Exploring the Physiological Multiplicity of Native Microalgae from the Ecuadorian Highland, Italian Lowland and Indoor Locations in Response to UV-B
Abstract
:1. Introduction
2. Results
2.1. Identification of the Strains PEC and ETI
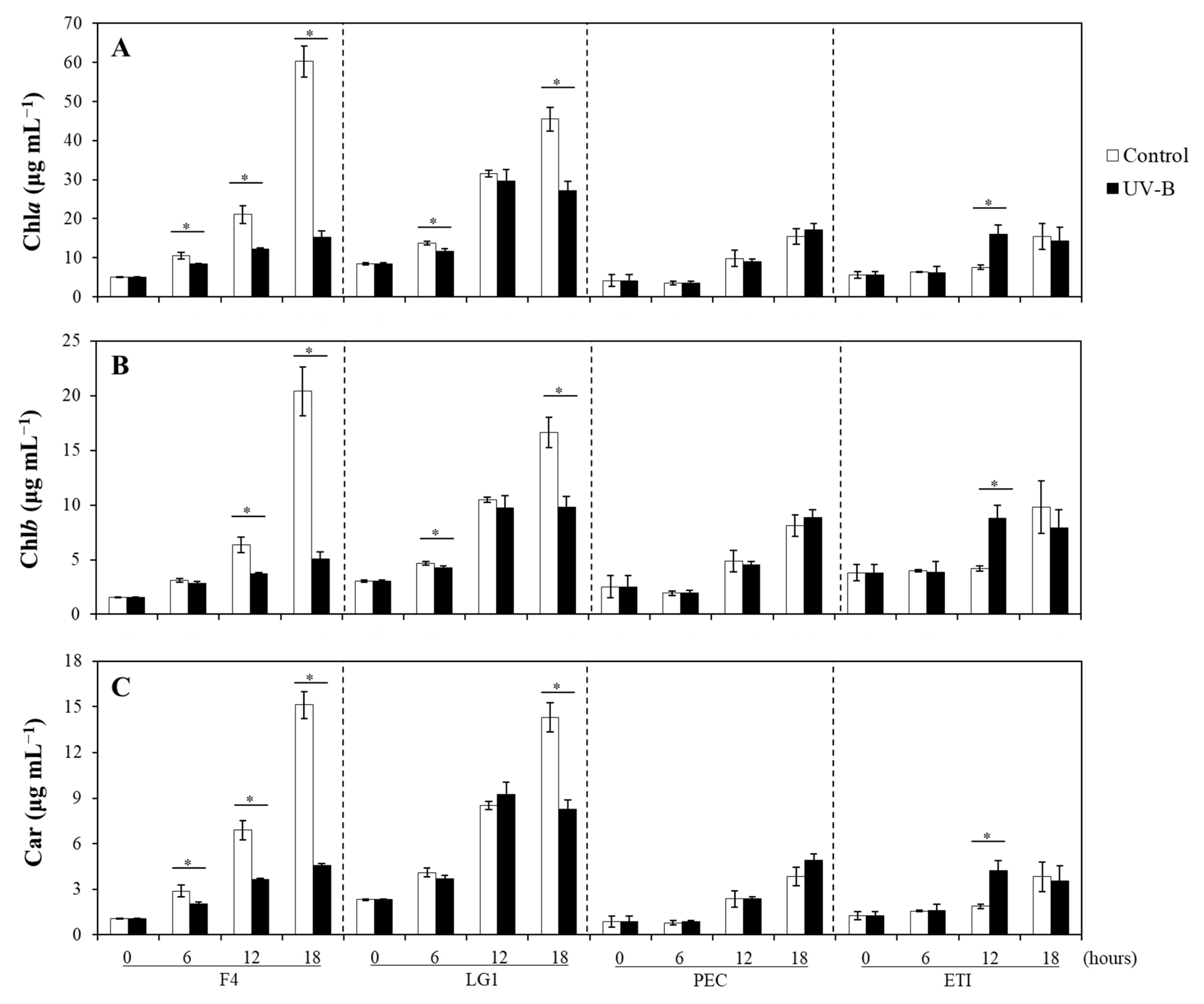
2.2. Effects of UV-B on the Photosynthetic Pigments of Microalgal Strains
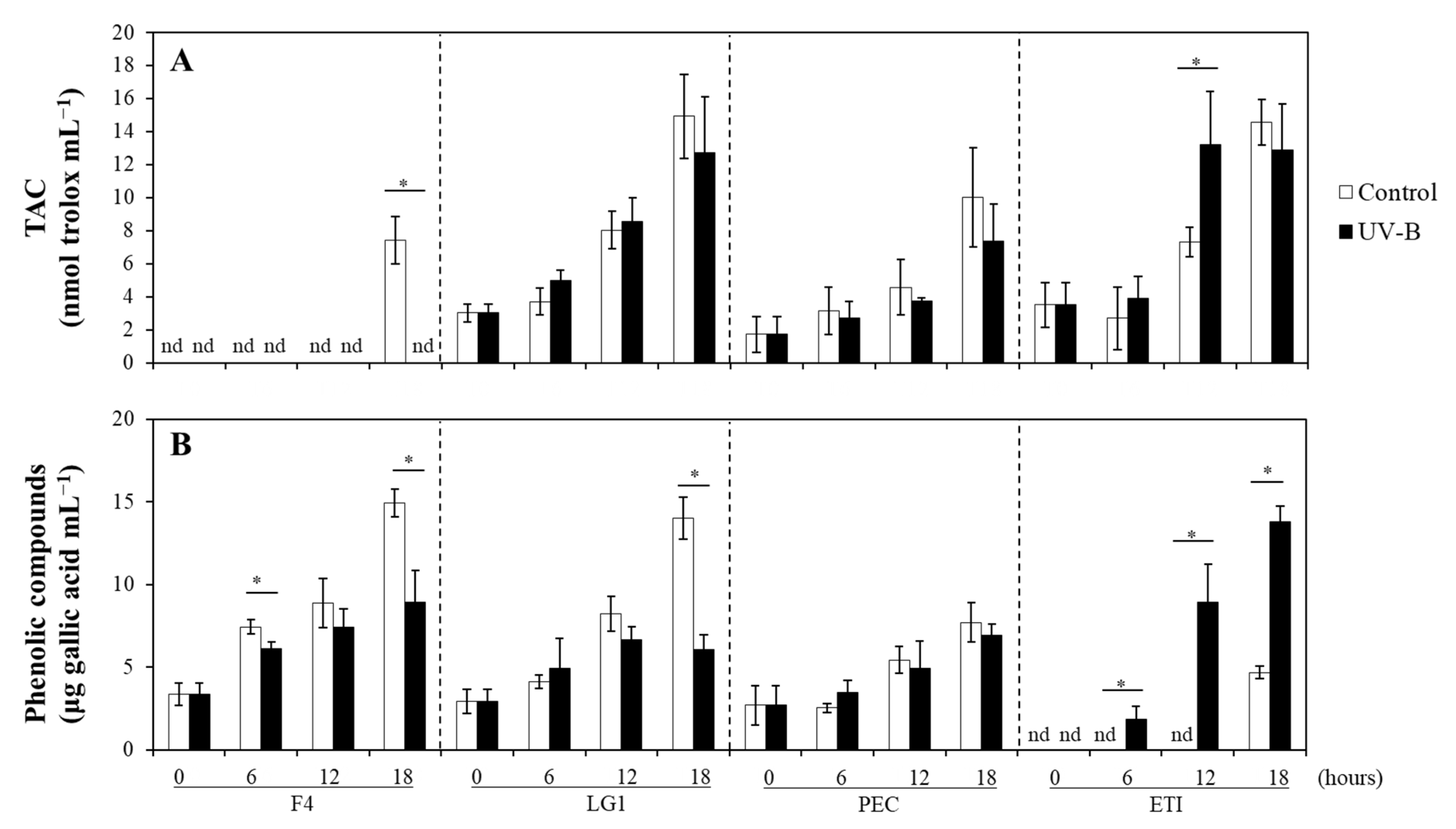
2.3. Effects of UV-B on the Non-Enzymatic Antioxidants of Microalgal Strains
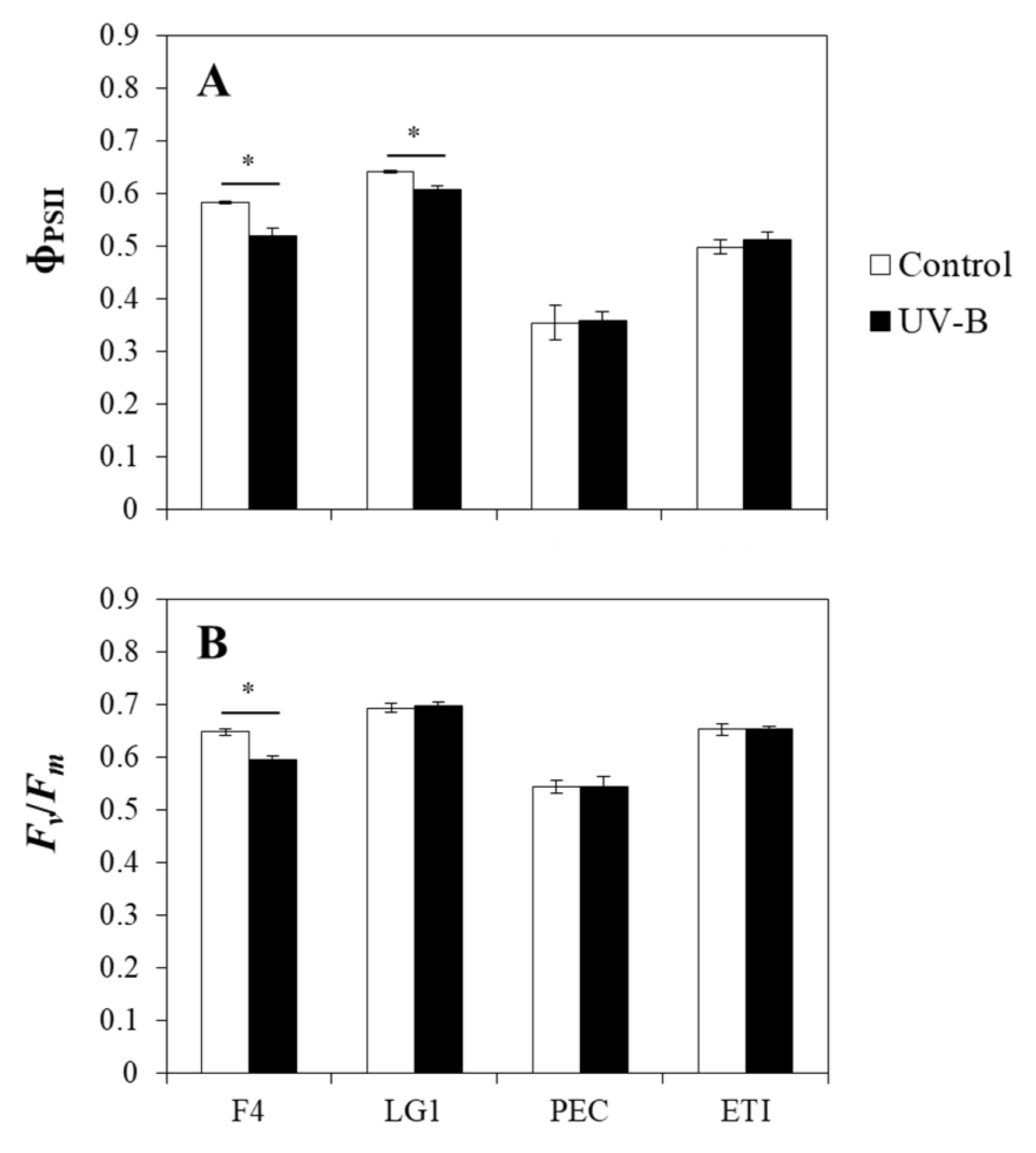
2.4. Effects of UV-B on the Chlorophyll a Fluorescence of Microalgal Strains
2.5. Multiple Factor Analysis
3. Discussion
4. Materials and Methods
4.1. Microalgal Strains and Growth Conditions
4.2. Characterization of PEC and ETI Strains
4.3. UV-B Radiation Treatment
4.4. Extraction and Determination of Photosynthetic Pigments
4.5. Extraction and Determination of Total Antioxidant Capacity and Phenolic Compounds
4.6. Chlorophyll a Fluorescence
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in Microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 21–36. [Google Scholar]
- Al-Qasmi, M.; Raut, N.; Talebi, S.; Al-Rajhi, S.; Al-Barwani, T. A Review of Effect of Light on Microalgae Growth. In Proceedings of the World Congress on Engineering, London, UK, 4–6 July 2012; Volume I, pp. 1–3. [Google Scholar]
- Hughes, K.A. Solar UV-B Radiation, Associated with Ozone Depletion, Inhibits the Antarctic Terrestrial Microalga, Stichococcus bacillaris. Polar Biol. 2006, 29, 327–336. [Google Scholar] [CrossRef]
- Bornman, J.F.; Barnes, P.W.; Robinson, S.A.; Ballaré, C.L.; Flint, S.D.; Caldwell, M.M. Solar Ultraviolet Radiation and Ozone Depletion-Driven Climate Change: Effects on Terrestrial Ecosystems. Photochem. Photobiol. Sci. 2015, 14, 88–107. [Google Scholar] [CrossRef] [Green Version]
