Concentration of Polycyclic Aromatic Hydrocarbons (PAHs) in Human Serum and Adipose Tissues and Stimulatory Effect of Naphthalene in Adipogenesis in 3T3-L1 Cells
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Bariatric Patients
2.2. PAHs Concentrations in Blood Plasma and AT
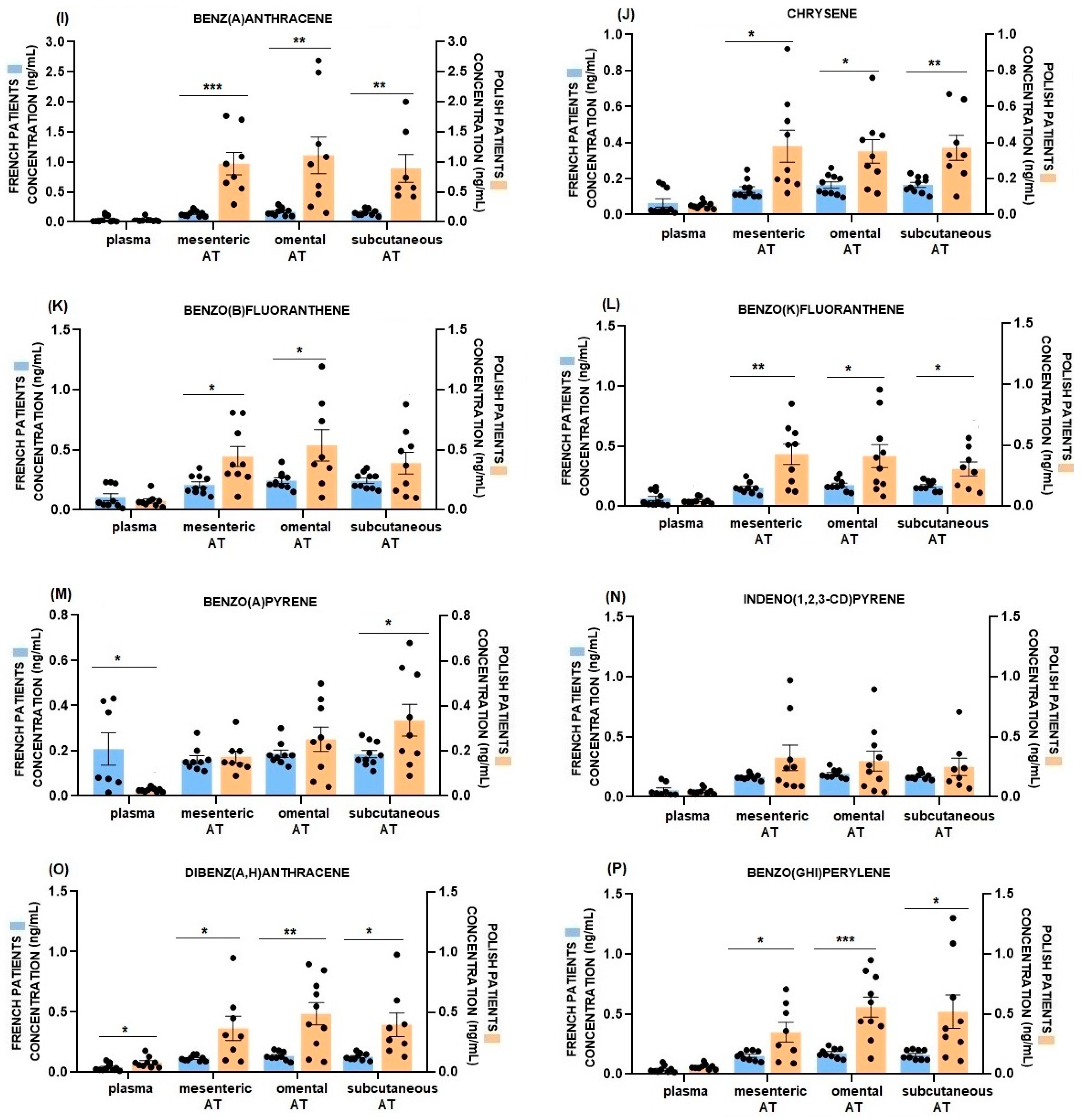
2.3. Comparison of Plasma and AT PAHs Concentrations between French and Polish Patients
2.4. Correlation between PAHs Concentration in ATs or Blood Plasma and BMI and Adipokines Level in French and Polish Patients
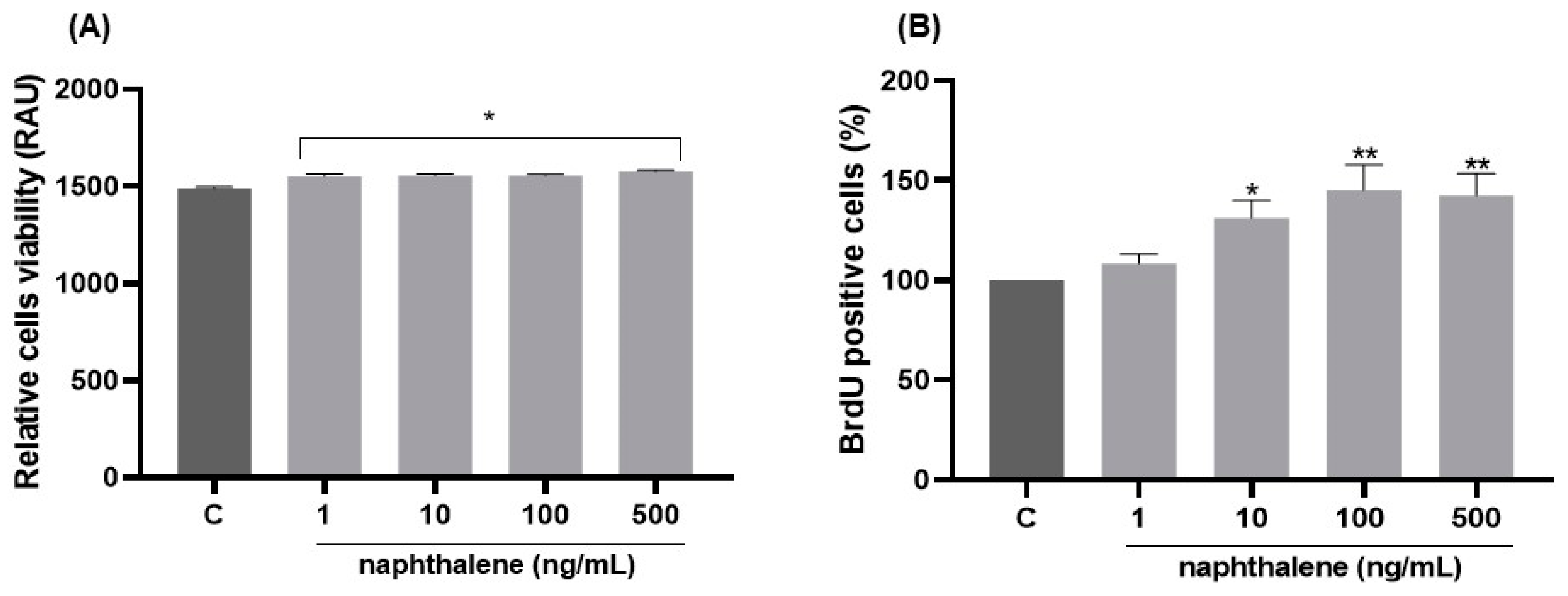
2.5. Dose-Dependent Effects of Naphthalene on 3T3-L1 Cell Viability and Proliferation
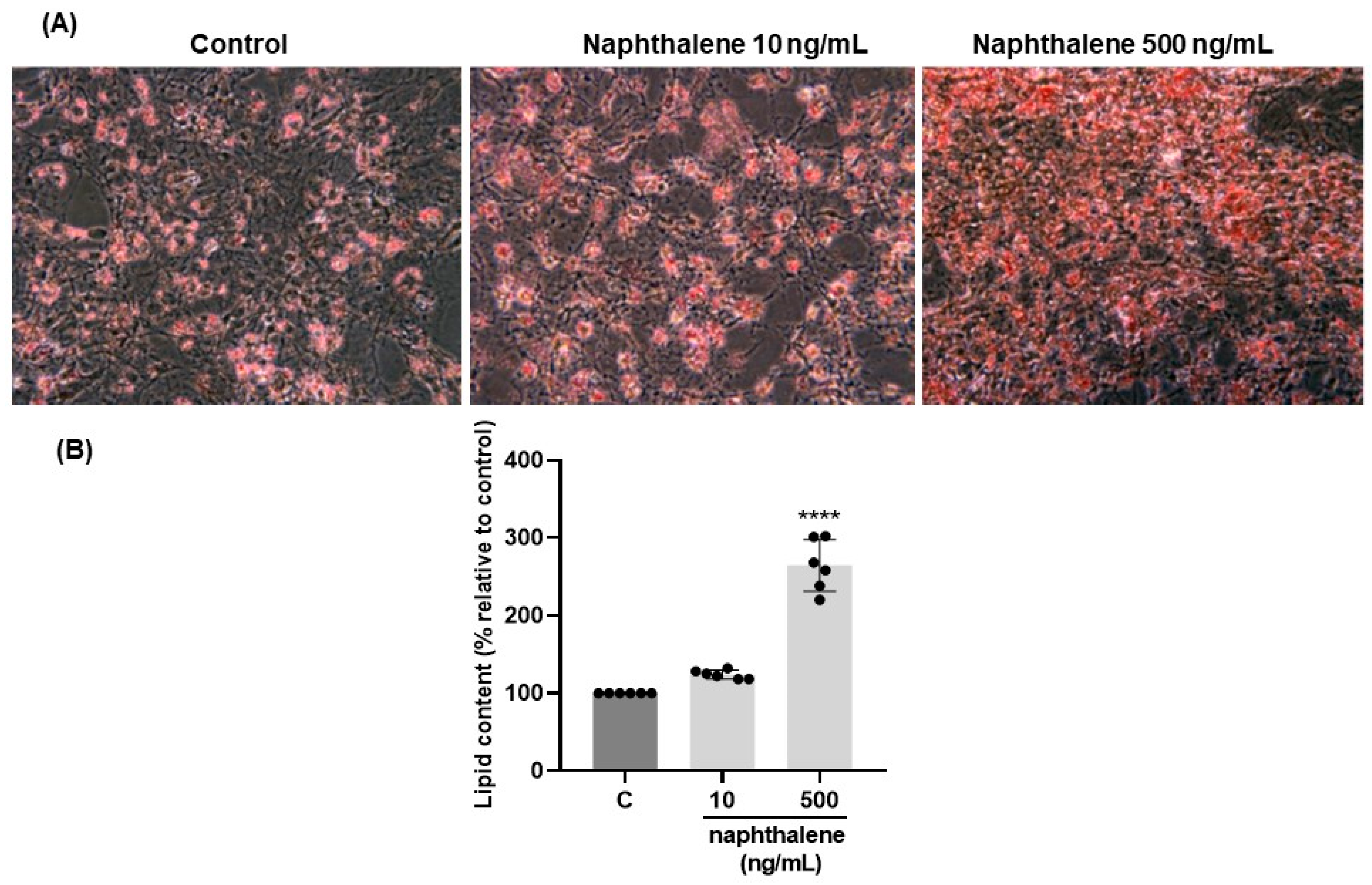
2.6. Effects of Naphthalene on 3T3-L1 Cell Differentiation
3. Discussion
3.1. Accumulation of PAHs in Human Adipose Tissues and Difference between French and Polish Patients
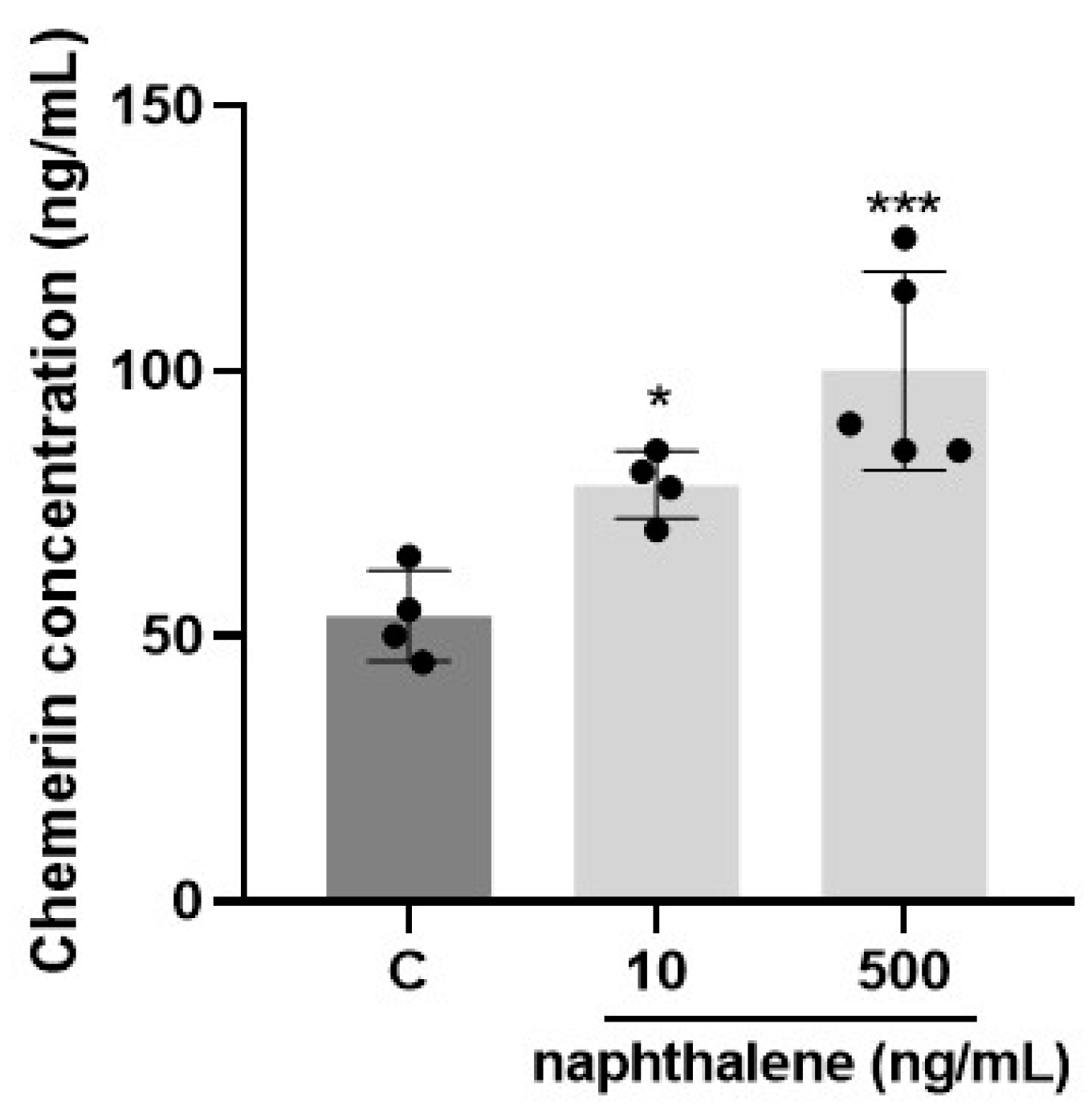
3.2. Naphthalene Is One of the Main PAHs Present in Human Blood Plasma and ATs and in French Patients Its Adipose Concentration Is Positively Correlated with BMI, Plasma Chemerin and Omental RARRES2 Gene Expression
3.3. Role of Naphthalene in Adipogenesis/Lipogenesis in 3T3-L1 Cells
3.4. Limitations of Study
- Due to the small number of enrolled French and Polish patients this should be considered a pilot study.
- The Polish patients studied had a higher BMI than French. It is impossible to exclude the influence of this BMI difference on the parameters studied.
- The absence of adipokine expression data in the Polish patients (due to insufficient ATs collection) made it impossible to compare these data with French patients.
- Finally, it will be interesting to measure PAHs metabolites since they could be used as biomarkers of recent exposure to these compounds [10].
4. Materials and Methods
4.1. Study Group and Ethics Approval
4.2. Human Samples Collection
4.3. PAHs Measurements in Blood Plasma and Adipose Tissues
4.4. Adipokines Concentration in Human Blood Plasma
4.5. The Culture and Treatment of 3T3-L1 Preadipocytes
4.6. Preadipocytes 3T3-L1 Cells Viability
4.7. Preadipocytes 3T3-L1 Cells Proliferation
4.8. Oil Red O Staining
4.9. Real-Time Fluorescence Quantitative PCR Analysis
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, H.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.; Wang, B.; Zhang, Y.; Chen, Y.; Lu, Y.; et al. Global Atmospheric Emissions of Polycyclic Aromatic Hydrocarbons from 1960 to 2008 and Future Predictions. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dat, N.-D.; Chang, M.B. Review on Characteristics of PAHs in Atmosphere, Anthropogenic Sources and Control Technologies. Sci. Total Environ. 2017, 609, 682–693. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Huo, R.; Shi, Z.; Sun, Y.; Feng, Y.; Harrison, R.M. Organic Compound Source Profiles of PM2.5 from Traffic Emissions, Coal Combustion, Industrial Processes and Dust. Chemosphere 2021, 278, 130429. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.H. The Source of U.S. EPA’s Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Vangrieken, R. Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef] [Green Version]
- Khairy, M.A.; Lohmann, R. Source Apportionment and Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Atmospheric Environment of Alexandria, Egypt. Chemosphere 2013, 91, 895–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, M.S.; Zhu, J.; Foster, W.G. Quantification of Benzo[a]Pyrene and Other PAHs in the Serum and Follicular Fluid of Smokers versus Non-Smokers. Reprod. Toxicol. 2008, 25, 100–106. [Google Scholar] [CrossRef]
