Exploring the Role of Serotonin as an Immune Modulatory Component in Cardiovascular Diseases
Abstract
1. Introduction
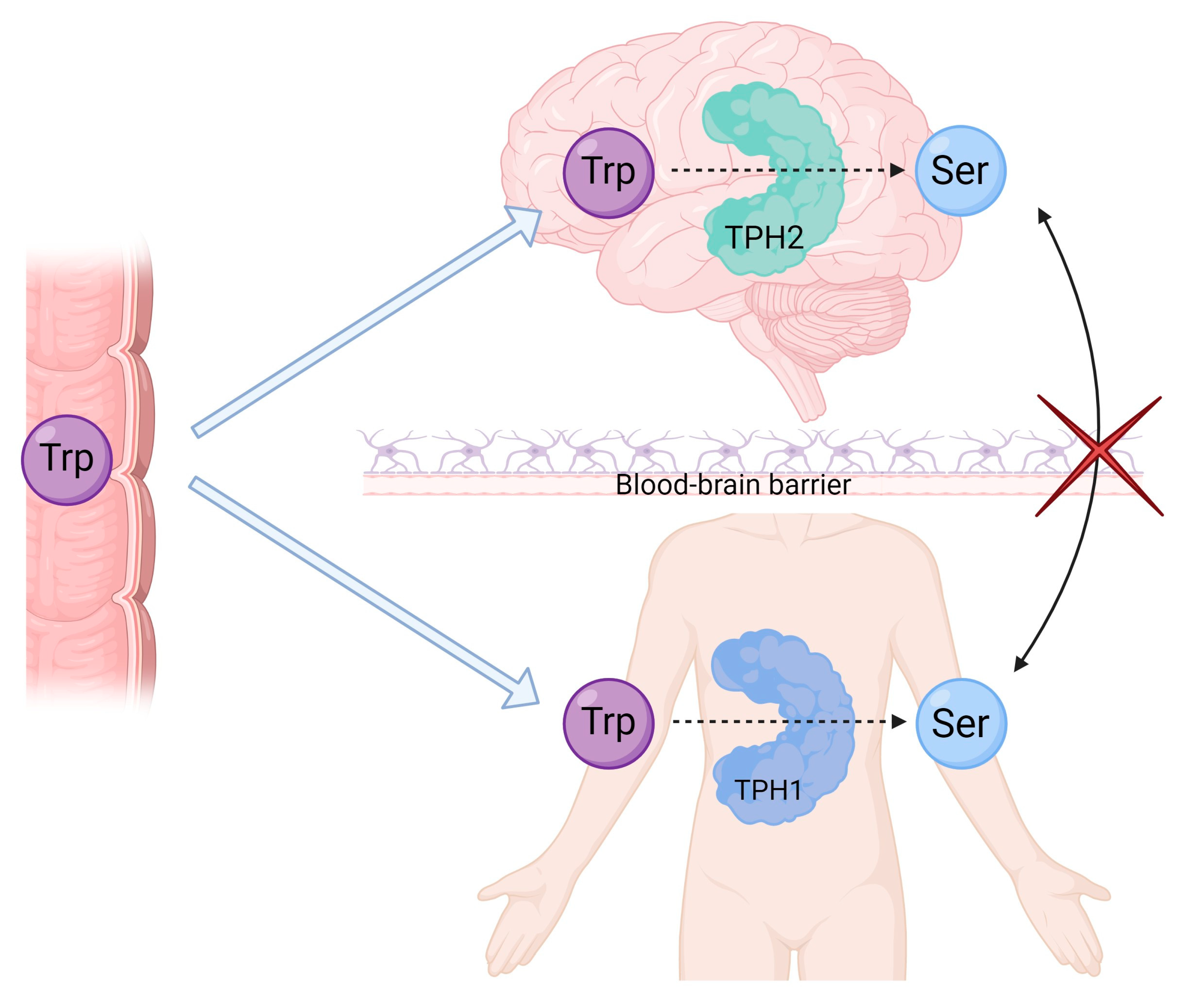
2. Physiological Production of Serotonin: Serotonin Formation and Regulation
3. Serotonin and Vascular Reactivity
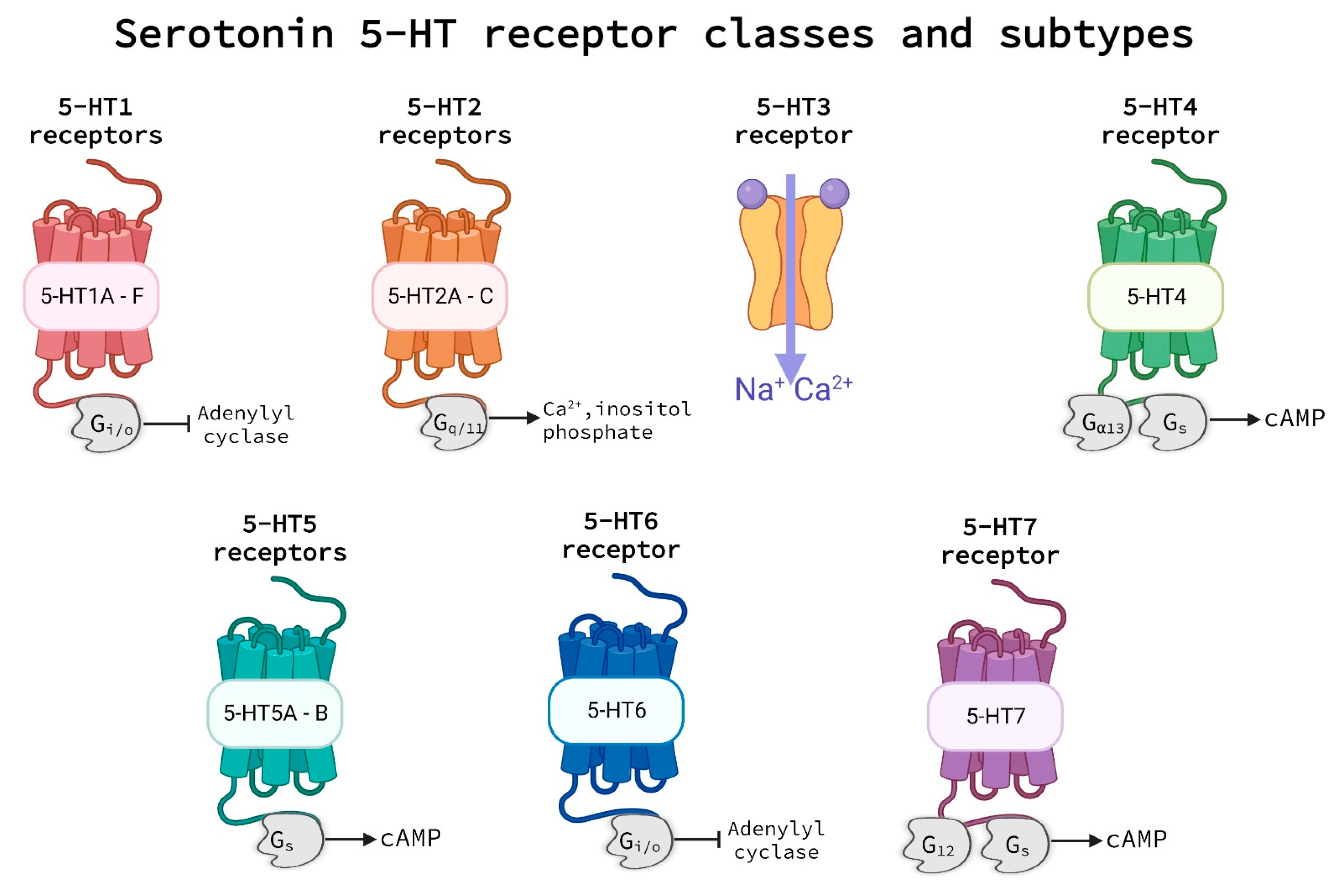
3.1. Serotonin Receptors
3.2. The Development and Progression of Atherosclerosis
3.3. Early Studies Involving Serotonin and Vasoreactivity
4. Serotonin and Immune Response in Chronic Inflammation and Atherosclerosis
4.1. B-Cells
4.2. T-Cells
4.3. Natural Killer Cells
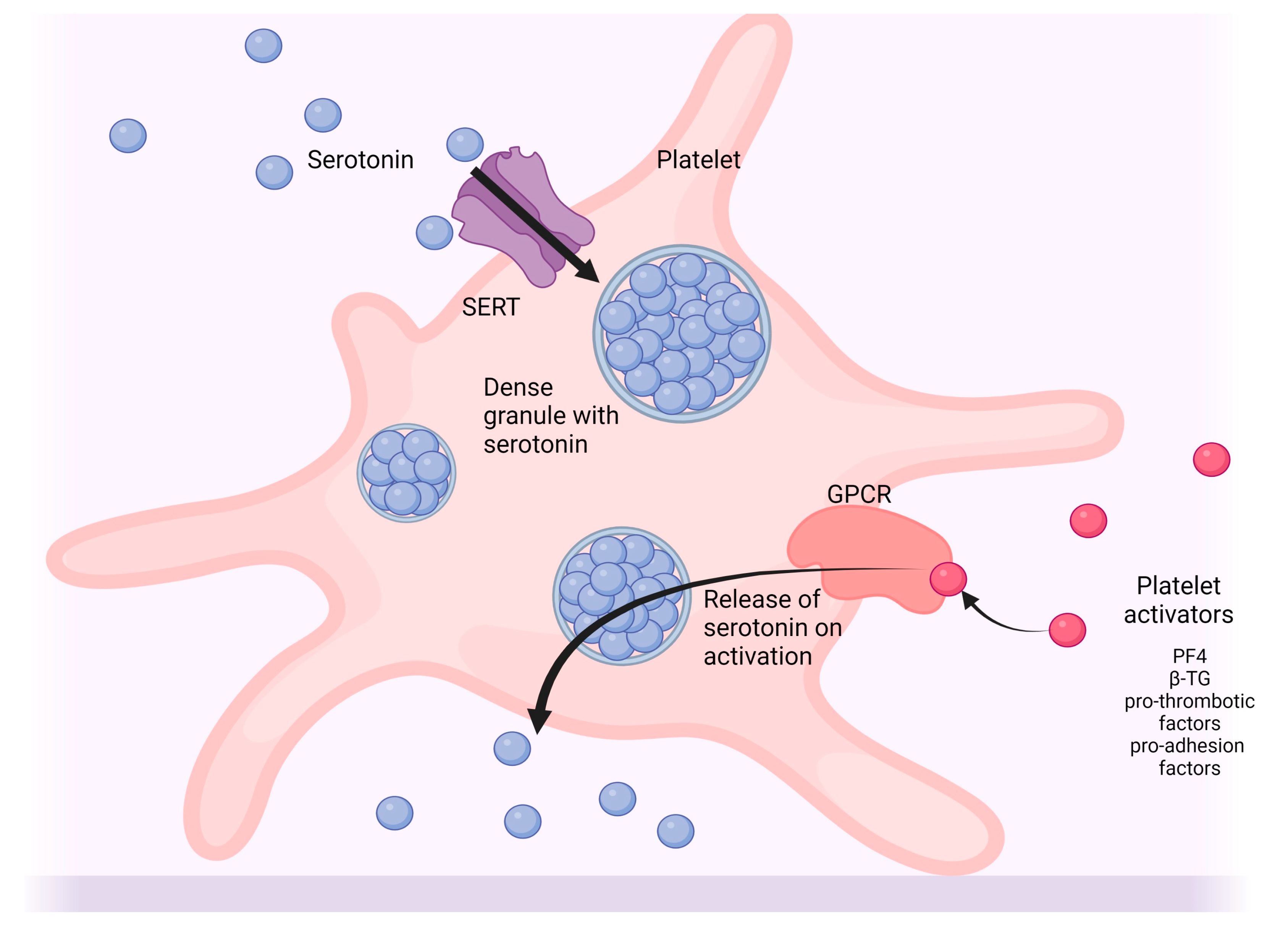
4.4. Platelets
4.5. Eosinophils
4.6. Neutrophils
4.7. Mast Cells
4.8. Monocytes
4.9. Macrophages
4.10. Dendritic Cells (DCs)
5. Current Knowledge about the Impact of Serotonin Modifying Drugs and Cardiovascular Events
6. Conclusions and Perspectives on Serotonin in Inflammatory Diseases
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frohlich, P.; Meston, C. Evidence that serotonin affects female sexual functioning via peripheral mechanisms. Physiol. Behav. 2000, 71, 383–393. [Google Scholar] [CrossRef]
- Houston, D.S.; Vanhoutte, P.M. Serotonin and the Vascular System. Drugs 1986, 31, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Page, I.H.; Rapport, M.M.; Green, A.A. The crystallization of serotonin. J. Lab. Clin. Med. 1948, 33, 1606. [Google Scholar]
- Rapport, M.M.; Green, A.A.; Page, I.H. Crystalline Serotonin. Science 1948, 108, 329–330. [Google Scholar] [CrossRef]
- Cohen, R.A.; Shepherd, J.T.; Vanhoutte, P.M. Endothelium and asymmetrical responses of the coronary arterial wall. Am. J. Physiol. Circ. Physiol. 1984, 247, H403–H408. [Google Scholar] [CrossRef]
- Keszthelyi, D.; Troost, F.J.; Masclee, A.A.M. Understanding the role of tryptophan and serotonin metabolism in gastrointestinal function. Neurogastroenterol. Motil. 2009, 21, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Developmental programming and reprogramming of hypertension and kidney disease: Impact of tryptophan metabolism. Int. J. Mol. Sci. 2020, 21, 8705. [Google Scholar] [CrossRef]
