UV Laser-Induced Phototransformations of Matrix-Isolated 5-Chloro-3-nitro-2-hydroxyacetophenone
Abstract
1. Introduction
2. Results and Discussion
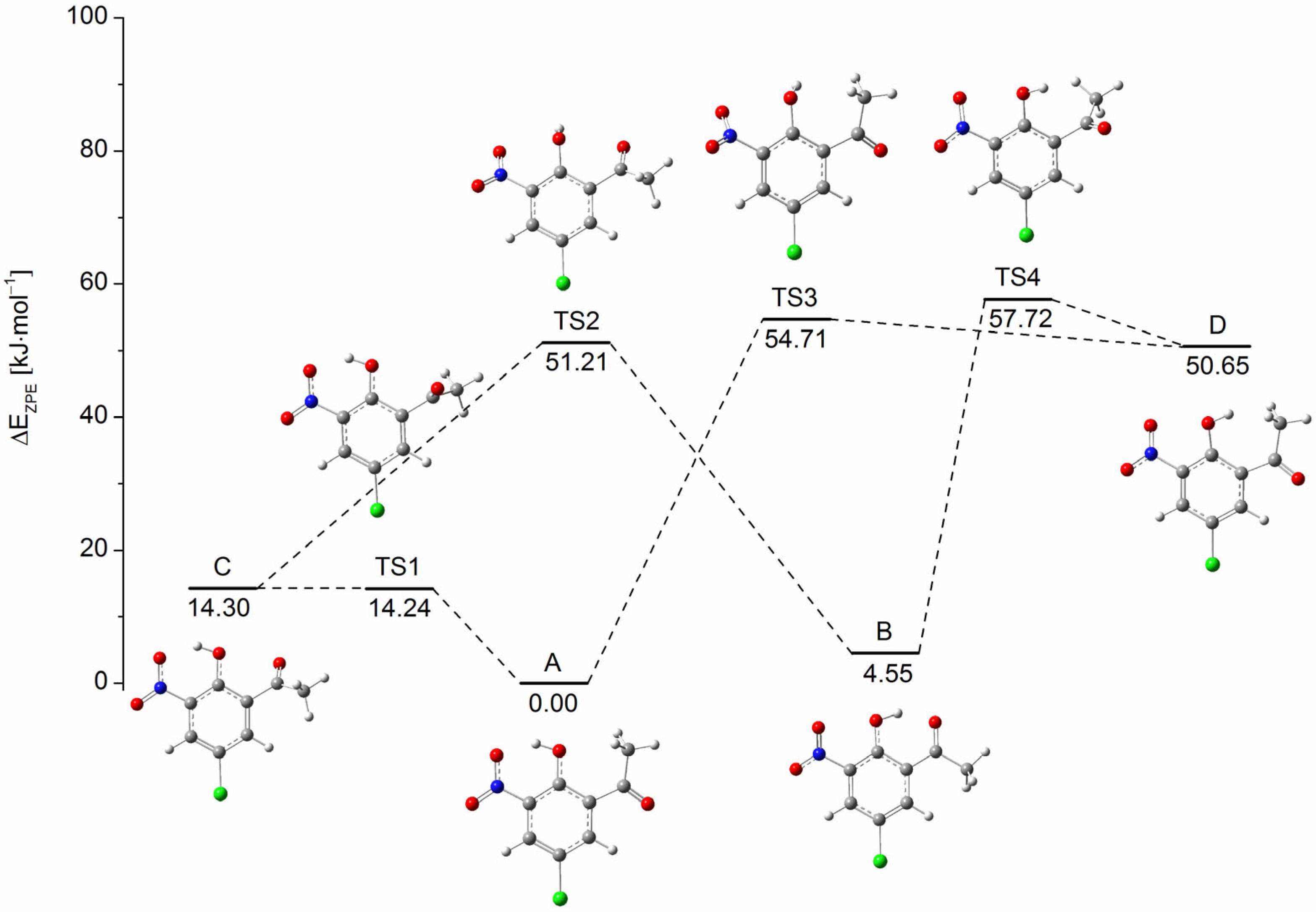
2.1. Energy Minima and Transition States of CNK on the S0 Potential Energy Surface (PES)
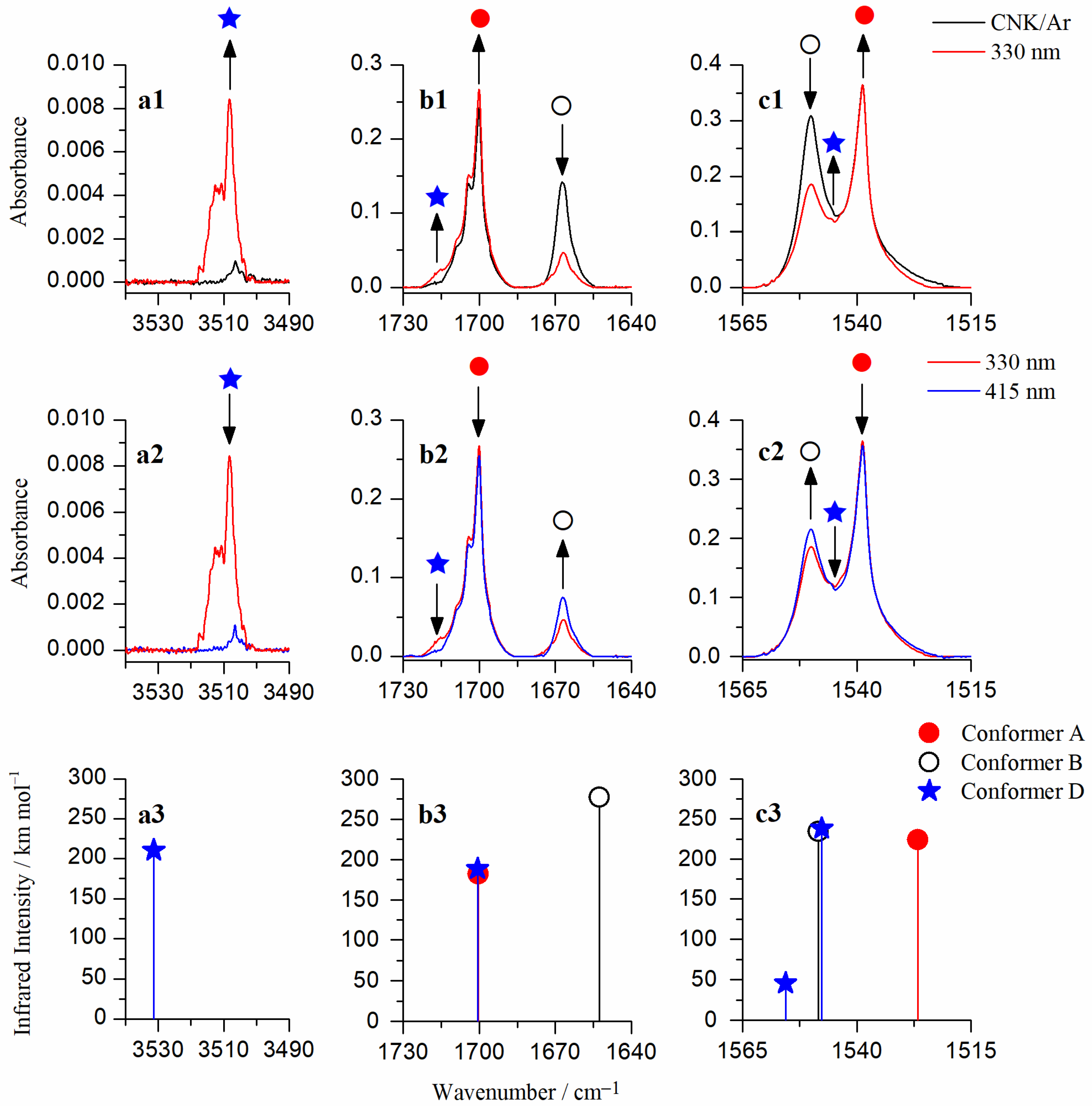
2.2. Matrix Isolation Spectrum of CNK and Its Comparison to the Theoretical Predictions
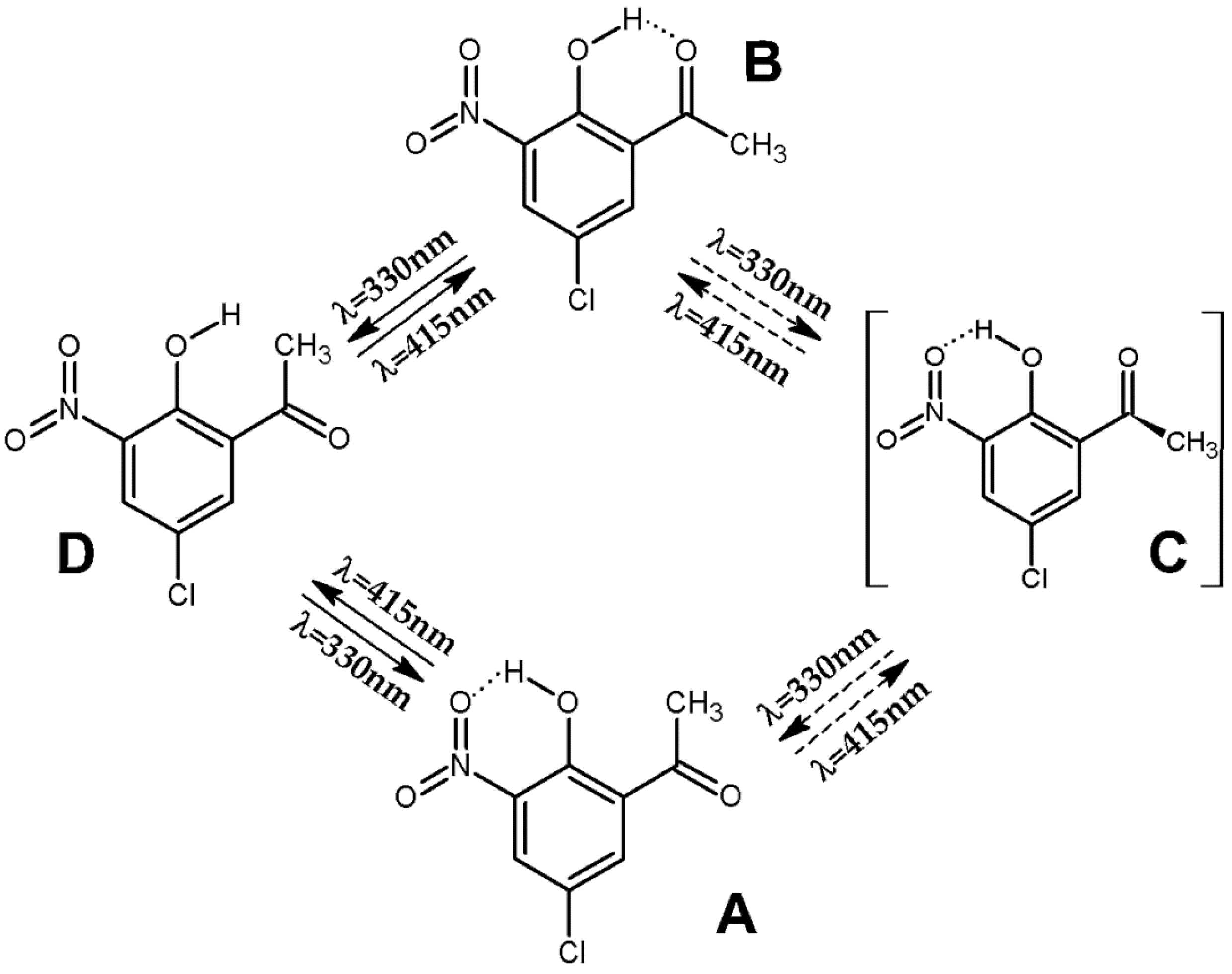
2.3. Phototransformations of CNK upon UV Laser Irradiation
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittle, E.; Dows, D.A.; Pimentel, G.C. Matrix Isolation Method for the Experimental Study of Unstable Species. J. Chem. Phys. 1954, 22, 1943. [Google Scholar] [CrossRef]
- Pimentel, G.C. Reaction Kinetics by the Matrix Isolation Method: Diffusion in Argon; cis-trans Isomerization of Nitrous Acid. J. Am. Chem. Soc. 1958, 80, 62–64. [Google Scholar] [CrossRef]
- Turner, J.J.; Pimentel, G.C. Krypton Fluoride: Preparation by the Matrix Isolation Technique. Science 1963, 140, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Bondybey, V.E.; Smith, A.M.; Agreiter, J. New Developments in Matrix Isolation Spectroscopy. Chem. Rev. 1996, 96, 2113–2134. [Google Scholar] [CrossRef] [PubMed]
- Gritsan, N.P. Study of photochemical transformations of organic azides by matrix isolation techniques and quantum chemistry. Russ. Chem. Rev. 2007, 76, 1139–1160. [Google Scholar] [CrossRef]
- Barnes, A.J.; Orville-Thomas, W.J.; Müller, A.; Gaufrès, R. Matrix Isolation Spectroscopy; Springer Publishing Company: Dordrecht, The Netherlands, 1981. [Google Scholar]