- Singh, V.K.; Patel, D.K.; Ram, S.; Mathur, N.; Siddiqui, M.K.J.; Behari, J.R. Blood Levels of Polycyclic Aromatic Hydrocarbons in Children of Lucknow, India. Arch. Environ. Contam. Toxicol. 2008, 54, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Kumar, A.; Aramandla, M.; Nyanda, A. Polycyclic Aromatic Hydrocarbon Residues in Serum Samples of Autopsied Individuals from Tennessee. Int. J. Environ. Res. Public Health 2014, 12, 322–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickland, P.; Kang, D.; Sithisarankul, P. Polycyclic Aromatic Hydrocarbon Metabolites in Urine as Biomarkers of Exposure and Effect. Environ. Health Perspect. 1996, 104, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Poursafa, P.; Dadvand, P.; Amin, M.M.; Hajizadeh, Y.; Ebrahimpour, K.; Mansourian, M.; Pourzamani, H.; Sunyer, J.; Kelishadi, R. Association of Polycyclic Aromatic Hydrocarbons with Cardiometabolic Risk Factors and Obesity in Children. Environ. Int. 2018, 118, 203–210. [Google Scholar] [CrossRef]
- Ratelle, M.; Khoury, C.; Adlard, B.; Laird, B. Polycyclic Aromatic Hydrocarbons (PAHs) Levels in Urine Samples Collected in a Subarctic Region of the Northwest Territories, Canada. Environ. Res. 2020, 182, 109112. [Google Scholar] [CrossRef]
- Bushnik, T.; Wong, S.L.; Holloway, A.C.; Thomson, E.M. Association of Urinary Polycyclic Aromatic Hydrocarbons and Obesity in Children Aged 3–18: Canadian Health Measures Survey 2009–2015. J. Dev. Orig. Health Dis. 2020, 11, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Gaspari, L.; Chang, S.-S.; Santella, R.M.; Garte, S.; Pedotti, P.; Taioli, E. Polycyclic Aromatic Hydrocarbon-DNA Adducts in Human Sperm as a Marker of DNA Damage and Infertility. Mutat. Res. Toxicol. Environ. Mutagen. 2003, 535, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Belda, M.; Campillo, N.; Arroyo-Manzanares, N.; Torres, C.; Pérez-Cárceles, M.D.; Hernández-Córdoba, M.; Viñas, P. Bioaccumulation of Polycyclic Aromatic Hydrocarbons for Forensic Assessment Using Gas Chromatography-Mass Spectrometry. Chem. Res. Toxicol. 2019, 32, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic Polycyclic Aromatic Hydrocarbon-DNA Adducts and Mechanism of Action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, S.; Wang, H.; Tao, S.; Kiyama, R. Biological Impact of Environmental Polycyclic Aromatic Hydrocarbons (EPAHs) as Endocrine Disruptors. Environ. Pollut. 2016, 213, 809–824. [Google Scholar] [CrossRef]
- Vondráček, J.; Pivnička, J.; Machala, M. Polycyclic Aromatic Hydrocarbons and Disruption of Steroid Signaling. Curr. Opin. Toxicol. 2018, 11–12, 27–34. [Google Scholar] [CrossRef]
- Marris, C.R.; Kompella, S.N.; Miller, M.R.; Incardona, J.P.; Brette, F.; Hancox, J.C.; Sørhus, E.; Shiels, H.A. Polyaromatic Hydrocarbons in Pollution: A Heart-breaking Matter. J. Physiol. 2020, 598, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hou, J.; Zhou, Y.; Sun, H.; Yin, W.; Zhang, Y.; Wang, X.; Wang, G.; Chen, W.; Yuan, J. Association of Polycyclic Aromatic Hydrocarbons Exposure with Atherosclerotic Cardiovascular Disease Risk: A Role of Mean Platelet Volume or Club Cell Secretory Protein. Environ. Pollut. 2018, 233, 45–53. [Google Scholar] [CrossRef]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic Aromatic Hydrocarbon and Its Effects on Human Health: An Overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef]
