Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer
Abstract
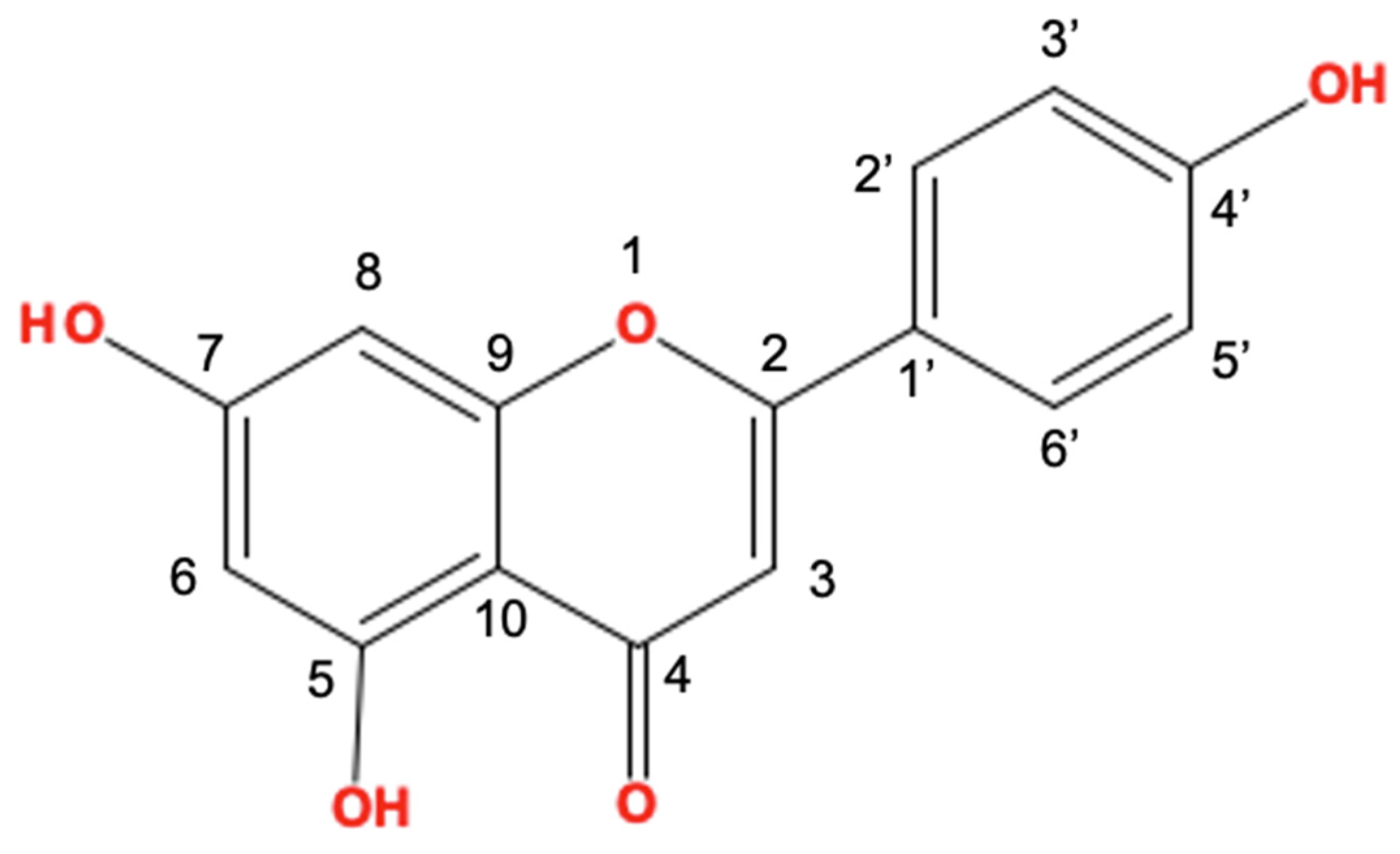
:1. Introduction
2. Anti-Inflammatory Effects of Apigenin on UV-Irradiated Skin
3. Effect of Apigenin on Attenuating AD
4. Treatment with Apigenin for Alleviating Pruritus
5. The Mechanism of Apigenin for the Amelioration of Psoriasis
6. The Suppressive Activity of Apigenin on Skin Cancer
6.1. NMSC
6.2. CMs
7. The Therapeutic Effects of Apigenin on Vitiligo
8. Conclusions
9. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC | Langerhans cells |
| UV | Ultraviolet radiation |
| Treg | Regulatory T cells |
| AD | Atopic dermatitis |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| COX-2 | Cyclooxygenase-2 |
| Src | Non-receptor tyrosine kinase |
| CPD | Cyclobutene pyrimidine dimers |
| NER | Nucleotide excision repair |
| MMP-1 | Metalloproteinases-1 |
| MAPK | Mitogen-activated protein kinase |
| AP-1 | Activator protein 1 |
| nHDF | Normal Human Dermal Fibroblasts |
| IgE | Immunoglobulin E |
| IFN-γ | Interferon-gamma |
| PiCl | Picrylchloride |
| STAT6 | Signal transducers and activators of transcription 6 |
| IL-4 | Interleukin-4 |
| IL-31 | Interleukin-31 |
| HMC-1 | Human mast cells |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| IKK | Inhibitor of NF-κB kinase |
| IκB | Inhibitor of kappaB |
| JNK | c-Jun N-terminal kinase |
| ERK | Extracellular signal-regulated kinase |
| CCL26 | Eosinophil chemokine |
| IL-31RA | Interleukin-31 receptor A |
| ILC2 | Innate lymphoid cells-2 |
| CXCR3 | C-X-C Motif Chemokine Receptor 3 |
| TSLP | Thymic stromal lymphopoietin |
| CCL | C-C motif chemokine ligand |
| NK cells | Natural killer cells |
| OVA | Ovalbumin |
| T-bet | T-box protein expressed in T cells |
| IL-23 | Interleukin-23 |
| LPS | Lipopolysaccharide |
| TLR4 | Toll-like receptor 4 |
| TME | Tumor microenvironments |
| BCCs | Basal cell carcinomas |
| CMs | Cutaneous malignant melanomas |
| NMSC | Nonmelanocytic skin cancer |
| SCCs | Squamous cell carcinomas |
| TRAIL | TNF-related apoptosis-inducing ligand |
| mTOR | Mammalian target of rapamycin |
| HEKs | Human epidermal keratinocytes |
| COLO-16 | Skin cutaneous squamous cell carcinoma cell line |
| LC3 | Light chain 3 |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| Bax | Bcl-2-associated X protein |
| PARP | Poly ADP-ribose polymerase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSH-Px | Glutathione peroxidase |
References
- Hsu, Y.-C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkaid, Y.; Tamoutounour, S. The influence of skin microorganisms on cutaneous immunity. Nat. Rev. Immunol. 2016, 16, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The applied anatomy of human skin: A model for regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Tang, A.; Amagai, M.; Granger, L.G.; Stanley, J.R.; Uddy, M.C. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature 1993, 361, 82–85. [Google Scholar] [CrossRef]
- Schwarz, A.; Noordegraaf, M.; Maeda, A.; Torii, K.; Clausen, B.E.; Schwarz, T. Langerhans cells are required for UVR-induced immunosuppression. J. Investig. Dermatol. 2010, 130, 1419–1427. [Google Scholar] [CrossRef] [Green Version]
