The Importance of the Immune System and Molecular Cell Signaling Pathways in the Pathogenesis and Progression of Lung Cancer
Abstract
1. Introduction
2. Results and Discussions
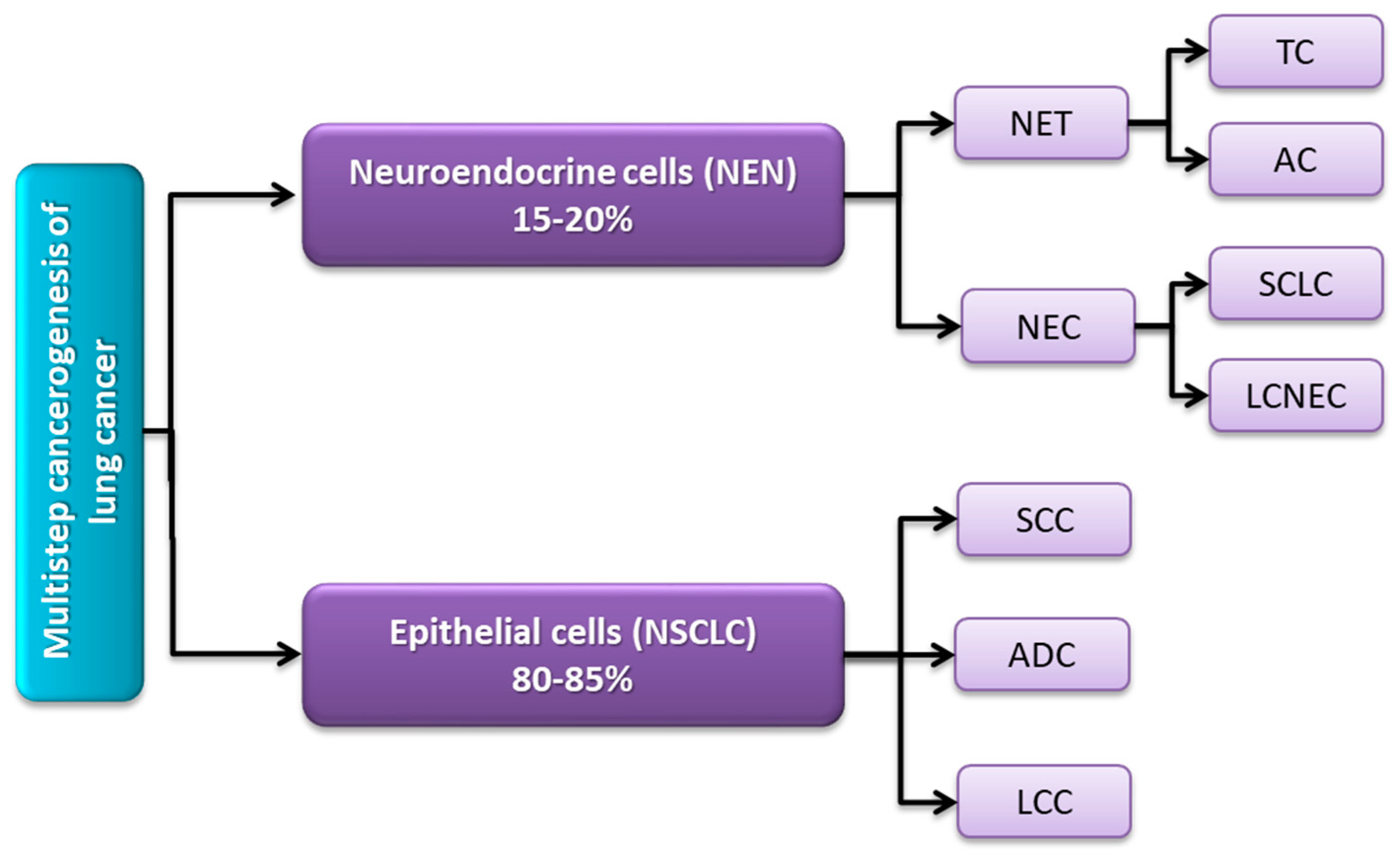
2.1. Molecular and Histological Classification of Lung Cancer
2.2. Disorders of Cell Signaling Pathways as an Element of Lung Cancer Development and Progression
2.3. Epigenetic Changes Involved in the Pathogenesis of Lung Cancer
- (1)
- A change in DNA methylation status within the CpG islands of tumor suppressor genes;
- (2)
- Covalent modifications of histone tails;
- (3)
- Regulation of genes by micro-RNAs (miRNAs).
2.4. The Importance of the Immune System in the Pathogenesis of Lung Cancer
2.4.1. Importance of Tumor Antigens and Neoantigens
2.4.2. Importance of Immune Checkpoints
PD-1/PD-L1 Pathway
CTLA4 Pathway
| Pathway | Molecules Found on Cancer Cells | Molecules Found on T Lymphocytes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Gene and Location | Amino Acid Length and Molecular Weight | Function | Name | Gene and Location | Amino acid Length and Molecular Weight | Function | References | |
| Inhibitory pathway | A2aR | ADORA2A, 22q11.23 | 412 aa; 44.707 kDa |
| No data | [362,363,364,365] | |||
| VISTA | C10orf54, 10q22.1 | 311 aa, 33.908 kDa |
| No data | [364,366,367] | ||||
| B7-H3 | CD276, 15q24.1 | 534 aa, 57.235 kDa |
| No data | [368,369,370,371] | ||||
| PD-L1 | CD274, 9p24.1 | 290 aa, 33.275 kDa |
| PD-1 | PDCD1, 2q37.3 | 288 aa, 31.647 kDa |
| [346,372,373,374,375,376,377,378] | |
| PD-L2 | PDCD1LG2, 9p24.1 | 273, 30,957 | |||||||
| CD80 | CD80, 13q13.3-q21 | 288 aa, 33.048 kDa |
| CTLA-4 | CTLA-4, 2q33.2 | 223 aa, 24.656 kDa |
| [379,380,381,382,383,384,385,386] | |
| CD86 | CD86, 3q21 | 323 aa, 37.021 kDa |
| ||||||
| Galectin 9 | LGALS9, 17q11.2 | 355 aa, 39.518 kDa |
| TIM3 | HAVCR2, 5q33.3, | 301 aa, 33.394 kDa |
| [387,388,389,390,391,392] | |
| HVEM | TNFRSF14, 1p36.32 | 283 aa, 30.392 kDa |
| BTLA | BTLA, 3q13.2 | 289 aa, 32.834 kDa |
| [393,394,395,396,397] | |
| MHC II | HLA-DP, -DQ and –DR, chromosome 6 | - |
| LAG3 | LAG3, 12p13.31 | 525 aa,57.449 kDa |
| [398,399,400,401,402] | |
| Stimulatory pathway | OX40L | TNFSF4, 1q25.1 | 183 aa, 21.050 kDa |
| OX40 | TNFRSF4, 1p36.33 | 277 aa, 29.341 kDa |
| [403,404,405,406] |
| CD40 | TNFRSF5, 20q12-q13.2 | 117 aa,13.158 kDa |
| CD40L | CD40LG, Xq26 | 261 aa, 29.274 kDa |
| [407,408,409,410] | |
| B7RP1 | ICOSG, 21q22.3 | 302 aa, 33.349 kDa |
| ICOS | ICOS, 2q33.2 | 199 aa, 22.625 kDa |
| [411,412,413,414,415] | |
| CD70 | CD70, 19p13.3 | 193 aa, 21.118 kDa |
| CD27 | CD27, 12p13.31 | 260 aa, 29.137 kDa |
| [416,417,418,419,420,421] | |
| GITRL | TNFSF18, 1q25.1 | 177 aa, 20.308 kDa |
| GITR | TNFRSF18, 1p36.33 | 241 aa, 26.000 kDa |
| [422,423,424,425] | |
| 4-1 BBL | TNFSF9, 19p13.3 | 254 aa, 26.625 kDa |
| 4-1 BB | TNFRSF9, 1p36.23 | 255 aa, 27.899 kDa |
| [426,427,428,429,430] | |
| CD155 | PVR, 19q13.31 | 417 aa, 45.303 k Da |
| TIGIT | TIGIT, 3q13.31 | 244 aa, 26.319 kDa |
| [431,432,433,434] | |
3. Materials and Methods
3.1. Search Strategy
3.2. Assessment of (Quality) Bias Risk and Data Synthesis Strategy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The Epidemiology of Lung Cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
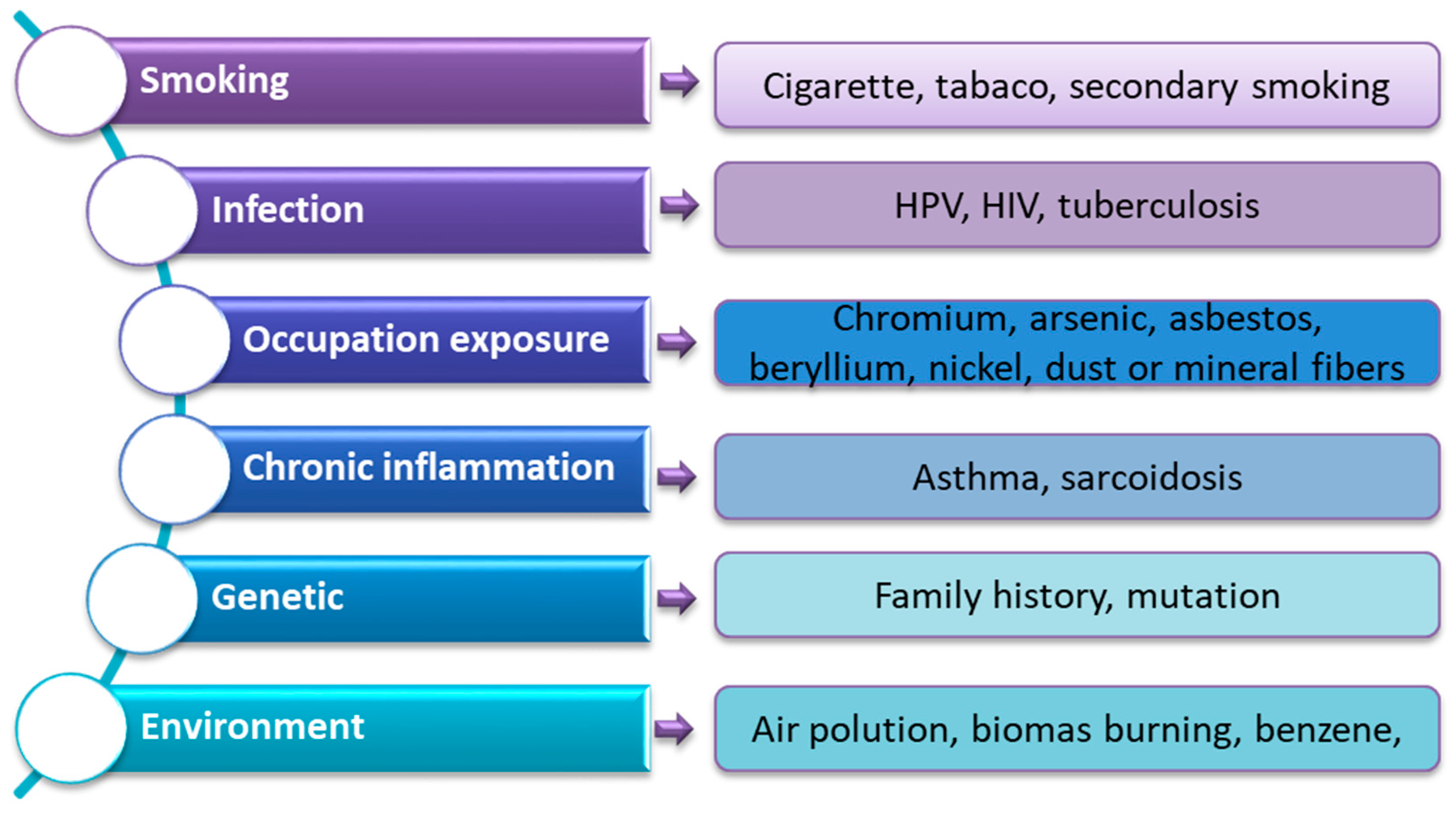
- Rahal, Z.; El Nemr, S.; Sinjab, A.; Chami, H.; Tfayli, A.; Kadara, H. Smoking and Lung Cancer: A Geo-Regional Perspective. Front Oncol. 2017, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and Occupational Determinants of Lung Cancer. Transl. Lung Cancer Res. 2019, 8, S31–S49. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Tsuang, B.-J.; Chiang, C.-J.; Ku, K.-C.; Tseng, J.-S.; Yang, T.-Y.; Hsu, K.-H.; Chen, K.-C.; Yu, S.-L.; Lee, W.-C.; et al. The Relationship Between Air Pollution and Lung Cancer in Nonsmokers in Taiwan. J. Thorac. Oncol. 2019, 14, 784–792. [Google Scholar] [CrossRef]
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 November 2022).
- Lung Cancer Statistics | World Cancer Research Fund International; WCRF International: Londin, UK, 2022.
- Lung Cancer Statistics | How Common Is Lung Cancer? Available online: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (accessed on 21 November 2022).
- Benusiglio, P.R.; Fallet, V.; Sanchis-Borja, M.; Coulet, F.; Cadranel, J. Lung Cancer Is Also a Hereditary Disease. Eur. Respir. Rev. 2021, 30, 210045. [Google Scholar] [CrossRef]
- Chen, X.; Xu, B.; Li, Q.; Xu, X.; Li, X.; You, X.; Yu, Z. Genetic Profile of Non-small Cell Lung Cancer (NSCLC): A Hospital-based Survey in Jinhua. Mol. Genet. Genomic. Med. 2020, 8, e1398. [Google Scholar] [CrossRef]
- Cline, M.J. The Role of Proto-Oncogenes in Human Cancer: Implications for Diagnosis and Treatment. Int. J. Radiat Oncol. Biol. Phys. 1987, 13, 1297–1301. [Google Scholar] [CrossRef]
- El-Telbany, A.; Ma, P.C. Cancer Genes in Lung Cancer. Genes Cancer 2012, 3, 467–480. [Google Scholar] [CrossRef]
- Kanwal, M.; Ding, X.-J.; Cao, Y. Familial Risk for Lung Cancer. Oncol. Lett. 2017, 13, 535–542. [Google Scholar] [CrossRef]
- Oxnard, G.R.; Binder, A.; Jänne, P.A. New Targetable Oncogenes in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2013, 31, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, P.; Raghu, P.S.; Reddy, V.D.; Bulle, S.; Marthadu, S.B.; Maturu, P.; Varadacharyulu, N.C. Chronic Cigarette Smoking-Induced Oxidative/Nitrosative Stress in Human Erythrocytes and Platelets. Mol. Cell Toxicol. 2018, 14, 27–34. [Google Scholar] [CrossRef]
- Robinson, L.A.; Jaing, C.J.; Pierce Campbell, C.; Magliocco, A.; Xiong, Y.; Magliocco, G.; Thissen, J.B.; Antonia, S. Molecular Evidence of Viral DNA in Non-Small Cell Lung Cancer and Non-Neoplastic Lung. Br. J. Cancer 2016, 115, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.-M.; Xu, Q.-P.; Li, X.; Xiao, R.-D.; Cai, L.; He, F. The Association between Human Papillomavirus Infection and Lung Cancer: A System Review and Meta-Analysis. Oncotarget 2017, 8, 96419–96432. [Google Scholar] [CrossRef]
- Barros-Filho, M.C.; Guisier, F.; Rock, L.D.; Becker-Santos, D.D.; Sage, A.P.; Marshall, E.A.; Lam, W.L.; Barros-Filho, M.C.; Guisier, F.; Rock, L.D.; et al. Tumour Suppressor Genes with Oncogenic Roles in Lung Cancer; IntechOpen: London, UK, 2019; ISBN 978-1-78984-427-6. [Google Scholar]
- Domagala-Kulawik, J. The Role of the Immune System in Non-Small Cell Lung Carcinoma and Potential for Therapeutic Intervention. Transl. Lung Cancer Res. 2015, 4, 177–190. [Google Scholar] [CrossRef]
- Domagala-Kulawik, J.; Osinska, I.; Hoser, G. Mechanisms of Immune Response Regulation in Lung Cancer. Transl. Lung Cancer Res. 2014, 3, 15–22. [Google Scholar] [CrossRef]
- Domagala-Kulawik, J.; Raniszewska, A. How to Evaluate the Immune Status of Lung Cancer Patients before Immunotherapy. Breathe 2017, 13, 291–296. [Google Scholar] [CrossRef]
- Kuramochi, M.; Fukuhara, H.; Nobukuni, T.; Kanbe, T.; Maruyama, T.; Ghosh, H.P.; Pletcher, M.; Isomura, M.; Onizuka, M.; Kitamura, T.; et al. TSLC1 Is a Tumor-Suppressor Gene in Human Non-Small-Cell Lung Cancer. Nat. Genet. 2001, 27, 427–430. [Google Scholar] [CrossRef]
- Lim, R.J.; Liu, B.; Krysan, K.; Dubinett, S.M. Lung Cancer and Immunity Markers. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2423–2430. [Google Scholar] [CrossRef]
- Pikor, L.A.; Ramnarine, V.R.; Lam, S.; Lam, W.L. Genetic Alterations Defining NSCLC Subtypes and Their Therapeutic Implications. Lung Cancer 2013, 82, 179–189. [Google Scholar] [CrossRef]
- Saab, S.; Zalzale, H.; Rahal, Z.; Khalifeh, Y.; Sinjab, A.; Kadara, H. Insights Into Lung Cancer Immune-Based Biology, Prevention, and Treatment. Front. Immunol. 2020, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, M.; Zhou, N.; Hou, H.; Li, T.; Yu, H.; Tan, Y.-D.; Zhang, X. Use Tumor Suppressor Genes as Biomarkers for Diagnosis of Non-Small Cell Lung Cancer. Sci. Rep. 2021, 11, 3596. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.-K. Non-Small-Cell Lung Cancers: A Heterogeneous Set of Diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Fisseler-Eckhoff, A.; Demes, M. Neuroendocrine Tumors of the Lung. Cancers 2012, 4, 777–798. [Google Scholar] [CrossRef] [PubMed]
- Niemira, M.; Collin, F.; Szalkowska, A.; Bielska, A.; Chwialkowska, K.; Reszec, J.; Niklinski, J.; Kwasniewski, M.; Kretowski, A. Molecular Signature of Subtypes of Non-Small-Cell Lung Cancer by Large-Scale Transcriptional Profiling: Identification of Key Modules and Genes by Weighted Gene Co-Expression Network Analysis (WGCNA). Cancers 2019, 12, 37. [Google Scholar] [CrossRef]
- Rodriguez-Canales, J.; Parra-Cuentas, E.; Wistuba, I.I. Diagnosis and Molecular Classification of Lung Cancer. In Lung Cancer: Treatment and Research; Reckamp, K.L., Ed.; Cancer Treatment and Research; Springer International Publishing: Cham, Switzerland, 2016; pp. 25–46. ISBN 978-3-319-40389-2. [Google Scholar]
- Savu, C.; Melinte, A.; Diaconu, C.; Stiru, O.; Gherghiceanu, F.; Tudorica Ștefan, D.O.; Dumitrașcu, O.C.; Bratu, A.; Balescu, I.; Bacalbasa, N. Lung Neuroendocrine Tumors: A Systematic Literature Review (Review). Exp. Ther. Med. 2022, 23, 176. [Google Scholar] [CrossRef]
- West, L.; Vidwans, S.J.; Campbell, N.P.; Shrager, J.; Simon, G.R.; Bueno, R.; Dennis, P.A.; Otterson, G.A.; Salgia, R. A Novel Classification of Lung Cancer into Molecular Subtypes. PLoS ONE 2012, 7, e31906. [Google Scholar] [CrossRef]
- Contributors, W.E. The Types of Lung Cancer. Available online: https://www.webmd.com/lung-cancer/lung-cancer-types (accessed on 21 November 2022).