- Bussmann, U.A.; Bussmann, L.E.; Barañao, J.L. An Aryl Hydrocarbon Receptor Agonist Amplifies the Mitogenic Actions of Estradiol in Granulosa Cells: Evidence of Involvement of the Cognate Receptors1. Biol. Reprod. 2006, 74, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Drwal, E.; Rak, A.; Grochowalski, A.; Milewicz, T.; Gregoraszczuk, E.L. Cell-Specific and Dose-Dependent Effects of PAHs on Proliferation, Cell Cycle, and Apoptosis Protein Expression and Hormone Secretion by Placental Cell Lines. Toxicol. Lett. 2017, 280, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bolden, A.L.; Rochester, J.R.; Schultz, K.; Kwiatkowski, C.F. Polycyclic Aromatic Hydrocarbons and Female Reproductive Health: A Scoping Review. Reprod. Toxicol. 2017, 73, 61–74. [Google Scholar] [CrossRef]
- Luderer, U.; Christensen, F.; Johnson, W.O.; She, J.; Ip, H.S.S.; Zhou, J.; Alvaran, J.; Krieg, E.F.; Kesner, J.S. Associations between Urinary Biomarkers of Polycyclic Aromatic Hydrocarbon Exposure and Reproductive Function during Menstrual Cycles in Women. Environ. Int. 2017, 100, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, M.; Rotondi, M.A.; Ardern, C.I.; Kuk, J.L. Urinary Biomarkers of Polycyclic Aromatic Hydrocarbons Are Associated with Cardiometabolic Health Risk. PLoS ONE 2015, 10, e0137536. [Google Scholar] [CrossRef]
- Hendryx, M.; Luo, J. Latent Class Analysis of the Association between Polycyclic Aromatic Hydrocarbon Exposures and Body Mass Index. Environ. Int. 2018, 121, 227–231. [Google Scholar] [CrossRef]
- Lee, I.; Park, H.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Cheon, G.J.; et al. Exposure to Polycyclic Aromatic Hydrocarbons and Volatile Organic Compounds Is Associated with a Risk of Obesity and Diabetes Mellitus among Korean Adults: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Int. J. Hyg. Environ. Health 2022, 240, 113886. [Google Scholar] [CrossRef] [PubMed]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western Diet: How We Got Here. Mo. Med. 2020, 117, 536–538. [Google Scholar]
- Lindstrom, M.B.; Barrowman, J.A.; Borgstrom, B. Fate of 7,12-Dimethylbenz(a)Anthracene Absorbed from the Rat Intestine and Transported in Chylomicrons. Lipids 1987, 22, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Hua, Q.; Liu, Y.; Wu, Z.; Xu, D.; Ren, Q.; Zhao, W.; Guo, X. Organ and Tissue-Specific Distribution of Selected Polycyclic Aromatic Hydrocarbons (PAHs) in ApoE-KO Mouse. Environ. Pollut. 2021, 286, 117219. [Google Scholar] [CrossRef] [PubMed]
- Ciganek, M.; Neca, J. Polycyclic Aromatic Hydrocarbons in Porcine and Bovine Organs and Tissues. Veter. Med. 2012, 51, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Guerranti, C.C.F.; Perra, G.; Moroni, A.; Moroni, A.; Focardi, S.; Sorbellini, F.; Azzara, A.; Cisternino, F.; Cisternino, F.; Malagnino, G.; et al. Polycyclic Aromatic Hydrocarbon (PAH) Levels in Various Food Components and in Human Adipose Tissue. A Comparison with PAH Values in the Atmosphere. GIMT-G. Ital. Delle Mal. Del. Torace 2009, 63, 397–404. [Google Scholar]