- Herndon, J.; Hoisington, R.; Whiteside, M. Deadly Ultraviolet UV-C and UV-B Penetration to Earth’s Surface: Human and Environmental Health Implications. J. Geogr. Environ. Earth Sci. Int. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Dahms, H.U.; Lee, J.S. UV Radiation in Marine Ectotherms: Molecular Effects and Responses. Aquat. Toxicol. 2010, 97, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D. Response of Terrestrial Microorganisms to Ultraviolet-B Radiation in Ecosystems. Res. Microbiol. 2003, 154, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.H. Effects of Increasing UV-B Radiation and Atmospheric CO2 on Photosynthesis and Growth: Implications for Terrestrial Ecosystems. Plant Ecol. 1997, 128, 195–206. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Wang, M.; Zhang, W.; Zhou, B.; Wang, Y. ROS and Calcium Signaling Mediated Pathways Involved in Stress Responses of the Marine Microalgae Dunaliella salina to Enhanced UV-B Radiation. J. Photochem. Photobiol. B Biol. 2017, 173, 360–367. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Scott, J.A.; Ross, G.M. Management of Oxidative Stress by Microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Moreira, J.B.; Izaguirres, G.G.; Teixeira, L.M.; Morais, M.G. Microalgae-based UV Protection Compounds. In Bioprospecting of Microorganism-Based Industrial Molecules; Singh, S.P., Upadhyay, S.K., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2021; pp. 201–224. [Google Scholar]
- Ugya, A.Y.; Imam, T.S.; Li, A.; Ma, J.; Hua, X. Antioxidant Response Mechanism of Freshwater Microalgae Species to Reactive Oxygen Species Production: A Mini Review. Chem. Ecol. 2020, 36, 174–193. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D.; Nakamoto, H.; Incharoensakdi, A. Resilience and Self-Regulation Processes of Microalgae under UV Radiation Stress. J. Photochem. Photobiol. C Photochem. Rev. 2020, 43, 100322. [Google Scholar] [CrossRef]
- Favory, J.-J.; Stec, A.; Gruber, H.; Rizzini, L.; Oravecz, A.; Funk, M.; Albert, A.; Cloix, C.; Jenkins, G.I.; Oakeley, E.J.; et al. Interaction of COP1 and UVR8 Regulates UV-B-Induced Photomorphogenesis and Stress Acclimation in Arabidopsis. EMBO J. 2009, 28, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Rizzini, L.; Favory, J.