- Cosmic Cancer Gene Census. Available online: http://cancer.sanger.ac.uk/census (accessed on 22 November 2022).
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front Oncol. 2017, 7, 193. [Google Scholar] [CrossRef]
- Joshi, A.; Mishra, R.; Desai, S.; Chandrani, P.; Kore, H.; Sunder, R.; Hait, S.; Iyer, P.; Trivedi, V.; Choughule, A.; et al. Molecular Characterization of Lung Squamous Cell Carcinoma Tumors Reveals Therapeutically Relevant Alterations. Oncotarget 2021, 12, 578–588. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cress, W.D.; Muñoz-Antonia, T. Racial and Ethnic Differences in the Epidemiology of Lung Cancer and the Lung Cancer Genome. Cancer Control. 2016, 23, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.; Toyooka, S.; Matsuo, K.; Yamamoto, H.; Wistuba, I.I.; Lam, S.; Fong, K.M.; Gazdar, A.F.; Miyoshi, S. Ethnicity Affects EGFR and KRAS Gene Alterations of Lung Adenocarcinoma. Oncol. Lett. 2015, 10, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. North Am. 2016, 25, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Furrukh, M. Tobacco Smoking and Lung Cancer. Sultan Qaboos Univ. Med. J. 2013, 13, 345–358. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, X.; Han, Y.; Gorlova, O.; Qian, D.; Leighl, N.; Johansen, J.S.; Barnett, M.; Chen, C.; Goodman, G.; et al. Genome-Wide Interaction Study of Smoking Behavior and Non-Small Cell Lung Cancer Risk in Caucasian Population. Carcinogenesis 2018, 39, 336–346. [Google Scholar] [CrossRef]
- Massion, P.P.; Carbone, D.P. The Molecular Basis of Lung Cancer: Molecular Abnormalities and Therapeutic Implications. Respir. Res. 2003, 4, 12. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, J.; Liu, N.; Zhao, L.; Xu, B. Prognostic Implications of Molecular Subtypes in Primary Small Cell Lung Cancer and Their Correlation With Cancer Immunity. Front. Oncol. 2022, 12, 424. [Google Scholar] [CrossRef]
- Ray, M.R.; Jablons, D.; He, B. Lung Cancer Therapeutics That Target Signaling Pathways: An Update. Expert Rev. Respir. Med. 2010, 4, 631–645. [Google Scholar] [CrossRef]
- Schwendenwein, A.; Megyesfalvi, Z.; Barany, N.; Valko, Z.; Bugyik, E.; Lang, C.; Ferencz, B.; Paku, S.; Lantos, A.; Fillinger, J.; et al. Molecular Profiles of Small Cell Lung Cancer Subtypes: Therapeutic Implications. Mol. Ther. Oncolytics 2021, 20, 470–483. [Google Scholar] [CrossRef]
- Shtivelman, E.; Hensing, T.; Simon, G.R.; Dennis, P.A.; Otterson, G.A.; Bueno, R.; Salgia, R. Molecular Pathways and Therapeutic Targets in Lung Cancer. Oncotarget 2014, 5, 1392–1433. [Google Scholar] [CrossRef]
- Gurguş, D.; Grigoraş, M.L.; Motoc, A.G.M.; Zamfir, C.L.; Cornianu, M.; Faur, C.I.; Pop, D.L.; Folescu, R. Clinical Relevance and Accuracy of P63 and TTF-1 for Better Approach of Small Cell Lung Carcinoma versus Poorly Differentiated Nonkeratinizing Squamous Cell Carcinoma. Rom. J. Morphol. Embryol. 2019, 60, 139–143. [Google Scholar] [PubMed]
- Nagashio, R.; Ueda, J.; Ryuge, S.; Nakashima, H.; Jiang, S.-X.; Kobayashi, M.; Yanagita, K.; Katono, K.; Satoh, Y.; Masuda, N.; et al. Diagnostic and Prognostic Significances of MUC5B and TTF-1 Expressions in Resected Non-Small Cell Lung Cancer. Sci. Rep. 2015, 5, 8649. [Google Scholar] [CrossRef] [PubMed]
- Veena, V.S.; Saritha, V.N.; George, P.S.; Rajan, K.; Jayasree, K.; Sujathan, K. Immunoexpression of Ttf1 and P63 Differentiates Lung Adenocarcinomas in Sputum Samples. J. Cytol. 2021, 38, 151. [Google Scholar] [CrossRef] [PubMed]
- Zito Marino, F.; Bianco, R.; Accardo, M.; Ronchi, A.; Cozzolino, I.; Morgillo, F.; Rossi, G.; Franco, R. Molecular Heterogeneity in Lung Cancer: From Mechanisms of Origin to Clinical Implications. Int. J. Med. Sci. 2019, 16, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Tanaka, N. The Hedgehog Signaling Networks in Lung Cancer: The Mechanisms and Roles in Tumor Progression and Implications for Cancer Therapy. BioMed Res. Int. 2016, 2016, e7969286. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Gazdar, A. Pathogenesis of Lung Cancer Signaling Pathways: Roadmap for Therapies. Eur. Respir. J. 2009, 33, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.C.; Stehr, H.; Liang, Y.; Das, M.; Huang, J.; Diehn, M.; Wakelee, H.A.; Neal, J.W. ERBB2-Mutated Metastatic Non-Small Cell Lung Cancer: Response and Resistance to Targeted Therapies. J. Thorac. Oncol. 2017, 12, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Varella-Garcia, M.; Bunn, P.A.; Di Maria, M.V.; Veve, R.; Bremnes, R.M.; Barón, A.E.; Zeng, C.; Franklin, W.A. Epidermal Growth Factor Receptor in Non–Small-Cell Lung Carcinomas: Correlation Between Gene Copy Number and Protein Expression and Impact on Prognosis. JCO 2003, 21, 3798–3807. [Google Scholar] [CrossRef]
- Reiter, J.L.; Maihle, N.J. Characterization and Expression of Novel 60-KDa and 110-KDa EGFR Isoforms in Human Placenta. Ann. New York Acad. Sci. 2003, 995, 39–47. [Google Scholar] [CrossRef]
- Roviello, G.; D’Angelo, A.; Sirico, M.; Pittacolo, M.; Conter, F.U.; Sobhani, N. Advances in Anti-BRAF Therapies for Lung Cancer. Investig. New Drugs 2021, 39, 879–890. [Google Scholar] [CrossRef]
- Rowinsky, E.K. The ErbB Family: Targets for Therapeutic Development Against Cancer and Therapeutic Strategies Using Monoclonal Antibodies and Tyrosine Kinase Inhibitors. Annu. Rev. Med. 2004, 55, 433–457. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.V.; Bell, D.W.; Settleman, J.; Haber, D.A. Epidermal Growth Factor Receptor Mutations in Lung Cancer. Nat. Rev. Cancer 2007, 7, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Feldman, R.; Sukari, A.; Kim, C.; Mamdani, H.; Spira, A.I.; Bepler, G.; Kim, E.S.; Raez, L.E.; Pai, S.G.; et al. Characterization of ERBB2 Alterations in Non-Small Cell Lung Cancer. JCO 2020, 38, e21553. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schreiner, J.; Müller, P.; Herzig, P.; Roller, A.; Belousov, A.; Umana, P.; Pisa, P.; Klein, C.; Bacac, M.; et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol. Res. 2015, 3, 1344–1355. [Google Scholar] [CrossRef]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef]
- BRAF-Serine/Threonine-Protein Kinase B-Raf-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P15056/entry (accessed on 22 November 2022).
- EGFR-Epidermal Growth Factor Receptor-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P00533/entry (accessed on 22 November 2022).
- ERBB2-Receptor Tyrosine-Protein Kinase ErbB-2-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P04626/entry (accessed on 22 November 2022).
- Cao, L.-H.; Qiao, J.-Y.; Huang, H.-Y.; Fang, X.-Y.; Zhang, R.; Miao, M.-S.; Li, X.-M. PI3K–AKT Signaling Activation and Icariin: The Potential Effects on the Perimenopausal Depression-Like Rat Model. Molecules 2019, 24, 3700. [Google Scholar] [CrossRef]
- Cheng, K.T.; Chakrabart, A.; Aruva, M.R.; Thakur, M.L.; Wickstrom, E. 64Cu-N,N’-Bis(S-Benzoyl-Thioglycoloyl)Diaminopropanoate-KRAS-PNA-d(Cys-Ser-Lys-Cys). In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Du, X.; Shao, Y.; Qin, H.; Tai, Y.; Gao, H. ALK-rearrangement in Non-small-cell Lung Cancer (NSCLC). Thorac. Cancer 2018, 9, 423–430. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The Evolution of Phosphatidylinositol 3-Kinases as Regulators of Growth and Metabolism. Nat. Rev. Genet 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Maeda, Y.; Davé, V.; Whitsett, J.A. Transcriptional Control of Lung Morphogenesis. Physiol. Rev. 2007, 87, 219–244. [Google Scholar] [CrossRef]
- Reck, M.; Carbone, D.P.; Garassino, M.; Barlesi, F. Targeting KRAS in Non-Small-Cell Lung Cancer: Recent Progress and New Approaches. Ann. Oncol. 2021, 32, 1101–1110. [Google Scholar] [CrossRef]
- Tanaka, H.; Yanagisawa, K.; Shinjo, K.; Taguchi, A.; Maeno, K.; Tomida, S.; Shimada, Y.; Osada, H.; Kosaka, T.; Matsubara, H.; et al. Lineage-Specific Dependency of Lung Adenocarcinomas on the Lung Development Regulator TTF-1. Cancer Res. 2007, 67, 6007–6011. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, L.; Zhou, C. Lung Cancer in China: Current and Prospect. Curr. Opin. Oncol. 2021, 33, 40–46. [Google Scholar] [CrossRef] [PubMed]
- ALK-ALK Tyrosine Kinase Receptor-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9UM73/entry (accessed on 22 November 2022).
- KRAS-GTPase KRas-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P01116/entry (accessed on 22 November 2022).
- PIK3CA-Phosphatidylinositol 4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha Isoform—Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P42336/entry (accessed on 22 November 2022).
- TITF1-Thyroid Transcription Factor 1-Canis Lupus Familiaris (Dog) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P43698/entry (accessed on 22 November 2022).
- Bhateja, P.; Chiu, M.; Wildey, G.; Lipka, M.B.; Fu, P.; Yang, M.C.L.; Ardeshir-Larijani, F.; Sharma, N.; Dowlati, A. Retinoblastoma Mutation Predicts Poor Outcomes in Advanced Non Small Cell Lung Cancer. Cancer Med. 2019, 8, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Farkas, M.; McMahon, S. Unlocking P53 Response Elements: DNA Shape Is the Key. Mol. Cell. Oncol. 2021, 8, 1905489. [Google Scholar] [CrossRef]
- Gazzeri, S.; Brambilla, E.; Chauvin, C.; Jacrot, M.; Benabid, A.L.; Brambilla, C. Analysis of the Activation of the Myc Family Oncogene and of Its Stability over Time in Xenografted Human Lung Carcinomas. Cancer Res. 1990, 50, 1566–1570. [Google Scholar]
- Offin, M.; Chan, J.M.; Tenet, M.; Rizvi, H.A.; Shen, R.; Riely, G.J.; Rekhtman, N.; Daneshbod, Y.; Quintanal-Villalonga, A.; Penson, A.; et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at Risk for Histologic Transformation and Inferior Clinical Outcomes. J. Thorac. Oncol. 2019, 14, 1784–1793. [Google Scholar] [CrossRef]
- Olivier, M.; Petitjean, A.; Marcel, V.; Pétré, A.; Mounawar, M.; Plymoth, A.; de Fromentel, C.C.; Hainaut, P. Recent Advances in P53 Research: An Interdisciplinary Perspective. Cancer Gene Ther. 2009, 16, 1–12. [Google Scholar] [CrossRef]
- Sung, H.-J.; Cho, J.-Y. Biomarkers for the Lung Cancer Diagnosis and Their Advances in Proteomics. BMB Reports 2008, 41, 615–625. [Google Scholar] [CrossRef]
- Wohlhieter, C.A.; Richards, A.L.; Uddin, F.; Hulton, C.H.; Quintanal-Villalonga, À.; Martin, A.; de Stanchina, E.; Bhanot, U.; Asher, M.; Shah, N.S.; et al. Concurrent Mutations in STK11 and KEAP1 Promote Ferroptosis Protection and SCD1 Dependence in Lung Cancer. Cell Reports 2020, 33, 108444. [Google Scholar] [CrossRef]
- MYC-Myc Proto-Oncogene Protein-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P01106/entry (accessed on 22 November 2022).
- MYCL-Protein L-Myc-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P12524/entry (accessed on 22 November 2022).
- MYCN-N-Myc Proto-Oncogene Protein-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P04198/entry (accessed on 22 November 2022).
- RB1-Retinoblastoma-Associated Protein-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P06400/entry (accessed on 22 November 2022).
- STK11-Serine/Threonine-Protein Kinase STK11-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q15831/entry (accessed on 22 November 2022).
- TP53-Cellular Tumor Antigen P53-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P04637/entry (accessed on 22 November 2022).
- Danial, N.N. BCL-2 Family Proteins: Critical Checkpoints of Apoptotic Cell Death. Clin. Cancer Res. 2007, 13, 7254–7263. [Google Scholar] [CrossRef]
- Gavory, G.; Farrow, M.; Balasubramanian, S. Minimum Length Requirement of the Alignment Domain of Human Telomerase RNA to Sustain Catalytic Activity in Vitro. Nucleic Acids Res. 2002, 30, 4470–4480. [Google Scholar] [CrossRef]
- Groeger, A.M.; Esposito, V.; De Luca, A.; Cassandro, R.; Tonini, G.; Ambrogi, V.; Baldi, F.; Goldfarb, R.; Mineo, T.C.; Baldi, A.; et al. Prognostic Value of Immunohistochemical Expression of P53, Bax, Bcl-2 and Bcl-XL in Resected Non-Small-Cell Lung Cancers. Histopathology 2004, 44, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Porębska, I.; Wyrodek, E.; Kosacka, M.; Adamiak, J.; Jankowska, R.; Harłozińska-Szmyrka, A. Apoptotic Markers P53, Bcl-2 and Bax in Primary Lung Cancer. In Vivo 2006, 20, 599–604. [Google Scholar]
- Qi, R.; Wang, D.; Wu, F.; Zhang, S. Correlations between Fas/FasL Expression and Apoptosis as Well as Clinicopathological Features in Non-Small Cell Lung Cancer. Int. J. Clin. Exp. Med. 2020, 6, 4251–4256. [Google Scholar]
- Viard-Leveugle, I.; Veyrenc, S.; French, L.E.; Brambilla, C.; Brambilla, E. Frequent Loss of Fas Expression and Function in Human Lung Tumours with Overexpression of FasL in Small Cell Lung Carcinoma. J. Pathol. 2003, 201, 268–277. [Google Scholar] [CrossRef] [PubMed]
- 12789-1-AP. Available online: https://www.ptglab.com/products/BCL2-Antibody-12789-1-AP.htm (accessed on 22 November 2022).
- 50599-2-Ig. Available online: https://www.ptglab.com/products/BAX-Antibody-50599-2-Ig.htm (accessed on 22 November 2022).
- BAX-Apoptosis Regulator BAX-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q07812/entry (accessed on 22 November 2022).
- BCL2-Apoptosis Regulator Bcl-2-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P10415/entry (accessed on 22 November 2022).
- E2F1-Transcription Factor E2F1-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q01094/entry (accessed on 22 November 2022).
- FASLG-Tumor Necrosis Factor Ligand Superfamily Member 6-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P48023/entry (accessed on 22 November 2022).
- STK11 Gene: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/gene/stk11/ (accessed on 22 November 2022).
- TERT-Telomerase Reverse Transcriptase-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/O14746/entry (accessed on 22 November 2022).