- Orville-Thomas, W.J. The History of Matrix Isolation Spectroscopy. In Matrix Isolation Spectroscopy; Barnes, A.J., Orville-Thomas, W.J., Müller, A., Gaufrès, R., Eds.; Springer Publishing Company: Dordrecht, The Netherlands, 1981. [Google Scholar]
- Barnes, A.J.; Orville-Thomas, W.J. Vibrational Spectroscopy: Modern Trends; Elsevier: New York, NY, USA, 1977. [Google Scholar]
- Fausto, R.; Borba, A.; Gómez-Zavaglia, A. Light Induced Reactions in Cryogenic Matrices (Highlights 2013–2014). In Photochemistry; Fasani, E., Albini, A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; Volume 43, pp. 20–82. [Google Scholar]
- Kołos, R. Carbon chain extension processes in cryogenic environments: UV-assisted growth of polyynic nitriles in solidified rare gases. Low Temp. Phys. 2019, 45, 585–588. [Google Scholar] [CrossRef]
- Saini, J.; Dubey, P.; Verma, K.; Karir, G.; Viswanathan, K.S. Intermolecular complexes and molecular conformations directed by hydrogen bonds: Matrix isolation and ab initio studies. J. Indian Inst. Sci. 2019, 100, 167–190. [Google Scholar] [CrossRef]
- Avadanei, M.; Kus’, N.; Cozan, V.; Fausto, R. Structure and Photochemistry of N-Salicylidene-p-carboxyaniline Isolated in Solid Argon. J. Phys. Chem. A 2015, 119, 9121–9132. [Google Scholar] [CrossRef]
- Barnes, A.J. Matrix isolation studies of hydrogen bonding—A historical perspective. J. Mol. Struct. 2018, 1163, 77–85. [Google Scholar] [CrossRef]
- Lapinski, L.; Rostkowska, H.; Reva, I.; Fausto, R.; Nowak, M.J. Positive Identification of UV-Generated, Non-Hydrogen-Bonded Isomers of o-Hydroxybenzaldehyde and o-Hydroxyacetophenone. J. Phys. Chem. A 2010, 114, 5588–5595. [Google Scholar] [CrossRef]