- Moon, H.-B.; Lee, D.-H.; Lee, Y.S.; Kannan, K. Occurrence and Accumulation Patterns of Polycyclic Aromatic Hydrocarbons and Synthetic Musk Compounds in Adipose Tissues of Korean Females. Chemosphere 2012, 86, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Asimakopoulos, A.G.; Kannan, K. Accumulation of 19 Environmental Phenolic and Xenobiotic Heterocyclic Aromatic Compounds in Human Adipose Tissue. Environ. Int. 2015, 78, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, Y.S.; Lee, D.H.; Kim, D.S. Polycyclic Aromatic Hydrocarbons Are Associated with Insulin Receptor Substrate 2 Methylation in Adipose Tissues of Korean Women. Environ. Res. 2016, 150, 47–51. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. AMS 2013, 9, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Luo, N.; Klein, R.L.; Garvey, W.T. Adiponectin Promotes Adipocyte Differentiation, Insulin Sensitivity, and Lipid Accumulation. J. Lipid Res. 2005, 46, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Zhang, N.; Song, L.; Xie, H.; Zhao, C.; Li, S.; Zhao, W.; Zhao, Y.; Gao, C.; Xu, G. Adipokines and Free Fatty Acids Regulate Insulin Sensitivity by Increasing MicroRNA-21 Expression in Human Mature Adipocytes. Mol. Med. Rep. 2017, 16, 2254–2258. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Takahashi, Y.; Takahashi, K.; Zolotaryov, F.N.; Hong, K.S.; Kitazawa, R.; Iida, K.; Okimura, Y.; Kaji, H.; Kitazawa, S.; et al. Chemerin Enhances Insulin Signaling and Potentiates Insulin-Stimulated Glucose Uptake in 3T3-L1 Adipocytes. FEBS Lett. 2008, 582, 573–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traczyk, P.; Gruszecka-Kosowska, A. The Condition of Air Pollution in Kraków, Poland, in 2005–2020, with Health Risk Assessment. Int. J. Environ. Res. Public Health 2020, 17, 6063. [Google Scholar] [CrossRef]
- Kobza, J.; Geremek, M.; Dul, L. Characteristics of Air Quality and Sources Affecting High Levels of PM10 and PM2.5 in Poland, Upper Silesia Urban Area. Environ. Monit. Assess. 2018, 190, 515. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile And Cut Off Points. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Merrill, M.; Emond, C.; Kim, M.J.; Antignac, J.-P.; Le Bizec, B.; Clément, K.; Birnbaum, L.S.; Barouki, R. Toxicological Function of Adipose Tissue: Focus on Persistent Organic Pollutants. Environ. Health Perspect. 2013, 121, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourez, S.; Le Lay, S.; Van den Daelen, C.; Louis, C.; Larondelle, Y.; Thomé, J.-P.; Schneider, Y.-J.; Dugail, I.; Debier, C. Accumulation of Polychlorinated Biphenyls in Adipocytes: Selective Targeting to Lipid Droplets and Role of Caveolin-1. PLoS ONE 2012, 7, e31834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-J.; Marchand, P.; Henegar, C.; Antignac, J.-P.; Alili, R.; Poitou, C.; Bouillot, J.-L.; Basdevant, A.; Le Bizec, B.; Barouki, R.; et al. Fate and Complex Pathogenic Effects of Dioxins and Polychlorinated Biphenyls in Obese Subjects before and after Drastic Weight Loss. Environ. Health Perspect. 2011, 119, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Hruska, P.; Kucera, J.; Pekar, M.; Holéczy, P.; Mazur, M.; Buzga, M.; Kuruczova, D.; Lenart, P.; Fialova Kucerova, J.; Potesil, D.; et al. Proteomic Signatures of Human Visceral and Subcutaneous Adipocytes. J. Clin. Endocrinol. Metab. 2022, 107, 755–775. [Google Scholar] [CrossRef]
