Shape-Memory-Reduced Graphene/Chitosan Cryogels for Non-Compressible Wounds
Abstract
:1. Introduction
2. Results and Discussion
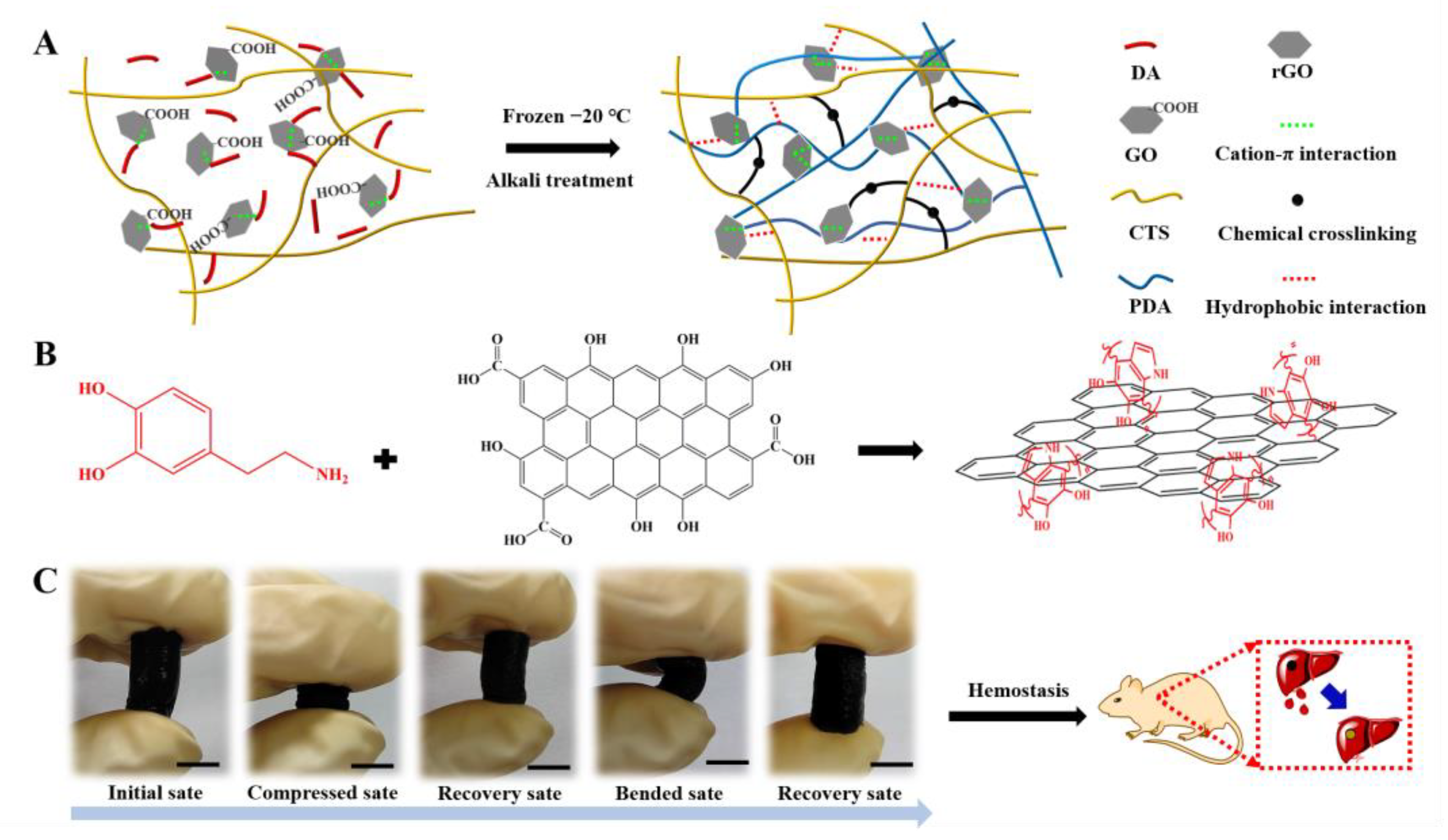
2.1. Preparation and Characteristics of the rGO-CTS Cryogel
2.2. Water-Triggered Shape Memory of the rGO-CTS Cryogel
2.3. In Vitro Blood Clotting Performance and Hemocompatibility of the rGO-CTS Cryogel
2.4. Antibacterial Activity of the rGO-CTS Cryogel
2.5. Cytocompatibility of the rGO-CTS Cryogel
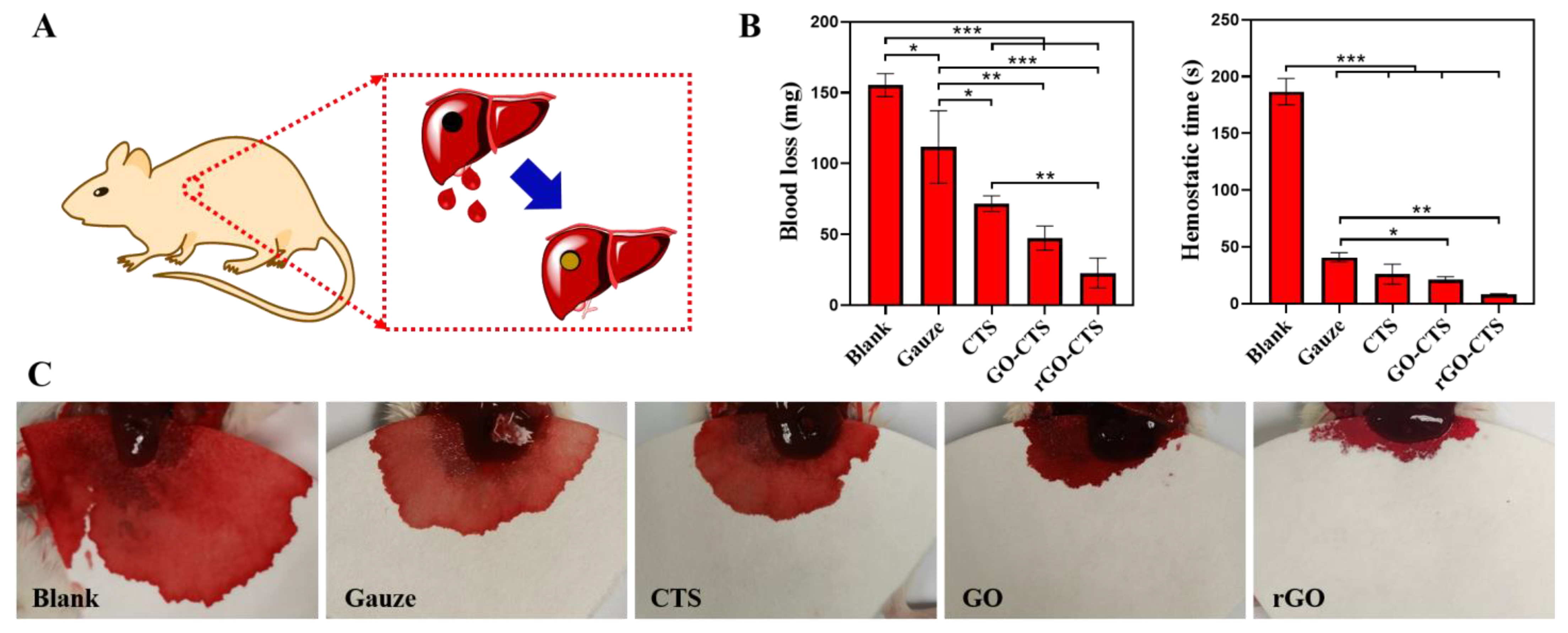
2.6. In Vivo Hemostatic Capacity of the rGO-CTS Cryogel
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Shape-Memory rGO-CTS Cryogels
3.3. Characterization
3.4. Shape-Memory Behavior
3.5. Hemolytic Activity Assay
3.6. Coagulation Test
3.7. Antibacterial Activity
3.8. Cell Cytotoxicity
3.9. In Vivo Hemostatic Performance
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Cheng, F.; Wei, X.; Yi, X.; Tang, S.; Wang, Z.; Zhang, Y.S.; He, J.; Huang, Y. Injectable, self-healing, antibacterial, and hemostatic N,O-carboxymethyl chitosan/oxidized chondroitin sulfate composite hydrogel for wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Qiao, L.; Zou, F.; Xie, Y.; Zheng, Y.; Chao, Y.; Yang, Y.; He, W.; Yang, S. Cellulose fibers-reinforced self-expanding porous composite with multiple hemostatic efficacy and shape adaptability for uncontrollable massive hemorrhage treatment. Bioact. Mater. 2021, 6, 2089–2104. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Hou, W.; Liu, K.; Yang, H.; Wei, W.; Kang, H.; Dai, H. A multifunctional chitosan hydrogel dressing for liver hemostasis and infected wound healing. Carbohydr. Polym. 2022, 291, 119631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, H.; Chen, L.; Hong, F.F. A Biodegradable Antibacterial nanocomposite based on oxidized bacterial nanocellulose for rapid hemostasis and wound healing. ACS Appl. Mater. Inter. 2020, 12, 3382–3392. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Liang, Y.; He, J.; Guo, B. Multifunctional tissue-adhesive cryogel wound dressing for rapid nonpressing surface hemorrhage and wound repair. ACS Appl. Mater. Interfaces 2020, 12, 35856–35872. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Gao, H.; Lin, Z. A shape memory and antibacterial cryogel with rapid hemostasis for noncompressible hemorrhage and wound healing. Chem. Eng. J. 2022, 428, 131005. [Google Scholar] [CrossRef]