- Idoyaga, J.; Fiorese, C.; Zbytnuik, L.; Lubkin, A.; Miller, J.; Malissen, B.; Mucida, D.; Merad, M.; Steinman, R.M. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Investig. 2013, 123, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Scharschmidt, T.C.; Fischbach, M.A. What lives on our skin: Ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discov. Today: Dis. Mech. 2013, 10, e83–e89. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Grice, E.A. The intersection of microbiome and host at the skin interface: Genomic-and metagenomic-based insights. Genome Res. 2015, 25, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, I.J.; Wright, E.M.; Tagg, J.R.; Jain, R.; Hale, J.D. Skin microbiome—The next frontier for probiotic intervention. Probiotics Antimicrob. Proteins 2022, 14, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M.; Mancini, C.; Cicerone, C.; Corazziari, E.; Pantanella, F. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol 2016, 39, 1–12. [Google Scholar]
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The multitasking organ: Recent insights into skin immune function. Immunity 2011, 35, 857–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Kupper, T.S. Inflammatory Skin Diseases, T Cells, and Immune Surveillance. New Engl. J. Med. 1999, 341, 1817–1828. [Google Scholar] [CrossRef]
- Balato, A.; Cacciapuoti, S.; Di Caprio, R.; Marasca, C.; Masarà, A.; Raimondo, A.; Fabbrocini, G. Human Microbiome: Composition and Role in Inflammatory Skin Diseases. Arch. Immunol. Et Ther. Exp. 2019, 67, 1–18. [Google Scholar] [CrossRef]
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469. [Google Scholar]
- Abdulla, A.; Adams, N.; Bone, M.; Elliott, A.M.; Gaffin, J.; Jones, D.; Knaggs, R.; Martin, D.; Sampson, L.; Schofield, P. Guidance on the management of pain in older people. Age Ageing 2013, 42, i1–i57. [Google Scholar] [CrossRef] [Green Version]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Conaghan, P.G. A turbulent decade for NSAIDs: Update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol. Int. 2012, 32, 1491–1502. [Google Scholar] [CrossRef] [Green Version]
- Melmed, S.; Polonsky, K.S.; Larsen, P.R.; Kronenberg, H.M. Williams Textbook of Endocrinology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Emery, P. Treatment of rheumatoid arthritis. Bmj 2006, 332, 152–155. [Google Scholar] [CrossRef] [Green Version]
- Borquaye, L.S.; Darko, G.; Laryea, M.K.; Roberts, V.; Boateng, R.; Gasu, E.N. Anti-inflammatory activities of extracts from Oliva sp., Patella rustica, and Littorina littorea collected from Ghana’s coastal shorelines. Cogent Biol. 2017, 3, 1364063. [Google Scholar] [CrossRef]
- Tang, D.; Chen, K.; Huang, L.; Li, J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin. Drug Metab. Toxicol. 2017, 13, 323–330. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Ornano, L.; Venditti, A.; Donno, Y.; Sanna, C.; Ballero, M.; Bianco, A. Phytochemical analysis of non-volatile fraction of Artemisia caerulescens subsp. densiflora (Viv.)(Asteraceae), an endemic species of La Maddalena Archipelago (Sardinia–Italy). Nat. Prod. Res. 2016, 30, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Maggi, F.; Vittori, S.; Papa, F.; Serrilli, A.M.; Di Cecco, M.; Ciaschetti, G.; Mandrone, M.; Poli, F.; Bianco, A. Antioxidant and α-glucosidase inhibitory activities of Achillea tenorii. Pharm. Biol. 2015, 53, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Sciubba, F.; Serafini, M.; Bianco, A.; Cianfaglione, K.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Maggi, F. Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip.). Ind. Crops Prod. 2018, 118, 225–235. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Lin, L.; Zhou, J. Apigenin suppresses the apoptosis of H9C2 rat cardiomyocytes subjected to myocardial ischemia-reperfusion injury via upregulation of the PI3K/Akt pathway. Mol. Med. Rep. 2018, 18, 1560–1570. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Y.-T.; Xiao, L.; Zhu, L.; Wang, Q.; Yan, T. Anti-Inflammatory Effects of Apigenin in Lipopolysaccharide-Induced Inflammatory in Acute Lung Injury by Suppressing COX-2 and NF-kB Pathway. Inflammation 2014, 37, 2085–2090. [Google Scholar] [CrossRef]