- Le Floc’H, N.; Seve, B. Biological roles of tryptophan and its metabolism: Potential implications for pig feeding. Livest. Sci. 2007, 112, 23–32. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Rukmini, R.; Mukherjee, S. Photophysics of a neurotransmitter: Ionization and spectroscopic properties of serotonin. Biophys. J. 1996, 71, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Gairhe, S.; Bauer, N.N.; Gebb, S.A.; McMurtry, I.F. Serotonin passes through myoendothelial gap junctions to promote pulmonary arterial smooth muscle cell differentiation. Am. J. Physiol. Cell. Mol. Physiol. 2012, 303, L767–L777. [Google Scholar] [CrossRef]
- Holmsen, H.; Weiss, H.J. Secretable storage pools in platelets. Annu. Rev. Med. 1979, 30, 119–134. [Google Scholar] [CrossRef]
- Duerschmied, D.; Suidan, G.L.; Demers, M.; Herr, N.; Carbo, C.; Brill, A.; Cifuni, S.M.; Mauler, M.; Cicko, S.; Bader, M.; et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013, 121, 1008–1015. [Google Scholar] [CrossRef]
- Moul, C.; Dobson-Stone, C.; Brennan, J.; Hawes, D.; Dadds, M. An Exploration of the Serotonin System in Antisocial Boys with High Levels of Callous-Unemotional Traits. PLoS ONE 2013, 8, e56619. [Google Scholar] [CrossRef]
- Benech, N.; Rolhion, N.; Sokol, H. Gut Microbiota Reprogramming of Tryptophan Metabolism During Pregnancy Shapes Host Insulin Resistance. Gastroenterology 2022, 162, 1587–1589. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V.; Asero, B. Identification of Enteramine, the Specific Hormone of the Enterochromaffin Cell System, as 5-Hydroxytryptamine. Nature 1952, 169, 800–801. [Google Scholar] [CrossRef]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut—Functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Almaça, J.; Molina, J.; Menegaz, D.; Pronin, A.N.; Tamayo, A.; Slepak, V.; Berggren, P.O.; Caicedo, A. Human beta cells produce and release serotonin to inhibit glucagon secre-tion from alpha cells. Cell Rep. 2016, 17, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Stunes, A.K.; Reseland, J.E.; Hauso, Ø.; Kidd, M.; Tømmerås, K.; Waldum, H.L.; Syversen, U.; Gustafsson, B.I. Adipocytes express a functional system for serotonin synthesis, reuptake and receptor activation. Diabetes Obes. Metab. 2011, 13, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rodríguez, A.; Morell-Azanza, L.; Azcona-Sanjulián, M.C.; Martínez, J.A.; Ramírez, M.J.; Marti, A. Reduced serotonin levels after a lifestyle intervention in obese children: Association with glucose and anthropometric measurements. Nutr. Hosp. 2018, 35, 279–285. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Carrillo, C. Blood serotonin levels in postmenopausal women: Effects of age and serum oestradiol levels. Maturitas 1993, 17, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Giulietti, M.; Vivenzio, V.; Piva, F.; Principato, G.; Bellantuono, C.; Nardi, B. How much do we know about the coupling of G-proteins to serotonin receptors? Mol. Brain 2014, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef]
- Chen, J.X.; Pan, H.; Rothman, T.P.; Wade, P.R.; Gershon, M.D. Guinea pig 5-HT transporter: Cloning, expression, distribution, and function in intestinal sensory reception. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G433–G448. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ding, D.; Bai, D.; Zhu, Y.; Sun, W.; Sun, Y.; Zhang, D. Melatonin biosynthesis pathways in nature and its production in engineered microorganisms. Synth. Syst. Biotechnol. 2022, 7, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Chen, K.; Shih, J.C. The Degradation of Serotonin: Role of MAO. In Handbook of Behavioral Neuroscience; Müller, C.P., Jacobs, B.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 21, pp. 203–218. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Mauler, M.; Braun, A.; Duerschmied, D. Autocrine and paracrine regulatory functions of platelet serotonin. Platelets 2018, 29, 541–548. [Google Scholar] [CrossRef]
- Lee, S.L.; Wang, W.W.; Lanzillo, J.J.; Fanburg, B.L. Serotonin produces both hyperplasia and hypertrophy of bovine pulmonary artery smooth muscle cells in culture. Am. J. Physiol. Cell. Mol. Physiol. 1994, 266, L46–L52. [Google Scholar] [CrossRef]
- Warden, S.J.; Haney, E.M. Skeletal effects of serotonin (5-hydroxytryptamine) transporter inhibition: Evidence from in vitro and animal-based studies. J. Musculoskelet. Neuronal Interact. 2008, 8, 121–132. [Google Scholar]
- Weinstein, H. Hallucinogen Actions on 5-HT Receptors Reveal Distinct Mechanisms of Activation and Signaling by G Protein-Coupled Receptors. In Drug Addiction: From Basic Research to Therapy; Rapaka, R.S., Sadée, W., Eds.; Springer: New York, NY, USA, 2008; pp. 265–286. [Google Scholar] [CrossRef]
