glasby, M.A.; Healy, D.M.; Kelly, G.; van Lenihan, D.; Mcdowall, K.L.; Millert, I.A.; Myles, L.M. In Vitro Nerve Repair-in Vivo. The Reconstruction of Peripheral Nerves by Entuhulation with Biodegradeable Glass Tubes-a Preliminary Report. Br. J. Plast. Surg. 1998, 51, 231–237. [Google Scholar] [CrossRef]
- Casañas, J.; de la Torre, J.; Soler, F.; García, F.; Rodellar, C.; Pumarola, M.; Climent, J.; Soler, R.; Orozco, L. Peripheral nerve regeneration after experimental section in ovine radial and tibial nerves using synthetic nerve grafts, including expanded bone marrow mesenchymal cells: Morphological and neurophysiological results. Injury 2014, 45 (Suppl. 4), S2–S6. [Google Scholar] [CrossRef]
- Radtke, C.; Allmeling, C.; Waldmann, K.H.; Reimers, K.; Thies, K.; Schenk, H.C.; Hillmer, A.; Guggenheim, M.; Brandes, G.; Vogt, P.M. Spider Silk Constructs Enhance Axonal Regeneration and Remyelination in Long Nerve Defects in Sheep. PLoS ONE 2011, 25, e16990. [Google Scholar] [CrossRef]