- Das, P.M.; Singal, R. DNA Methylation and Cancer. JCO 2004, 22, 4632–4642. [Google Scholar] [CrossRef]
- Duruisseaux, M.; Esteller, M. Lung Cancer Epigenetics: From Knowledge to Applications. Semin. Cancer Biol. 2018, 51, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.M.; Kratzke, R.A.; Kelsey, K.T. Epigenetics of Lung Cancer. Transl. Res. 2015, 165, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Sauerwald, A.; Sandin, S.; Cristofari, G.; Scheres, S.H.W.; Lingner, J.; Rhodes, D. Structure of Active Dimeric Human Telomerase. Nat. Struct Mol. Biol. 2013, 20, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-X.; Sheng, D.-Q.; Cheng, L.; Song, X.-Y. Current Landscape of Epigenetics in Lung Cancer: Focus on the Mechanism and Application. J. Oncol. 2019, 2019, e8107318. [Google Scholar] [CrossRef]
- Wajed, S.A.; Laird, P.W.; DeMeester, T.R. DNA Methylation: An Alternative Pathway to Cancer. Ann. Surg 2001, 234, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Debernardi, C.; Libera, L.; Berrino, E.; Sahnane, N.; Chiaravalli, A.M.; Laudi, C.; Berselli, M.; Sapino, A.; Sessa, F.; Venesio, T.; et al. Evaluation of Global and Intragenic Hypomethylation in Colorectal Adenomas Improves Patient Stratification and Colorectal Cancer Risk Prediction. Clin. Epigenetics 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA Hypomethylation in Cancer Cells. Epigenomics 2009, 1, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Lujambio, A. CpG Island Hypermethylation of Tumor Suppressor MicroRNAs in Human Cancer. Cell Cycle 2007, 6, 1454–1458. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA Methylation Landscape of Cancer. Trends Genet. 2021, 37, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, W.; Claret, F.X. Mutual Regulation of MicroRNAs and DNA Methylation in Human Cancers. Epigenetics 2017, 12, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Wedge, E.; Hansen, J.W.; Garde, C.; Asmar, F.; Tholstrup, D.; Kristensen, S.S.; Munch-Petersen, H.D.; Ralfkiaer, E.; Brown, P.; Grønbæk, K.; et al. Global Hypomethylation Is an Independent Prognostic Factor in Diffuse Large B Cell Lymphoma. Am. J. Hematol. 2017, 92, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Wong, N.C.; Murone, C.; John, T.; Solomon, B.; Mitchell, P.L.; Dobrovic, A. A Critical Re-Assessment of DNA Repair Gene Promoter Methylation in Non-Small Cell Lung Carcinoma. Sci. Rep. 2014, 4, 4186. [Google Scholar] [CrossRef]
- Feng, L.; Chen, C.; Li, L. Hypermethylation of Tumor Suppressor Genes Is a Risk Factor for Poor Prognosis in Ovarian Cancer: A Meta-Analysis. Medicine 2019, 98, e14588. [Google Scholar] [CrossRef]
- Ng, J.M.-K.; Yu, J. Promoter Hypermethylation of Tumour Suppressor Genes as Potential Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2015, 16, 2472–2496. [Google Scholar] [CrossRef]
- Tam, K.W.; Zhang, W.; Soh, J.; Stastny, V.; Chen, M.; Sun, H.; Thu, K.; Rios, J.J.; Yang, C.; Marconett, C.N.; et al. CDKN2A/P16 Inactivation Mechanisms and Their Relationship to Smoke Exposure and Molecular Features in Non-Small-Cell Lung Cancer. J. Thorac. Oncol. 2013, 8, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.F.H.; Rozynek, P.; Casjens, S.; Werner, R.; Mairinger, F.D.; Speel, E.J.M.; Zur Hausen, A.; Meier, S.; Wohlschlaeger, J.; Theegarten, D.; et al. Methylation of L1RE1, RARB, and RASSF1 Function as Possible Biomarkers for the Differential Diagnosis of Lung Cancer. PLoS ONE 2018, 13, e0195716. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, K.H.; Szmyd, B.; Barańska, M.; Kaszkowiak, M.; Kordiak, J.; Antczak, A.; Pastuszak-Lewandoska, D.; Brzeziańska-Lasota, E. A Strong Decrease in TIMP3 Expression Mediated by the Presence of MiR-17 and 20a Enables Extracellular Matrix Remodeling in the NSCLC Lesion Surroundings. Front. Oncol. 2019, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lu, J.; Cui, T.; Lu, C.; Shi, H.; Xu, W.; Yuan, X.; Yang, X.; Huang, Y.; Lu, M. Association between MGMT Promoter Methylation and Non-Small Cell Lung Cancer: A Meta-Analysis. PLoS ONE 2013, 8, e72633. [Google Scholar] [CrossRef]
- Su, C.-W.; Chang, Y.-C.; Chien, M.-H.; Hsieh, Y.-H.; Chen, M.-K.; Lin, C.-W.; Yang, S.-F. Loss of TIMP3 by Promoter Methylation of Sp1 Binding Site Promotes Oral Cancer Metastasis. Cell Death Dis. 2019, 10, 793. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, M.; Zhang, X.; Cheng, D.; Ma, X. Clinical Significance of DAPK Promoter Hypermethylation in Lung Cancer: A Meta-Analysis. Drug Des. Devel. Ther. 2015, 9, 1785–1796. [Google Scholar] [CrossRef]
- Pu, W.; Geng, X.; Chen, S.; Tan, L.; Tan, Y.; Wang, A.; Lu, Z.; Guo, S.; Chen, X.; Wang, J. Aberrant Methylation of CDH13 Can Be a Diagnostic Biomarker for Lung Adenocarcinoma. J. Cancer 2016, 7, 2280. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Yang, J.; Li, B.; Wang, J. CDH13 Promoter Methylation Regulates Cisplatin Resistance of Non-Small Cell Lung Cancer Cells. Oncol. Lett. 2018, 16, 5715–5722. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, Q.; Chen, L.; Liu, S. Clinicopathological Significance and Potential Drug Targeting of CDH1 in Lung Cancer: A Meta-Analysis and Literature Review. Drug Des. Devel. Ther. 2015, 9, 2171–2178. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Huang, G.; Xu, S. Clinicopathological Significance of DAPK Promoter Methylation in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Cancer Manag. Res. 2018, 10, 6897–6904. [Google Scholar] [CrossRef]
- Bajbouj, K.; Al-Ali, A.; Ramakrishnan, R.K.; Saber-Ayad, M.; Hamid, Q. Histone Modification in NSCLC: Molecular Mechanisms and Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 11701. [Google Scholar] [CrossRef]
- Barlési, F.; Giaccone, G.; Gallegos-Ruiz, M.I.; Loundou, A.; Span, S.W.; Lefesvre, P.; Kruyt, F.A.E.; Rodriguez, J.A. Global Histone Modifications Predict Prognosis of Resected Non Small-Cell Lung Cancer. J. Clin. Oncol. 2007, 25, 4358–4364. [Google Scholar] [CrossRef] [PubMed]
- Seligson, D.B.; Horvath, S.; McBrian, M.A.; Mah, V.; Yu, H.; Tze, S.; Wang, Q.; Chia, D.; Goodglick, L.; Kurdistani, S.K. Global Levels of Histone Modifications Predict Prognosis in Different Cancers. Am. J. Pathol. 2009, 174, 1619–1628. [Google Scholar] [CrossRef]
- Van Den Broeck, A.; Brambilla, E.; Moro-Sibilot, D.; Lantuejoul, S.; Brambilla, C.; Eymin, B.; Gazzeri, S. Loss of Histone H4K20 Trimethylation Occurs in Preneoplasia and Influences Prognosis of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2008, 14, 7237–7245. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Li, J.; Sun, Z.; Liu, D.; Zeng, B.; Zhao, Q.; Wang, J.; Xing, H.R. Cdh1 Functions as an Oncogene by Inducing Self-Renewal of Lung Cancer Stem-like Cells via Oncogenic Pathways. Int. J. Biol. Sci. 2020, 16, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shilatifard, A. Epigenetic Modifications of Histones in Cancer. Genome Biol. 2019, 20, 245. [Google Scholar] [CrossRef] [PubMed]
- Asakura, K.; Kadota, T.; Matsuzaki, J.; Yoshida, Y.; Yamamoto, Y.; Nakagawa, K.; Takizawa, S.; Aoki, Y.; Nakamura, E.; Miura, J.; et al. A MiRNA-Based Diagnostic Model Predicts Resectable Lung Cancer in Humans with High Accuracy. Commun. Biol. 2020, 3, 134. [Google Scholar] [CrossRef]
- Gajda, E.; Grzanka, M.; Godlewska, M.; Gawel, D. The Role of MiRNA-7 in the Biology of Cancer and Modulation of Drug Resistance. Pharmaceuticals (Basel) 2021, 14, 149. [Google Scholar] [CrossRef]
- Zhao, J.; Tao, Y.; Zhou, Y.; Qin, N.; Chen, C.; Tian, D.; Xu, L. MicroRNA-7: A Promising New Target in Cancer Therapy. Cancer Cell Int. 2015, 15, 103. [Google Scholar] [CrossRef]
- Zhong, S.; Golpon, H.; Zardo, P.; Borlak, J. MiRNAs in Lung Cancer. A Systematic Review Identifies Predictive and Prognostic MiRNA Candidates for Precision Medicine in Lung Cancer. Transl. Res. 2021, 230, 164–196. [Google Scholar] [CrossRef]
- Zhu, X.; Kudo, M.; Huang, X.; Sui, H.; Tian, H.; Croce, C.M.; Cui, R. Frontiers of MicroRNA Signature in Non-Small Cell Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 643942. [Google Scholar] [CrossRef] [PubMed]
- Acunzo, M.; Romano, G.; Palmieri, D.; Laganá, A.; Garofalo, M.; Balatti, V.; Drusco, A.; Chiariello, M.; Nana-Sinkam, P.; Croce, C.M. Cross-Talk between MET and EGFR in Non-Small Cell Lung Cancer Involves MiR-27a and Sprouty2. Proc. Natl. Acad. Sci. USA 2013, 110, 8573–8578. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Rao, Z.; Chen, C. MiR-30a Suppresses Lung Cancer Progression by Targeting SIRT1. Oncotarget 2017, 9, 4924–4934. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, M.; Ding, L.; Tang, J. MiR-27a: A Novel Biomarker and Potential Therapeutic Target in Tumors. J. Cancer 2019, 10, 2836–2848. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Z.; Yang, G.; You, L.; Zhang, T.; Zhao, Y. MicroRNA-27a (MiR-27a) in Solid Tumors: A Review Based on Mechanisms and Clinical Observations. Front. Oncol. 2019, 9, 893. [Google Scholar] [CrossRef]
- MIR27A MicroRNA 27a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/407018 (accessed on 22 November 2022).
- MIR30A MicroRNA 30a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/407029 (accessed on 22 November 2022).
- Hu, J.; Cheng, Y.; Li, Y.; Jin, Z.; Pan, Y.; Liu, G.; Fu, S.; Zhang, Y.; Feng, K.; Feng, Y. MicroRNA-128 Plays a Critical Role in Human Non-Small Cell Lung Cancer Tumourigenesis, Angiogenesis and Lymphangiogenesis by Directly Targeting Vascular Endothelial Growth Factor-C. Eur. J. Cancer 2014, 50, 2336–2350. [Google Scholar] [CrossRef]
- Li, L.; Wang, D. MicroRNA-128-b Regulates Epidermal Growth Factor Receptor Expression in Non-Small Cell Lung Cancer. Mol. Med. Rep. 2019, 20, 4803–4810. [Google Scholar] [CrossRef]
- PubChem MIR128-1-MicroRNA 128-1 (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/MIR128-1/human (accessed on 22 November 2022).
- Rupaimoole, R.; Yoon, B.; Zhang, W.C.; Adams, B.D.; Slack, F.J. A High-Throughput Small Molecule Screen Identifies Ouabain as Synergistic with MiR-34a in Killing Lung Cancer Cells. iScience 2020, 23, 100878. [Google Scholar] [CrossRef]
- Song, K.; Jiang, Y.; Zhao, Y.; Xie, Y.; Zhou, J.; Yu, W.; Wang, Q. Members of the MiR-30 Family Inhibit the Epithelial-to-Mesenchymal Transition of Non-Small-Cell Lung Cancer Cells by Suppressing XB130 Expression Levels. Oncol. Lett. 2020, 20, 68. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Tang, L. MicroRNA-34 Family: A Potential Tumor Suppressor and Therapeutic Candidate in Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 53. [Google Scholar] [CrossRef]
- MIR34A MicroRNA 34a [ Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/407040 (accessed on 22 November 2022).
- Liao, Y.; Wu, X.; Wu, M.; Fang, Y.; Li, J.; Tang, W. Non-Coding RNAs in Lung Cancer: Emerging Regulators of Angiogenesis. J. Transl. Med. 2022, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Zheng, M.; Ge, J.; Li, J.; Yu, P. MiR-542-3p Exerts Tumor Suppressive Functions in Non-Small Cell Lung Cancer Cells by Upregulating FTSJ2. Life Sci. 2017, 188, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.-Y.; Zhang, F.; Sun, C.-C.; Li, S.-J.; Li, G.; Gong, F.-Y.; Bo, T.; He, J.; Hua, R.-X.; Hu, W.-D.; et al. MiR-134: A Human Cancer Suppressor? Mol. Ther. Nucleic Acids 2017, 6, 140–149. [Google Scholar] [CrossRef] [PubMed]
- PubChem MIR542-MicroRNA 542 (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/MIR542/human (accessed on 22 November 2022).
- Qin, Q.; Wei, F.; Zhang, J.; Li, B. MiR-134 Suppresses the Migration and Invasion of Non-small Cell Lung Cancer by Targeting ITGB1. Oncol. Rep. 2017, 37, 823–830. [Google Scholar] [CrossRef] [PubMed]
- MIR134 MicroRNA 134 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406924 (accessed on 22 November 2022).
- Chu, D.; Li, J.; Lin, H.; Zhang, X.; Pan, H.; Liu, L.; Yu, T.; Yan, M.; Yao, M. Quantitative Proteomic Analysis of the MiR-148a-Associated Mechanisms of Metastasis in Non-Small Cell Lung Cancer. Oncol. Lett. 2018, 15, 9941–9952. [Google Scholar] [CrossRef]
- He, R.; Li, X.; Liang, L.; Xie, Y.; Luo, D.; Ma, J.; Peng, Z.; Hu, X.; Chen, G. The Suppressive Role of MiR-542-5p in NSCLC: The Evidence from Clinical Data and in Vivo Validation Using a Chick Chorioallantoic Membrane Model. BMC Cancer 2017, 17, 655. [Google Scholar] [CrossRef]
- Liang, L.; Xu, W.; Shen, A.; Cen, H.; Chen, Z.; Tan, L.; Zhang, L.; Zhang, Y.; Fu, J.; Qin, A.; et al. Promoter Methylation-Regulated MiR-148a-3p Inhibits Lung Adenocarcinoma (LUAD) Progression by Targeting MAP3K9. Acta Pharmacol. Sin. 2022, 43, 2946–2955. [Google Scholar] [CrossRef]
- Qian, M.; Xia, Y.; Zhang, G.; Yu, H.; Cui, Y. Research Progress on MicroRNA-1258 in the Development of Human Cancer. Front. Oncol. 2022, 12, 1024234. [Google Scholar] [CrossRef]
- MicroRNA-1258 Suppresses Tumour Progression via GRB2/Ras/Erk Pathway in Non-small-cell Lung Cancer; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022. [CrossRef]
- MIR148A MicroRNA 148a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406940 (accessed on 22 November 2022).
- MIR1258 MicroRNA 1258 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/100302172 (accessed on 22 November 2022).
- Braicu, C.; Gulei, D.; Cojocneanu, R.; Raduly, L.; Jurj, A.; Knutsen, E.; Calin, G.A.; Berindan-Neagoe, I. MiR-181a/b Therapy in Lung Cancer: Reality or Myth? Mol. Oncol. 2019, 13, 9–25. [Google Scholar] [CrossRef]
- Ding, H.; Chu, M.; Yue, J.; Huang, H.; Wang, J.; Zhu, L. MiR-96 Induced Non-Small-Cell Lung Cancer Progression through Competing Endogenous RNA Network and Affecting EGFR Signaling Pathway. Iran J. Basic Med. Sci. 2019, 22, 908–914. [Google Scholar] [CrossRef]
- Fan, Q.; Hu, X.; Zhang, H.; Wang, S.; Zhang, H.; You, C.; Zhang, C.-Y.; Liang, H.; Chen, X.; Ba, Y. MiR-193a-3p Is an Important Tumour Suppressor in Lung Cancer and Directly Targets KRAS. Cell Physiol. Biochem. 2017, 44, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tang, R.; Xie, Q.; Lin, J.; Shi, H.; Chen, G.; Li, Z. The Clinical Value of MiR-193a-3p in Non-small Cell Lung Cancer and Its Potential Molecular Mechanism Explored in Silico Using RNA-sequencing and Microarray Data. FEBS Open Bio. 2018, 8, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wang, L.; Chen, W.; Wang, L. Mechanism of MiR-760 Reversing Lung Cancer Immune Escape by Downregulating IDO1 and Eliminating Regulatory T Cells Based on Mathematical Biology. Comput. Math Methods Med. 2022, 2022, 2960773. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhou, Z.; Ye, N.; Chen, Q.; Zheng, X.; Fang, M. MiR-181a Inhibits Non-Small Cell Lung Cancer Cell Proliferation by Targeting CDK1. Cancer Biomark 2017, 20, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, J.; Mei, S.; Wu, D.; Mu, Z.; Chen, B.; Xie, Y.; Ye, Y.; Liu, J. Circulating Exosomal MicroRNA-96 Promotes Cell Proliferation, Migration and Drug Resistance by Targeting LMO7. J. Cell Mol. Med. 2017, 21, 1228–1236. [Google Scholar] [CrossRef]
- Xue, B.; Chuang, C.-H.; Prosser, H.M.; Fuziwara, C.S.; Chan, C.; Sahasrabudhe, N.; Kühn, M.; Wu, Y.; Chen, J.; Biton, A.; et al. MiR-200 Deficiency Promotes Lung Cancer Metastasis by Activating Notch Signaling in Cancer-Associated Fibroblasts. Genes Dev. 2021, 35, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, W.; Shi, X.; Zheng, J.; Jin, X.; Huo, J. MiR-760 Suppresses Non-Small Cell Lung Cancer Proliferation and Metastasis by Targeting ROS1. Environ Sci Pollut Res Int 2018, 25, 18385–18391. [Google Scholar] [CrossRef]
- MIR96 MicroRNA 96 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/407053 (accessed on 22 November 2022).