- Jung, U.J.; Cho, Y.-Y.; Choi, M.-S. Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients 2016, 8, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa-Arango, A.C.; Flórez-Muñoz, S.V.; Sanclemente, G. Mechanisms of skin aging. Iatreia 2017, 30, 160–170. [Google Scholar] [CrossRef]
- Lephart, E.D. Equol’s anti-aging effects protect against environmental assaults by increasing skin antioxidant defense and ECM proteins while decreasing oxidative stress and inflammation. Cosmetics 2018, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Barker, J.N.; Griffiths, C.E.; Nickoloff, B.J.; Mitra, R.; Dixit, V.M. Keratinocytes as initiators of inflammation. Lancet 1991, 337, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Dross, R.T.V.; Abu-Yousif, A.; Morrison, A.R.; Pelling, J.C. Apigenin Prevents UVB-Induced Cyclooxygenase 2 Expression: Coupled mRNA Stabilization and Translational Inhibition. Mol. Cell. Biol. 2007, 27, 283–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, S.; Park, J.; Lee, E.; Lim, S.; Yu, J.G.; Lee, S.J.; Chen, H.; Dong, Z.; Lee, K.W.; Lee, H.J. Src kinase is a direct target of apigenin against UVB-induced skin inflammation. Carcinogenesis 2012, 34, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Britto, S.M.; Shanthakumari, D.; Agilan, B.; Radhiga, T.; Kanimozhi, G.; Prasad, N.R. Apigenin prevents ultraviolet-B radiation induced cyclobutane pyrimidine dimers formation in human dermal fibroblasts. Mutat. Res. /Genet. Toxicol. Environ. Mutagen. 2017, 821, 28–35. [Google Scholar] [CrossRef]
- García Forero, A.; Villamizar Mantilla, D.A.; Núñez, L.A.; Ocazionez, R.E.; Stashenko, E.E.; Fuentes, J.L. Photoprotective and Antigenotoxic Effects of the Flavonoids Apigenin, Naringenin and Pinocembrin. Photochem. Photobiol. 2019, 95, 1010–1018. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J Derm. Sci 2011, 61, 23–31. [Google Scholar] [CrossRef]
- Choi, S.; Youn, J.; Kim, K.; Joo da, H.; Shin, S.; Lee, J.; Lee, H.K.; An, I.S.; Kwon, S.; Youn, H.J.; et al. Apigenin inhibits UVA-induced cytotoxicity in vitro and prevents signs of skin aging in vivo. Int J Mol Med 2016, 38, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Meisel, J.S.; Sfyroera, G.; Bartow-McKenney, C.; Gimblet, C.; Bugayev, J.; Horwinski, J.; Kim, B.; Brestoff, J.R.; Tyldsley, A.S.; Zheng, Q. Commensal microbiota modulate gene expression in the skin. Microbiome 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.H.; Oh, J.; Deming, C.; Conlan, S.; Grice, E.A.; Beatson, M.A.; Nomicos, E.; Polley, E.C.; Komarow, H.D.; Murray, P.R. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012, 22, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Bangayan, N.J.; Curd, E.; Taylor, P.A.; Gallo, R.L.; Leung, D.Y.; Li, H. The skin microbiome is different in pediatric versus adult atopic dermatitis. J. Allergy Clin. Immunol. 2016, 138, 1233–1236. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.E.; Leung, D.Y. Epidermal barrier in atopic dermatitis. Allergy Asthma Immunol. Res. 2012, 4, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm. Regen. 2017, 37, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic dermatitis: Pathophysiology. Manag. Atopic Dermat. 2017, 21–37. [Google Scholar]