- Millan, M.J.; Marin, P.; Bockaert, J.; La Cour, C.M. Signaling at G-protein-coupled serotonin receptors: Recent advances and future research directions. Trends Pharmacol. Sci. 2008, 29, 454–464. [Google Scholar] [CrossRef]
- Peroutka, S.; Howell, T. The molecular evolution of G protein-coupled receptors: Focus on 5-hydroxytryptamine receptors. Neuropharmacology 1994, 33, 319–324. [Google Scholar] [CrossRef]
- Watanabe, S.; Matsumoto, T.; Oda, M.; Yamada, K.; Takagi, J.; Taguchi, K.; Kobayashi, T. Insulin augments serotonin-induced contraction via activation of the IR/PI3K/PDK1 pathway in the rat carotid artery. Pflügers Arch. Eur. J. Physiol. 2015, 468, 667–677. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Kobayashi, M.; Kikuta, K.; Matsuda, S. Roles of PI3K/AKT/GSK3/mTOR pathway in cell signaling of mental illnesses. Depress. Res. Treat. 2012, 2012, 752563. [Google Scholar] [CrossRef] [PubMed]
- Dizeyi, N.; Hedlund, P.; Bjartell, A.; Tinzl, M.; Austild-Taskén, K.; Abrahamsson, P.A. Serotonin activates MAP kinase and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol. Oncol. 2011, 29, 436–445. [Google Scholar] [CrossRef]
- Lin, S.L.; Setya, S.; Johnson-Farley, N.N.; Cowen, D.S. Differential coupling of 5-HT1receptors to G proteins of the Gifamily. Br. J. Pharmacol. 2002, 136, 1072–1078. [Google Scholar] [CrossRef]
- Francken, B.J.; Josson, K.; Lijnen, P.; Jurzak, M.; Luyten, W.H.; E Leysen, J. Human 5-hydroxytryptamine(5A) receptors activate coexpressed G(i) and G(o) proteins in Spodoptera frugiperda 9 cells. Mol. Pharmacol. 2000, 57, 1034. [Google Scholar]
- Day, M.; Olson, P.A.; Platzer, J.; Striessnig, J.; Surmeier, D.J. Stimulation of 5-HT2Receptors in Prefrontal Pyramidal Neurons Inhibits Cav1.2 L-Type Ca2+Currents Via a PLCβ/IP3/Calcineurin Signaling Cascade. J. Neurophysiol. 2002, 87, 2490–2504. [Google Scholar] [CrossRef]
- Kaumann, A.J.; Sanders, L.; Brown, A.M.; Murray, K.J.; Brown, M.J. A 5-hydroxytryptamine receptor in human atrium. J. Cereb. Blood Flow Metab. 1990, 100, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Bockaert, J.; Sebben, M.; Dumuis, A. Pharmacological characterization of 5-hydroxytryptamine4(5-HT4) receptors positively coupled to adenylate cyclase in adult guinea pig hippocampal membranes: Effect of substituted benzamide derivatives. Mol. Pharmacol. 1990, 37, 408. [Google Scholar] [PubMed]