- Botan, M.; Hamm, P. Temperature dependence of the IR driven cis—Trans isomerization of nitrous acid (HONO). J. Chem. Phys. 2008, 129, 114510. [Google Scholar] [CrossRef] [PubMed]
- Hamm, P. The infrared-driven cis–trans isomerization of nitrous acid HONO III: A mixed quantum–classical simulation. Chem. Phys. 2008, 347, 503–513. [Google Scholar] [CrossRef]
- Mielke, Z.; Latajka, Z.; Olbert-Majkut, A.; Wieczorek, R. Matrix infrared spectra and ab initio calculations of the nitrous acid complexes with nitrogen monoxide. J. Phys. Chem. A 2000, 104, 3764–3769. [Google Scholar] [CrossRef]
- Pagacz-Kostrzewa, M.; Mucha, K.; Gul, W.; Wierzejewska, M. FTIR spectroscopic evidence for new isomers of 3-aminopyrazine-2-carboxylic acid formed in argon matrices upon UV irrdiations. Spectrochim. Acta A 2021, 263, 120158. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, B.A.; Ildiz, G.O.; Canotilho, J.; Eusebio, M.E.S.; Henriques, M.S.C.; Paixao, J.A.; Fausto, R. 5-Methylhydantoin: From Isolated Molecules in a Low-Temperature Argon Matrix to Solid State Polymorphs Characterization. J. Phys. Chem. A 2017, 121, 5267–5279. [Google Scholar] [CrossRef]
- Benković, T.; Kendel, A.; Parlov-Vuković, J.; Kontrec, D.; Chiş, V.; Miljanić, S.; Galić, N. Multiple dynamics of aroylhydrazone induced by mutual effect of solvent and light—Spectroscopic and computational study. J. Mol. Liq. 2018, 255, 18–25. [Google Scholar] [CrossRef]