- Tsakok, T.; Woolf, R.; Smith, C.; Weidinger, S.; Flohr, C. Atopic dermatitis: The skin barrier and beyond. Br. J. Dermatol. 2019, 180, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Umeda, D.; Yamashita, S.; Yamada, K.; Tachibana, H. Dietary apigenin attenuates the development of atopic dermatitis-like skin lesions in NC/Nga mice. J. Nutr. Biochem. 2009, 20, 876–881. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.-S.; Oh, H.; Kim, Y.-S.; Jang, S.I. Apigenin Inhibits IL-31 Cytokine in Human Mast Cell and Mouse Skin Tissues. Molecules 2019, 24, 1290. [Google Scholar] [CrossRef] [Green Version]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017, 171, 217–228. e213. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, C.; Marquardt, Y.; Czaja, K.; Wenzel, J.; Frank, J.; Lüscher-Firzlaff, J.; Lüscher, B.; Baron, J.M. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J. Allergy Clin. Immunol. 2012, 129, 426–433. e428. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Berger, T.; Fassett, M. Neuroimmune interactions in chronic itch of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kezic, S.; O’Regan, G.M.; Lutter, R.; Jakasa, I.; Koster, E.S.; Saunders, S.; Caspers, P.; Kemperman, P.M.J.H.; Puppels, G.J.; Sandilands, A.; et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J. Allergy Clin. Immunol. 2012, 129, 1031–1039. e1031. [Google Scholar] [CrossRef] [Green Version]
- Archer, N.K.; Jo, J.-H.; Lee, S.K.; Kim, D.; Smith, B.; Ortines, R.V.; Wang, Y.; Marchitto, M.C.; Ravipati, A.; Cai, S.S. Injury, dysbiosis, and filaggrin deficiency drive skin inflammation through keratinocyte IL-1α release. J. Allergy Clin. Immunol. 2019, 143, 1426–1443. e1426. [Google Scholar] [CrossRef] [Green Version]
- Roan, F.; Obata-Ninomiya, K.; Ziegler, S.F. Epithelial cell–derived cytokines: More than just signaling the alarm. J. Clin. Investig. 2019, 129, 1441–1451. [Google Scholar] [CrossRef] [Green Version]
- Ryu, W.-I.; Lee, H.; Bae, H.C.; Ryu, H.J.; Son, S.W. IL-33 down-regulates filaggrin expression by inducing STAT3 and ERK phosphorylation in human keratinocytes. J. Dermatol. Sci. 2016, 82, 131–134. [Google Scholar] [CrossRef]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef] [Green Version]
- Albanesi, C.; Scarponi, C.; Sebastiani, S.; Cavani, A.; Federici, M.; De Pità, O.; Puddu, P.; Girolomoni, G. IL-4 enhances keratinocyte expression of CXCR3 agonistic chemokines. J. Immunol. 2000, 165, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Liu, Y.J. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin. Exp. Allergy 2009, 39, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Cianferoni, A.; Spergel, J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev. Clin. Immunol. 2014, 10, 1463–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Takai, T.; Chen, X.; Okumura, K.; Ogawa, H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J. Dermatol. Sci. 2012, 66, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Ogawa, H.; Niyonsaba, F. Current insights into the role of human β-defensins in atopic dermatitis. Clin. Exp. Immunol. 2017, 190, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Steinhoff, M.; Schmelz, M.; Weisshaar, E.; Metze, D.; Luger, T. Neurophysiology of pruritus: Cutaneous elicitation of itch. Arch. Dermatol. 