- Maillet, M.; Robert, S.J.; Cacquevel, M.; Gastineau, M.; Vivien, D.; Bertoglio, J.; Zugaza, J.L.; Fischmeister, R.; Lezoualc’H, F. Crosstalk between Rap1 and Rac regulates secretion of sAPPα. Nat. Cell Biol. 2003, 5, 633–639. [Google Scholar] [CrossRef]
- Baker, L.P.; Nielsen, M.D.; Impey, S.; Metcalf, M.A.; Poser, S.W.; Chan, G.; Obrietan, K.; Hamblin, M.W.; Storm, D.R. Stimulation of Type 1 and Type 8 Ca2+/Calmodulin-sensitive Adenylyl Cyclases by the Gs-coupled 5-Hydroxytryptamine Subtype 5-HT7AReceptor. J. Biol. Chem. 1998, 273, 17469–17476. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Nawoschik, S.; Kowal, D.; Smith, D.; Spangler, T.; Ochalski, R.; Schechter, L.; Dunlop, J. Characterization of the 5-HT6 receptor coupled to Ca2+ signaling using an enabling chimeric G-protein. Eur. J. Pharmacol. 2003, 472, 33–38. [Google Scholar] [CrossRef]
- Ponimaskin, E.G.; Profirovic, J.; Vaiskunaite, R.; Richter, D.W.; Voyno-Yasenetskaya, T.A. 5-Hydroxytryptamine 4(a) Receptor Is Coupled to the Gα Subunit of Heterotrimeric G13 Protein. J. Biol. Chem. 2002, 277, 20812–20819. [Google Scholar] [CrossRef]
- Robson, M.J.; Quinlan, M.A.; Blakely, R.D. Immune System Activation and Depression: Roles of Serotonin in the Central Nervous System and Periphery. ACS Chem. Neurosci. 2017, 8, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Okaty, B.W.; Commons, K.G.; Dymecki, S.M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 2019, 20, 397–424. [Google Scholar] [CrossRef]
- Żmudzka, E.; Sałaciak, K.; Sapa, J.; Pytka, K. Serotonin receptors in depression and anxiety: Insights from animal studies. Life Sci. 2018, 210, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Olivier, B. Serotonin: A never-ending story. Eur. J. Pharmacol. 2014, 753, 2–18. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E. Atherosclerosis: The interplay between lipids and immune cells. Curr. Opin. Lipidol. 2016, 27, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, L.H. Mechanisms of plaque rupture mechanical and biologic interactions. Cardiovasc. Res. 1999, 41, 369–375. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Bodelsson, M.; Törnebrandt, K.; Bertilsson, I.-L.; Arneklo-Nobin, B. Heterogeneity of contractile 5-HT receptors in human hand veins. Eur. J. Pharmacol. 1992, 219, 455–460. [Google Scholar] [CrossRef]