- MIR193A MicroRNA 193a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406968 (accessed on 22 November 2022).
- MIR200C MicroRNA 200c [ Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/406985 (accessed on 22 November 2022).
- MIR760 MicroRNA 760 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/100126348 (accessed on 22 November 2022).
- Chen, J.; Cui, J.; Guo, X.; Cao, X.; Li, Q. Increased Expression of MiR-641 Contributes to Erlotinib Resistance in Non-Small-Cell Lung Cancer Cells by Targeting NF1. Cancer Med. 2018, 7, 1394–1403. [Google Scholar] [CrossRef]
- Fang, X.; Shi, H.; Sun, F. The MicroRNA-520a-3p Inhibits Invasion and Metastasis by Targeting NF-KappaB Signaling Pathway in Non-Small Cell Lung Cancer. Clin. Transl. Oncol. 2022, 24, 1569–1579. [Google Scholar] [CrossRef]
- Kong, Q.; Shu, N.; Li, J.; Xu, N. MiR-641 Functions as a Tumor Suppressor by Targeting MDM2 in Human Lung Cancer. Oncol. Res. 2018, 26, 735–741. [Google Scholar] [CrossRef]
- Li, J.; Tan, Q.; Yan, M.; Liu, L.; Lin, H.; Zhao, F.; Bao, G.; Kong, H.; Ge, C.; Zhang, F.; et al. MiRNA-200c Inhibits Invasion and Metastasis of Human Non-Small Cell Lung Cancer by Directly Targeting Ubiquitin Specific Peptidase 25. Mol. Cancer 2014, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-L.; Liu, W.-L.; Chang, J.-M.; Chen, Y.-H.; Liu, Y.-P.; Kuo, H.-F.; Hsieh, C.-C.; Ding, Y.-S.; Chen, W.-W.; Chong, I.-W. MicroRNA-200c Inhibits Epithelial-Mesenchymal Transition, Invasion, and Migration of Lung Cancer by Targeting HMGB1. PLoS ONE 2017, 12, e0180844. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xue, C.; Guo, S.; Yang, L. MicroRNA-520a Suppresses Pathogenesis and Progression of Non-Small-Cell Lung Cancer through Targeting the RRM2/Wnt Axis. Anal. Cell Pathol. (Amst) 2021, 2021, 9652420. [Google Scholar] [CrossRef]
- MicroRNA-520a-3p Inhibits Cell Growth and Metastasis of Non-Small Cell Lung Cancer through PI3K/AKT/MTOR Signaling Pathway. Eur. Rev. 2018, 22, 2321–2327.
- MIR520A MicroRNA 520a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/574467 (accessed on 22 November 2022).
- MIR641 MicroRNA 641 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/693226 (accessed on 22 November 2022).
- MIR660 MicroRNA 660 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/724030 (accessed on 22 November 2022).
- Andriani, F.; Majorini, M.T.; Mano, M.; Landoni, E.; Miceli, R.; Facchinetti, F.; Mensah, M.; Fontanella, E.; Dugo, M.; Giacca, M.; et al. MiR-16 Regulates the pro-Tumorigenic Potential of Lung Fibroblasts through the Inhibition of HGF Production in an FGFR-1- and MEK1-Dependent Manner. J. Hematol. Oncol. 2018, 11, 45. [Google Scholar] [CrossRef]
- Bandi, N.; Zbinden, S.; Gugger, M.; Arnold, M.; Kocher, V.; Hasan, L.; Kappeler, A.; Brunner, T.; Vassella, E. MiR-15a and MiR-16 Are Implicated in Cell Cycle Regulation in a Rb-Dependent Manner and Are Frequently Deleted or down-Regulated in Non-Small Cell Lung Cancer. Cancer Res. 2009, 69, 5553–5559. [Google Scholar] [CrossRef]
- Chatterjee, A.; Chattopadhyay, D.; Chakrabarti, G. MiR-16 Targets Bcl-2 in Paclitaxel-Resistant Lung Cancer Cells and Overexpression of MiR-16 along with MiR-17 Causes Unprecedented Sensitivity by Simultaneously Modulating Autophagy and Apoptosis. Cell Signal. 2015, 27, 189–203. [Google Scholar] [CrossRef]
- Chen, T.; Xiao, Q.; Wang, X.; Wang, Z.; Hu, J.; Zhang, Z.; Gong, Z.; Chen, S. MiR-16 Regulates Proliferation and Invasion of Lung Cancer Cells via the ERK/MAPK Signaling Pathway by Targeted Inhibition of MAPK Kinase 1 (MEK1). J. Int. Med. Res. 2019, 47, 5194–5204. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and MiR-16 Induce Apoptosis by Targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Fortunato, O.; Boeri, M.; Moro, M.; Verri, C.; Mensah, M.; Conte, D.; Caleca, L.; Roz, L.; Pastorino, U.; Sozzi, G. Mir-660 Is Downregulated in Lung Cancer Patients and Its Replacement Inhibits Lung Tumorigenesis by Targeting MDM2-P53 Interaction. Cell Death Dis. 2014, 5, e1564. [Google Scholar] [CrossRef]
- Guo, S.; Li, M.; Li, J.; Lv, Y. Inhibition Mechanism of Lung Cancer Cell Metastasis through Targeted Regulation of Smad3 by MiR-15a. Oncol. Lett. 2020, 19, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Huang, B.; Li, Z.; Li, H.; Sun, L.; Zhang, Q.; Qiu, X.; Wang, E. MicroRNA-449a Is Downregulated in Non-Small Cell Lung Cancer and Inhibits Migration and Invasion by Targeting c-Met. PLoS ONE 2013, 8, e64759. [Google Scholar] [CrossRef] [PubMed]
- PubChem MIR15A-MicroRNA 15a (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/MIR15A/human (accessed on 22 November 2022).
- MIR449A MicroRNA 449a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/554213 (accessed on 22 November 2022).
- Hu, S.; Cao, P.; Kong, K.; Han, P.; Deng, Y.; Li, F.; Zhao, B. MicroRNA-449a Delays Lung Cancer Development through Inhibiting KDM3A/HIF-1α Axis. J. Transl. Med. 2021, 19, 224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, X.; Zhang, T.; Yao, F. MiR-512-5p Suppresses the Progression of Non-small Cell Lung Cancer by Targeting Β-catenin. Oncol. Lett. 2020, 19, 415–423. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Chen, J.; He, H.; Ma, H.; Lv, X. MiR-449a Suppresses Tumor Growth, Migration, and Invasion in Non-Small Cell Lung Cancer by Targeting a HMGB1-Mediated NF-ΚB Signaling Pathway. Oncol. Res. 2019, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ji, A.; Zhang, Z.; Li, J.; Li, P. MiR-491-5p Inhibits the Proliferation and Migration of A549 Cells by FOXP4. Exp. Ther. Med. 2021, 21, 622. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chen, X.; Yuan, B.; Hu, Y. Effect of Mir-299-3p on the Biological Function of Lung Adenocarcinoma Cell H1299 through Targeting PTPRD. Food Sci. Technol. 2022, 42, e43321. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, H.; Wang, L. MiR-491-3p Functions as a Tumor Suppressor in Non-Small Cell Lung Cancer by Targeting Fibroblast Growth Factor 5. Oncol. Rep. 2022, 48, 164. [Google Scholar] [CrossRef]
- Zheng, D.; Dai, Y.; Wang, S.; Xing, X. MicroRNA-299-3p Promotes the Sensibility of Lung Cancer to Doxorubicin through Directly Targeting ABCE1. Int. J. Clin. Exp. Pathol. 2015, 8, 10072–10081. [Google Scholar]
- MIR299 MicroRNA 299 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/407023 (accessed on 22 November 2022).
- MIR491 MicroRNA 491 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/574444 (accessed on 22 November 2022).
- MIR512-1 MicroRNA 512-1 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/574458 (accessed on 22 November 2022).
- Bica-Pop, C.; Cojocneanu-Petric, R.; Magdo, L.; Raduly, L.; Gulei, D.; Berindan-Neagoe, I. Overview upon MiR-21 in Lung Cancer: Focus on NSCLC. Cell Mol. Life Sci. 2018, 75, 3539–3551. [Google Scholar] [CrossRef]
- Chu, K.; Gao, G.; Yang, X.; Ren, S.; Li, Y.; Wu, H.; Huang, Y.; Zhou, C. MiR-512-5p Induces Apoptosis and Inhibits Glycolysis by Targeting P21 in Non-Small Cell Lung Cancer Cells. Int. J. Oncol. 2016, 48, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C.; et al. MicroRNA-29 Family Reverts Aberrant Methylation in Lung Cancer by Targeting DNA Methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, B.; Li, R.; Wang, F.; Wang, N.; Zhang, M.; Bai, Y.; Wu, J.; Liu, L.; Han, D.; et al. MiR-146a-5p Plays an Oncogenic Role in NSCLC via Suppression of TRAF6. Front Cell Dev. Biol. 2020, 8, 847. [Google Scholar] [CrossRef]
- Liu, X.; Lv, X.; Yang, Q.; Jin, H.; Zhou, W.; Fan, Q. MicroRNA-29a Functions as a Tumor Suppressor and Increases Cisplatin Sensitivity by Targeting NRAS in Lung Cancer. Technol. Cancer Res. Treat 2018, 17, 1533033818758905. [Google Scholar] [CrossRef]
- Markou, A.; Zavridou, M.; Lianidou, E.S. MiRNA-21 as a Novel Therapeutic Target in Lung Cancer. Lung Cancer (Auckl) 2016, 7, 19–27. [Google Scholar] [CrossRef]
- PubChem MIR29A-MicroRNA 29a (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/MIR29A/human (accessed on 22 November 2022).
- PubChem MIR146A-MicroRNA 146a (Human). Available online: https://pubchem.ncbi.nlm.nih.gov/gene/MIR146A/human (accessed on 22 November 2022).
- Wani, J.A.; Majid, S.; Khan, A.; Arafah, A.; Ahmad, A.; Jan, B.L.; Shah, N.N.; Kazi, M.; Rehman, M.U. Clinico-Pathological Importance of MiR-146a in Lung Cancer. Diagnostics (Basel) 2021, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, J.; Tao, Y.; Guo, M.; Ya, Z.; Chen, C.; Qin, N.; Zheng, J.; Luo, J.; Xu, L. MicroRNA-21: A Promising Biomarker for the Prognosis and Diagnosis of Non-small Cell Lung Cancer (Review). Oncol. Lett. 2018, 16, 2777–2782. [Google Scholar] [CrossRef]
- MIR21 MicroRNA 21 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406991 (accessed on 22 November 2022).
- Fan, X.; Tao, S.; Li, Q.; Deng, B.; Tan, Q.-Y.; Jin, H. The MiR-23a/27a/24-2 Cluster Promotes Postoperative Progression of Early-Stage Non-Small Cell Lung Cancer. Mol. Ther. Oncolytics 2022, 24, 205–217. [Google Scholar] [CrossRef]
- Feliciano, A.; Garcia-Mayea, Y.; Jubierre, L.; Mir, C.; Hummel, M.; Castellvi, J.; Hernández-Losa, J.; Paciucci, R.; Sansano, I.; Sun, Y.; et al. MiR-99a Reveals Two Novel Oncogenic Proteins E2F2 and EMR2 and Represses Stemness in Lung Cancer. Cell Death Dis. 2017, 8, e3141. [Google Scholar] [CrossRef]
- Hetta, H.F.; Zahran, A.M.; Shafik, E.A.; El-Mahdy, R.I.; Mohamed, N.A.; Nabil, E.E.; Esmaeel, H.M.; Alkady, O.A.; Elkady, A.; Mohareb, D.A.; et al. Circulating MiRNA-21 and MiRNA-23a Expression Signature as Potential Biomarkers for Early Detection of Non-Small-Cell Lung Cancer. Microrna 2019, 8, 206–215. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.-A.; Lin, Y.-S.; Pan, Y.-C.; Tsai, P.-H.; Wu, C.-Y.; Kuo, P.-L. Hypoxic Lung Cancer-Secreted Exosomal MiR-23a Increased Angiogenesis and Vascular Permeability by Targeting Prolyl Hydroxylase and Tight Junction Protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, C.; Dong, F.; Zhang, Y. MiR-99a Suppresses the Metastasis of Human Non-Small Cell Lung Cancer Cells by Targeting AKT1 Signaling Pathway. J. Cell Biochem. 2015, 116, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Zhao, F.; Liu, Q.; Jiang, K.; Yang, G. MicroRNA-21 (MiR-21) Represses Tumor Suppressor PTEN and Promotes Growth and Invasion in Non-Small Cell Lung Cancer (NSCLC). Clin. Chim. Acta 2010, 411, 846–852. [Google Scholar] [CrossRef] [PubMed]
- MIR99A MicroRNA 99a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/407055 (accessed on 22 November 2022).
- MIR155 MicroRNA 155 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406947 (accessed on 23 November 2022).
- Xue, X.; Liu, Y.; Wang, Y.; Meng, M.; Wang, K.; Zang, X.; Zhao, S.; Sun, X.; Cui, L.; Pan, L.; et al. MiR-21 and MiR-155 Promote Non-Small Cell Lung Cancer Progression by Downregulating SOCS1, SOCS6, and PTEN. Oncotarget 2016, 7, 84508–84519. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Liu, L.; Shen, A.; Zheng, W. MicroRNA-155-5p Suppresses the Migration and Invasion of Lung Adenocarcinoma A549 Cells by Targeting Smad2. Oncol. Lett. 2018, 16, 2444–2452. [Google Scholar] [CrossRef]
- The Value of MiR-155 as a Biomarker for the Diagnosis and Prognosis of Lung Cancer: A Systematic Review with Meta-Analysis | BMC Cancer | Full Text. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-019-6297-6 (accessed on 23 November 2022).
- MIR192 MicroRNA 192 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406967 (accessed on 23 November 2022).
- Li, Y.; Zu, L.; Wu, H.; Zhang, F.; Fan, Y.; Pan, H.; Du, X.; Guo, F.; Zhou, Q. MiR-192/NKRF Axis Confers Lung Cancer Cell Chemoresistance to Cisplatin via the NF-κB Pathway. Thorac. Cancer 2022, 13, 430–441. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Lyu, C.; Buchner, A.; Pohla, H. Diagnostic and Prognostic Role of MiR-192 in Different Cancers: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2021, 2021, 8851035. [Google Scholar] [CrossRef]
- Zou, P.; Zhu, M.; Lian, C.; Wang, J.; Chen, Z.; Zhang, X.; Yang, Y.; Chen, X.; Cui, X.; Liu, J.; et al. MiR-192-5p Suppresses the Progression of Lung Cancer Bone Metastasis by Targeting TRIM44. Sci. Rep. 2019, 9, 19619. [Google Scholar] [CrossRef]
- Xu, S.; Shi, L. High Expression of MiR-155 and MiR-21 in the Recurrence or Metastasis of Non-Small Cell Lung Cancer. Oncol. Lett. 2019, 18, 758–763. [Google Scholar] [CrossRef]
- Yang, M.; Shen, H.; Qiu, C.; Ni, Y.; Wang, L.; Dong, W.; Liao, Y.; Du, J. High Expression of MiR-21 and MiR-155 Predicts Recurrence and Unfavourable Survival in Non-Small Cell Lung Cancer. Eur. J. Cancer 2013, 49, 604–615. [Google Scholar] [CrossRef]
- MIR520B MicroRNA 520b [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/574473 (accessed on 23 November 2022).
- Zhang, L.; Yu, S. Role of MiR-520b in Non-Small Cell Lung Cancer. Exp. Ther. Med. 2018, 16, 3987–3995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shen, S. MiR-520f Acts as a Biomarker for the Diagnosis of Lung Cancer. Medicine (Baltimore) 2019, 98, e16546. [Google Scholar] [CrossRef] [PubMed]
- MIR497 MicroRNA 497 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/574456 (accessed on 23 November 2022).
- Zhao, W.; Wang, Y.; An, Z.; Shi, C.; Zhu, G.; Wang, B.; Lu, M.; Pan, C.; Chen, P. Downregulation of MiR-497 Promotes Tumor Growth and Angiogenesis by Targeting HDGF in Non-Small Cell Lung Cancer. Biochem. Biophys. Res. Commun. 2013, 435, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, L.; Liu, W.; Li, F. MicroRNA-497-5p Inhibits Proliferation and Invasion of Non-Small Cell Lung Cancer by Regulating FGF2. Oncol. Lett. 2019, 17, 3425–3431. [Google Scholar] [CrossRef]
- Chae, D.-K.; Park, J.; Cho, M.; Ban, E.; Jang, M.; Yoo, Y.S.; Kim, E.E.; Baik, J.-H.; Song, E.J. MiR-195 and MiR-497 Suppress Tumorigenesis in Lung Cancer by Inhibiting SMURF2-Induced TGF-β Receptor I Ubiquitination. Mol. Oncol. 2019, 13, 2663–2678. [Google Scholar] [CrossRef]
- MIR494 MicroRNA 494 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/574452 (accessed on 23 November 2022).
- Zhang, J.; Wang, T.; Zhang, Y.; Wang, H.; Wu, Y.; Liu, K.; Pei, C. Upregulation of Serum MiR-494 Predicts Poor Prognosis in Non-Small Cell Lung Cancer Patients. Cancer Biomark 2018, 21, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.; Liao, Y.; Chen, N.; Liu, T.; Zhang, H.; Zhang, H. Expression and Clinical Evidence of MiR-494 and PTEN in Non-Small Cell Lung Cancer. Tumour. Biol. 2015, 36, 6965–6972. [Google Scholar] [CrossRef]
- Xu, F.; Liu, G.; Wang, L.; Wang, X.; Jin, X.; Bo, W. MiR-494 Promotes Progression of Retinoblastoma via PTEN through PI3K/AKT Signaling Pathway. Oncol. Lett. 2020, 20, 1952–1960. [Google Scholar] [CrossRef]
- MIR126 MicroRNA 126 [ Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/406913 (accessed on 23 November 2022).