2003, 139, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Weisshaar, E.; Apfelbacher, C.; Jäger, G.; Zimmermann, E.; Bruckner, T.; Diepgen, T.; Gollnick, H. Pruritus as a leading symptom: Clinical characteristics and quality of life in German and Ugandan patients. Br. J. Dermatol. 2006, 155, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Lyell, A. The itching patient: A review of the causes of pruritus. Scott. Med. J. 1972, 17, 334–347. [Google Scholar] [CrossRef]
- Lavery, M.J.; Kinney, M.O.; Mochizuki, H.; Craig, J.; Yosipovitch, G. Pruritus: An overview. What drives people to scratch an itch? Ulst. Med. J. 2016, 85, 164. [Google Scholar]
- Schmelz, M. Itch—Mediators and mechanisms. J. Dermatol. Sci. 2002, 28, 91–96. [Google Scholar] [CrossRef]
- Shibuya, R.; Takimoto-Ito, R.; Kambe, N.; Kabashima, K. A new era with the development of cytokine-based therapy for pruritus. J. Investig. Dermatol. 2022, 142, 47–52. [Google Scholar] [CrossRef]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Takamori, A.; Nambu, A.; Sato, K.; Yamaguchi, S.; Matsuda, K.; Numata, T.; Sugawara, T.; Yoshizaki, T.; Arae, K.; Morita, H. IL-31 is crucial for induction of pruritus, but not inflammation, in contact hypersensitivity. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Hudson, C.A.; Christophi, G.P.; Gruber, R.C.; Wilmore, J.R.; Lawrence, D.A.; Massa, P.T. Induction of IL-33 expression and activity in central nervous system glia. J. Leukoc. Biol. 2008, 84, 631–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuoka, S.; Kawanokuchi, J.; Parajuli, B.; Jin, S.; Doi, Y.; Noda, M.; Sonobe, Y.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Production and functions of IL-33 in the central nervous system. Brain Res. 2011, 1385, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; He, D.; Yan, B. Apigenin Attenuates Allergic Responses of Ovalbumin-Induced Allergic Rhinitis Through Modulation of Th1/Th2 Responses in Experimental Mice. Dose-Response 2020, 18. [Google Scholar] [CrossRef]
- Che, D.N.; Cho, B.O.; Kim, J.-S.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Effect of Luteolin and Apigenin on the Production of Il-31 and Il-33 in Lipopolysaccharides-Activated Microglia Cells and Their Mechanism of Action. Nutrients 2020, 12, 811. [Google Scholar] [CrossRef] [Green Version]
- Che, D.N.; Cho, B.O.; Kim, J.-S.; Shin, J.Y.; Kang, H.J.; Jang, S.I. Luteolin and Apigenin Attenuate LPS-Induced Astrocyte Activation and Cytokine Production by Targeting MAPK, STAT3, and NF-κB Signaling Pathways. Inflammation 2020, 43, 1716. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, M.; Sun, R.; Hupe, M.; Kim, P.L.; Park, K.; Crumrine, D.; Lin, T.-K.; Santiago, J.L.; Mauro, T.M.; Elias, P.M.; et al. Topical apigenin improves epidermal permeability barrier homoeostasis in normal murine skin by divergent mechanisms. Exp. Dermatol. 2013, 22, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Ginwala, R.; Bhavsar, R.; Moore, P.; Bernui, M.; Singh, N.; Bearoff, F.; Nagarkatti, M.; Khan, Z.K.; Jain, P. Apigenin Modulates Dendritic Cell Activities and Curbs Inflammation Via RelB Inhibition in the Context of Neuroinflammatory Diseases. J Neuroimmune Pharm. 2021, 16, 403–424. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Naylor, M.; Stamp, G.W.; Foulkes, W.D.; Eccles, D.; Balkwill, F.R. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J. Clin. Investig. 1993, 91, 2194–2206. [Google Scholar] [CrossRef] [Green Version]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Hinckley, M.R.; Feldman, S.R.; Fleischer, A.B.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch. Dermatol. 2010, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.C.; Annas, G.D. Epidemiology of Melanoma and Nonmelanoma Skin Cancer. In Seminars in Oncology Nursing; Elsevier: Amsterdam, The Netherlands, 2003; pp. 2–11. [Google Scholar]