- Scinicariello, F.; Buser, M.C. Urinary Polycyclic Aromatic Hydrocarbons and Childhood Obesity: NHANES (2001-2006). Environ. Health Perspect. 2014, 122, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Irigaray, P.; Ogier, V.; Jacquenet, S.; Notet, V.; Sibille, P.; Méjean, L.; Bihain, B.E.; Yen, F.T. Benzo[a]Pyrene Impairs Beta-Adrenergic Stimulation of Adipose Tissue Lipolysis and Causes Weight Gain in Mice. A Novel Molecular Mechanism of Toxicity for a Common Food Pollutant. FEBS J. 2006, 273, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, P.; Konstandi, M.; Pappas, P.; Marselos, M. Alterations in Central Monoaminergic Neurotrasmission Induced by Polycyclic Aromatic Hydrocarbons in Rats. Eur. J. Drug Metab. Pharmacokinet. 1998, 23, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Irigaray, P.; Belpomme, D. Basic Properties and Molecular Mechanisms of Exogenous Chemical Carcinogens. Carcinogenesis 2010, 31, 135–148. [Google Scholar] [CrossRef] [Green Version]
- de Souza Batista, C.M.; Yang, R.-Z.; Lee, M.-J.; Glynn, N.M.; Yu, D.-Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin Plasma Levels and Gene Expression Are Decreased in Obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Alkaabi, J.; Yasin, J.; Al Essa, A. Total Adiponectin in Overweight and Obese Subjects and Its Response to Visceral Fat Loss. BMC Endocr. Disord. 2019, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a Novel Adipokine That Regulates Adipogenesis and Adipocyte Metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, S.; Song, S.-H.; Choi, K.-C.; Katoh, K.; Wittamer, V.; Parmentier, M.; Sasaki, S. Chemerin—A New Adipokine That Modulates Adipogenesis via Its Own Receptor. Biochem. Biophys. Res. Commun. 2007, 362, 1013–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körner, A.; Wabitsch, M.; Seidel, B.; Fischer-Posovszky, P.; Berthold, A.; Stumvoll, M.; Blüher, M.; Kratzsch, J.; Kiess, W. Adiponectin Expression in Humans Is Dependent on Differentiation of Adipocytes and Down-Regulated by Humoral Serum Components of High Molecular Weight. Biochem. Biophys. Res. Commun. 2005, 337, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The Hormone Resistin Links Obesity to Diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Audano, M.; Pedretti, S.; Caruso, D.; Crestani, M.; De Fabiani, E.; Mitro, N. Regulatory Mechanisms of the Early Phase of White Adipocyte Differentiation: An Overview. Cell Mol. Life Sci. CMLS 2022, 79, 139. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARγ and the Global Map of Adipogenesis and Beyond. Trends Endocrinol. Metab. TEM 2014, 25, 293–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Cruz, M.M.; Lopes, A.B.; Crisma, A.R.; de Sá, R.C.C.; Kuwabara, W.M.T.; Curi, R.; de Andrade, P.B.M.; Alonso-Vale, M.I.C. Palmitoleic acid (16, 1n7) increases oxygen consumption, fatty acid oxidation and ATP content in white adipocytes. Lipids Health Dis. 2018, 17, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estienne, A.; Brossaud, A.; Rame, C.; Bernardi, O.; Reverchon, M.; Rat, C.; Delaveau, J.; Chambellon, E.; Helloin, E.; Froment, P.; et al. Chemerin is secreted by the chicken oviduct, accumulates in egg albumen and could promote embryo development. Sci. Rep. 2022, 12, 8989. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]

| French Patients (n = 10) | Polish Patients (n = 10) | p Value | |
|---|---|---|---|
| Age (years) | 45 ± 4.6 | 37 ± 2 | p = 0.065 |
| BMI (kg/m2) | 40.2 ± 1.7 | 49 ± 2.5 | p = 0.011 |
| plasma glucose (mmol/L) | 4.93 ± 0.20 | 5.37 ± 0.26 | p = 0.193 |
| plasma insulin (pmol/L) | 70.9 ± 6.63 | 73.90 ± 4.42 | p = 0.711 |
| plasma chemerin (ng/mL) | 220 ± 22.81 | 235.65 ± 34.66 | p = 0.710 |
| plasma adiponectin (µg/mL) | 4.98 ± 0.38 | 4.15 ± 0.51 | p = 0.208 |
