Mechanistic Insights, Treatment Paradigms, and Clinical Progress in Neurological Disorders: Current and Future Prospects
Abstract
1. Introduction
| NDs | Drug/Bioactive Molecule | Mechanism of Action | Ref. |
|---|---|---|---|
| PD | Donepezil | Acetylcholinesterase inhibition | [52] |
| L-dopa and carbidopa | Dopaminergic agonism | [53,54] | |
| AD | Bexarotene | Dissolution of Aβ plaques | [55] |
| Memantine | N-methyl D-aspartate receptor antagonism | [56] | |
| Lacenemab | Reduce Aβ protofibrils in the brain and CSF | [57] | |
| Ischemic stroke | Semagacestat | γ-Secretase inhibitor | [58] |
| Aspirin and Statin | COX inhibition and cholesterol synthesis, respectively | [59,60] | |
| Multiple sclerosis | Alteplase | Tissue plasminogen activation | [61] |
| Mitoxantrone | Lymphocyte apoptosis | [62] | |
| Laquinimod | Shifting the T cell response to T helper 2 type | [63] | |
| Glioblastoma | Temozolomide | DNA alkylation followed by apoptosis | [64] |
| Spinal muscular atrophy | ZD1839 (gefitinib), OSI774 (erlotinib) | Protein kinase inhibition | [65] |
| Zolgensma | Replacing the non-working survival motor neuron 1 (SMN1) gene | [66] |