- Armstrong, B.K.; Kricker, A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B Biol. 2001, 63, 8–18. [Google Scholar] [CrossRef]
- Saladi, R.N.; Persaud, A.N. The causes of skin cancer: A comprehensive review. Drugs Today 2005, 41, 37–54. [Google Scholar] [CrossRef]
- Nikolaou, V.; Stratigos, A. Emerging trends in the epidemiology of melanoma. Br. J. Dermatol. 2014, 170, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R. Skin Cancer: An overview of Epidemiology and Risk Factors. In Seminars in Oncology Nursing: 2013; Elsevier: Amsterdam, The Netherlands, 2003; pp. 160–169. [Google Scholar]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Frew, A.J.; Smyth, M.J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 2008, 8, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.J.; Putoczki, T. JAK-STAT Signalling Pathway in Cancer. Cancers 2020, 12, 1971. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridgeman, B.B.; Wang, P.; Ye, B.; Pelling, J.C.; Volpert, O.V.; Tong, X. Inhibition of mTOR by apigenin in UVB-irradiated keratinocytes: A new implication of skin cancer prevention. Cell. Signal. 2016, 28, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jangdey, M.S.; Gupta, A.; Saraf, S.; Saraf, S. Development and optimization of apigenin-loaded transfersomal system for skin cancer delivery: In vitro evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1452–1462. [Google Scholar] [CrossRef] [Green Version]
- Gilardini Montani, M.S.; Cecere, N.; Granato, M.; Romeo, M.A.; Falcinelli, L.; Ciciarelli, U.; D’orazi, G.; Faggioni, A.; Cirone, M. Mutant p53, stabilized by its interplay with HSP90, activates a positive feed-back loop between NRF2 and p62 that induces chemo-resistance to apigenin in pancreatic cancer cells. Cancers 2019, 11, 703. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, S.; Tong, X.; Bridgeman, B.B.; Plebanek, M.P.; Volpert, O.V. Apigenin Inhibits UVB-Induced Skin Carcinogenesis: The Role of Thrombospondin-1 as an Anti-Inflammatory Factor. Neoplasia 2018, 20, 930–942. [Google Scholar] [CrossRef]
- Berenblum, I. A re-evaluation of the concept of cocarcinogenesis. Carcinog. Carcinog. Test. 1969, 11, 21–30. [Google Scholar]
- Boutwell, R.K. The function and mechanism of promoters of carcinogenesis. CRC Crit. Rev. Toxicol. 1974, 2, 419–443. [Google Scholar] [CrossRef]
- Wei, H.; Tye, L.; Bresnick, E.; Birt, D.F. Inhibitory effect of apigenin, a plant flavonoid, on epidermal ornithine decarboxylase and skin tumor promotion in mice. Cancer Res 1990, 50, 499–502. [Google Scholar]
- Huang, Y.T.; Kuo, M.L.; Liu, J.Y.; Huang, S.Y.; Lin, J.K. Inhibitions of protein kinase C and proto-oncogene expressions in NIH 3T3 cells by apigenin. Eur. J. Cancer 1996, 32a, 146–151. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.-H.; Chu, J.-H.; Kwan, H.-Y.; Su, T.; Yu, H.; Cheng, C.-Y.; Fu, X.-Q.; Guo, H.; Li, T.; Tse, A.K.-W.; et al. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci. Rep. 2016, 6, 21731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, J.S.; Choo, G.S.; Yoo, E.S.; Kim, S.H.; Lee, J.H.; Han, S.H.; Kim, H.J.; Jung, S.H.; Park, Y.S.; Kim, B.S.; et al. Apigenin induces apoptosis by regulating Akt and MAPK pathways in human melanoma cell A375SM. Mol Med Rep 2020, 22, 4877–4889. [Google Scholar] [CrossRef] [PubMed]