-J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Tilbrook, K.; Dubois, M.; Crocco, C.D.; Yin, R.; Chappuis, R.; Allorent, G.; Schmid-Siegert, E.; Goldschmidt-Clermont, M.; Ulma, R. UV-B Perception and Acclimation in Chlamydomonas reinhardtii. Plant Cell 2016, 28, 966–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xu, C.; Zhang, S.; Shi, C.; Cheng, H.; Liu, H.; Zhong, B. Origin and Adaptive Evolution of UV RESISTANCE LOCUS 8-Mediated Signaling during Plant Terrestrialization. Plant Physiol. 2022, 188, 332–346. [Google Scholar] [CrossRef]
- Chandra, R.; Das, P.; Vishal, G.; Nagra, S. Factors Affecting the Induction of UV Protectant and Lipid Productivity in Lyngbya for Sequential Biorefinery Product Recovery. Bioresour. Technol. 2019, 278, 303–310. [Google Scholar] [CrossRef] [PubMed]
- John, S.L.; Williams, G.P.; Brijithlal, N.D.; Namitha, L.H.; Krishnakumar, S. Effect of UV-β Radiation on the Growth, Pigment Production and Macromolecular Contents in Marine Microalgae. Res. J. Pharm. Technol. 2019, 12, 5888–5892. [Google Scholar] [CrossRef]
- Benito, X.; Luethje, M.; Schneider, T.; Fritz, S.C.; Baker, P.A.; Pedersen, E.J.; Gaüzère, P.; de Novaes Nascimento, M.; Bush, M.; Ruhi, A. Ecological Resilience in Tropical Andean Lakes: A Paleolimnological Perspective. Limnol. Oceanogr. 2022, 67, S23–S37. [Google Scholar] [CrossRef]
- Chiellini, C.; Mariotti, L.; Huarancca Reyes, T.; de Arruda, E.J.; Fonseca, G.G.; Guglielminetti, L. Remediation Capacity of Different Microalgae in Effluents Derived from the Cigarette Butt Cleaning Process. Plants 2022, 11, 1770. [Google Scholar] [CrossRef]
- Chiellini, C.; Serra, V.; Gammuto, L.; Ciurli, A.; Longo, V.; Gabriele, M. Evaluation of Nutraceutical Properties of Eleven Microalgal Strains Isolated from Different Freshwater Aquatic Environments: Perspectives for Their Application as Nutraceuticals. Foods 2022, 11, 654. [Google Scholar] [CrossRef]
- Morales, E.; Luna, V.; Navarro, L.; Santana, V.; Gordillo, A.; Arévalo, A. Diversidad de Microalgas y Cianobacterias En Muestras Provenientes de Diferentes Provincias Del Ecuador, Destinadas a Una Colección de Cultivos. Rev. Ecuat. Med. Cienc. Biol. 2017, 34, 129–149. [Google Scholar] [CrossRef]