- Blauw, G.J.; Van Brummelen, P.; Chang, P.C.; Vermeij, P.; Van Zwieten, P.A. 5HT3 receptor-mediated vasodilation in the human forearm. J. Hypertens. 1988, 6, S33–S35. [Google Scholar] [CrossRef] [PubMed]
- Chester, A.H.; Martin, G.R.; Bodelsson, M.; Arneklo-Nobin, B.; Tadjkarimi, S.; Tornebrandt, K.; Yacoub, M.H. 5-Hydroxytryptamine receptor profile in healthy and diseased human epicardial coronary arteries. Cardiovasc. Res. 1990, 24, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Takacs, L.; Vajda, V. Effect of serotonin on cardiac output and organ blood flow of rats. Am. J. Physiol. Content 1963, 204, 301–303. [Google Scholar] [CrossRef]
- Dedeoğlu, A.; Fisher, A.L. Central and peripheral injections of the 5-HT2 agonist, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane, modify cardiovascular function through different mechanisms. J. Pharmacol. Exp. Ther. 1991, 259, 1027–1034. [Google Scholar] [PubMed]
- Wright, C.E.; Angus, J.A. Diverse Vascular Responses to Serotonin in the Conscious Rabbit. J. Cardiovasc. Pharmacol. 1987, 10, 415–423. [Google Scholar] [CrossRef]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Immune-inflammatory responses in atherosclerosis: Role of an adaptive immunity mainly driven by T and B cells. Immunobiology 2016, 221, 1014–1033. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Herbin, O.; Bouaziz, J.-D.; Binder, C.J.; Uyttenhove, C.; Laurans, L.; Taleb, S.; Van Vré, E.; Esposito, B.; Vilar, J.; et al. B cell depletion reduces the development of atherosclerosis in mice. J. Exp. Med. 2010, 207, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Iken, K.; Chheng, S.; Fargin, A.; Goulet, A.-C.; Kouassi, E. Serotonin Upregulates Mitogen-Stimulated B Lymphocyte Proliferation through 5-HT1AReceptors. Cell. Immunol. 1995, 163, 1–9. [Google Scholar] [CrossRef]
- Abdouh, M.; Storring, J.M.; Riad, M.; Paquette, Y.; Albert, P.R.; Drobetsky, E.; Kouassi, E. Transcriptional Mechanisms for Induction of 5-HT1AReceptor mRNA and Protein in Activated B and T Lymphocytes. J. Biol. Chem. 2001, 276, 4382–4388. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.M.; McGrath, K.M.; Sarr, T.; Bombara, M.P.; Kelley, A.K. Expression of 5HT1a receptors on activated human T cells. Regulation of cyclic AMP levels and T cell proliferation by 5-hydroxytryptamine. J. Immunol. 