- Whitton, M.E.; Pinart, M.; Batchelor, J.; Leonardi-Bee, J.; González, U.; Jiyad, Z.; Eleftheriadou, V.; Ezzedine, K. Interventions for vitiligo. Cochrane Database Syst. Rev. 2015, 2, Cd003263. [Google Scholar] [CrossRef]
- Halder, R.M.; Chappell, J.L. Vitiligo Update. In Seminars in Cutaneous Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2009; pp. 86–92. [Google Scholar]
- Mahmoud, F.; Abul, H.; Al-Saleh, Q.; Haines, D.; Burleson, J.; Morgan, G. Peripheral T-cell activation in non-segmental vitiligo. J. Dermatol. 1998, 25, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.; Abul, H.; Haines, D.; Al-Saleh, C.; Khajeji, M.; Whaley, K. Decreased total numbers of peripheral blood lymphocytes with elevated percentages of CD4+ CD45RO+ and CD4+ CD25+ of T-helper cells in non-segmental vitiligo. J. Dermatol. 2002, 29, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Taïeb, A. Vitiligo as an inflammatory skin disorder: A therapeutic perspective. Pigment Cell Melanoma Res. 2012, 25, 9–13. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Zhao, G.; Lin, M.; Lang, Y.; Zhang, D.; Feng, D.; Tu, C. Apigenin protects human melanocytes against oxidative damage by activation of the Nrf2 pathway. Cell Stress Chaperones 2020, 25, 277–285. [Google Scholar] [CrossRef]
- Lin, M.; Lu, S.-s.; Wang, A.-x.; Qi, X.-y.; Zhao, D.; Wang, Z.-h.; Man, M.-Q.; Tu, C.-x. Apigenin attenuates dopamine-induced apoptosis in melanocytes via oxidative stress-related p38, c-Jun NH2-terminal kinase and Akt signaling. J. Dermatol. Sci. 2011, 63, 10–16. [Google Scholar] [CrossRef]
- Malik, S.; Suchal, K.; Khan, S.I.; Bhatia, J.; Kishore, K.; Dinda, A.K.; Arya, D.S. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK-NF-κB-TNF-α and TGF-β1-MAPK-fibronectin pathways. Am. J. Physiol. -Ren. Physiol. 2017, 313, F414–F422. [Google Scholar] [CrossRef] [Green Version]
- Torkin, R.; Lavoie, J.-F.; Kaplan, D.R.; Yeger, H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol. Cancer Ther. 2005, 4, 1–11. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popović, M.; Caballero-Bleda, M.; Benavente-García, O.; Castillo, J. The flavonoid apigenin delays forgetting of passive avoidance conditioning in rats. J. Psychopharmacol. 2014, 28, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharm. Rev 2011, 5, 120–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drew, A.K.; Myers, S.P. Safety issues in herbal medicine: Implications for the health professions. Med. J. Aust. 1997, 166, 538–541. [Google Scholar] [CrossRef] [PubMed]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 1477. [Google Scholar] [CrossRef]
- Barnes, J.S.; Foss, F.W., Jr.; Schug, K.A. Thermally accelerated oxidative degradation of quercetin using continuous flow kinetic electrospray-ion trap-time of flight mass spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 1513–1522. [Google Scholar] [CrossRef]








| Cytokines | Classification | Role | Reference |
|---|---|---|---|
| IL-4 | Th2 cell-derived cytokines |
| [51,52] |
| IL-13 | |||
| IL-31 | Th2 cell-derived cytokines |
| [53,54] |
| IL-1α | IL-1 family |
| [55,56] |
| IL-1β | |||
| IL-33 | IL-1 family |
| [57,58,59] |