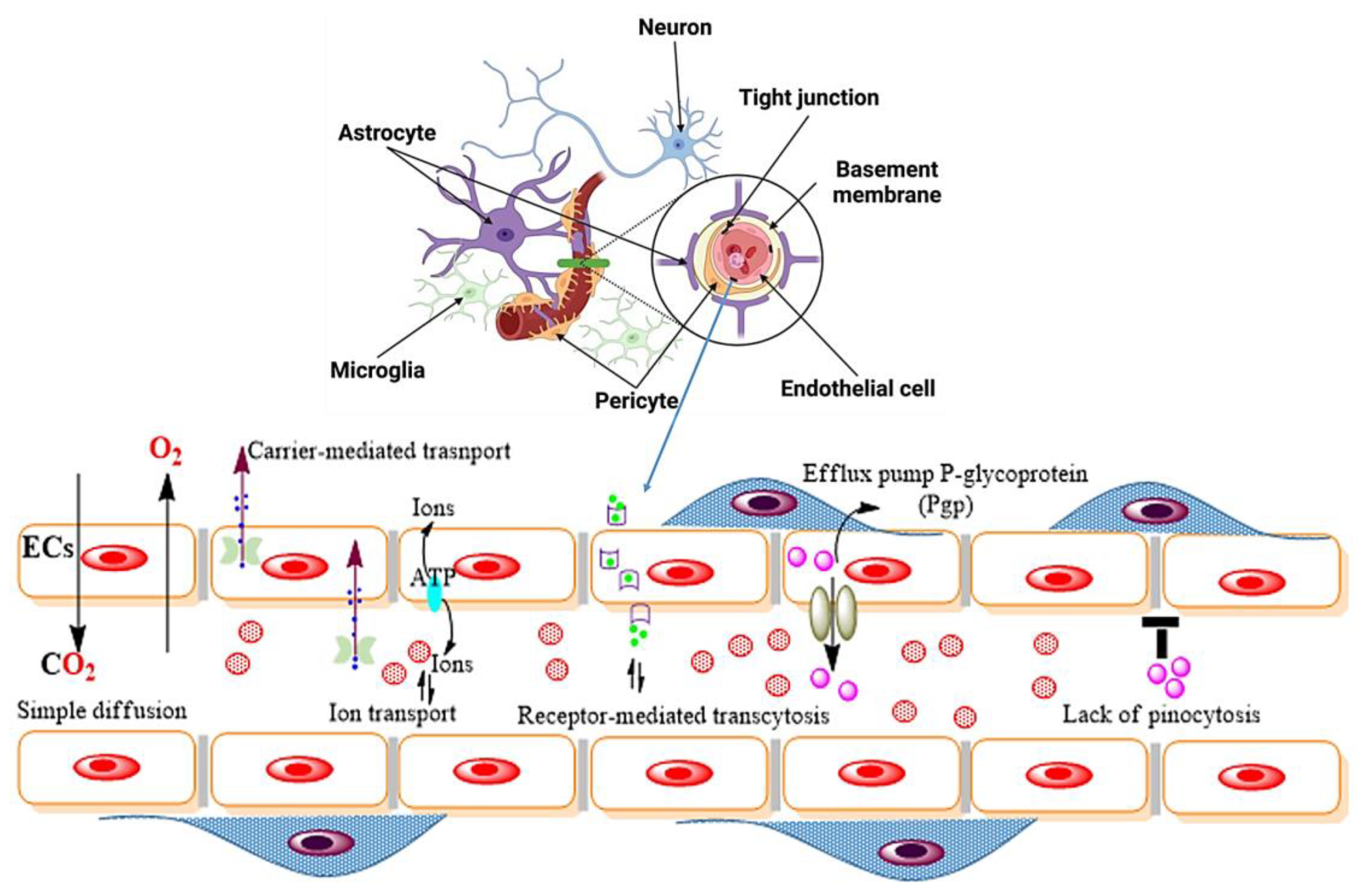
2. Origin of NDs in Relation to the Blood–Brain Barrier
2.1. Endothelial Cells (ECs)
2.2. Pericytes
2.3. Astrocytes
2.4. Microglia
3. Types of Brain Disorders
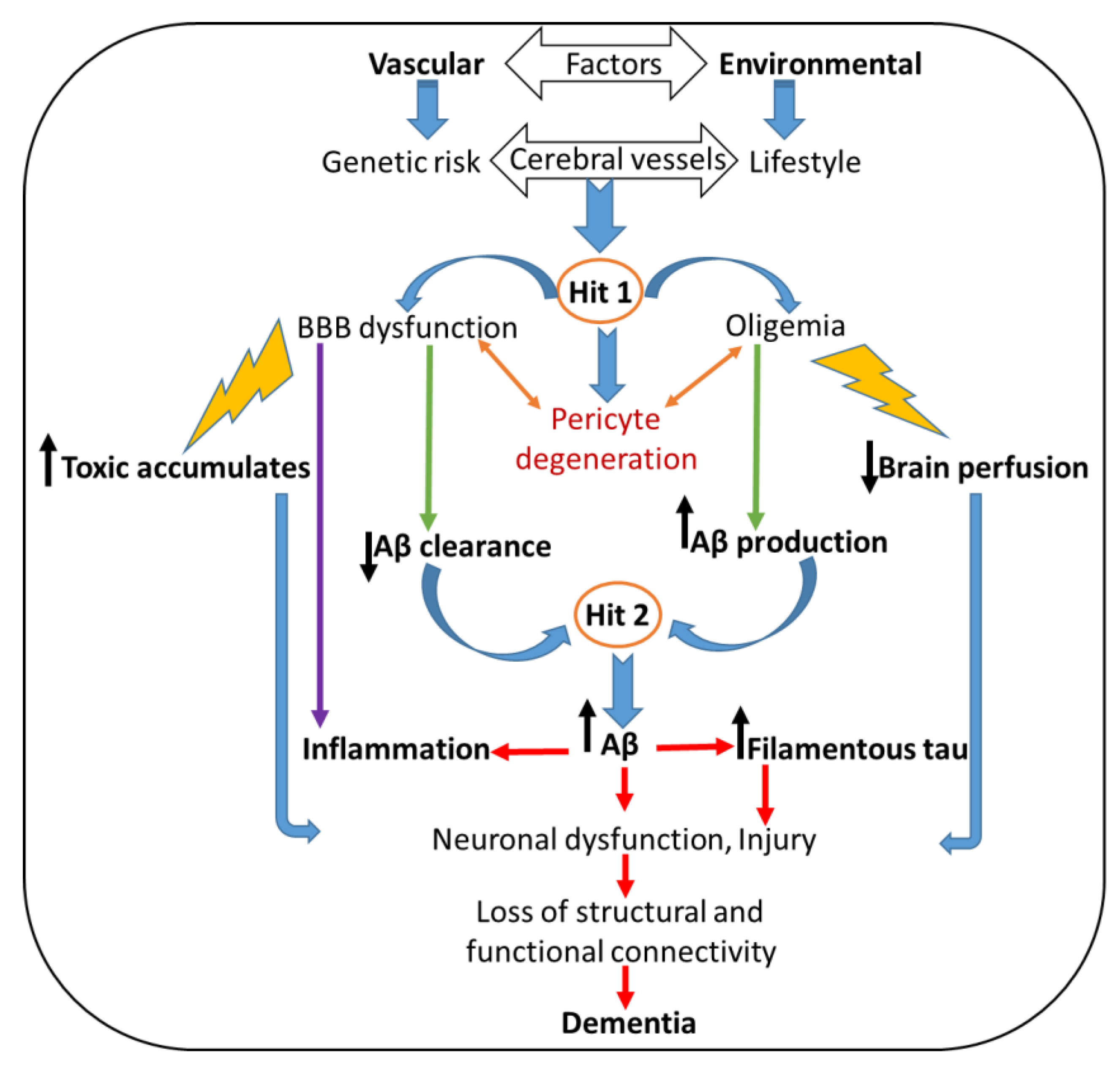
3.1. Alzheimer’s Disease (AD)
3.2. Treatment of Ads
- (i)
- Cholinesterase inhibitors (ChEIs)
- (ii)
- Amyloid-β-targeted drugs
3.3. Parkinson’s Disease (PD)
3.4. Treatment of PD
- (i)
- Neurotrophic factors (NTFs)
- (ii)
- Proteins that work to overdraw the dopamine production
3.5. Huntington’s Disease (HD)
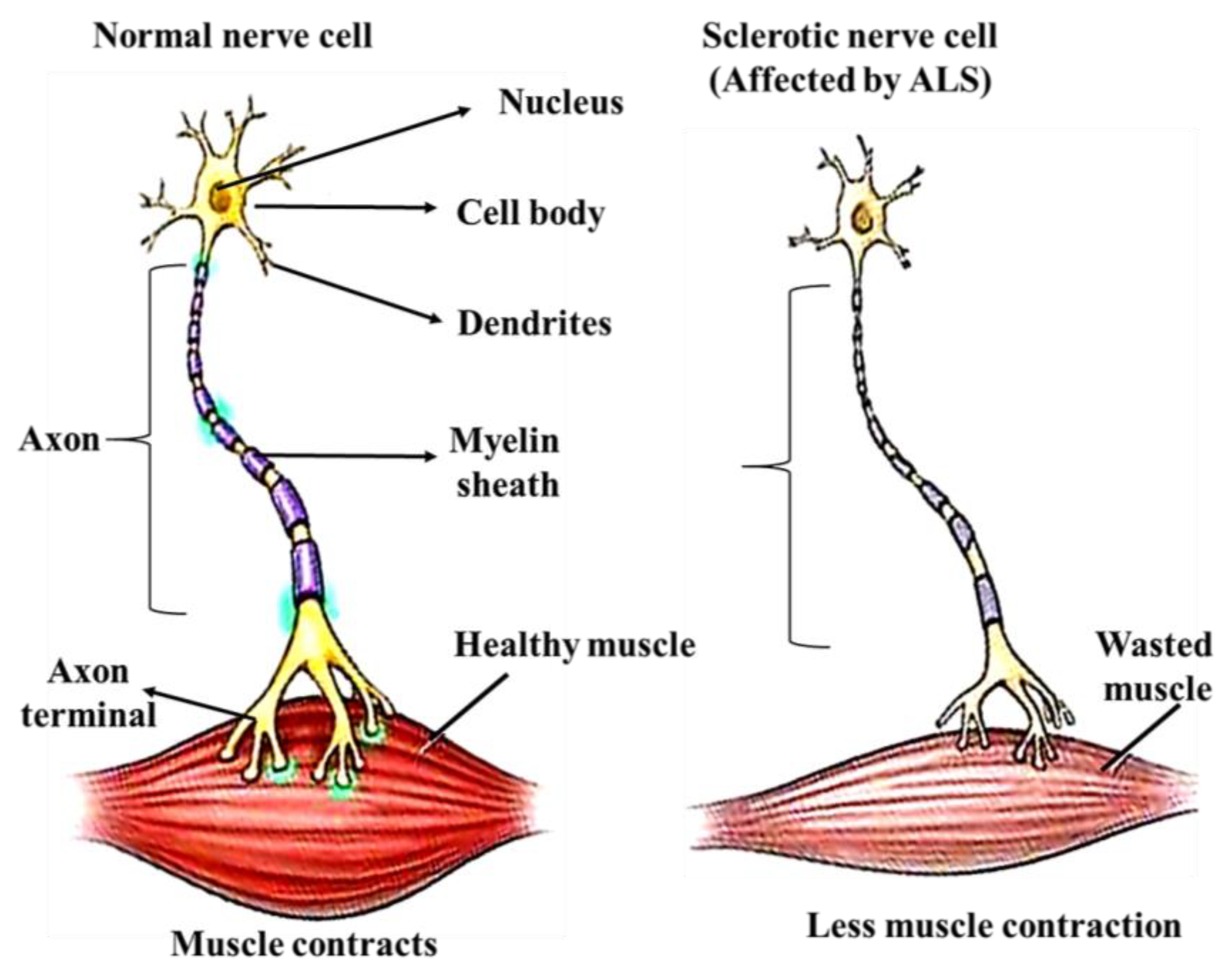
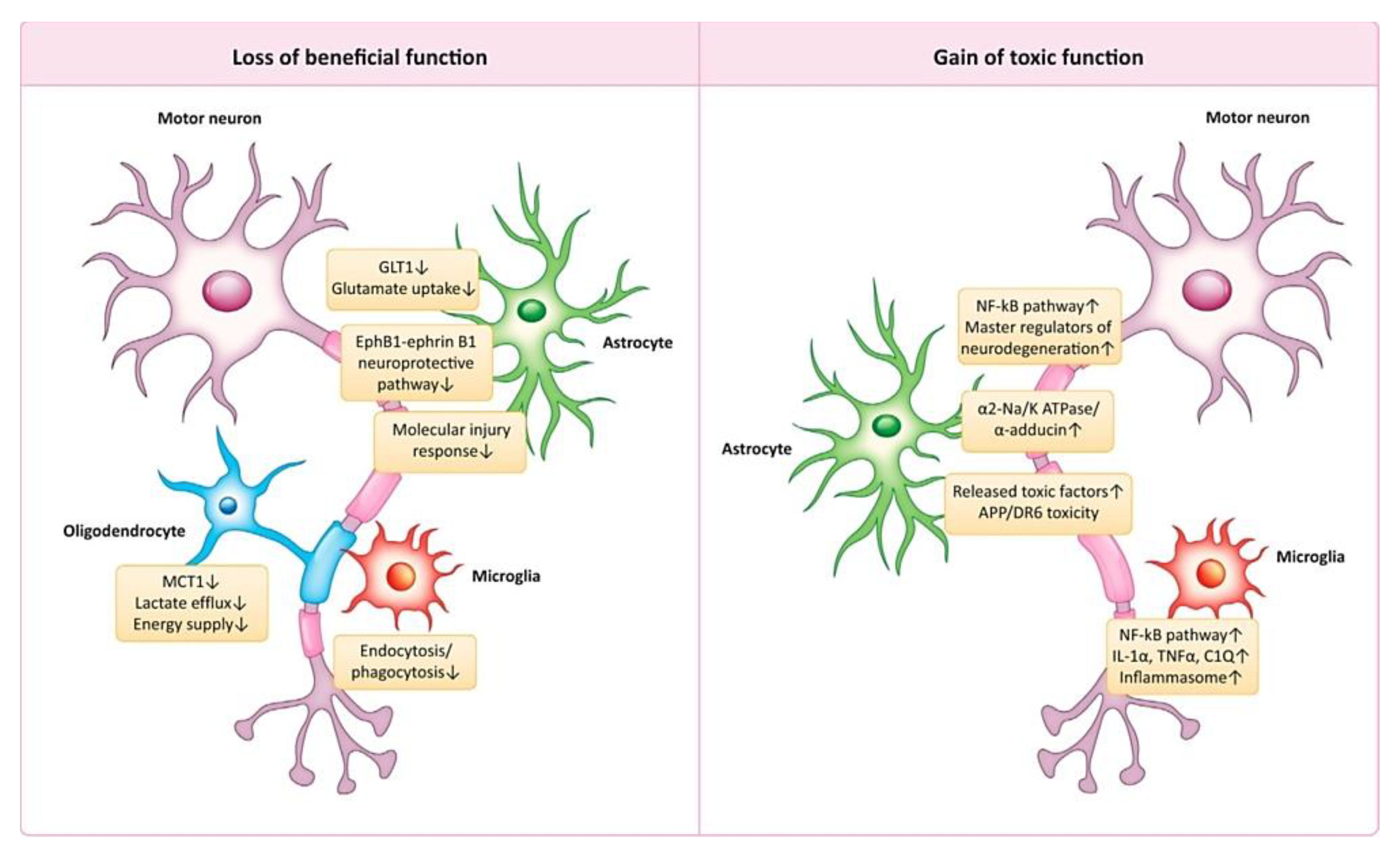
3.6. Amyotrophic Lateral Sclerosis (ALS)
4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putera, A.M.; Irwanto, I.; Maramis, M.M.; Prasetyo, R.V.; Soemyarso, N.A.; Noer, M.S. Effect of Mental Health Problems on the Quality of Life in Children with Lupus Nephritis. Neuropsychiatr. Dis. Treat. 2020, 16, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.; Brazier, J.; O’Cathain, A.; Lloyd-Jones, M.; Paisley, S. Quality of life of people with mental health problems: A synthesis of qualitative research. Health Qual. Life Outcomes 2012, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Haro, J.M.; Heeringa, S.G.; Pennell, B.