| plasma visfatin (ng/mL) | 7.79 ± 1.05 | 7.55 ± 0.40 | p = 0.833 |
| plasma resistin (ng/mL) | 10.11 ± 1.13 | 5.78 ± 0.47 | p = 0.002 |
| Plasma | Mesenteric AT | Omental AT | Subcutaneous AT | p Value | |
|---|---|---|---|---|---|
| naphthalene | 0.52 ± 0.09 b,A | 14.76 ± 0.96 c,B | 15.10 ± 1.14 c,B | 15.60 ± 1.05 c,B | p < 0.0001 |
| acenaphthylene | 0.04 ± 0.01 a,A | 0.33 ± 0.04 a,B | 0.34 ± 0.03 a,B | 0.34 ± 0.04 a,B | p < 0.0001 |
| acenaphthene | 0.10 ± 0.03 a,A | 0.49 ± 0.05 a,B | 0.57 ± 0.07 a,B | 0.58 ± 0.07 a,B | p < 0.0001 |
| fluorene | 0.28 ± 0.05 a,A | 0.81 ± 0.12 a,B | 0.91 ± 0.15 a,B | 0.92 ± 0.16 a,B | p = 0.0032 |
| phenanthrene | 1.36 ± 0.07 d,A | 3.05 ± 0.28 b,B | 2.95 ± 0.36 b,B | 4.43 ± 0.25 b,C | p < 0.0001 |
| anthracene | 0.15 ± 0.03 a,A | 0.38 ± 0.09 a,A | 0.35 ± 0.06 a,A | 0.35 ± 0.07 a,A | p = 0.0689 |
| fluoranthene | 0.90 ± 0.06 c,A | 0.61 ± 0.06 a,A | 0.71 ± 0.09 a,A | 0.76 ± 0.09 a,A | p = 0.1366 |
| pyrene | 0.91 ± 0.04 c,B | 0.40 ± 0.05 a,A | 0.42 ± 0.05 a,A | 0.42 ± 0.04 a,A | p < 0.0001 |
| benz(a)anthracene | 0.05 ± 0.02 a,A | 0.14 ± 0.02 a,B | 0.17 ± 0.02 a,B | 0.16 ± 0.02 a,B | p < 0.0001 |
| chrysene | 0.06 ± 0.02 a,A | 0.14 ± 0.02 a,B | 0.16 ± 0.02 a,B | 0.17 ± 0.01 a,B | p = 0.0007 |
| benzo(b)fluoranthene | 0.11 ± 0.03 a,A | 0.21 ± 0.02 a,B | 0.24 ± 0.02 a,B | 0.24 ± 0.02 a,B | p = 0.0012 |
| benzo(k)fluoranthene | 0.06 ± 0.02 a,A | 0.15 ± 0.02 a,B | 0.18 ± 0.02 a,B | 0.17 ± 0.01 a,B | p < 0.0001 |
| benzo(a)pyrene | 0.21 ± 0.06 a,A | 0.16 ± 0.02 a,A | 0.19 ± 0.02 a,A | 0.19 ± 0.02 a,A | p = 0.8091 |
| indeno(1,2,3-cd)pyrene | 0.06 ± 0.02 a,A | 0.16 ± 0.01 a,B | 0.19 ± 0.01 a,B | 0.17 ± 0.01 a,B | p < 0.0001 |
| dibenz(a,h)anthracene | 0.04 ± 0.01 a,A | 0.11 ± 0.01 a,B | 0.14 ± 0.01 a,B | 0.13 ± 0.01 a,B | p < 0.0001 |
| benzo(g,h,i)perylene | 0.04 ± 0.01 a,A | 0.15 ± 0.01 a,B | 0.17 ±0.01 a,B | 0.16 ± 0.01 a,B | p < 0.0001 |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Plasma | Mesenteric AT | Omental AT | Subcutaneous AT | p Value | |
|---|---|---|---|---|---|
| naphthalene | 1.07 ± 0.18 c,A | 44.19 ± 10.28 d,B | 31.56 ± 4.66 d,B | 50.02 ± 16.75 e,B | p = 0.0101 |
| acenaphthylene | 0.07 ± 0.02 a,A | 3.90 ± 0.31 ab,B | 4.38 ± 0.72 b,B | 4.31 ± 0.70 b,B | p < 0.0001 |
| acenaphthene | 0.24 ± 0.08 a,A | 7.47 ± 0.63 b,B | 7.29 ± 1.16 bc,B | 10.25 ± 1.51 bc,B | p < 0.0001 |
| fluorene | 0.22 ± 0.04 a,A | 19.49 ± 5.05 c,B | 10.68 ± 1.74 c,B | 16.13 ± 3.64 c,B | p = 0.0007 |
| phenanthrene | 1.26 ± 0.22 c,A | 109.94 ± 14.37 e,B | 93.99 ± 16.31 e,B | 139.43 ± 31.47 f,B | p < 0.0001 |
| anthracene | 0.11 ± 0.02 a,A | 29.69 ± 4.89 c,B | 24.97 ± 5.39 d,B | 42.00 ± 4.38 e,B | p < 0.0001 |
| fluoranthene | 0.78 ± 0.13 b,A | 27.52 ± 3.40 c,B | 25.63 ± 4.80 d,B | 49.44 ± 10.31 e,B | p < 0.0001 |
| pyrene | 0.63 ± 0.11 b,A | 20.08 ± 2.74 c,BC | 19.39 ± 4.20 d,B | 28.43 ± 4.63 d,C | p < 0.0001 |
| benz(a)anthracene | 0.03 ± 0.01 a,A | 0.97 ± 0.18 a,B | 1.11 ± 0.29 ab,B | 0.89 ± 0.19 a,B | p = 0.0022 |
| chrysene | 0.05 ± 0.01 a,A | 0.38 ± 0.09 a,B | 0.35 ± 0.06 a,B | 0.37 ± 0.06 a,B | p = 0.0023 |
| benzo(b)fluoranthene | 0.08 ± 0.02 a,A | 0.44 ± 0.08 a,B | 0.54 ± 0.12 a,B | 0.39 ± 0.09 a,AB | p = 0.0044 |
| benzo(k)fluoranthene | 0.05 ± 0.01 a,A | 0.43 ± 0.08 a,B | 0.41 ± 0.09 a,B | 0.31 ± 0.05 a,AB | p = 0.0022 |
| benzo(a)pyrene | 0.03 ± 0.003 a,A | 0.17 ± 0.01 a,AB | 0.25 ± 0.05 a,B | 0.34 ± 0.07 a,B | p = 0.0004 |