- Hegewald, E.; Bock, C.; Krienitz, L. A Phylogenetic Study on Scenedesmaceae with the Description of a New Species of Pectinodesmus and the New Genera Verrucodesmus and Chodatodesmus (Chlorophyta, Chlorophyceae). Fottea 2013, 13, 149–164. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of Light Intensity on Growth and Lipid Production in Microalgae Grown in Wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, L.; Zhang, Y.; Zhang, T.; An, L.; Wang, X. Effects of Enhanced Ultraviolet-B Radiation on Algae and Cyanobacteria. Crit. Rev. Microbiol. 2005, 31, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yu, J. Changes in Ultrastructure and Responses of Antioxidant Systems of Algae (Dunaliella salina) during Acclimation to Enhanced Ultraviolet-B Radiation. J. Photochem. Photobiol. B Biol. 2009, 97, 152–160. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Zhou, B.; Hu, S.; Wang, Y. Effect of Enhanced UV-B Radiation on Photosynthetic Characteristics of Marine Microalgae Dunaliella salina (Chlorophyta, Chlorophyceae). J. Exp. Mar. Biol. Ecol. 2015, 469, 27–35. [Google Scholar] [CrossRef]
- Baneschi, I. Geochemical and Environmental Study of a Coastal Ecosystem: Massaciuccoli Lake (Northen Tuscany, Italy). Ph.D. Thesis, Università Ca’ Foscari Venezia, Venice, Italy, 2007. [Google Scholar]
- Jansen, M.A.K.; Bilger, W.; Hideg, É.; Strid, Å.; Urban, O.; Aphalo, P.; Brelsford, C.; Klem, K.; Mátai, A.; Llorens, L.; et al. Editorial: Interactive Effects of UV-B Radiation in a Complex Environment. Plant Physiol. Biochem. 2019, 134, 1–8. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Mariotti, L.; Chiellini, C.; Guglielminetti, L.; Fonseca, G.G. UV-B Irradiation Effect on Microalgae Performance in the Remediation of Effluent Derived from the Cigarette Butt Cleaning Process. Plants 2022, 11, 2356. [Google Scholar] [CrossRef]