-E.; Bedirhan Üstün, T. The World Health Organization World Mental Health Survey Initiative. Epidemiol. Psichiatr. Soc. 2006, 15, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A. Mental illness in Saudi Arabia: An overview. Psychol. Res. Behav. Manag. 2015, 47, 47–49. [Google Scholar] [CrossRef]
- Alenezi, S.; Temsah, M.-H.; Alyahya, A.S.; Almadani, A.H.; Almarshedi, A.; Algazlan, M.S.; Alnemary, F.; Bashiri, F.A.; Alkhawashki, S.H.; Altuwariqi, M.H.; et al. Mental health impact of COVID-19 on Saudi families and children with special educational needs and disabilities in Saudi Arabia: A national perspective. Front. Public Health 2022, 10, 992658. [Google Scholar] [CrossRef]
- Al-Subaie, A.S.; Al-Habeeb, A.; Altwaijri, Y.A. Overview of the Saudi National Mental Health Survey. Int. J. Methods Psychiatr. Res. 2020, 29, e1835. [Google Scholar] [CrossRef]
- Koenig, H.G.; Al Zaben, F.; Sehlo, M.G.; Khalifa, D.A.; Al Ahwal, M.S.; Qureshi, N.A.; Al-Habeeb, A.A. Mental Health Care in Saudi Arabia: Past, Present and Future. Open J. Psychiatry 2014, 04, 113–130. [Google Scholar] [CrossRef]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef]
- Gómez-Gómez, M.E.; Zapico, S.C. Frailty, Cognitive Decline, Neurodegenerative Diseases and Nutrition Interventions. Int. J. Mol. Sci. 2019, 20, 2842. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood–brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Small, G.W.; Lee, J.; Kaufman, A.; Jalil, J.; Siddarth, P.; Gaddipati, H.; Moody, T.D.; Bookheimer, S.Y. Brain health consequences of digital technology use. Dialogues Clin. Neurosci. 2020, 22, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Disease Statistics, Alzheimer’s News Today. Available online: https://alzheimersnewstoday.com/alzheimers-disease-statistics/?cn-reloaded=1 (accessed on 20 December 2022).
- Launch of WHO’s Parkinson Disease Technical Brief. 14 June 2022. Available online: https://www.who.int/news/item/14-06-2022-launch-of-who-s-parkinson-disease-technical-brief (accessed on 14 June 2022).
- Report, Alzheimer’s Disease International. Available online: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/ (accessed on 21 September 2022).