| indeno(1,2,3-cd)pyrene | 0.05 ± 0.01 a,A | 0.33 ± 0.11 a,A | 0.30 ± 0.08 a,A | 0.25 ± 0.07 a,A | p = 0.0662 |
| dibenz(a,h)anthracene | 0.08 ± 0.01 a,A | 0.37 ± 0.09 a,AB | 0.49 ± 0.09 a,B | 0.40 ± 0.09 a,AB | p = 0.0091 |
| benzo(g,h,i)perylene | 0.06 ± 0.01 a,A | 0.35 ± 0.08 a,AB | 0.56 ± 0.08 a,B | 0.52 ± 0.13 a,B | p = 0.0019 |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
| Patients | Naphthalene | Phenanthrene | Fluoranthene | Pyrene | |
|---|---|---|---|---|---|
| Plasma | French | r = −0.303 p = 0.395 | r = 0.293 p = 0.411 | r = 0.273 p = 0.445 | r = 0.254 p = 0.479 |
| Polish | r = 0.613 p = 0.106 | r = 0.504 p = 0.202 | r = 0.442 p = 0.273 | r = 0.389 p = 0.341 | |
| Mesenteric AT | French | r = 0.743 p = 0.014 | r = −0.141 p = 0.698 | r = −0.418 p = 0.229 | r = −0.366 p = 0.298 |
| Polish | r = 0.299 p = 0.515 | r = −0.664 p = 0.104 | r = −0.701 p = 0.079 | r = −0.675 p = 0.096 | |
| Omental AT | French | r = 0.904 p = 0.0003 | r = −0.198 p = 0.583 | r = −0.368 p = 0.295 | r = −0.425 p = 0.221 |
| Polish | r = 0.508 p = 0.199 | r = −0.426 p = 0.292 | r = −0.379 p = 0.354 | r = −0.362 p = 0.378 | |
| Subcutaneous AT | French | r = 0.906 p = 0.0003 | r = −0.228 p = 0.527 | r = −0.285 p = 0.425 | r = −0.393 p = 0.262 |
| Polish | r = 0.355 p = 0.489 | r = −0.241 p = 0.602 | r = −0.383 p = 0.396 | r = −0.314 p = 0.493 |
| Patients | Naphthalene | Phenanthrene | Fluoranthene | Pyrene | |
|---|---|---|---|---|---|
| Chemerin | French | r = 0.979 p < 0.0001 | r = −0.313 p = 0.379 | r = −0.435 p = 0.209 | r = −0.309 p = 0.385 |
| Polish | r = 0.086 p = 0.812 | r = 0.510 p = 0.132 | r = 0.427 p = 0.218 | r = 0.352 p = 0.319 | |
| Adiponectin | French | r = −0.938 p < 0.0001 | r = 0.277 p = 0.439 | r = 0.424 p = 0.222 | r = 0.461 p = 0.180 |
| Polish | r = −0.078 p = 0.831 | r = −0.490 p = 0.150 | r = −0.436 p = 0.208 | r = −0.424 p = 0.222 | |
| Visfatin | French | r = 0.174 p = 0.630 | r = 0.171 p = 0.636 | r = 0.042 p = 0.908 | r = −0.118 p = 0.745 |
| Polish | r = 0.186 p = 0.608 | r = −0.363 p = 0.302 | r = −0.406 p = 0.244 | r = −0.422 p = 0.224 | |
| Resistin | French | r = 0.838 p = 0.002 | r = −0.158 p = 0.663 | r = −0.317 p = 0.372 | r = −0.408 p = 0.241 |
| Polish | r = 0.098 p = 0.787 | r = 0.327 p = 0.356 | r = 0.295 p = 0.408 | r = 0.285 p = 0.424 |
| Naphthalene | Phenanthrene | Fluoranthene | Pyrene | |
|---|---|---|---|---|
| RARRES2 | r = 0.679 p = 0.031 | r = 0.070 p = 0.848 | r = −0.179 p = 0.645 | r = −0.419 p = 0.229 |
| ADIPOQ | r = −0.635 p = 0.049 | r = 0.107 p = 0.768 | r = −0.165 p = 0.671 | r = −0.379 p = 0.280 |
| NAMPT | r = 0.323 p = 0.362 | r = −0.199 p = 0.582 | r = −0.439 p = 0.237 | r = −0.427 p = 0.218 |
| RETN | r = 0.667 p = 0.035 | r = 0.047 p = 0.897 | r = −0.264 p = 0.492 | r = −0.427 p = 0.218 |
| Naphthalene (ng/mL) | ||||
|---|---|---|---|---|
| Gene | C | 10 | 500 | |
| Lipogenic enzymes | Fasn | 1.22 ± 0.06 A | 21.08 ± 2.86 B | 135.46 ± 10.35 C |
| Acaca | 1.48 ± 0.09 A | 8.22 ± 0.47 B | 34.32 ± 2.30 C | |
| Pro-adipogenesis factor | Cebpa | 2.12 ± 0.20 A | 6.16 ± 0.27 B | 51.06 ± 3.64 C |
| Pparg | 1.38 ± 0.11 A | 32.66 ± 3.64 B | 113.16 ± 4.72 C | |
| Fabp4 | 1.68 ± 0.14 A | 5.38 ± 0.28 B | 20.46 ± 1.62 C | |
| Plin1 | 1.78 ± 0.15 A | 4.02 ± 0.50 B | 15.36 ± 0.72 C | |
| Adipokines | Rarres2 | 1.22 ± 0.06 A | 2.52 ± 0.22 B | 11.88 ± 0.51 C |
| Adipoq | 1.22 ± 0.06 A | 4.54 ± 0.47 B | 10.42 ± 0.62 C | |
| Retn | 1.22 ± 0.06 A | 4.18 ± 0.59 B | 9.84 ± 0.92 C | |