- Czégény, G.; Mátai, A.; Hideg, É. UV-B Effects on Leaves—Oxidative Stress and Acclimation in Controlled Environments. Plant Sci. 2016, 248, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Duan, P.; Huang, T.; Xiong, W.; Shu, L.; Yang, Y.; Shao, C.; Xu, X.; Ma, W.; Tang, R. Protection of Photosynthetic Algae against Ultraviolet Radiation by One-Step CeO2 Shellization. Langmuir 2017, 33, 2454–2459. [Google Scholar] [CrossRef]
- Saber, H.; El-Sheekh, M.M.; Ibrahim, A.; Alwaleed, E.A. Effect of UV-B Radiation on Amino Acids Profile, Antioxidant Enzymes and Lipid Peroxidation of Some Cyanobacteria and Green Algae. Int. J. Radiat. Biol. 2020, 3002, 1192–1206. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, J.; Zhao, X.; Qu, T.; Guan, C.; Hou, C.; Tang, X.; Wang, Y. Balancing Damage via Non-Photochemical Quenching, Phenolic Compounds and Photorespiration in Ulva prolifera Induced by Low-Dose and Short-Term UV-B Radiation. Int. J. Mol. Sci. 2022, 23, 2693. [Google Scholar] [CrossRef]
- Xing, T.; Gao, K.; Beardall, J. Response of Growth and Photosynthesis of Emiliania huxleyi to Visible and UV Irradiances under Different Light Regimes. Photochem. Photobiol. 2015, 91, 343–349. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Scartazza, A.; Castagna, A.; Cosio, E.G.; Ranieri, A.; Guglielminetti, L. Physiological Effects of Short Acute UVB Treatments in Chenopodium Quinoa Willd. Sci. Rep. 2018, 8, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malerba, M.E.; Palacios, M.M.; Palacios Delgado, Y.M.; Beardall, J.; Marshall, D.J. Cell Size, Photosynthesis and the Package Effect: An Artificial Selection Approach. New Phytol. 2018, 219, 449–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Ye, T.; Li, C.; Li, X.; Chen, L.; Wang, G. Cell Damage Repair Mechanism in a Desert Green Algae Chlorella sp. against UV-B Radiation. Ecotoxicol. Environ. Saf. 2022, 242, 113916. [Google Scholar] [CrossRef] [PubMed]
- Rubio Camacho, F.; García Camacho, F.; Fernández Sevilla, J.M.; Chisti, Y.; Molina Grima, E. A Mechanistic Model of Photosynthesis in Microalgae. Biotechnol. Bioeng. 