- Kornfeld, T.; Borger, A.; Radtke, C. Reconstruction of Critical Nerve Defects Using Allogenic Nerve Tissue: A Review of Current Approaches. Int. J. Mol. Sci. 2021, 22, 3515. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Montoya, F.E.; Tamez-Mata, Y.A.; Simental-Mendía, M.; Soto-Domínguez, A.; García-Pérez, M.M.; Said-Fernández, S.; Montes-de-Oca-Luna, R.; González-Flores, J.R.; Martínez-Rodríguez, H.G.; Vilchez-Cavazos, F. Repair of Ovine Peripheral Nerve Injuries with Xenogeneic Human Acellular Sciatic Nerves Prerecellularized with Allogeneic Schwann-like Cells—An Innovative and Promising Approach. Regen. Ther. 2022, 19, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Tamez-Mata, Y.; Pedroza-Montoya, F.E.; Martínez-Rodríguez, H.G.; García-Pérez, M.M.; Ríos-Cantú, A.A.; González-Flores, J.R.; Soto-Domínguez, A.; Montes-de-Oca-Luna, R.; Simental-Mendía, M.; Peña-Martínez, V.M.; et al. Nerve Gaps Repaired with Acellular Nerve Allografts Recellularized with Schwann-like Cells: Preclinical Trial. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 296–306. [Google Scholar] [CrossRef]