- Chen, Q.; Chen, S.; Zhao, J.; Zhou, Y.; Xu, L. MicroRNA-126: A New and Promising Player in Lung Cancer. Oncol. Lett. 2021, 21, 35. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Zhang, X.; Yan, N.; Li, X. Exosomal MiR-126 Blocks the Development of Non-Small Cell Lung Cancer through the Inhibition of ITGA6. Cancer Cell Int. 2020, 20, 574. [Google Scholar] [CrossRef]
- Liu, B.; Peng, X.-C.; Zheng, X.-L.; Wang, J.; Qin, Y.-W. MiR-126 Restoration down-Regulate VEGF and Inhibit the Growth of Lung Cancer Cell Lines in Vitro and in Vivo. Lung Cancer 2009, 66, 169–175. [Google Scholar] [CrossRef] [PubMed]
- MIR33B MicroRNA 33b [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/693120 (accessed on 23 November 2022).
- Zhai, S.; Zhao, L.; Lin, T.; Wang, W. Downregulation of MiR-33b Promotes Non-small Cell Lung Cancer Cell Growth through Reprogramming Glucose Metabolism MiR-33b Regulates Non-small Cell Lung Cancer Cell Growth. J. Cell Biochem. 2019, 120, 6651–6660. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.I.H.N.; Othman, I.; Naidu, R. The Role of MicroRNAs in Lung Cancer Metabolism. Cancers 2021, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- MIR144 MicroRNA 144 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406936 (accessed on 23 November 2022).
- Morais, M.; Dias, F.; Nogueira, I.; Leão, A.; Gonçalves, N.; Araújo, L.; Granja, S.; Baltazar, F.; Teixeira, A.L.; Medeiros, R. Cancer Cells’ Metabolism Dynamics in Renal Cell Carcinoma Patients’ Outcome: Influence of GLUT-1-Related Hsa-MiR-144 and Hsa-MiR-186. Cancers 2021, 13, 1733. [Google Scholar] [CrossRef]
- LIU, M.; GAO, J.; HUANG, Q.; JIN, Y.; WEI, Z. Downregulating MicroRNA-144 Mediates a Metabolic Shift in Lung Cancer Cells by Regulating GLUT1 Expression. Oncol. Lett. 2016, 11, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- MIR124-1 MicroRNA 124-1 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406907 (accessed on 23 November 2022).
- Zhao, X.; Lu, C.; Chu, W.; Zhang, B.; Zhen, Q.; Wang, R.; Zhang, Y.; Li, Z.; Lv, B.; Li, H.; et al. MicroRNA-124 Suppresses Proliferation and Glycolysis in Non-Small Cell Lung Cancer Cells by Targeting AKT-GLUT1/HKII. Tumour. Biol. 2017, 39, 1010428317706215. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, Q.; Cao, F.; Wang, S.-N.; Wang, R.-T.; Wang, Y.; Tan, Q.-Y.; Li, C.-R.; Zou, H.; Wang, D.; et al. MiR-124 Inhibits Lung Tumorigenesis Induced by K-Ras Mutation and NNK. Mol. Ther. Nucleic Acids 2017, 9, 145–154. [Google Scholar] [CrossRef]
- MIR199A1 MicroRNA 199a-1 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406976 (accessed on 23 November 2022).
- Yang, X.; Zheng, Y.; Tan, J.; Tian, R.; Shen, P.; Cai, W.; Liao, H. MiR-199a-5p–HIF-1α-STAT3 Positive Feedback Loop Contributes to the Progression of Non-Small Cell Lung Cancer. Front Cell Dev. Biol. 2021, 8, 620615. [Google Scholar] [CrossRef]
- Wang, L.-M.; Zhang, L.-L.; Wang, L.-W.; Zhu, L.; Ma, X.-X. Influence of MiR-199a on Rats with Non-Small Cell Lung Cancer via Regulating the HIF-1α/VEGF Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10363–10369. [Google Scholar] [CrossRef]
- MIR31 MicroRNA 31 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/407035 (accessed on 23 November 2022).
- Davenport, M.L.; Echols, J.B.; Silva, A.D.; Anderson, J.C.; Owens, P.; Yates, C.; Wei, Q.; Harada, S.; Hurst, D.R.; Edmonds, M.D. MiR-31 Displays Subtype Specificity in Lung Cancer. Cancer Res. 2021, 81, 1942–1953. [Google Scholar] [CrossRef]
- Zhu, B.; Cao, X.; Zhang, W.; Pan, G.; Yi, Q.; Zhong, W.; Yan, D. MicroRNA-31-5p Enhances the Warburg Effect via Targeting FIH. FASEB J. 2019, 33, 545–556. [Google Scholar] [CrossRef] [PubMed]
- MIR28 MicroRNA 28 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/407020 (accessed on 23 November 2022).
- Cui, F.; Zhou, Q.; Xiao, K.; Qian, H. MicroRNA-28 Promotes the Proliferation of Non-small-cell Lung Cancer Cells by Targeting PTEN. Mol. Med. Rep. 2020, 21, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, W.; Tang, X.; Li, S.; Guo, L.; Lin, Y.; Kwok, H.F. The Roles of MicroRNAs in Regulating the Expression of PD-1/PD-L1 Immune Checkpoint. Int. J. Mol. Sci. 2017, 18, 2540. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Xu, Q.; Fan, X.; Wu, S.; Tian, F. Downregulation of MiR-138-5p Promotes Non-small Cell Lung Cancer Progression by Regulating CDK8. Mol. Med. Rep. 2019, 20, 5272–5278. [Google Scholar] [CrossRef]
- Song, N.; Li, P.; Song, P.; Li, Y.; Zhou, S.; Su, Q.; Li, X.; Yu, Y.; Li, P.; Feng, M.; et al. MicroRNA-138-5p Suppresses Non-Small Cell Lung Cancer Cells by Targeting PD-L1/PD-1 to Regulate Tumor Microenvironment. Front. Cell Dev. Biol. 2020, 8, 540. [Google Scholar] [CrossRef]
- MIR197 MicroRNA 197 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406974 (accessed on 23 November 2022).
- Fujita, Y.; Yagishita, S.; Hagiwara, K.; Yoshioka, Y.; Kosaka, N.; Takeshita, F.; Fujiwara, T.; Tsuta, K.; Nokihara, H.; Tamura, T.; et al. The Clinical Relevance of the MiR-197/CKS1B/STAT3-Mediated PD-L1 Network in Chemoresistant Non-Small-Cell Lung Cancer. Mol. Ther. 2015, 23, 717–727. [Google Scholar] [CrossRef]
- Mavridis, K.; Gueugnon, F.; Petit-Courty, A.; Courty, Y.; Barascu, A.; Guyetant, S.; Scorilas, A. The OncomiR MiR-197 Is a Novel Prognostic Indicator for Non-Small Cell Lung Cancer Patients. Br. J. Cancer 2015, 112, 1527–1535. [Google Scholar] [CrossRef]
- MIR513A1 MicroRNA 513a-1 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/574509 (accessed on 23 November 2022).
- Zhang, X.; Zhu, J.; Xing, R.; Tie, Y.; Fu, H.; Zheng, X.; Yu, B. MiR-513a-3p Sensitizes Human Lung Adenocarcinoma Cells to Chemotherapy by Targeting GSTP1. Lung Cancer 2012, 77, 488–494. [Google Scholar] [CrossRef]
- Wang, J.; Sheng, Z.; Cai, Y. Effects of MicroRNA-513b on Cell Proliferation, Apoptosis, Invasion, and Migration by Targeting HMGB3 through Regulation of MTOR Signaling Pathway in Non-Small-Cell Lung Cancer. J. Cell. Physiol. 2019, 234, 10934–10941. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Lee, J.-M.; Jang, K.-J. Mechanistic Insights of Anti-Immune Evasion by Nobiletin through Regulating MiR-197/STAT3/PD-L1 Signaling in Non-Small Cell Lung Cancer (NSCLC) Cells. Int. J. Mol. Sci. 2021, 22, 9843. [Google Scholar] [CrossRef]
- MIR20A MicroRNA 20a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406982 (accessed on 23 November 2022).
- Gong, J.; Shen, Y.; Jiang, F.; Wang, Y.; Chu, L.; Sun, J.; Shen, P.; Chen, M. MicroRNA-20a Promotes Non-Small Cell Lung Cancer Proliferation by Upregulating PD-L1 by Targeting PTEN. Oncol. Lett. 2022, 23, 148. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Fan, X.-X.; Wang, M.-F.; Duan, F.-G.; Wei, C.-L.; Li, R.-Z.; Jiang, Z.-B.; Wang, Y.-W.; Yao, X.-J.; Chen, M.-W.; et al. MiR-20b Promotes Growth of Non-Small Cell Lung Cancer through a Positive Feedback Loop of the Wnt/β-Catenin Signaling Pathway. Int. J. Oncol. 2019, 56, 470–479. [Google Scholar] [CrossRef] [PubMed]
- MIR130A MicroRNA 130a [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406919 (accessed on 23 November 2022).
- Zhang, Q.; Zhang, B.; Sun, L.; Yan, Q.; Zhang, Y.; Zhang, Z.; Su, Y.; Wang, C. MicroRNA-130b Targets PTEN to Induce Resistance to Cisplatin in Lung Cancer Cells by Activating Wnt/Β-catenin Pathway. Cell Biochem. Funct 2018, 36, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.-C.; Wang, Y.-M.; Wang, D.-W. miR-130a-Mediated KLF3 Can Inhibit the Growth of Lung Cancer Cells. CMAR 2021, 13, 2995–3004. [Google Scholar] [CrossRef] [PubMed]
- MIR424 MicroRNA 424 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/494336 (accessed on 23 November 2022).
- Wang, Y.; Lv, Z.; Fu, J.; Wang, Z.; Fan, Z.; Lei, T. Endogenous MicroRNA-424 Predicts Clinical Outcome and Its Inhibition Acts as Cancer Suppressor in Human Non-Small Cell Lung Cancer. Biomed. Pharmacother. 2017, 89, 208–214. [Google Scholar] [CrossRef]
- Richardsen, E.; Andersen, S.; Al-Saad, S.; Rakaee, M.; Nordby, Y.; Pedersen, M.I.; Ness, N.; Ingebriktsen, L.M.; Fassina, A.; Taskén, K.A.; et al. Low Expression of MiR-424-3p Is Highly Correlated with Clinical Failure in Prostate Cancer. Sci. Rep. 2019, 9, 10662. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Zhang, J.; Sun, H.; Wang, X. Role of MiRNA-424 in Cancers. Onco Targets Ther. 2020, 13, 9611–9622. [Google Scholar] [CrossRef]
- MIR136 MicroRNA 136 [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/406927 (accessed on 23 November 2022).
- Shen, S.; Yue, H.; Li, Y.; Qin, J.; Li, K.; Liu, Y.; Wang, J. Upregulation of MiR-136 in Human Non-Small Cell Lung Cancer Cells Promotes Erk1/2 Activation by Targeting PPP2R2A. Tumour. Biol. 2014, 35, 631–640. [Google Scholar] [CrossRef]
- Li, T.; Gao, X.; Gao, L.; Gan, B.; Xie, Z.; Zeng, J.; Chen, G. Role of Upregulated MiR-136-5p in Lung Adenocarcinoma: A Study of 1242 Samples Utilizing Bioinformatics Analysis. Pathol. Res. Pract. 2018, 214, 750–766. [Google Scholar] [CrossRef]
- Gao, L.; Li, T.; Gan, B.; Gao, X.; Xie, Z.; He, R.; Mo, W.; Chi, X. MiR-136-5p Is Involved in the Pathogenesis of LUSC through Targeting MTDH: A Study Based on RT-QPCR, IHC, Public Database and Dual-Luciferase Reporter Assay. Int. J. Clin. Exp. Med. 2018, 10, 10417–10432. [Google Scholar]
- MIR301B MicroRNA 301b [Homo Sapiens (Human)]-Gene-NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/100126318 (accessed on 23 November 2022).
- Liu, H.; Ma, X.; Niu, N.; Zhao, J.; Lu, C.; Yang, F.; Qi, W. MIR-301b-3p Promotes Lung Adenocarcinoma Cell Proliferation, Migration and Invasion by Targeting DLC1. Technol. Cancer Res. Treat. 2021, 20, 1533033821990036. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yao, B.; Zhu, Q.; Xiao, Z.; Hu, L.; Liu, X.; Li, L.; Wang, J.; Xu, Q.; Yang, L.; et al. MicroRNA-301b-3p Contributes to Tumour Growth of Human Hepatocellular Carcinoma by Repressing Vestigial like Family Member 4. J. Cell. Mol. Med. 2019, 23, 5037–5047. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xing, W.; Xu, J.; Yuan, D.; Liang, G.; Liu, B.; Ma, H. MicroRNA-301b-3p Downregulation Underlies a Novel Inhibitory Role of Long Non-Coding RNA MBNL1-AS1 in Non-Small Cell Lung Cancer. Stem Cell Res. Ther. 2019, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Hirono, T.; Jingushi, K.; Kitae, K.; Nagata, T.; Sato, M.; Minami, K.; Aoki, M.; Harada Takeda, A.; Umehara, T.; Egawa, H.; et al. MiR-301a/b Function as OncomiRs in Non-Small-Cell Lung Cancer. Integr. Mol. Med. 2018, 5, 1–6. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Weston, A.; Harris, C.C. Multistage Carcinogenesis. In Holland-Frei Cancer Medicine, 6th ed.; PMPH USA, Ltd.: Raleigh, NC, USA, 2003. [Google Scholar]
- Stankovic, B.; Bjørhovde, H.A.K.; Skarshaug, R.; Aamodt, H.; Frafjord, A.; Müller, E.; Hammarström, C.; Beraki, K.; Bækkevold, E.S.; Woldbæk, P.R.; et al. Immune Cell Composition in Human Non-Small Cell Lung Cancer. Front. Immunol. 2019, 9, 3101. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kang, J.; Park, H.; Sohn, I.; Lee, S.-H.; Lee, H.Y. Deciphering the Tumor Microenvironment through Radiomics in Non-Small Cell Lung Cancer: Correlation with Immune Profiles. PLoS ONE 2020, 15, e0231227. [Google Scholar] [CrossRef]
- Pennycuick, A.; Teixeira, V.H.; AbdulJabbar, K.; Raza, S.E.A.; Lund, T.; Akarca, A.U.; Rosenthal, R.; Kalinke, L.; Chandrasekharan, D.P.; Pipinikas, C.P.; et al. Immune Surveillance in Clinical Regression of Preinvasive Squamous Cell Lung Cancer. Cancer Discov. 2020, 10, 1489–1499. [Google Scholar] [CrossRef]
- Kunimasa, K.; Goto, T. Immunosurveillance and Immunoediting of Lung Cancer: Current Perspectives and Challenges. Int. J. Mol. Sci. 2020, 21, 597. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Gure, A.O.; Chua, R.; Williamson, B.; Gonen, M.; Ferrera, C.A.; Gnjatic, S.; Ritter, G.; Simpson, A.J.G.; Chen, Y.-T.; Old, L.J.; et al. Cancer-Testis Genes Are Coordinately Expressed and Are Markers of Poor Outcome in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2005, 11, 8055–8062. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, C.; Koslowski, M.; Arsiray, T.; Dhaene, K.; Praet, M.; Victor, A.; Morresi-Hauf, A.; Lindner, M.; Passlick, B.; Lehr, H.-A.; et al. Expression of Multiple Epigenetically Regulated Cancer/Germline Genes in Nonsmall Cell Lung Cancer. Int. J. Cancer 2006, 118, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Palata, O.; Podzimkova Hradilova, N.; Mysiková, D.; Kutna, B.; Mrazkova, H.; Lischke, R.; Spisek, R.; Adkins, I. Detection of Tumor Antigens and Tumor-Antigen Specific T Cells in NSCLC Patients: Correlation of the Quality of T Cell Responses with NSCLC Subtype. Immunol. Lett. 2020, 219, 46–53. [Google Scholar] [CrossRef]
- Hanash, S.M.; Ostrin, E.J.; Fahrmann, J.F. Blood Based Biomarkers beyond Genomics for Lung Cancer Screening. Transl. Lung Cancer Res. 2018, 7, 327–335. [Google Scholar] [CrossRef]
- Li, N.; Holden, V.K.; Deepak, J.; Todd, N.W.; Jiang, F. Autoantibodies against Tumor-Associated Antigens in Sputum as Biomarkers for Lung Cancer. Transl. Oncol. 2021, 14, 100991. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.A.; O’Leary, J.J.; Cahill, D.J. Assessment of the Humoral Immune Response to Cancer. J. Proteom. 2012, 75, 4573–4579. [Google Scholar] [CrossRef]
- Zaenker, P.; Gray, E.S.; Ziman, M.R. Autoantibody Production in Cancer-The Humoral Immune Response toward Autologous Antigens in Cancer Patients. Autoimmun. Rev. 2016, 15, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, X.; Powell, C.A.; Ni, J.; Wang, B.; Zhang, J.; Zhang, Y.; Wang, L.; Xu, Z.; Zhang, L.; et al. Probability of Cancer in High-Risk Patients Predicted by the Protein-Based Lung Cancer Biomarker Panel in China: LCBP Study. Cancer 2018, 124, 262–270. [Google Scholar] [CrossRef]
- Li, X.; Hayward, C.; Fong, P.-Y.; Dominguez, M.; Hunsucker, S.W.; Lee, L.W.; McLean, M.; Law, S.; Butler, H.; Schirm, M.; et al. A Blood-Based Proteomic Classifier for the Molecular Characterization of Pulmonary Nodules. Sci. Transl. Med. 2013, 5, 207ra142. [Google Scholar] [CrossRef]
- Pan, Y.W.; Zhou, Z.G.; Wang, M.; Dong, J.Q.; Du, K.P.; Li, S.; Liu, Y.L.; Lv, P.J.; Gao, J.B. Combination of IL-6, IL-10, and MCP-1 with Traditional Serum Tumor Markers in Lung Cancer Diagnosis and Prognosis. Genet Mol. Res. 2016, 15, 4210–4238. [Google Scholar] [CrossRef]
- Ostroff, R.M.; Bigbee, W.L.; Franklin, W.; Gold, L.; Mehan, M.; Miller, Y.E.; Pass, H.I.; Rom, W.N.; Siegfried, J.M.; Stewart, A.; et al. Unlocking Biomarker Discovery: Large Scale Application of Aptamer Proteomic Technology for Early Detection of Lung Cancer. PLoS ONE 2010, 5, e15003. [Google Scholar] [CrossRef]