| IFN-γ | Th1 cytokines |
| [60] |
| TNF-α | |||
| TSLP | IL-7-like cytokines |
| [61,62,63,64] |
| The Type of Skin Disease | Mechanisms of Apigenin | Test Model | Dose | References |
|---|---|---|---|---|
| UV-mediated inflammation | Downregulates Src and COX-2 levels. | In vitro | 10, 20, 40, 50 μM | [36,37] |
| Regulates the level of apoptotic proteins and anti-apoptotic proteins. | In vitro | 7, 15 μM | [38,39] | |
| Inhibits MMP-1 expression by suppressing Ca2+ influx. | [40,41] | |||
| Suppresses the MAPK and AP-1 signaling pathways. | In vitro | 1, 5, 10, 20 μM | ||
| Atopic dermatitis | Suppresses phosphorylation of STAT6 in IL-4 stimulated mouse spleen cells. | Ex vivo | 25 μM | [49] |
| Ameliorates damaged skin lesions induced by picrylchloride(piCl). | In vivo | 0.05% feed to mice | ||
| Downregulates the protein levels of the NF-κB, MAPK pathways. | In vitro | 10, 20, 30 μM | [50] | |
| Pruritus | Suppresses IL-31 levels by inhibiting the NF-κB and MAPK signaling pathways. | In vitro | 10, 30 µM | [50] |
| Regulates Th1/Th2 balance by inhibiting the NF-κB pathway, and levels of histamine, IgE, and STAT1 expression. | In vivo | 5, 10, 20 mg/kg of mice | [75] | |
| Enhances the Th1 response by decreasing the expression of IFN-γ, and T-box proteins in T cells. | ||||
| Shows low expression of IL-31, IL-33 in apigenin-treated microglial cells via downregulating ERK and JNK expression. | In vitro | 5, 10, 20, 40, 60, 80, 100 µM | [76] | |
| Inactivates MAPK and NF-κB proteins. | In vitro | 30, 60 µM | [77] | |
| Psoriasis | Promotes the synthesis of skin barrier factors. | In vivo | 60 µL of 0.1% apigenin in 100% ethanol | [80] |
| Downregulates the mRNA expression of inflammatory cytokines in LPS-treated DCs. | In vitro | 8, 20 μM | [81] | |
| Skin cancer | Downregulates mTOR and AKT signaling pathways. | In vitro | 25 μM | [95,96] |
| In vivo | 5 μM in 0.2 mL DMSO/acetone (1:9) vehicle mix of mice | |||
| Induces autophagy by inhibiting mTOR expression and the conversion of LC3. | In vitro | 6, 12, 25, 50 μM | [97] | |
| Decreases carcinogenesis in TPA-mediated mouse skin and PKC activity,. | In vivo | 5, 25 μM to mice 10, 50, 100 μM | [101,102] | |
| In vitro | ||||
| Attenuates melanoma metastases to the lung by decreasing STAT3 levels. | In vivo | 150 mg/kg of mice | [104] | |
| Promotes the expression of apoptotic proteins in A375SM cells. Inactivates the Akt and MAPK pathway proteins. | In vitro | 25, 50, 75, 100 µM | [103,105] | |
| In vivo | 25, 50 mg/kg of mice | |||
| Vitiligo | Promotes antioxidant enzyme activity in dose-dependent ways. Increases the expression of antioxidant genes at the mRNA and protein levels. | In vitro | 1, 5, 10, 20 µM | [111] |
| Protects pigment cells from DA-induced apoptosis by decreasing the level of apoptotic agents. | In vitro | 10 µM | [112] | |
| Inactivates p38, JNK, and Akt levels in the presence of DA. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.H.; Kim, M.-Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. https://doi.org/10.3390/ijms24021498
Yoon JH, Kim M-Y, Cho JY. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. International Journal of Molecular Sciences. 2023; 24(2):1498. https://doi.org/10.3390/ijms24021498
Chicago/Turabian StyleYoon, Ji Hye, Mi-Yeon Kim, and Jae Youl Cho. 2023. "Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer" International Journal of Molecular Sciences 24, no. 2: 1498. https://doi.org/10.3390/ijms24021498