- Parry, G.J. Electrodiagnostic Studies in the Evaluation of Peripheral Nerve and Brachial Plexus Injuries. Neurol. Clin. 1992, 10, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X. Functional Evaluation of Peripheral Nerve Regeneration and Target Reinnervation in Animal Models: A Critical Overview. Eur. J. Neurosci. 2016, 43, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.C.; Browne, K.D.; Dutton, J.L.; Laimo, F.A.; Das, S.; Brown, D.P.; Roberts, S.; Petrov, D.; Ali, Z.; Ledebur, H.C.; et al. A Porcine Model of Peripheral Nerve Injury Enabling Ultra-Long Regenerative Distances: Surgical Approach, Recovery Kinetics, and Clinical Relevance. Neurosurgery 2020, 87, 833–846. [Google Scholar] [CrossRef]
- Valero-Cabré, A.; Navarro, X. Functional Impact of Axonal Misdirection after Peripheral Nerve Injuries Followed by Graft or Tube Repair. J. Neurotrauma 2002, 19, 1475–1485. [Google Scholar] [CrossRef]
- De Ruiter, G.C.W.; Malessy, M.J.A.; Alaid, A.O.; Spinner, R.J.; Engelstad, J.N.K.; Sorenson, E.J.; Kaufman, K.R.; Dyck, P.J.; Windebank, A.J. Misdirection of Regenerating Motor Axons after Nerve Injury and Repair in the Rat Sciatic Nerve Model. Exp. Neurol. 2008, 211, 339–350. [Google Scholar] [CrossRef]