- Farlow, E.C.; Patel, K.; Basu, S.; Lee, B.-S.; Kim, A.W.; Coon, J.S.; Faber, L.P.; Bonomi, P.; Liptay, M.J.; Borgia, J.A. Development of a Multiplexed Tumor-Associated Autoantibody-Based Blood Test for the Detection of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2010, 16, 3452–3462. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Bitterlich, N.; Velcovsky, H.-G.; Morr, H.; Katz, N.; Eigenbrodt, E. Fuzzy Logic-Based Tumor-Marker Profiles Improved Sensitivity in the Diagnosis of Lung Cancer. Int. J. Clin. Oncol. 2002, 7, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Borgia, J.A.; Basu, S.; Faber, L.P.; Kim, A.W.; Coon, J.S.; Kaiser-Walters, K.A.; Fhied, C.; Thomas, S.; Rouhi, O.; Warren, W.H.; et al. Establishment of a Multi-Analyte Serum Biomarker Panel to Identify Lymph Node Metastases in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, Y.; Wang, J.; Yan, Z.; Qu, L.; Xiang, B.; Zhang, Y. An Optimal Tumor Marker Group-Coupled Artificial Neural Network for Diagnosis of Lung Cancer. Expert Syst. Appl. 2011, 38, 11329–11334. [Google Scholar] [CrossRef]
- Feng, F.; Wu, Y.; Wu, Y.; Nie, G.; Ni, R. The Effect of Artificial Neural Network Model Combined with Six Tumor Markers in Auxiliary Diagnosis of Lung Cancer. J. Med. Syst. 2012, 36, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Flores-Fernández, J.M.; Herrera-López, E.J.; Sánchez-Llamas, F.; Rojas-Calvillo, A.; Cabrera-Galeana, P.A.; Leal-Pacheco, G.; González-Palomar, M.G.; Femat, R.; Martínez-Velázquez, M. Development of an Optimized Multi-Biomarker Panel for the Detection of Lung Cancer Based on Principal Component Analysis and Artificial Neural Network Modeling. Expert Syst. Appl. 2012, 39, 10851–10856. [Google Scholar] [CrossRef]
- Louis, E.; Adriaensens, P.; Guedens, W.; Bigirumurame, T.; Baeten, K.; Vanhove, K.; Vandeurzen, K.; Darquennes, K.; Vansteenkiste, J.; Dooms, C.; et al. Detection of Lung Cancer through Metabolic Changes Measured in Blood Plasma. J. Thorac. Oncol. 2016, 11, 516–523. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Kim, K.; DeFelice, B.C.; Taylor, S.L.; Gandara, D.R.; Yoneda, K.Y.; Cooke, D.T.; Fiehn, O.; Kelly, K.; Miyamoto, S. Investigation of Metabolomic Blood Biomarkers for Detection of Adenocarcinoma Lung Cancer. Cancer Epidemiol Biomark. Prev. 2015, 24, 1716–1723. [Google Scholar] [CrossRef]
- Flores, J.M.; Herrera, E.; Leal, G.; González, M.G.; Sánchez, F.; Rojas, A.; Cabrera, P.A.; Femat, R.; Martínez-Velázquez, M. Artificial Neural Network-Based Serum Biomarkers Analysis Improves Sensitivity in the Diagnosis of Lung Cancer. In Proceedings of the V Latin American Congress on Biomedical Engineering CLAIB 2011, 16–21 May 2011,Habana, Cuba; Folgueras Méndez, J., Aznielle Rodríguez, T.Y., Calderón Marín, C.F., Llanusa Ruiz, S.B., Castro Medina, J., Vega Vázquez, H., Carballo Barreda, M., Rodríguez Rojas, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 882–885. [Google Scholar]
- Chapman, C.J.; Healey, G.F.; Murray, A.; Boyle, P.; Robertson, C.; Peek, L.J.; Allen, J.; Thorpe, A.J.; Hamilton-Fairley, G.; Parsy-Kowalska, C.B.; et al. EarlyCDT®-Lung Test: Improved Clinical Utility through Additional Autoantibody Assays. Tumor Biol. 2012, 33, 1319–1326. [Google Scholar] [CrossRef]
- Li, P.; Shi, J.-X.; Xing, M.-T.; Dai, L.-P.; Li, J.-T.; Zhang, J.-Y. Evaluation of Serum Autoantibodies against Tumor-Associated Antigens as Biomarkers in Lung Cancer. Tumour Biol. 2017, 39, 1010428317711662. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, J.; Tsay, J.-C.J.; Yie, T.-A.; Munger, J.S.; Pass, H.; Rom, W.N.; Tan, E.M.; Zhang, J.-Y. Identification of Autoantibodies to ECH1 and HNRNPA2B1 as Potential Biomarkers in the Early Detection of Lung Cancer. OncoImmunology 2017, 6, e1310359. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Tsay, J.-C.J.; Li, J.; Yie, T.-A.; Munger, J.S.; Pass, H.; Rom, W.N.; Zhang, Y.; Tan, E.M.; Zhang, J.-Y. Autoantibodies against Tumor-Associated Antigens in the Early Detection of Lung Cancer. Lung Cancer 2016, 99, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Full Article: Serum Anti-MDM2 and Anti-c-Myc Autoantibodies as Biomarkers in the Early Detection of Lung Cancer. Available online: https://www.tandfonline.com/doi/full/10.1080/2162402X.2016.1138200 (accessed on 23 November 2022).
- Li, P.; Shi, J.-X.; Dai, L.-P.; Chai, Y.-R.; Zhang, H.-F.; Kankonde, M.; Kankonde, P.; Yu, B.-F.; Zhang, J.-Y. Serum Anti-MDM2 and Anti-c-Myc Autoantibodies as Biomarkers in the Early Detection of Lung Cancer. OncoImmunology 2016, 5, e1138200. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Taylor, S.L.; Barupal, D.K.; Taguchi, A.; Wohlgemuth, G.; Wikoff, W.R.; Yoneda, K.Y.; Gandara, D.R.; Hanash, S.M.; Kim, K.; et al. Systemic Metabolomic Changes in Blood Samples of Lung Cancer Patients Identified by Gas Chromatography Time-of-Flight Mass Spectrometry. Metabolites 2015, 5, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Boyle, P.; Healey, G.F.; Maddison, P.; Peek, L.; Murray, A.; Chapman, C.J.; Allen, J.; Wood, W.C.; Sewell, H.F.; et al. EarlyCDT-Lung: An Immunobiomarker Test as an Aid to Early Detection of Lung Cancer. Cancer Prev. Res. (Phila) 2011, 4, 1126–1134. [Google Scholar] [CrossRef]
- Ros-Mazurczyk, M.; Jelonek, K.; Marczyk, M.; Binczyk, F.; Pietrowska, M.; Polanska, J.; Dziadziuszko, R.; Jassem, J.; Rzyman, W.; Widlak, P. Serum Lipid Profile Discriminates Patients with Early Lung Cancer from Healthy Controls. Lung Cancer 2017, 112, 69–74. [Google Scholar] [CrossRef]
- Klupczynska, A.; Dereziński, P.; Garrett, T.J.; Rubio, V.Y.; Dyszkiewicz, W.; Kasprzyk, M.; Kokot, Z.J. Study of Early Stage Non-Small-Cell Lung Cancer Using Orbitrap-Based Global Serum Metabolomics. J. Cancer Res. Clin. Oncol. 2017, 143, 649–659. [Google Scholar] [CrossRef]
- Campa, M.J.; Gottlin, E.B.; Herndon, J.E.; Patz, E.F. Rethinking Autoantibody Signature Panels for Cancer Diagnosis. J. Thorac. Oncol. 2017, 12, 1011–1014. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Lu, Y.-C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. New Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.-C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer Immunotherapy Based on Mutation-Specific CD4+ T Cells in a Patient with Epithelial Cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Creaney, J.; Redwood, A.; Robinson, B. The Current Lung Cancer Neoantigen Landscape and Implications for Therapy. J. Thorac. Oncol. 2021, 16, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.; Nardin, A.; Jhunjhunwala, S. Late-differentiated effector neoantigen-specific CD8+ T cells are enriched in non-small cell lung carcinoma patients responding to atezolizumab treatment. Eur. J. Cancer 2019, 110, S2–S3. [Google Scholar] [CrossRef]
- McGranahan, N.; Furness, A.J.S.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal Neoantigens Elicit T Cell Immunoreactivity and Sensitivity to Immune Checkpoint Blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational Landscape Determines Sensitivity to PD-1 Blockade in Non-Small Cell Lung Cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Wells, D.K.; van Buuren, M.M.; Dang, K.K.; Hubbard-Lucey, V.M.; Sheehan, K.C.F.; Campbell, K.M.; Lamb, A.; Ward, J.P.; Sidney, J.; Blazquez, A.B.; et al. Key Parameters of Tumor Epitope Immunogenicity Revealed Through a Consortium Approach Improve Neoantigen Prediction. Cell 2020, 183, 818–834. [Google Scholar] [CrossRef]
- Karasaki, T.; Nagayama, K.; Kawashima, M.; Hiyama, N.; Murayama, T.; Kuwano, H.; Nitadori, J.; Anraku, M.; Sato, M.; Miyai, M.; et al. Identification of Individual Cancer-Specific Somatic Mutations for Neoantigen-Based Immunotherapy of Lung Cancer. J. Thorac. Oncol. 2016, 11, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Shimizu, K.; Iyoda, T.; Ueda, S.; Shinga, J.; Mochizuki, Y.; Watanabe, T.; Ohara, O.; Fujii, S. PD-L1 Expression Affects Neoantigen Presentation. iScience 2020, 23, 101238. [Google Scholar] [CrossRef]
- Niu, M.; Yi, M.; Li, N.; Luo, S.; Wu, K. Predictive Biomarkers of Anti-PD-1/PD-L1 Therapy in NSCLC. Exp. Hematol. Oncol. 2021, 10, 18. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune Checkpoint Signaling and Cancer Immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- McBride, M.A.; Patil, T.K.; Bohannon, J.K.; Hernandez, A.; Sherwood, E.R.; Patil, N.K. Immune Checkpoints: Novel Therapeutic Targets to Attenuate Sepsis-Induced Immunosuppression. Front. Immunol. 2021, 11, 624272. [Google Scholar] [CrossRef] [PubMed]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Zhang, Q.; Wu, J.; Xiao, Y.; Song, L.; Gong, H.; Li, Y. The Landscape of Immune Checkpoint Inhibitor Therapy in Advanced Lung Cancer. BMC Cancer 2021, 21, 968. [Google Scholar] [CrossRef]
- Domagała-Kulawik, J. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer—Towards Daily Practice. Adv. Respir. Med. 2018, 86, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Su, W.; Lu, T.; Wang, Y.; Dong, Y.; Qin, Y.; Liu, D.; Sun, L.; Jiao, W. First-Line Immune-Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Current Landscape and Future Progress. Front. Pharmacol. 2020, 11, 578091. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Y.; Du, H.; Guo, X. Current Status of Immune Checkpoint Inhibitor Immunotherapy for Lung Cancer. Front. Oncol. 2021, 11, 704336. [Google Scholar] [CrossRef]
- Ma, L.-R.; Li, J.-X.; Tang, L.; Li, R.-Z.; Yang, J.-S.; Sun, A.; Leung, E.L.-H.; Yan, P.-Y. Immune Checkpoints and Immunotherapy in Non-small Cell Lung Cancer: Novel Study Progression, Challenges and Solutions (Review). Oncol. Lett. 2021, 22, 787. [Google Scholar] [CrossRef]
- Tsoukalas, N.; Kiakou, M.; Tsapakidis, K.; Tolia, M.; Baxevanos, P.; Kyrgias, G.; Theocharis, S. PD-1 and PD-L1 as Immunotherapy Targets and Biomarkers in Non-Small Cell Lung Cancer. J. Buon 2019, 24, 883–888. [Google Scholar]
- Abu Hejleh, T.; Furqan, M.; Ballas, Z.; Clamon, G. The Clinical Significance of Soluble PD-1 and PD-L1 in Lung Cancer. Crit. Rev. Oncol. /Hematol. 2019, 143, 148–152. [Google Scholar] [CrossRef]
- Xia, L.; Liu, Y.; Wang, Y. PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions. Oncologist 2019, 24, S31–S41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, L.; Zhou, Y.; He, W.; Li, W. Immune Checkpoint Inhibitor-Associated Pneumonitis in Non-Small Cell Lung Cancer: Current Understanding in Characteristics, Diagnosis, and Management. Front. Immunol. 2021, 12, 1889. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Borghaei, H.; O’Byrne, K.J. Nivolumab plus Ipilimumab in Non-Small-Cell Lung Cancer. Future Oncol. 2019, 15, 2287–2302. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- ADORA2A-Adenosine Receptor A2a-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P29274/entry (accessed on 23 November 2022).
- Leone, R.D.; Lo, Y.-C.; Powell, J.D. A2aR Antagonists: Next Generation Checkpoint Blockade for Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2015, 13, 265–272. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Hinz, S.; Navarro, G.; Borroto-Escuela, D.; Seibt, B.F.; Ammon, Y.-C.; de Filippo, E.; Danish, A.; Lacher, S.K.; Červinková, B.; Rafehi, M.; et al. Adenosine A2A Receptor Ligand Recognition and Signaling Is Blocked by A2B Receptors. Oncotarget 2018, 9, 13593–13611. [Google Scholar] [CrossRef]
- VSIR-V-Type Immunoglobulin Domain-Containing Suppressor of T-Cell Activation-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9H7M9/entry (accessed on 23 November 2022).
- Huang, X.; Zhang, X.; Li, E.; Zhang, G.; Wang, X.; Tang, T.; Bai, X.; Liang, T. VISTA: An Immune Regulatory Protein Checking Tumor and Immune Cells in Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 83. [Google Scholar] [CrossRef]
- CD276-CD276 Antigen-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q5ZPR3/entry (accessed on 23 November 2022).
- Aicher, W.K.; Korn, M.; Reitnauer, L.; Maurer, F.B.; Hennenlotter, J.; Black, P.C.; Todenhofer, T.; Bedke, J.; Stenzl, A. Expression Patterns of the Immune Checkpoint Ligand CD276 in Urothelial Carcinoma. BMC Urol. 2021, 21, 60. [Google Scholar] [CrossRef]
- Li, F.; Chen, H.; Wang, D. Silencing of CD276 Suppresses Lung Cancer Progression by Regulating Integrin Signaling. J. Thorac. Dis. 2020, 12, 2137–2145. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, X. Prognostic Significance of CD276 in Non-Small Cell Lung Cancer. Open Med. (Wars) 2019, 14, 805–812. [Google Scholar] [CrossRef] [PubMed]
- CD274-Programmed Cell Death 1 Ligand 1-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9NZQ7/entry (accessed on 23 November 2022).
- PDCD1LG2-Programmed Cell Death 1 Ligand 2-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9BQ51/entry (accessed on 23 November 2022).
- Larsen, T.V.; Hussmann, D.; Nielsen, A.L. PD-L1 and PD-L2 Expression Correlated Genes in Non-Small-Cell Lung Cancer. Cancer Commun. (Lond) 2019, 39, 30. [Google Scholar] [CrossRef] [PubMed]
- Ludovini, V.; Bianconi, F.; Siggillino, A.; Vannucci, J.; Baglivo, S.; Berti, V.; Tofanetti, F.R.; Reda, M.S.; Bellezza, G.; Mandarano, M.; et al. High PD-L1/IDO-2 and PD-L2/IDO-1 Co-Expression Levels Are Associated with Worse Overall Survival in Resected Non-Small Cell Lung Cancer Patients. Genes (Basel) 2021, 12, 273. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, I.; Rutkowska, E.; Polubiec-Kownacka, M.; Raniszewska, A.; Rzepecki, P.; Domagała-Kulawik, J. Identification of PD-1 Ligands: PD-L1 and PD-L2 on Macrophages in Lung Cancer Milieu by Flow Cytometry. Transl. Lung Cancer Res. 2021, 10, 1679. [Google Scholar] [CrossRef]
- PDCD1-Programmed Cell Death Protein 1-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q15116/entry (accessed on 23 November 2022).
- Santini, F.C.; Hellmann, M.D. PD-1/PD-L1 Axis in Lung Cancer. Cancer J. 2018, 24, 15–19. [Google Scholar] [CrossRef] [PubMed]
- CD80-CD80 Antigen (CD28 Antigen Ligand 1, B7-1 Antigen), Isoform CRA_a-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/A0N0P2/entry (accessed on 23 November 2022).
- Sato, T.; Takagi, K.; Higuchi, M.; Abe, H.; Kojimahara, M.; Sagawa, M.; Tanaki, M.; Miki, Y.; Suzuki, T.; Hojo, H. Immunolocalization of CD80 and CD86 in Non-Small Cell Lung Carcinoma: CD80 as a Potent Prognostic Factor. Acta Histochem. Cytochem. 2022, 55, 25–35. [Google Scholar] [CrossRef]
- Vackova, J.; Polakova, I.; Johari, S.D.; Smahel, M. CD80 Expression on Tumor Cells Alters Tumor Microenvironment and Efficacy of Cancer Immunotherapy by CTLA-4 Blockade. Cancers 2021, 13, 1935. [Google Scholar] [CrossRef]
- Qiu, H.; Tian, W.; He, Y.; Li, J.; He, C.; Li, Y.; Liu, N.; Li, J. Integrated Analysis Reveals Prognostic Value and Immune Correlates of CD86 Expression in Lower Grade Glioma. Front. Oncol. 2021, 11, 654350. [Google Scholar] [CrossRef]
- CD86-CD86 Antigen-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/A0A0X9R4E0/entry (accessed on 23 November 2022).
- CTLA4-Cytotoxic T-Lymphocyte Protein 4-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P16410/entry (accessed on 23 November 2022).