- Erickson, M.; Banks, W. Age-Associated Changes in the Immune System and Blood–Brain Barrier Functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood–brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Pasinelli, P.; Brown, R.H. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat. Rev. Neurosci. 2006, 7, 710–723. [Google Scholar] [CrossRef]
- Bentham Science Publisher Bentham Science Publisher ERRATUM. Curr. Neuropharmacol. 2015, 13, 846. [CrossRef]
- Wang, X.; Hou, Y.; Ai, X.; Sun, J.; Xu, B.; Meng, X.; Zhang, Y.; Zhang, S. Potential applications of microfluidics based blood brain barrier (BBB)-on-chips for in vitro drug development. Biomed. Pharmacother. 2020, 132, 110822. [Google Scholar] [CrossRef]
- Haddad, F.; Sawalha, M.; Khawaja, Y.; Najjar, A.; Karaman, R. Dopamine and Levodopa Prodrugs for the Treatment of Parkinson’s Disease. Molecules 2017, 23, 40. [Google Scholar] [CrossRef]
- Nutt, J.G.; Woodward, W.R.; Beckner, R.M.; Stone, C.K.; Berggren, K.; Carter, J.H.; Gancher, S.T.; Hammerstad, J.P.; Gordin, A. Effect of peripheral catechol-O-methyltransferase inhibition on the pharmacokinetics and pharmacodynamics of levodopa in parkinsonian patients. Neurology 1994, 44, 913. [Google Scholar] [CrossRef]
- Hagan, J.J.; Middlemiss, D.N.; Sharpe, P.C.; Poste, G.H. Parkinson’s disease: Prospects for improved drug therapy. Trends Pharmacol. Sci. 1997, 18, 156–163. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting Brain Uptake of a Therapeutic Antibody by Reducing Its Affinity for a Transcytosis Target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef] [PubMed]
- Khawli, L.A.; Prabhu, S. Drug Delivery across the Blood–Brain Barrier. Mol. Pharm. 2013, 10, 1471–1472. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M. Epidemiology of Parkinson’s Disease. Neurol. Clin. 1992, 10, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63. [Google Scholar] [CrossRef]
- VanItallie, T.B. Alzheimer’s disease: Innate immunity gone awry? Metabolism 2017, 69, S41–S49. [Google Scholar] [CrossRef]
- Gan, L.; Cookson, M.R.; Petrucelli, L.; La Spada, A.R. Converging pathways in neurodegeneration, from genetics to mechanisms. Nat. Neurosci. 2018, 21, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.-Z. Nature of Tau-Associated Neurodegeneration and the Molecular Mechanisms. J. Alzheimer’s Dis. 2018, 62, 1305–1317. [Google Scholar] [CrossRef]
- Linsley, J.W.; Reisine, T.; Finkbeiner, S. Cell death assays for neurodegenerative disease drug discovery. Expert Opin. Drug Discov. 2019, 14, 901–913. [Google Scholar] [CrossRef]
- Taylor, J.P.; Hardy, J.; Fischbeck, K.H. Toxic Proteins in Neurodegenerative Disease. Science 2002, 296, 1991–1995. [Google Scholar] [CrossRef]
- Goedert, M.; Eisenberg, D.S.; Crowther, R.A. Propagation of Tau Aggregates and Neurodegeneration. Annu. Rev. Neurosci. 2017, 40, 189–210. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Rothhammer, V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front. Immunol. 2020, 11, 573256. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 2016, 4, e1138017. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; He, C.; Liu, H.; Liao, X.; Dai, B.; Chen, Y.; Yang, Y.; Zhao, B.; Bihl, J.; Ma, X. Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow. Mol. Brain 2016, 9, 63. [Google Scholar] [CrossRef]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the blood–brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Bicker, J.; Alves, G.; Fortuna, A.; Falcão, A. Blood–brain barrier models and their relevance for a successful development of CNS drug delivery systems: A review. Eur. J. Pharm. Biopharm. 2014, 87, 409–432. [Google Scholar] [CrossRef]
- McCabe, S.M.; Zhao, N. The Potential Roles of Blood–Brain Barrier and Blood–Cerebrospinal Fluid Barrier in Maintaining Brain Manganese Homeostasis. Nutrients 2021, 13, 1833. [Google Scholar] [CrossRef]
- Małkiewicz, M.A.; Szarmach, A.; Sabisz, A.; Cubała, W.J.; Szurowska, E.; Winklewski, P.J. Blood-brain barrier permeability and physical exercise. J. Neuroinflammation 2019, 16, 15. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood–Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 1605. [Google Scholar] [CrossRef]
- Head, E. Oxidative Damage and Cognitive Dysfunction: Antioxidant Treatments to Promote Healthy Brain Aging. Neurochem. Res. 2009, 34, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, M.; Betancourt, C.E.; Liu, L.; Sitikov, A.; Sladojevic, N.; Zhao, Q.; Zhang, J.H.; Liao, J.K.; Wu, R. Increase in Blood-Brain Barrier (BBB) Permeability Is Regulated by MMP3 via the ERK Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6655122. [Google Scholar] [CrossRef]
- Luo, S.; Levine, R.L. Methionine in proteins defends against oxidative stress. FASEB J. 2009, 23, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Villaça, Y.; Levin, E.D. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Int. 2017, 99, 55–77. [Google Scholar] [CrossRef]
- Zhang, X.; Thompson, M.; Xu, Y. Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab. Investig. 2016, 96, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Safia; Haque, E.; Mir, S.S. Neurodegenerative Diseases: Multifactorial Conformational Diseases and Their Therapeutic Interventions. J. Neurodegener. Dis. 2013, 2013, 563481. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Lleó, A.; Greenberg, S.M.; Growdon, J.H. Current Pharmacotherapy for Alzheimer’s Disease. Annu. Rev. Med. 2006, 57, 513–533. [Google Scholar] [CrossRef]