- Witzel, C.; Rohde, C.; Brushart, T.M. Pathway Sampling by Regenerating Peripheral Axons. J. Comp. Neurol. 2005, 485, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, S.E.; Dellon, A.L.; O’Brien, J.P. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve 1991, 14, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
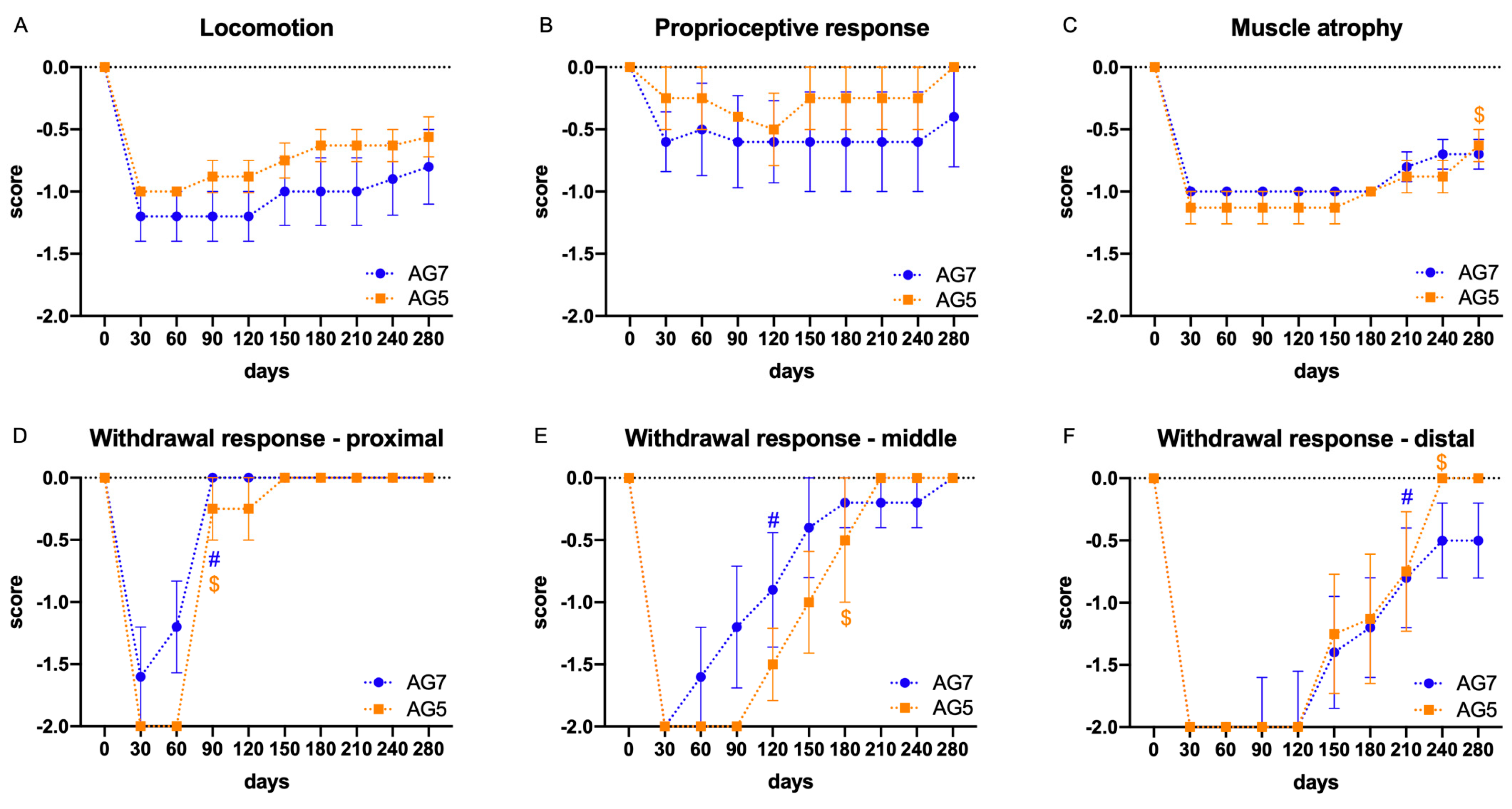

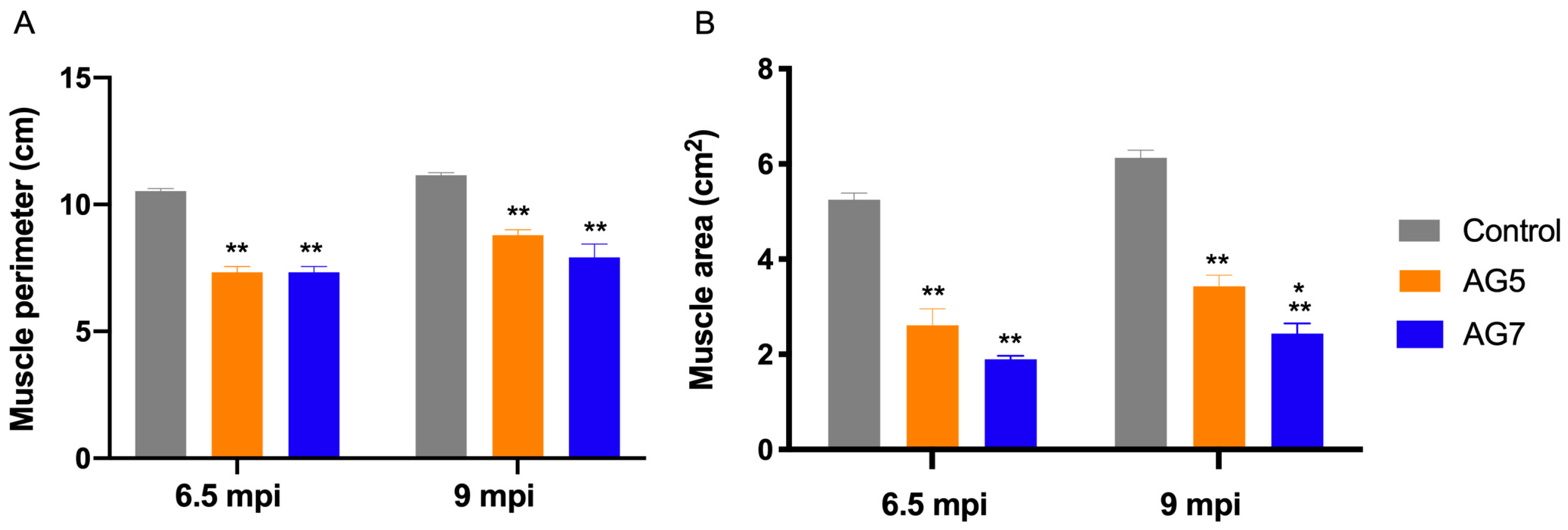
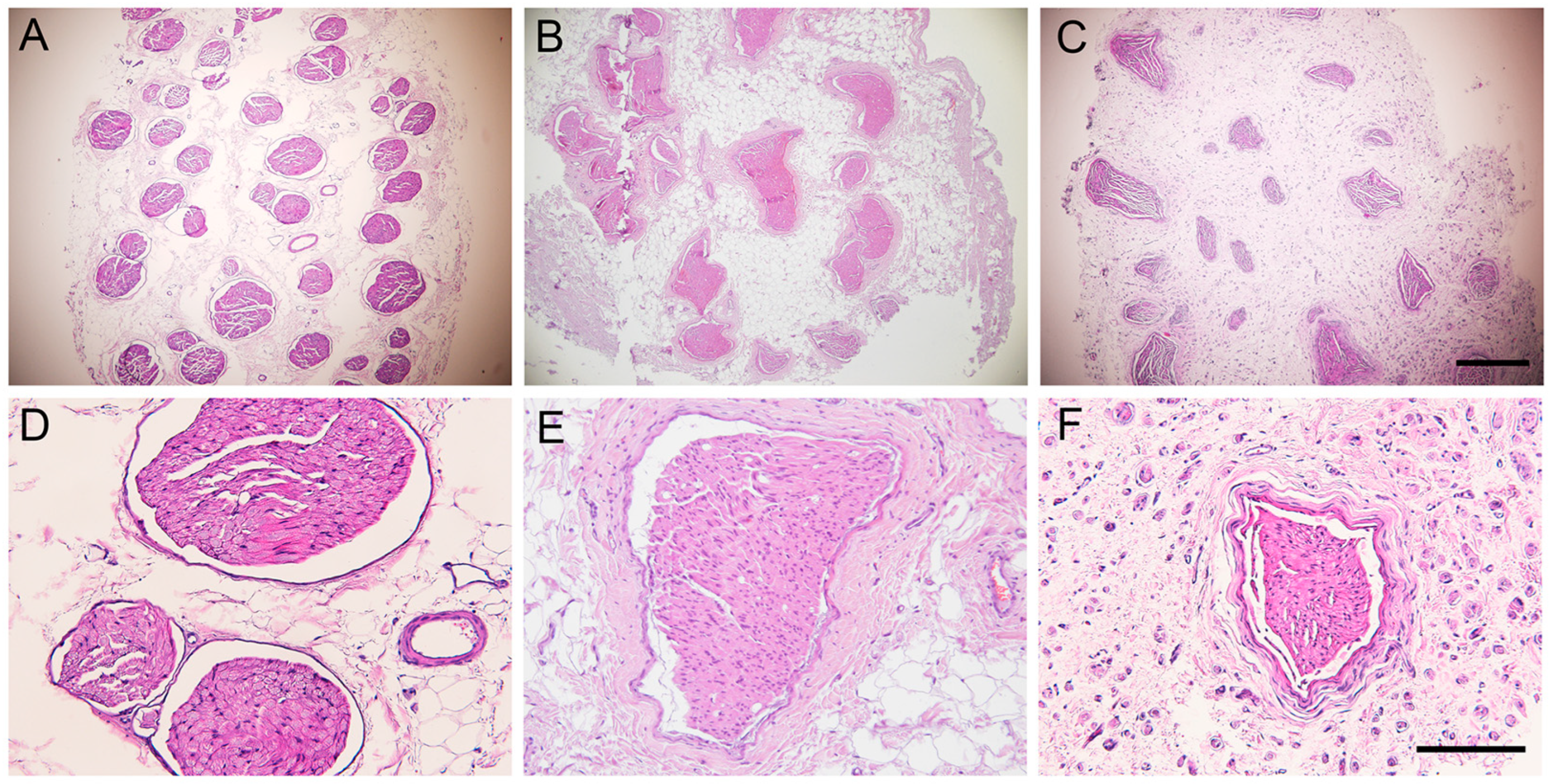
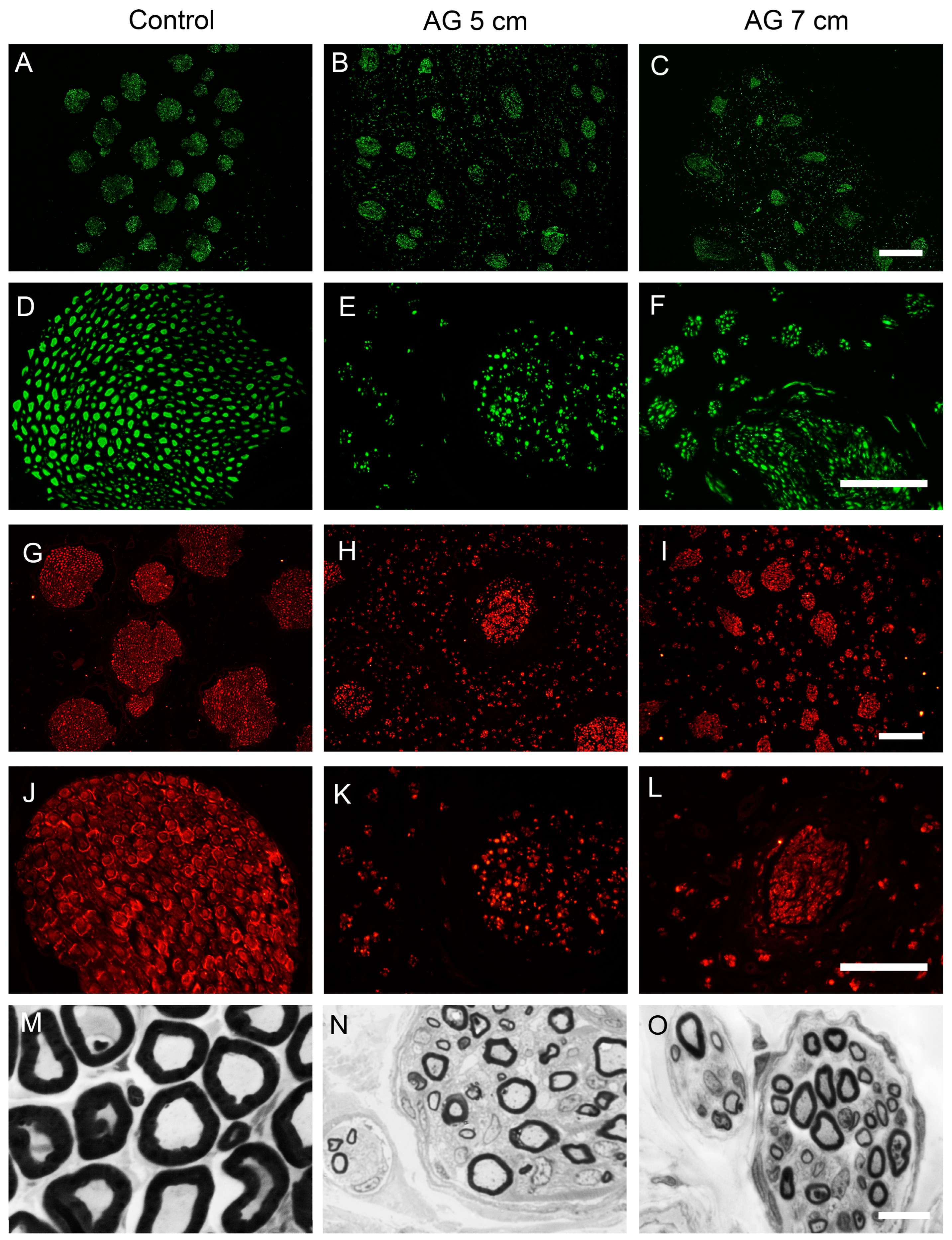

| Parameter | 0 | −1 | −2 |
|---|---|---|---|
| Locomotion | Normal gait | Limp | Drag the limb |
| Muscle loss (TA) | No loss | Reduced | Atrophy |
| Proprioception | Present | Decreased | Absent |
| Flexor withdrawal reflex (proximal point) | Present | Decreased | Absent |
| Flexor withdrawal reflex (middle point) | Present | Decreased | Absent |
| Flexor withdrawal reflex (distal point) | Present | Decreased | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, E.; Traserra, S.; Bolívar, S.; Forés, J.; Jose-Cunilleras, E.; Delgado-Martínez, I.; García, F.; Udina, E.; Navarro, X. Repair of Long Peripheral Nerve Defects in Sheep: A Translational Model for Nerve Regeneration. Int. J. Mol. Sci. 2023, 24, 1333. https://doi.org/10.3390/ijms24021333
Contreras E, Traserra S, Bolívar S, Forés J, Jose-Cunilleras E, Delgado-Martínez I, García F, Udina E, Navarro X. Repair of Long Peripheral Nerve Defects in Sheep: A Translational Model for Nerve Regeneration. International Journal of Molecular Sciences. 2023; 24(2):1333. https://doi.org/10.3390/ijms24021333
Chicago/Turabian StyleContreras, Estefanía, Sara Traserra, Sara Bolívar, Joaquím Forés, Eduard Jose-Cunilleras, Ignacio Delgado-Martínez, Félix García, Esther Udina, and Xavier Navarro. 2023. "Repair of Long Peripheral Nerve Defects in Sheep: A Translational Model for Nerve Regeneration" International Journal of Molecular Sciences 24, no. 2: 1333. https://doi.org/10.3390/ijms24021333
APA StyleContreras, E., Traserra, S., Bolívar, S., Forés, J., Jose-Cunilleras, E., Delgado-Martínez, I., García, F., Udina, E., & Navarro, X. (2023). Repair of Long Peripheral Nerve Defects in Sheep: A Translational Model for Nerve Regeneration. International Journal of Molecular Sciences, 24(2), 1333. https://doi.org/10.3390/ijms24021333