- Grabowski, S.J. Understanding Hydrogen Bonds: Theoretical and Experimental Views; The Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- Chou, P.; Martinez, M.; Clements, J. Reversal of excitation behavior of proton-transfer vs. charge-transfer by dielectric perturbation of electronic manifolds. J. Phys. Chem. 1993, 97, 2618–2622. [Google Scholar] [CrossRef]
- Catalan, J.; Fabero, F.; Claramunt, R.; Santa, M.; Foces-Foces, M.; Cano, F. New ultraviolet stabilizers: 3- and 5-(2′-hydroxyphenyl)pyrazoles. J. Am. Chem. Soc. 1992, 114, 5039–5048. [Google Scholar] [CrossRef]
- Scherl, M.; Haarer, D.; Fischer, J.; DeCian, A.; Lehn, J.-L.; Eichen, Y. Proton-Transfer Processes in Well-Defined Media: Experimental Investigation of Photoinduced and Thermal Proton-Transfer Processes in Single Crystals of 2-(2,4-Dinitrobenzyl)pyridine Derivatives. J. Phys. Chem. 1996, 100, 16175–16186. [Google Scholar] [CrossRef]
- Demchenko, A.P. Visualization and sensing of intermolecular interactions with two-color fluorescent probes. FEBS Lett. 2006, 580, 2951–2957. [Google Scholar] [CrossRef]
- Martin, R. Aromatic Hydroxyketones: Preparation and Physical Properties; Springer Publishing Company: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Gray, K.A.; Kamat, P.; Stafford, U.; Dieckmann, M. Mechanistic Studies of Chloro- and Nitrophenolic Degradation on Semiconductor Surfaces. In Aquatic and Surface Photochemistry; Helz, G.R., Zepp, R.G., Crosby, D.G., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 399–408. [Google Scholar]
- Konopacka, A.; Filarowski, A.; Pawełka, Z. Solvent influence on the transformation of intramolecular hydrogen bonds in 2-hydroxy-5-methyl-3-nitroacetophenone. J. Solut. Chem. 2005, 34, 929–945. [Google Scholar] [CrossRef]