- Paulsen, E.-E.; Kilvaer, T.K.; Rakaee, M.; Richardsen, E.; Hald, S.M.; Andersen, S.; Busund, L.-T.; Bremnes, R.M.; Donnem, T. CTLA-4 Expression in the Non-Small Cell Lung Cancer Patient Tumor Microenvironment: Diverging Prognostic Impact in Primary Tumors and Lymph Node Metastases. Cancer Immunol. Immunother. 2017, 66, 1449–1461. [Google Scholar] [CrossRef]
- Shen, K.; Cui, J.; Wei, Y.; Chen, X.; Liu, G.; Gao, X.; Li, W.; Lu, H.; Zhan, P.; Lv, T.; et al. Effectiveness and Safety of PD-1/PD-L1 or CTLA4 Inhibitors Combined with Chemotherapy as a First-Line Treatment for Lung Cancer: A Meta-Analysis. J. Thorac. Dis. 2018, 10, 6636. [Google Scholar] [CrossRef]
- LGALS9-Galectin-9-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/O00182/entry (accessed on 23 November 2022).
- He, Y.; Jia, K.; Dziadziuszko, R.; Zhao, S.; Zhang, X.; Deng, J.; Wang, H.; Hirsch, F.R.; Zhou, C. Galectin-9 in Non-Small Cell Lung Cancer. Lung Cancer 2019, 136, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhang, L.; Zhang, W.; Sun, C.; Wu, C.; He, Y.; Zhou, C. Galectin-9-Based Immune Risk Score Model Helps to Predict Relapse in Stage I-III Small Cell Lung Cancer. J. Immunother. Cancer 2020, 8, e001391. [Google Scholar] [CrossRef] [PubMed]
- HAVCR2-Hepatitis A Virus Cellular Receptor 2-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q8TDQ0/entry (accessed on 23 November 2022).
- Zhuang, X.; Zhang, X.; Xia, X.; Zhang, C.; Liang, X.; Gao, L.; Zhang, X.; Ma, C. Ectopic Expression of TIM-3 in Lung Cancers: A Potential Independent Prognostic Factor for Patients with NSCLC. Am. J. Clin. Pathol. 2012, 137, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Zang, K.; Hui, L.; Wang, M.; Huang, Y.; Zhu, X.; Yao, B. TIM-3 as a Prognostic Marker and a Potential Immunotherapy Target in Human Malignant Tumors: A Meta-Analysis and Bioinformatics Validation. Front. Oncol. 2021, 11, 579351. [Google Scholar] [CrossRef] [PubMed]
- TNFRSF14-Tumor Necrosis Factor Receptor Superfamily Member 14-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q92956/entry (accessed on 23 November 2022).
- Ren, S.; Tian, Q.; Amar, N.; Yu, H.; Rivard, C.J.; Caldwell, C.; Ng Terry, L.; Tu, M.; Liu, Y.; Gao, D.; et al. The Immune Checkpoint, HVEM May Contribute to Immune Escape in Non-Small Cell Lung Cancer Lacking PD-L1 Expression. Lung Cancer (Amsterdam, Netherlands) 2018, 125, 115–120. [Google Scholar] [CrossRef]
- Demerlé, C.; Gorvel, L.; Olive, D. BTLA-HVEM Couple in Health and Diseases: Insights for Immunotherapy in Lung Cancer. Front Oncol. 2021, 11, 682007. [Google Scholar] [CrossRef]
- BTLA-B-and T-Lymphocyte Attenuator-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q7Z6A9/entry (accessed on 23 November 2022).
- Li, X.; Xu, Z.; Cui, G.; Yu, L.; Zhang, X. BTLA Expression in Stage I–III Non–Small-Cell Lung Cancer and Its Correlation with PD-1/PD-L1 and Clinical Outcomes. Onco Targets Ther. 2020, 13, 215–224. [Google Scholar] [CrossRef]
- LAG3-Lymphocyte Activation Gene 3 Protein-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P18627/entry (accessed on 23 November 2022).
- Sun, H.; Dai, J.; Zhao, L.; Zhu, J.; Wang, H.; Chen, P.; Lu, H.; Chen, Q.; Zhang, Z. Lymphocyte Activation Gene-3 Is Associated with Programmed Death-Ligand 1 and Programmed Cell Death Protein 1 in Small Cell Lung Cancer. Ann. Transl. Med. 2021, 9, 1468. [Google Scholar] [CrossRef]
- He, Y.; Yu, H.; Rozeboom, L.; Rivard, C.J.; Ellison, K.; Dziadziuszko, R.; Suda, K.; Ren, S.; Wu, C.; Hou, L.; et al. LAG-3 Protein Expression in Non-Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J. Thorac. Oncol. 2017, 12, 814–823. [Google Scholar] [CrossRef]
- He, Y.; Rozeboom, L.; Rivard, C.J.; Ellison, K.; Dziadziuszko, R.; Yu, H.; Zhou, C.; Hirsch, F.R. MHC Class II Expression in Lung Cancer. Lung Cancer 2017, 112, 75–80. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Zhang, X.; Jia, K.; Deng, J.; Zhou, C.; He, Y. Major Histocompatibility Complex Class II Molecule in Non-Small Cell Lung Cancer Diagnosis, Prognosis and Treatment. Onco Targets Ther. 2019, 12, 7281–7288. [Google Scholar] [CrossRef] [PubMed]
- TNFSF4-Tumor Necrosis Factor Ligand Superfamily Member 4-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P23510/entry (accessed on 23 November 2022).
- Chen, P.; Wang, H.; Zhao, L.; Guo, H.; Zhang, L.; Zhang, W.; Sun, C.; Zhao, S.; Li, W.; Zhu, J.; et al. Immune Checkpoints OX40 and OX40L in Small-Cell Lung Cancer: Predict Prognosis and Modulate Immune Microenvironment. Front Oncol. 2021, 11, 713853. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Hirsch, F.R.; Zhou, C. OX40/OX40L Protein Expression in Non-Small Cell Lung Cancer and Its Role in Clinical Outcome and Relationships with Other Immune Biomarkers. Ann. Oncol. 2019, 30, v51. [Google Scholar] [CrossRef]
- TNFRSF4-Tumor Necrosis Factor Receptor Superfamily Member 4-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P43489/entry (accessed on 23 November 2022).
- CD40-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q09LL4/entry (accessed on 23 November 2022).
- CD40LG-CD40 Ligand-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P29965/entry (accessed on 23 November 2022).
- Roselli, M.; Mineo, T.C.; Basili, S.; Martini, F.; Mariotti, S.; Aloe, S.; Del Monte, G.; Ambrogi, V.; Spila, A.; Palmirotta, R.; et al. Soluble CD40 Ligand Plasma Levels in Lung Cancer. Clin. Cancer Res. 2004, 10, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Ke, D.; Min, L.; Lin, W.; Jiahui, Z.; Fang, L.; Zhaowei, G.; Zhe, Z.; Xi, C.; Huizhong, Z. Incorporation of CD40 Ligand Enhances the Immunogenicity of Tumor-associated Calcium Signal Transducer 2 Virus-like Particles against Lung Cancer. Int. J. Mol. Med. 2018, 41, 3671–3679. [Google Scholar] [CrossRef] [PubMed]
- ICOSLG-ICOS Ligand-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/O75144/entry (accessed on 23 November 2022).
- Zang, X.; Allison, J.P. The B7 Family and Cancer Therapy: Costimulation and Coinhibition. Clin. Cancer Res. 2007, 13, 5271–5279. [Google Scholar] [CrossRef]
- Wu, G.; He, M.; Ren, K.; Ma, H.; Xue, Q. Inducible Co-Stimulator ICOS Expression Correlates with Immune Cell Infiltration and Can Predict Prognosis in Lung Adenocarcinoma. Int. J. Gen. Med. 2022, 15, 3739–3751. [Google Scholar] [CrossRef]
- ICOS-Inducible T-Cell Costimulator-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9Y6W8/entry (accessed on 23 November 2022).
- Marinelli, O.; Nabissi, M.; Morelli, M.B.; Torquati, L.; Amantini, C.; Santoni, G. ICOS-L as a Potential Therapeutic Target for Cancer Immunotherapy. Curr. Protein Pept. Sci. 2018, 19, 1107–1113. [Google Scholar] [CrossRef]
- CD70-CD70 Antigen-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P32970/entry (accessed on 23 November 2022).
- Flieswasser, T.; Van den Eynde, A.; Van Audenaerde, J.; De Waele, J.; Lardon, F.; Riether, C.; de Haard, H.; Smits, E.; Pauwels, P.; Jacobs, J. The CD70-CD27 Axis in Oncology: The New Kids on the Block. J. Exp. Clin. Cancer Res. 2022, 41, 12. [Google Scholar] [CrossRef]
- Jacobs, J.; Zwaenepoel, K.; Rolfo, C.; Van den Bossche, J.; Deben, C.; Silence, K.; Hermans, C.; Smits, E.; Van Schil, P.; Lardon, F.; et al. Unlocking the Potential of CD70 as a Novel Immunotherapeutic Target for Non-Small Cell Lung Cancer. Oncotarget 2015, 6, 13462–13475. [Google Scholar] [CrossRef]
- CD27-CD27 Antigen-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P26842/entry (accessed on 23 November 2022).
- Starzer, A.M.; Berghoff, A.S. New Emerging Targets in Cancer Immunotherapy: CD27 (TNFRSF7). ESMO Open 2020, 4, e000629. [Google Scholar] [CrossRef]
- Kashima, J.; Okuma, Y.; Hosomi, Y.; Hishima, T. High Serum Soluble CD27 Level Correlates with Poor Performance Status and Reduced Survival in Patients with Advanced Lung Cancer. Oncology 2019, 97, 365–372. [Google Scholar] [CrossRef]
- TNFSF18-Tumor Necrosis Factor Ligand Superfamily Member 18-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9UNG2/entry (accessed on 23 November 2022).
- TNFRSF18-Tumor Necrosis Factor Receptor Superfamily Member 18-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9Y5U5/entry (accessed on 23 November 2022).
- Zhu, L.X.; Davoodi, M.; Srivastava, M.K.; Kachroo, P.; Lee, J.M.; St. John, M.; Harris-White, M.; Huang, M.; Strieter, R.M.; Dubinett, S.; et al. GITR Agonist Enhances Vaccination Responses in Lung Cancer. Oncoimmunology 2015, 4, e992237. [Google Scholar] [CrossRef]
- Kopru, C.Z.; Cagnan, I.; Akar, I.; Esendagli, G.; Korkusuz, P.; Gunel-Ozcan, A. Dual Effect of Glucocorticoid-Induced Tumor Necrosis Factor–Related Receptor Ligand Carrying Mesenchymal Stromal Cells on Small Cell Lung Cancer: A Preliminary in Vitro Study. Cytotherapy 2018, 20, 930–940. [Google Scholar] [CrossRef]
- TNFSF9-Tumor Necrosis Factor Ligand Superfamily Member 9-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P41273/entry (accessed on 23 November 2022).
- Qu, Q.; Zhu, X.; Du, W.; Wang, H.; Shen, Y.; Zhu, Y.; Chen, C. 4-1BB Agonism Combined With PD-L1 Blockade Increases the Number of Tissue-Resident CD8+ T Cells and Facilitates Tumor Abrogation. Front. Immunol. 2020, 11, 577. [Google Scholar] [CrossRef]
- Vinay, D.S.; Kwon, B.S. 4-1BB (CD137), an Inducible Costimulatory Receptor, as a Specific Target for Cancer Therapy. BMB Rep. 2014, 47, 122–129. [Google Scholar] [CrossRef]
- Shah, P.; Forget, M.-A.; Frank, M.L.; Jiang, P.; Sakellariou-Thompson, D.; Federico, L.; Khairullah, R.; Neutzler, C.A.; Wistuba, I.; Chow, C.-W.B.; et al. Combined IL-2, Agonistic CD3 and 4-1BB Stimulation Preserve Clonotype Hierarchy in Propagated Non-Small Cell Lung Cancer Tumor-Infiltrating Lymphocytes. J. Immunother. Cancer 2022, 10, e003082. [Google Scholar] [CrossRef]
- TNFRSF9-Tumor Necrosis Factor Receptor Superfamily Member 9-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q07011/entry (accessed on 23 November 2022).
- PVR-Poliovirus Receptor-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P15151/entry (accessed on 23 November 2022).
- Oyama, R.; Kanayama, M.; Mori, M.; Matsumiya, H.; Taira, A.; Shinohara, S.; Takenaka, M.; Yoneda, K.; Kuroda, K.; Tanaka, F. CD155 Expression and Its Clinical Significance in Non-Small Cell Lung Cancer. Oncol. Lett. 2022, 23, 166. [Google Scholar] [CrossRef]
- TIGIT-T-Cell Immunoreceptor with Ig and ITIM Domains-Homo Sapiens (Human) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/Q495A1/entry (accessed on 23 November 2022).