- Moradi, S.; Hamedi, H.; Tonelli, A.E.; King, M.W. Chitosan/graphene oxide composite films and their biomedical and drug delivery applications: A review. Appl. Sci. 2021, 11, 7776. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Napiwocki, B.N.; Peng, X.F.; Turng, L.S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Li, B.; Feng, B.; He, X.; Fu, W.; Yuan, H.; Xu, Z. Enhanced chondrogenic differentiation of human mesenchymal stems cells on citric acid-modified chitosan hydrogel for tracheal cartilage regeneration applications. RSC Adv. 2018, 8, 16910–16917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, C.; Ni, Z.; Yang, C.; Ni, Y.; Lv, Y. Gelatin-oxidized nanocellulose hydrogels suitable for extrusion-based 3D bioprinting. Processes 2022, 10, 2216. [Google Scholar] [CrossRef]
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on production methods, structure, properties and performance relationship. J. Membr. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Fang, Y.; Xu, Y.; Wang, Z.; Zhou, W.; Yan, L.; Fan, X.; Liu, H. 3D porous chitin sponge with high absorbency, rapid shape recovery, and excellent antibacterial activities for noncompressible wound. Chem. Eng. J. 2020, 388, 124169. [Google Scholar] [CrossRef]
- Nath, J.; Chowdhury, A.; Dolui, S.K. Chitosan/graphene oxide-based multifunctional pH-responsive hydrogel with significant mechanical strength, self-healing property, and shape memory effect. Adv. Polym. Technol. 2018, 37, 3665–3679. [Google Scholar] [CrossRef] [Green Version]
- Ying, G.; Jiang, N.; Parra, C.; Tang, G.; Zhang, J.; Wang, H.; Chen, S.; Huang, N.P.; Xie, J.; Zhang, Y.S. Bioprinted injectable hierarchically porous gelatin methacryloyl hydrogel constructs with shape-memory properties. Adv. Funct. Mater. 2020, 30, 2003740. [Google Scholar] [CrossRef] [PubMed]