- Hetmańczyk, Ł.; Szklarz, P.; Kwocz, A.; Wierzejewska, M.; Pagacz-Kostrzewa, M.; Melnikov, M.Y.; Tolstoy, P.M.; Filarowski, A. Polymorphism and Conformational Equilibrium of Nitro-Acetophenone in Solid State and under Matrix Conditions. Molecules 2021, 26, 3109. [Google Scholar] [CrossRef] [PubMed]
- Siegel, H.; Eggersdorfer, M. Ketones. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Wienheim, Germany, 2002. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Raghavachari, K.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. Basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagacz-Kostrzewa, M.; Mucha, K.; Wierzejewska, M.; Filarowski, A. UV Laser-Induced Phototransformations of Matrix-Isolated 5-Chloro-3-nitro-2-hydroxyacetophenone. Int. J. Mol. Sci. 2023, 24, 1546. https://doi.org/10.3390/ijms24021546
Pagacz-Kostrzewa M, Mucha K, Wierzejewska M, Filarowski A. UV Laser-Induced Phototransformations of Matrix-Isolated 5-Chloro-3-nitro-2-hydroxyacetophenone. International Journal of Molecular Sciences. 2023; 24(2):1546. https://doi.org/10.3390/ijms24021546
Chicago/Turabian StylePagacz-Kostrzewa, Magdalena, Karolina Mucha, Maria Wierzejewska, and Aleksander Filarowski. 2023. "UV Laser-Induced Phototransformations of Matrix-Isolated 5-Chloro-3-nitro-2-hydroxyacetophenone" International Journal of Molecular Sciences 24, no. 2: 1546. https://doi.org/10.3390/ijms24021546
APA StylePagacz-Kostrzewa, M., Mucha, K., Wierzejewska, M., & Filarowski, A. (2023). UV Laser-Induced Phototransformations of Matrix-Isolated 5-Chloro-3-nitro-2-hydroxyacetophenone. International Journal of Molecular Sciences, 24(2), 1546. https://doi.org/10.3390/ijms24021546