2003, 81, 459–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro Estvāo, D.M. Production of UV-B Screens and Changes in Photosynthetic Efficiency in Antarctic Nostoc Commune Colonies and a Lichen Xanthoria Elegans Depend on a Dose and Duration of UV-B Stress. Czech Polar Rep. 2015, 5, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Juneau, P.; El Berdey, A.; Popovic, R. PAM Fluorometry in the Determination of the Sensitivity of Chlorella vulgaris, Selenastrum capricornutum, and Chlamydomonas reinhardtii to Copper. Arch. Environ. Contam. Toxicol. 2002, 42, 155–164. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic Engineering of Algae for Enhanced Biofuel Production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.B.; Tossi, V.; Lamattina, L.; Cassia, R. A Comprehensive Phylogeny Reveals Functional Conservation of the UV-B Photoreceptor UVR8 from Green Algae to Higher Plants. Front. Plant Sci. 2016, 7, 1698. [Google Scholar] [CrossRef]
- Tokutsu, R.; Fujimura-Kamada, K.; Yamasaki, T.; Okajima, K.; Minagawa, J. UV-A/B Radiation Rapidly Activates Photoprotective Mechanisms in Chlamydomonas reinhardtii. Plant Physiol. 2021, 185, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Allorent, G.; Lefebvre-Legendre, L.; Chappuis, R.; Kuntz, M.; Truong, T.B.; Niyogi, K.K.; Ulm, R.; Goldschmidt-Clermont, M. UV-B Photoreceptor-Mediated Protection of the Photosynthetic Machinery in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2016, 113, 14864–14869. [Google Scholar] [CrossRef] [Green Version]
- Segovia, M.; Mata, T.; Palma, A.; García-Gõmez, C.; Lorenzo, R.; Rivera, A.; Figueroa, F.L. Dunaliella tertiolecta (Chlorophyta) Avoids Cell Death under Ultraviolet Radiation by Triggering Alternative Photoprotective Mechanisms. Photochem. Photobiol. 2015, 91, 1389–1402. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B Exposure, ROS, and Stress: Inseparable Companions or Loosely Linked Associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Andersen, R.A.; Kawachi, M. Traditional Microalgae Isolation Techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier Academic Press: Burlington, MA, USA, 2005; pp. 83–100. ISBN 0-12-088426-7. [Google Scholar]