| Carrier Gas Type | Helium |
|---|---|
| Dispenser operating temperature | 260 °C |
| Detector operating temperature | 260 °C |
| Volume of dispensed calibration standard PAH 50 ng | 1 µL |
| Dispensed sample volume | 1 µL |
| Transfer line | 280 °C |
| Ion source | EI; 280 °C |
| Chromatography column | DB5 MS 30 m, diameter 0.250 mm, phase thickness 0.25 µm |
| Temperature programme | 60 °C for 3 min, 15 °C/min ramp rate up to 280 °C, 280 °C for 25 min |
| Gene Symbol | Primers Sequences | Product Length | GenBank ID |
|---|---|---|---|
| GAPDH | 5′-TGCACCACCAACTGCTTAGC-3′ 5′-GGATGCAGGGATGATGTTCT-3′ | 177 | NM_008084.2 |
| BACTIN | 5′-CCTGTGCTGCTCACCGAGGC-3′ 5′-GACCCCGTCTCTCCGGATCCATC-3′ | 174 | NM_007393 |
| PPIA | 5′-CGCTTGCTGCAGCCATGGTC-3′ 5′-CAGCTCGAAGGAGACGCGGC-3′ | 86 | NM_008907.1 |
| FASN | 5′-GGAGGTGGTGATAGCCGGTAT-3′ 5′-TGGGTAATCCATAGAGCCCAG-3′ | 139 | XM030245556.1 |
| ACACA | 5′-CGATCTATCCGTCGGTGGTC-3′ 5′-TATTCTGCATTGGCTTTAAG-3′ | 100 | XM011248667.2 |
| CEBPA | 5′-CAAGAACAGCAACGAGTACCG-3′ 5′-GTCACTGGTCAACTCCAGCAC-3 | 128 | NM001287514.1 |
| PPARG | 5′-GGAAGACCACTCGCATTCCTT-3′ 5′-GTAATCAGCAACCATTGGGTCA-3′ | 120 | XR001785108.2 |
| FABP4 | 5′-AAGGTGAAGAGCATCATAACCCT-3′ 5′-TCACGCCTTTCATAACACATTCC-3′ | 129 | NM001409513.1 |
| PLIN1 | 5′-CACTCTCTGGCCATGTGGA-3′ 5′-AGCCAGGGCACCCGCACCTC-3′ | 118 | NM175640.2 |
| RARRES2 | 5′-GACCAACTGCCCCAAGAA-3′ 5′-GTCCATTTTAATGCAGGCCAG-3′ | 93 | NM001347168.1 |
| NAMPT | 5′-GAATGTCTCCTTCGGTTCTGG-3′ 5′-TCAGCAACTGGGTCCTTAAAC-3′ | 111 | NM021524.2 |
| ADIPOQ | 5′-TGTCTGTACGATTGTCAGTGG-3′ 5′-GCAGGATTAAGAGGAACAGGAG-3′ | 86 | NM 009605.5 |
| RETN | 5′-AGACTGCTGTGCCTTCTGGG-3′ 5′-CCCTCCTTTTCCTTTTCTTCCTTG-3′ | 200 | NM022984.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlyczyńska, E.; Bongrani, A.; Rame, C.; Węgiel, M.; Maślanka, A.; Major, P.; Zarzycki, P.; Ducluzeau, P.-H.; De Luca, A.; Bourbao-Tournois, C.; et al. Concentration of Polycyclic Aromatic Hydrocarbons (PAHs) in Human Serum and Adipose Tissues and Stimulatory Effect of Naphthalene in Adipogenesis in 3T3-L1 Cells. Int. J. Mol. Sci. 2023, 24, 1455. https://doi.org/10.3390/ijms24021455
Mlyczyńska E, Bongrani A, Rame C, Węgiel M, Maślanka A, Major P, Zarzycki P, Ducluzeau P-H, De Luca A, Bourbao-Tournois C, et al. Concentration of Polycyclic Aromatic Hydrocarbons (PAHs) in Human Serum and Adipose Tissues and Stimulatory Effect of Naphthalene in Adipogenesis in 3T3-L1 Cells. International Journal of Molecular Sciences. 2023; 24(2):1455. https://doi.org/10.3390/ijms24021455
Chicago/Turabian StyleMlyczyńska, Ewa, Alice Bongrani, Christelle Rame, Małgorzata Węgiel, Anna Maślanka, Piotr Major, Piotr Zarzycki, Pierre-Henri Ducluzeau, Arnaud De Luca, Celine Bourbao-Tournois, and et al. 2023. "Concentration of Polycyclic Aromatic Hydrocarbons (PAHs) in Human Serum and Adipose Tissues and Stimulatory Effect of Naphthalene in Adipogenesis in 3T3-L1 Cells" International Journal of Molecular Sciences 24, no. 2: 1455. https://doi.org/10.3390/ijms24021455
APA StyleMlyczyńska, E., Bongrani, A., Rame, C., Węgiel, M., Maślanka, A., Major, P., Zarzycki, P., Ducluzeau, P.-H., De Luca, A., Bourbao-Tournois, C., Froment, P., Rak, A., & Dupont, J. (2023). Concentration of Polycyclic Aromatic Hydrocarbons (PAHs) in Human Serum and Adipose Tissues and Stimulatory Effect of Naphthalene in Adipogenesis in 3T3-L1 Cells. International Journal of Molecular Sciences, 24(2), 1455. https://doi.org/10.3390/ijms24021455