- Thao, K.; Huynh, P. Anti-TIGIT and ICI Combinations Supported by Early-Phase Research. Target. Ther. Oncol. 2021, 10. [Google Scholar]

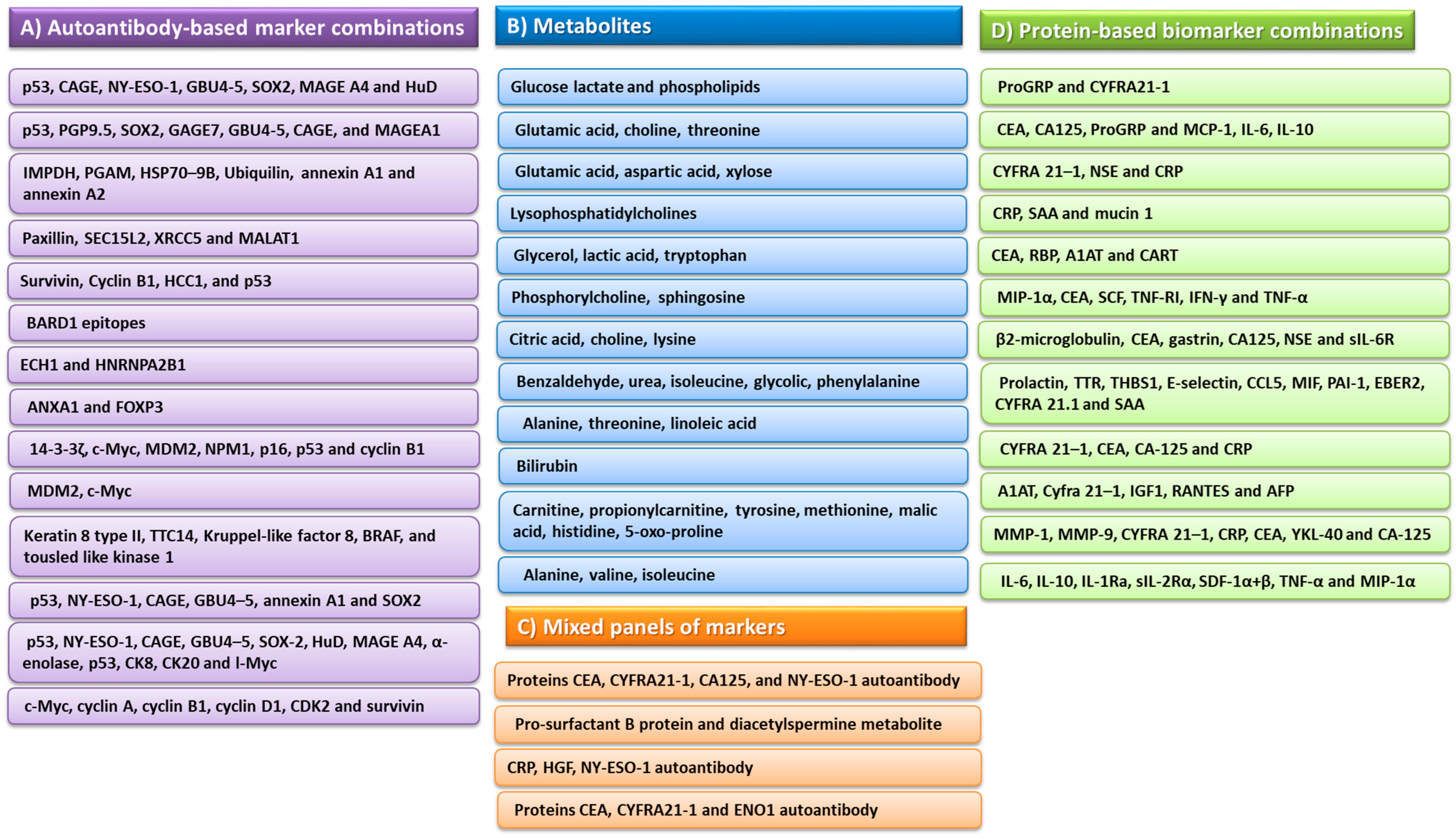
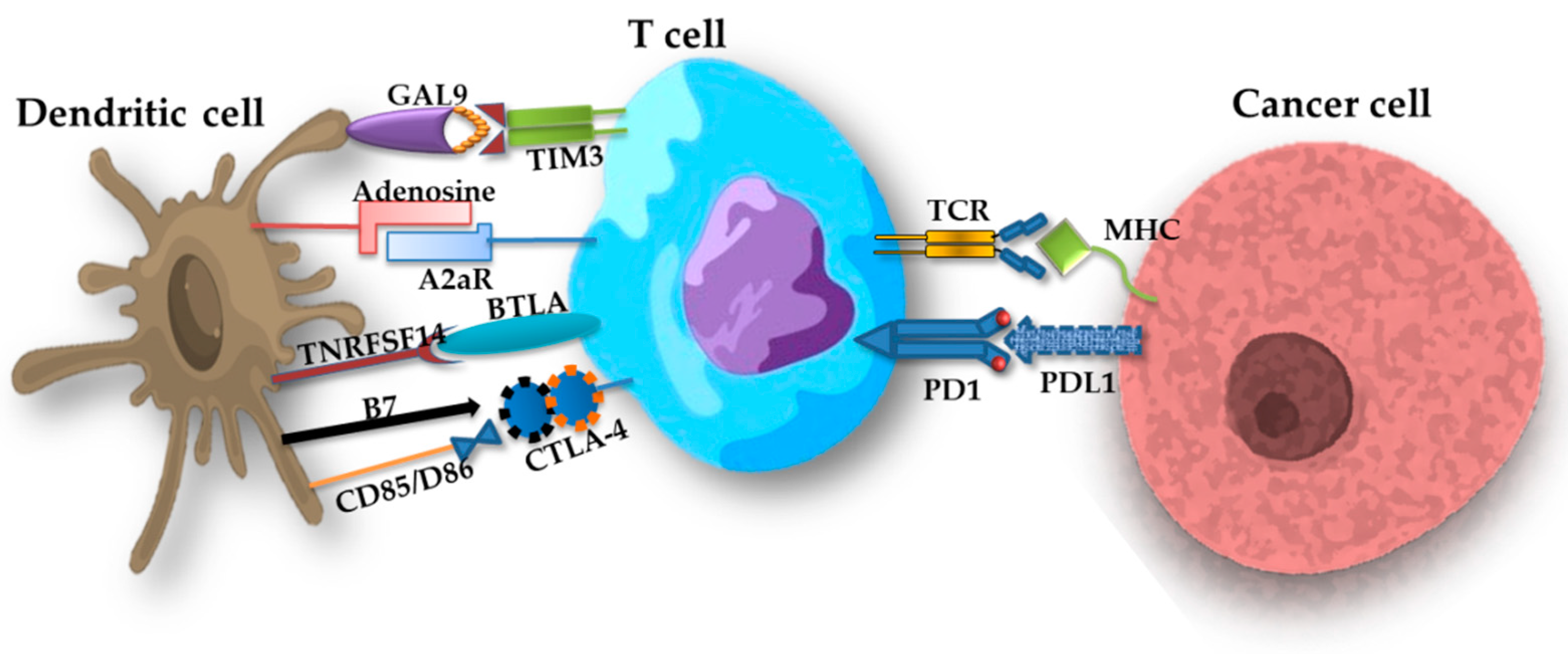
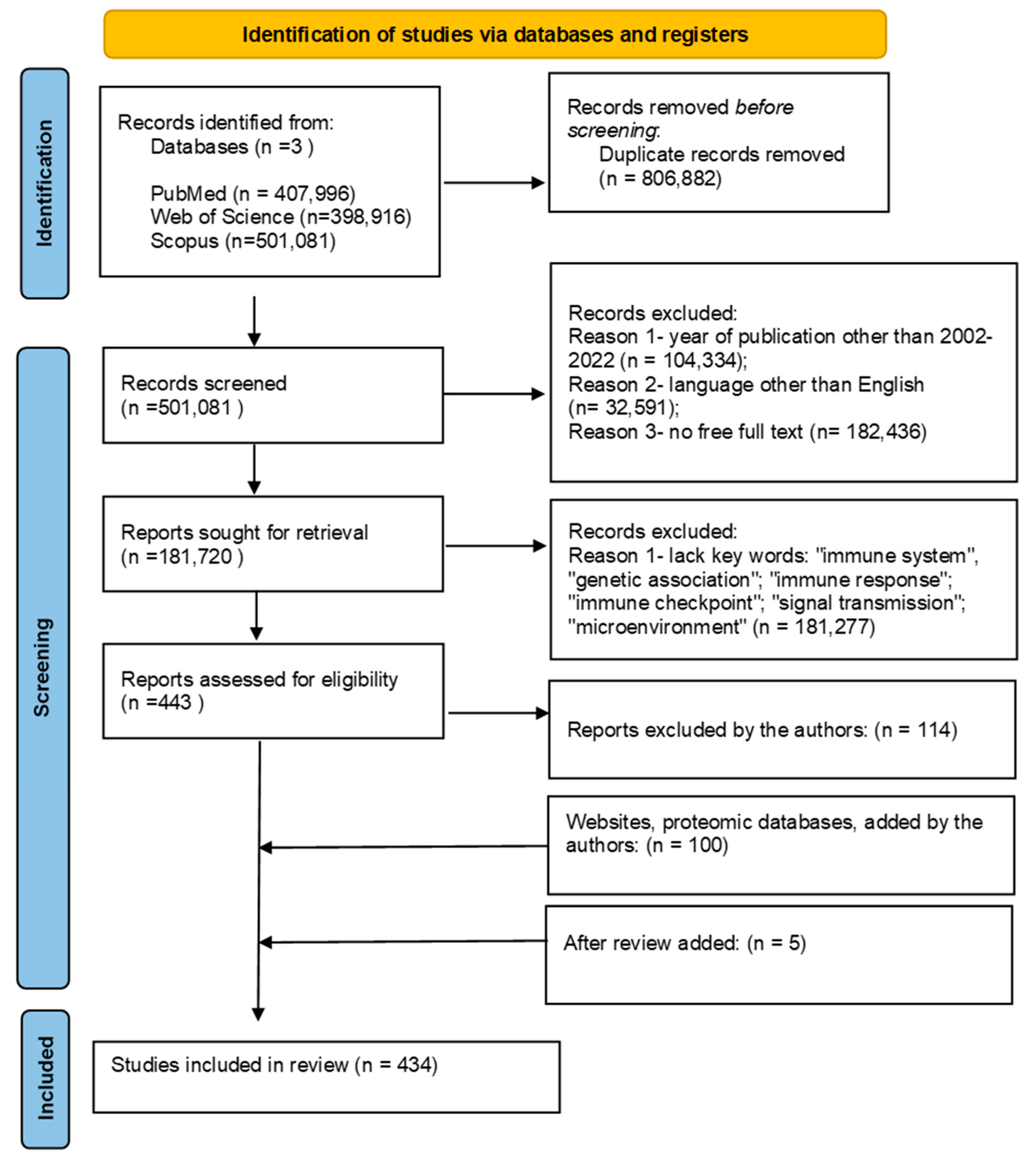
| AC | LCC | NCSLC | SCLC | ADC |
|---|---|---|---|---|
| EGFR (31%) | TP53 (68%) | EGFR (25%) | TP53 (63%) | TP53 (57%) |
| KRAS (17%) | KRAS (22%) | KRAS (16%) | RB1 (46%) | EGFR (7%) |
| TP53 (42%) | EGFR (5%) | TP53 (31%) | LRP1B (25%) | LRP1B (37%) |
| STK11 (11%) | STK11 (8%) | ARID1A (25%) | KMT2D (16%) | PIK3CA (8%) |
| BRAF (3%) | PIK3CA (4%) | PIK3CA (4%) | EGFR (6%) | NFE2L2 (12%) |
| KEAP1 (15%) | NRAS (3%) | ALK (17%) | NOTCH1 (9%) | KRAS (4%) |
| MET (3%) | MET (3%) | BRAF (2%) | PIK3CA (7%) | KMT2D (17%) |
| LRP1B (28%) | KEAP1 (21%) | MET (2%) | PTEN (6%) | CDKN2A (11%) |
| ERBB2 (2%) | AR (32%) | STK11 (7%) | EP300 (8%) | FAT4 (16%) |
| PIK3CA (4%) | BRAF (2%) | KEAP1 (16%) | FAT1 (9%) | KMT2C (15%) |
| SMARCA4 (9%) | PTEN (4%) | CDKN2A (4%) | ATRX (8%) | KEAP1 (9%) |
| NF1 (8%) | CDKN2A (5%) | ATM (13%) | KMT2C (8%) | NF1 (11%) |
| ATM (7%) | LRP1B (28%) | KMT2D (24%) | CREBBP (8%) | PTEN (7%) |
| FAT1 (8%) | POLQ (28%) | SMARCA4 (10%) | GRIN2A (8%) | ERBB4 (8%) |
| PTPRT (9%) | HRAS (4%) | NF1 (9%) | FAT4 (10%) | FAT1 (12%) |
| KMT2C (9%) | GNAS (4%) | SETBP1 (10%) | ROS1 (8%) | ROS1 (10%) |
| RBM10 (8%) | ERBB4 (5%) | FAT1 (15%) | ERBB4 (6%) | PREX2 (13%) |
| KMT2D (7%) | ATM (6%) | ERBB2 (2%) | ARID1A (6%) | PDE4DIP (11%) |
| FAT4 (16%) | FLT3 (7%) | TET2 (8%) | ZNF521 (7%) | ZFHX3 (9%) |
| ERBB4 (5%) | ZFHX3 (21%) | NOTCH1 (3%) | MTOR (5%) | GRIN2A (10%) |
| Type | Gene and Location | Protein Name | Amino Acid Length | Molar Weight [kDa] | Function | Function in Lung Cancer | References |
|---|---|---|---|---|---|---|---|
| Cell signaling pathway stimulating cell growth | EGFR, 7p11.2 | EGFR | 1210 | 134.277 |
|
| [55,56,57,58,59] |
| BRAF, 7q34 | BRAF | 766 | 84.437 |
|
| [60,61,62] | |
| ERBB2, 17q12 | HER2 | 1255 | 137.910 |
|
| [63,64,65] | |
| KRAS, 12p12.1 | KRAS | 189 | 21.656 |
|
| [66,67,68,69] | |
| PIK3CA, 3q25 | PIK3CA | 1068 | 124.284 |
|
| [70,71,72] | |
| ALK, 2p32.2 | ALK | 1620 | 176.442 |
|
| [73,74] | |
| TITF1, 14q13.3 | TITF1 | 371 | 38.596 |
|
| [75,76,77] | |
| c-MYC, 8q24.21 | MYC | 439 | 48.804 |
|
| [78,79,80] | |
| l-MYC, 1p34.2 | MYC | 364 | 40.327 | [80,81] | |||
| n-MYC, 2p24.3 | MYC | 464 | 49.561 | [79,80,82] | |||
| Szlak genów supresorowych guza | TP53, 17p13 | P53 | 393 | 43.653 |
|
| [83,84,85] |
| RB1, 13q14.2 | Rb1 | 928 | 106.159 |
|
| [86,87,88] | |
| STK11, 19p13.3 | STK11 | 433 | 48.636 |
|
| [89,90,91] | |
| Szlak związany z unikaniem apoptozy | Bax, 19q13.33 | Bax | 192 | 21.184 |
|
| [92,93,94,95,96] |
| Bcl-2, 18q21.33 | Bcl-2 | 239 | 26.266 | [93,94,96,97,98] | |||
| FASL, 1q24.3 | TNFL6 | 281 | 31.485 |
|
| [99,100,101] | |
| E2F1, 20q11.22 | E2F1 | 437 | 46.920 |
|
| [102] | |
| TERT, 5p15.33 | TERT | 1132 | 126.997 |
|
| [103,104,105] |
| miRNA | Genetic Location | Target | Mechasims | References |
|---|---|---|---|---|
| miR-7 | miR-7 is derived from three precursors: pri-miR-7-1 (9q21), pri-miR-7-2 (15q26) and andpri-miR-7-3 (19q13) | EGFR | Sustaining proliferative signaling | [139,140] |
| miR-27 | 19p13.12 | EGFR | Sustaining proliferative signaling | [141,142,143,144] |
| miR-30 | 6q13 | EGFR | Sustaining proliferative signaling | [145,146,147] |
| miR-34 | 1p36.22 | EGFR | Sustaining proliferative signaling | [148,149,150] |
| PD-L1 | Avoiding immune destruction and tumors promoting inflammation | |||
| miR-128 | 2q21.3 | EGFR | Sustaining proliferative signaling | [151,152,153,154] |
| VEGF | Inducing angiogenesis | |||
| miR-134 | 14q32.31 | EGFR | Sustaining proliferative signaling | [155,156,157] |
| miR-542 | Xq26.3 | EGFR | Sustaining proliferative signaling | [158,159,160] |
| miR-1258 | 2q31.3 | Grb2 | Sustaining proliferative signaling | [161,162,163] |
| miR-148a | 7p15.2 | SOS | Sustaining proliferative signaling | [164,165,166] |
| miR-181a | chromosome 1 (37.p5) | RAS | Sustaining proliferative signaling | [167,168] |
| miR-193a | 17q11.2 | RAS | Sustaining proliferative signaling | [169,170,171] |
| miR-760 | 1p22.1 | ROS1 | Sustaining proliferative signaling | [172,173,174] |
| miR-96 | 7q32.2 | EML4-ALK | Sustaining proliferative signaling | [175,176,177] |
| miR-200c/ | 12p13.31 | PI3K | Sustaining proliferative signaling | [178,179,180,181,182] |
| chromosome 1 | ZEB1/2 | Activating invasion and metastasis | ||
| miR-200 | VEGF | Inducing angiogenesis | ||
| PD-L1 | Avoiding immune destruction and tumors promoting inflammation | |||
| miR-520a | 19q13.42 | PI3K | Sustaining proliferative signaling | [183,184,185] |
| miR-641 | 19q13.2 | MDM2 | Evading growth suppressors | [186,187,188] |
| miR-660 | Xp11.23 | MDM2 | Evading growth suppressors | [189,190] |
| miR-15a | 13q14.2 | CDK/Cyclin | Evading growth suppressors | [191,192,193] |
| miR-16 | 13q14 | CDK/Cyclin | Evading growth suppressors | [194,195,196,197] |
| Bcl | Resisting cell death | |||
| miR-449a | 5q11.2 | E2F | Evading growth suppressors | [198,199,200,201] |
| miR-299 | 14q32.31 | hTERT | Enabling replicative immortality | [202,203,204] |
| miR-491 | 9p21.3 | hTERT | Enabling replicative immortality | [205,206,207] |
| miR-512 | 19q13.42 | hTERT | Enabling replicative immortality | [208,209,210] |
| miR-29 | 7q32.3 | DNMT | Enabling replicative immortality | [211,212,213] |
| miR-146A | 5q33.3 | Smad | Activating invasion and metastasis | [214,215,216] |
| miR-21 | 17q23.1 | TGF-β | Activating invasion and metastasis | [217,218,219,220,221] |
| PTEN | Avoiding immune destruction and Tumors promoting inflammation | |||
| miR-23 | no data | TGF-β | Activating invasion and metastasis | [222,223,224] |
| miR-99A | 21q21.1 | TGF-β | Activating invasion and metastasis | [225,226,227] |
| miR-155 | 21q21.3 | TGF-β | Activating invasion and metastasis | [228,229,230,231] |
| miR-192 | 11q13.1 | TGF-β | Activating invasion and metastasis | [232,233,234,235] |
| miR-115 | no data | TGF-β | Activating invasion and metastasis | [236,237] |
| miR-520 | 19q13.42 | TGF-β | Activating invasion and metastasis | [238,239,240] |
| miR-497 | 17p13.1 | HDGF | Inducing angiogenesis | [241,242,243,244] |
| miR-494 | 14q32.31 | PTEN | Inducing angiogenesis | [245,246,247,248] |
| miR-126 | 9q34.3 | VEGF | Inducing angiogenesis | [249,250,251,252] |
| miR-33b | 17p11.2 | LDHA | Deregulating cellular energetics | [253,254,255] |
| miR-144 | 17q11.2 | GLUT | Deregulating cellular energetics | [256,257,258] |
| miR-124 | 8p23.1 | Akt | Deregulating cellular energetics | [259,260,261] |
| miR-199a | 19p13.2 | HIF-1 | Deregulating cellular energetics | [262,263,264] |
| miR-31-5p | 9p21.3 | FIH | Deregulating cellular energetics | [265,266,267] |
| miR-28 | 3q28 | PD-1PTEN | Avoiding immune destruction and tumors promoting inflammation | [268,269,270] |
| miR-138 | 16q13 | PD-1 | Avoiding immune destruction and tumors promoting inflammation | [271,272] |
| PD-L1 | ||||
| miR-197 | 1p13.3 | CKS1B | Avoiding immune destruction and tumors promoting inflammation | [273,274,275] |
| miR-513 | Xq27.3 | PD-L1 | Avoiding immune destruction and tumors promoting inflammation | [276,277,278,279] |
| miR-20 | 13q31.3 | PTEN | Avoiding immune destruction and tumors promoting inflammation | [280,281,282] |
| miR-130 | 11q12.1 | PTEN | Avoiding immune destruction and tumors promoting inflammation | [283,284,285] |
| miR-424 | Xq26.3 | CD80 | Avoiding immune destruction and tumors promoting inflammation | [286,287,288,289] |
| miR-136 | 14q32.2 | CTLA-4 | Avoiding immune destruction and tumors promoting inflammation | [290,291,292,293] |
| miR-301b | 22q11.21 | BH3 | Resisting cell death | [294,295,296,297,298] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smok-Kalwat, J.; Mertowska, P.; Mertowski, S.; Smolak, K.; Kozińska, A.; Koszałka, F.; Kwaśniewski, W.; Grywalska, E.; Góźdź, S. The Importance of the Immune System and Molecular Cell Signaling Pathways in the Pathogenesis and Progression of Lung Cancer. Int. J. Mol. Sci. 2023, 24, 1506. https://doi.org/10.3390/ijms24021506
Smok-Kalwat J, Mertowska P, Mertowski S, Smolak K, Kozińska A, Koszałka F, Kwaśniewski W, Grywalska E, Góźdź S. The Importance of the Immune System and Molecular Cell Signaling Pathways in the Pathogenesis and Progression of Lung Cancer. International Journal of Molecular Sciences. 2023; 24(2):1506. https://doi.org/10.3390/ijms24021506
Chicago/Turabian StyleSmok-Kalwat, Jolanta, Paulina Mertowska, Sebastian Mertowski, Konrad Smolak, Aleksandra Kozińska, Filip Koszałka, Wojciech Kwaśniewski, Ewelina Grywalska, and Stanisław Góźdź. 2023. "The Importance of the Immune System and Molecular Cell Signaling Pathways in the Pathogenesis and Progression of Lung Cancer" International Journal of Molecular Sciences 24, no. 2: 1506. https://doi.org/10.3390/ijms24021506
APA StyleSmok-Kalwat, J., Mertowska, P., Mertowski, S., Smolak, K., Kozińska, A., Koszałka, F., Kwaśniewski, W., Grywalska, E., & Góźdź, S. (2023). The Importance of the Immune System and Molecular Cell Signaling Pathways in the Pathogenesis and Progression of Lung Cancer. International Journal of Molecular Sciences, 24(2), 1506. https://doi.org/10.3390/ijms24021506