- Ferro, L.; Gentili, F.G.; Funk, C. Isolation and Characterization of Microalgal Strains for Biomass Production and Wastewater Reclamation in Northern Sweden. Algal Res. 2018, 32, 44–53. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Pompeiano, A.; Ranieri, A.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Photosynthetic Performance of Five Cool-Season Turfgrasses under UV-B Exposure. Plant Physiol. Biochem. 2020, 151, 181–187. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Moles, T.M.; Guglielminetti, L.; Huarancca Reyes, T. Differential Effects of Sodium Chloride on Germination and Post-Germination Stages of Two Tomato Genotypes. Sci. Hortic. 2019, 257, 108730. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Esparza, E.; Crestani, G.; Limonchi, F.; Cruz, R.; Salinas, N.; Scartazza, A.; Guglielminetti, L.; Cosio, E. Physiological Responses of Maca (Lepidium meyenii Walp.) Plants to UV Radiation in Its High-Altitude Mountain Ecosystem. Sci. Rep. 2020, 10, 2654. [Google Scholar] [CrossRef]
- Pompeiano, A.; Huarancca Reyes, T.; Moles, T.M.; Guglielminetti, L.; Scartazza, A. Photosynthetic and Growth Responses of Arundo Donax L. Plantlets under Different Oxygen Deficiency Stresses and Reoxygenation. Front. Plant Sci. 2019, 10, 408. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.Y.; Teoh, M.L.; Phang, S.M.; Lim, P.E.; Beardall, J. Interactive Effects of Temperature and Uv Radiation on Photosynthesis of Chlorella Strains from Polar, Temperate and Tropical Environments: Differential Impacts on Damage and Repair. PLoS ONE 2015, 10, e0139469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, R.P.; Machado, M.; Vaz, M.G.M.V.; Vinson, C.C.; Leite, M.; Richard, R.; Mendes, L.B.B.; Araujo, W.L.; Caldana, C.; Martins, M.A.; et al. Exploring the Metabolic and Physiological Diversity of Native Microalgal Strains (Chlorophyta) Isolated from Tropical Freshwater Reservoirs. Algal Res. 2017, 28, 139–150. [Google Scholar] [CrossRef]
- Chen, L.; Deng, S.; De Philippis, R.; Tian, W.; Wu, H.; Wang, J. UV-B Resistance as a Criterion for the Selection of Desert Microalgae to Be Utilized for Inoculating Desert Soils. J. Appl. Phycol. 2013, 25, 1009–1015. [Google Scholar] [CrossRef]