- Keast, D.H.; Janmohammad, A. The hemostatic and wound healing effect of chitosan following debridement of chronic ulcers. Wounds 2021, 33, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, M.; Zheng, X.; Li, W.; Ren, X. Chitosan/mesoporous silica hybrid aerogel with bactericidal properties as hemostatic material. Eur. Polym. J. 2021, 142, 110132. [Google Scholar] [CrossRef]
- ASTM F756-17; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Khan, M.U.A.; Haider, S.; Raza, M.A.; Shah, S.A.; Razak, S.I.A.; Kadir, M.R.A.; Subhan, F.; Haider, A. Smart and pH-sensitive rGO/Arabinoxylan/chitosan composite for wound dressing: In-vitro drug delivery, antibacterial activity, and biological activities. Int. J. Biol. Macromol. 2021, 192, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, X.; Wang, C.; Chen, J.; Guo, B. High-strength anti-bacterial composite cryogel for lethal noncompressible hemorrhage hemostasis:synergistic physical hemostasis and chemical hemostasis. Chem. Eng. J. 2021, 427, 131977. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, H.; Du, Q.; Li, R.; Shen, X.; Zhou, J.; Li, B.; Jin, Y.; Yuan, H. Shape-Memory-Reduced Graphene/Chitosan Cryogels for Non-Compressible Wounds. Int. J. Mol. Sci. 2023, 24, 1389. https://doi.org/10.3390/ijms24021389
Xuan H, Du Q, Li R, Shen X, Zhou J, Li B, Jin Y, Yuan H. Shape-Memory-Reduced Graphene/Chitosan Cryogels for Non-Compressible Wounds. International Journal of Molecular Sciences. 2023; 24(2):1389. https://doi.org/10.3390/ijms24021389
Chicago/Turabian StyleXuan, Hongyun, Qian Du, Ruimeng Li, Xiaoni Shen, Jiao Zhou, Biyun Li, Yan Jin, and Huihua Yuan. 2023. "Shape-Memory-Reduced Graphene/Chitosan Cryogels for Non-Compressible Wounds" International Journal of Molecular Sciences 24, no. 2: 1389. https://doi.org/10.3390/ijms24021389
APA StyleXuan, H., Du, Q., Li, R., Shen, X., Zhou, J., Li, B., Jin, Y., & Yuan, H. (2023). Shape-Memory-Reduced Graphene/Chitosan Cryogels for Non-Compressible Wounds. International Journal of Molecular Sciences, 24(2), 1389. https://doi.org/10.3390/ijms24021389