- Annic, A.; Devos, D.; Seguy, D.; Dujardin, K.; Destée, A.; Defebvre, L. Intérêts de la stimulation dopaminergique continue par Duodopa® dans la maladie de Parkinson évoluée: Efficacité et tolérance. Rev. Neurol. 2009, 165, 718–727. [Google Scholar] [CrossRef]
- Md, S.; Haque, S.; Sahni, J.K.; Baboota, S.; Ali, J. New non-oral drug delivery systems for Parkinson’s disease treatment. Expert Opin. Drug Deliv. 2011, 8, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Lee, C.Y.D.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-Directed Therapeutics Rapidly Clear β-Amyloid and Reverse Deficits in AD Mouse Models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Rondi-Reig, L.; Libbey, M.; Eichenbaum, H.; Tonegawa, S. CA1-specific N -methyl- d -aspartate receptor knockout mice are deficient in solving a nonspatial transverse patterning task. Proc. Natl. Acad. Sci. USA 2001, 98, 3543–3548. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Möller, C.; Lannfelt, L. Lecanemab, Aducanumab, and Gantenerumab—Binding Profiles to Different Forms of Amyloid-Beta Might Explain Efficacy and Side Effects in Clinical Trials for Alzheimer’s Disease. Neurotherapeutics 2022. [Google Scholar] [CrossRef]
- Fleisher, A.S.; Raman, R.; Siemers, E.R.; Becerra, L.; Clark, C.M.; Dean, R.A.; Farlow, M.R.; Galvin, J.E.; Peskind, E.R.; Quinn, J.F.; et al. Phase 2 Safety Trial Targeting Amyloid β Production With a γ-Secretase Inhibitor in Alzheimer Disease. Arch. Neurol. 2008, 65, 1031–1038. [Google Scholar] [CrossRef]
- Crouse III, J.R.; Byington, R.P.; Furberg, C.D. HMG-CoA reductase inhibitor therapy and stroke risk reduction: An analysis of clinical trials data. Atherosclerosis 1998, 138, 11–24. [Google Scholar] [CrossRef]
- Weyrich, A.S.; Skalabrin, E.J.; Kraiss, L.W. Targeting the Inflammatory Response in Secondary Stroke Prevention: A Role for Combining Aspirin and Extended-release Dipyridamole. Am. J. Ther. 2009, 16, 164–170. [Google Scholar] [CrossRef]
- Adibhatla, R.; Hatcher, J. Tissue Plasminogen Activator (tPA) and Matrix Metalloproteinases in the Pathogenesis of Stroke: Therapeutic Strategies. CNS Neurol. Disord.-Drug Targets 2008, 7, 243–253. [Google Scholar] [CrossRef]
- Chan, A.; Weilbach, F.X.; Toyka, K.V.; Gold, R. Mitoxantrone induces cell death in peripheral blood leucocytes of multiple sclerosis patients. Clin. Exp. Immunol. 2004, 139, 152–158. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xu, L.-Y.; Xiao, B.-G.; Hedlund, G.; Link, H. Laquinimod (ABR-215062) suppresses the development of experimental autoimmune encephalomyelitis, modulates the Th1/Th2 balance and induces the Th3 cytokine TGF-β in Lewis rats. J. Neuroimmunol. 2004, 156, 3–9. [Google Scholar] [CrossRef]
- Villano, J.L.; Seery, T.E.; Bressler, L.R. Temozolomide in malignant gliomas: Current use and future targets. Cancer Chemother. Pharmacol. 2009, 64, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Mischel, P.S.; Cloughesy, T.F. Targeted Molecular Therapy of GBM. Brain Pathol. 2006, 13, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Dangouloff, T.; Servais, L. Clinical Evidence Supporting Early Treatment Of Patients With Spinal Muscular Atrophy: Current Perspectives. Ther. Clin. Risk Manag. 2019, 15, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Neurological Diseases in Relation to the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef]
- Sato, Y.; Falcone-Juengert, J.; Tominaga, T.; Su, H.; Liu, J. Remodeling of the Neurovascular Unit Following Cerebral Ischemia and Hemorrhage. Cells 2022, 11, 764. [Google Scholar] [CrossRef]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.-E.; Sleeboom, J.J.F.; Ingber, D.E. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 764. [Google Scholar] [CrossRef]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef]
- Kozma, M.; Mészáros, Á.; Nyúl-Tóth, Á.; Molnár, K.; Costea, L.; Hernádi, Z.; Fazakas, C.; Farkas, A.E.; Wilhelm, I.; Krizbai, I.A. Cerebral Pericytes and Endothelial Cells Communicate through Inflammasome-Dependent Signals. Int. J. Mol. Sci. 2021, 22, 6122. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1–H18. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, N.; Zhong, J.; Yu, B.; Wan, J.; Zhang, W. Age-associated changes in microglia and astrocytes ameliorate blood-brain barrier dysfunction. Mol. Ther.-Nucleic Acids 2021, 26, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Page, S.; Patel, R.; Raut, S.; Al-Ahmad, A. Neurological diseases at the blood-brain barrier: Stemming new scientific paradigms using patient-derived induced pluripotent cells. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165358. [Google Scholar] [CrossRef]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front. Pharmacol. 2020, 10, 1568. [Google Scholar] [CrossRef]
- Wang, F.; Cao, Y.; Ma, L.; Pei, H.; Rausch, W.D.; Li, H. Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia. Front. Aging Neurosci. 2018, 10, 376. [Google Scholar] [CrossRef]
- Khan, A.I.; Liu, J.; Dutta, P. Bayesian inference for parameter estimation in lactoferrin-mediated iron transport across blood-brain barrier. Biochim. Biophys. Acta-Gen. Subj. 2020, 1864, 129459. [Google Scholar] [CrossRef]