- Bischof, K.; Hanelt, D.; Wiencke, C. Effects of Ultraviolet Radiation on Photosynthesis and Related Enzyme Reactions of Marine Macroalgae. Planta 2000, 211, 555–562. [Google Scholar] [CrossRef] [PubMed]

| Strain | Division | GenBank Name | GenBank Accession Number | Identity | Reference |
|---|---|---|---|---|---|
| PEC | Chlorophyta | Pectinodesmus pectinatus (KU847995.1) | OP136070 | 99% | [24] |
| ETI | Chlorophyta | Ettlia pseudoalveolaris (LR215808.1) | OP136137 | 100% | [25] |
| Strain | Isolation Source | Taxonomic Affiliation | Accession Number | Reference |
|---|---|---|---|---|
| F4 | “Le Morette”, Fucecchio Marshland | Chlorella sorokiniana | OM311005 and OM311000 | [22] |
| LG1 | Recycled cigarette butts substrate | Chlorella sorokiniana | ON065975 | [21] |
| PEC | Yahuarcocha Lake | Pectinodesmus pectinatus | OP136070 | This work |
| ETI | Yahuarcocha Lake | Ettliapseudoalveolaris | OP136137 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huarancca Reyes, T.; Chiellini, C.; Barozzi, E.; Sandoval, C.; Echeverría, C.; Guglielminetti, L. Exploring the Physiological Multiplicity of Native Microalgae from the Ecuadorian Highland, Italian Lowland and Indoor Locations in Response to UV-B. Int. J. Mol. Sci. 2023, 24, 1346. https://doi.org/10.3390/ijms24021346
Huarancca Reyes T, Chiellini C, Barozzi E, Sandoval C, Echeverría C, Guglielminetti L. Exploring the Physiological Multiplicity of Native Microalgae from the Ecuadorian Highland, Italian Lowland and Indoor Locations in Response to UV-B. International Journal of Molecular Sciences. 2023; 24(2):1346. https://doi.org/10.3390/ijms24021346
Chicago/Turabian StyleHuarancca Reyes, Thais, Carolina Chiellini, Emilio Barozzi, Carla Sandoval, Cristina Echeverría, and Lorenzo Guglielminetti. 2023. "Exploring the Physiological Multiplicity of Native Microalgae from the Ecuadorian Highland, Italian Lowland and Indoor Locations in Response to UV-B" International Journal of Molecular Sciences 24, no. 2: 1346. https://doi.org/10.3390/ijms24021346