- Khan, A.I.; Lu, Q.; Du, D.; Lin, Y.; Dutta, P. Quantification of kinetic rate constants for transcytosis of polymeric nanoparticle through blood-brain barrier. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 2779–2787. [Google Scholar] [CrossRef]
- Yamanaka, G.; Takata, F.; Kataoka, Y.; Kanou, K.; Morichi, S.; Dohgu, S.; Kawashima, H. The Neuroinflammatory Role of Pericytes in Epilepsy. Biomedicines 2021, 9, 759. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Cerebrospinal Fluid Biomarkers of Neurovascular Dysfunction in Mild Dementia and Alzheimer’S Disease. J. Cereb. Blood Flow Metab. 2015, 35, 1055–1068. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Girolamo, F.; de Trizio, I.; Errede, M.; Longo, G.; D’Amati, A.; Virgintino, D. Neural crest cell-derived pericytes act as pro-angiogenic cells in human neocortex development and gliomas. Fluids Barriers CNS 2021, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The role of extracellular vesicles in the physiological and pathological regulation of the blood–brain barrier. FASEB BioAdvances 2021, 3, 665–675. [Google Scholar] [CrossRef]
- Makrygianni, E.A.; Chrousos, G.P. Extracellular vesicles and the Stress System. Neuroendocrinology 2022, volume, page. [Google Scholar] [CrossRef]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- MacVicar, B.A.; Newman, E.A. Astrocyte Regulation of Blood Flow in the Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a020388. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Dossi, E.; Rouach, N. Human astrocytes: Structure and functions in the healthy brain. Brain Struct. Funct. 2017, 222, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Villabona-Rueda, A.; Erice, C.; Pardo, C.A.; Stins, M.F. The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Front. Cell. Neurosci. 2019, 13, 405. [Google Scholar] [CrossRef] [PubMed]
- Skovronsky, D.M.; Lee, V.M.-Y.; Trojanowski, J.Q. NEURODEGENERATIVE DISEASES: New Concepts of Pathogenesis and Their Therapeutic Implications. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.; Brennan, S.E.; Keon, M.; Saksena, N.K. Prionoid Proteins in the Pathogenesis of Neurodegenerative Diseases. Front. Mol. Neurosci. 2019, 12, 271. [Google Scholar] [CrossRef]
- 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [CrossRef]
- Wang, S.; Liu, H.-Y.; Cheng, Y.-C.; Su, C.-H. Exercise Dosage in Reducing the Risk of Dementia Development: Mode, Duration, and Intensity—A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 13331. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.d.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef]
- Kaur, D.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Bungau, S. Multifaceted Alzheimer’s Disease: Building a Roadmap for Advancement of Novel Therapies. Neurochem. Res. 2021, 46, 2832–2851. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Banks, W.A.; Farr, S.A.; Morley, J.E.; Wolf, K.M.; Geylis, V.; Steinitz, M. Anti-amyloid beta protein antibody passage across the blood–brain barrier in the SAMP8 mouse model of Alzheimer’s disease: An age-related selective uptake with reversal of learning impairment. Exp. Neurol. 2007, 206, 248–256. [Google Scholar] [CrossRef]
- Bagaria, J.; Bagyinszky, E.; An, S.S.A. Genetics, Functions, and Clinical Impact of Presenilin-1 (PSEN1) Gene. Int. J. Mol. Sci. 2022, 23, 10970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.T.D.; Le, T.T.; Vo, V.G. Role of Insulin Resistance in the Alzheimer’s Disease Progression. Neurochem. Res. 2020, 45, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining cholinergic therapy for Alzheimer’s disease. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef]
- Vecchio, I.; Sorrentino, L.; Paoletti, A.; Marra, R.; Arbitrio, M. The State of The Art on Acetylcholinesterase Inhibitors in the Treatment of Alzheimer’s Disease. J. Cent. Nerv. Syst. Dis. 2021, 13, 117957352110291. [Google Scholar] [CrossRef]
- Saeedi, M.; Mehranfar, F. Challenges and Approaches of Drugs Such as Memantine, Donepezil, Rivastigmine, and Aducanumab in the Treatment, Control and Management of Alzheimer’s Disease. Recent Pat. Biotechnol. 2022, 16, 102–121. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, R.; Cheng, C.; Li, S.; Le, W. New therapeutics beyond amyloid-β and tau for the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 2021, 42, 1382–1389. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Dung Nguyen, T.T.; Vo, T.K.; Tran, N.-M.-A.; Nguyen, M.K.; Van Vo, T.; Van Vo, G. Nanotechnology-based drug delivery for central nervous system disorders. Biomed. Pharmacother. 2021, 143, 112117. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, H.; Lei, L.; Tong, X.; Yang, L.; Yang, Y.; Li, S.; Zhou, Y.; Luo, L.; Huang, J.; et al. Tight junctions and their regulation by non-coding RNAs. Int. J. Biol. Sci. 2021, 17, 712–727. [Google Scholar] [CrossRef]
- Re, F.; Salvati, E.; Sesana, S.; Cambianica; Sancini, G.; Masserini, M.; Gregori, M. Liposomes functionalized to overcome the blood-brain barrier and to target amyloid-beta peptide: The chemical design affects the permeability across an in vitro model. Int. J. Nanomed. 2013, 8, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M.J.; Bastus, N.G.; Amigo, R.; Grillo-Bosch, D.; Araya, E.; Turiel, A.; Labarta, A.; Giralt, E.; Puntes, V.F. Nanoparticle-Mediated Local and Remote Manipulation of Protein Aggregation. Nano Lett. 2006, 6, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427. [Google Scholar] [CrossRef]
- Pollanen, M.S.; Dickson, D.W.; Bergeron, C. Pathology and Biology of the Lewy Body. J. Neuropathol. Exp. Neurol. 1993, 52, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, S.; Mori, H.; Izumiyama, N.; Yoshimura, M.; Ihara, Y. Lewy bodies are ubiquitinated. Acta Neuropathol. 1988, 75, 345–353. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Kincses, Z.T.; Vecsei, L. Pharmacological Therapy in Parkinson’s Disease: Focus on Neuroprotection. CNS Neurosci. Ther. 2011, 17, 345–367. [Google Scholar] [CrossRef]
- Axelsen, T.M.; Woldbye, D.P.D. Gene Therapy for Parkinson’s Disease, An Update. J. Parkinsons. Dis. 2018, 8, 195–215. [Google Scholar] [CrossRef]
- Bondarenko, O.; Saarma, M. Neurotrophic Factors in Parkinson’s Disease: Clinical Trials, Open Challenges and Nanoparticle-Mediated Delivery to the Brain. Front. Cell. Neurosci. 2021, 15, 178. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Huang, R.-Y.; Liao, E.-C.; Lin, Y.-C.; Ho, Y.-J.; Chang, C.-W.; Chan, H.-L.; Huang, Y.-Z.; Hsieh, T.-H.; Fan, C.-H.; et al. A preliminary study of Parkinson’s gene therapy via sono-magnetic sensing gene vector for conquering extra/intracellular barriers in mice. Brain Stimul. 2020, 13, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Zhou, Q.-H.; Hui, E.K.-W.; Lu, J.Z.; Boado, R.J.; Pardridge, W.M. Intravenous treatment of experimental Parkinson’s disease in the mouse with an IgG-GDNF fusion protein that penetrates the blood–brain barrier. Brain Res. 2010, 1352, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.; Yang, T.; Knowles, J.; Xie, Y.; Moore, L.; Massa, S. Small Molecule Neurotrophin Receptor Ligands: Novel Strategies for Targeting Alzheimers Disease Mechanisms. Curr. Alzheimer Res. 2007, 4, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Mekky, G.; van der Meer, S.B.; Seeds, M.C.; Atala, A.J.; Epple, M. Transport of ultrasmall gold nanoparticles (2 nm) across the blood–brain barrier in a six-cell brain spheroid model. Sci. Rep. 2020, 10, 18033. [Google Scholar] [CrossRef]
- Xia, C.-F.; Chu, C.; Li, J.; Wang, Y.; Zhang, Y.; Boado, R.J.; Pardridge, W.M. Comparison of cDNA and genomic forms of tyrosine hydroxylase gene therapy of the brain with Trojan horse liposomes. J. Gene Med. 2007, 9, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.; Johnson, M.; Walker, M.; Riley, K.; Sims, C. Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Cao, Z.; Chu, L. The Antioxidant Effect of the Metal and Metal-Oxide Nanoparticles. Antioxidants 2022, 11, 791. [Google Scholar] [CrossRef] [PubMed]
- Bunner, K.D.; Rebec, G.V. Corticostriatal Dysfunction in Huntington’s Disease: The Basics. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Parsons, M.P.; Vanni, M.P.; Woodard, C.L.; Kang, R.; Murphy, T.H.; Raymond, L.A. Real-time imaging of glutamate clearance reveals normal striatal uptake in Huntington disease mouse models. Nat. Commun. 2016, 7, 11251. [Google Scholar] [CrossRef]
- Cepeda, C.; Tong, X.-P. Huntington’s disease: From basic science to therapeutics. CNS Neurosci. Ther. 2018, 24, 247–249. [Google Scholar] [CrossRef]
- Van Harten, A.C.M.; Phatnani, H.; Przedborski, S. Non-cell-autonomous pathogenic mechanisms in amyotrophic lateral sclerosis. Trends Neurosci. 2021, 44, 658–668. [Google Scholar] [CrossRef] [PubMed]
- McAlary, L.; Plotkin, S.S.; Yerbury, J.J.; Cashman, N.R. Prion-Like Propagation of Protein Misfolding and Aggregation in Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2019, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Stroehlein, M. ALS. Nurs. Made Incred. Easy 2018, 16, 9–12. [Google Scholar] [CrossRef]
- Larkin, H.D. New Research Partnership for ALS, Rare Neurodegenerative Diseases. JAMA 2022, 328, 1680. [Google Scholar] [CrossRef] [PubMed]
- Mentis, A.-F.A.; Bougea, A.M.; Chrousos, G.P. Amyotrophic lateral sclerosis (ALS) and the endocrine system: Are there any further ties to be explored? Aging Brain 2021, 1, 100024. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkahtani, S.; AL-Johani, N.S.; Alarifi, S. Mechanistic Insights, Treatment Paradigms, and Clinical Progress in Neurological Disorders: Current and Future Prospects. Int. J. Mol. Sci. 2023, 24, 1340. https://doi.org/10.3390/ijms24021340
Alkahtani S, AL-Johani NS, Alarifi S. Mechanistic Insights, Treatment Paradigms, and Clinical Progress in Neurological Disorders: Current and Future Prospects. International Journal of Molecular Sciences. 2023; 24(2):1340. https://doi.org/10.3390/ijms24021340
Chicago/Turabian StyleAlkahtani, Saad, Norah S. AL-Johani, and Saud Alarifi. 2023. "Mechanistic Insights, Treatment Paradigms, and Clinical Progress in Neurological Disorders: Current and Future Prospects" International Journal of Molecular Sciences 24, no. 2: 1340. https://doi.org/10.3390/ijms24021340
APA StyleAlkahtani, S., AL-Johani, N. S., & Alarifi, S. (2023). Mechanistic Insights, Treatment Paradigms, and Clinical Progress in Neurological Disorders: Current and Future Prospects. International Journal of Molecular Sciences, 24(2), 1340. https://doi.org/10.3390/ijms24021340