1993, 151, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Meredith, E.J.; Holder, M.J.; Chamba, A.; Challa, A.; Lee, A.D.; Bunce, C.M.; Drayson, M.T.; Pilkington, G.; Blakely, R.D.; Dyer, M.J.S.; et al. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: Probing a potential antitumor target for psychotropics. FASEB J. 2005, 19, 1187–1189. [Google Scholar] [CrossRef]
- Serafeim, A.; Grafton, G.; Chamba, A.; Gregory, C.D.; Blakely, R.D.; Bowery, N.G.; Barnes, N.M.; Gordon, J. 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: Reversal by selective serotonin reuptake inhibitors. Blood J. Am. Soc. Hematolog. 2002, 99, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Martinez-Fong, D.; Perez-Tapia, M.; Estrada-Garcia, I.; Estrada-Parra, S.; Pavón, L. Evaluation of the effect of selective serotonin-reuptake inhibitors on lymphocyte subsets in patients with a major depressive disorder. Eur. Neuropsychopharmacol. 2010, 20, 88–95. [Google Scholar] [CrossRef] [PubMed]
- León-Ponte, M.; Ahern, G.P.; O’Connell, P.J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 2006, 109, 3139–3146. [Google Scholar] [CrossRef]
- Urbina, M.; Arroyo, R.; Lima, L. 5-HT7 Receptors and Tryptophan Hydroxylase in Lymphocytes of Rats: Mitogen Activation, Physical Restraint or Treatment with Reserpine. Neuroimmunomodulation 2014, 21, 240–249. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, L.; Muñoz, E.; Calzado, M.A.; Lieb, K.; Candelario-Jalil, E.; Gschaidmeir, H.; Färber, L.; Mueller, W.; Stratz, T.; Fiebich, B.L. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem. Pharmacol. 2005, 70, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Albert, R.H.; Tretiakova, A.P.; Jameson, B.A. 5-HT1B receptors play a prominent role in the proliferation of T-lymphocytes. J. Neuroimmunol. 2006, 181, 68–81. [Google Scholar] [CrossRef]
- Wu, H.; Herr, D.; MacIver, N.J.; Rathmell, J.C.; Gerriets, V.A. CD4 T cells differentially express cellular machinery for serotonin signaling, synthesis, and metabolism. Int. Immunopharmacol. 2020, 88, 106922. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Conti, P.; Dempsey, R.A.; Reale, M.; Barbacane, R.C.; Panara, M.R.; Bongrazio, M.; Mier, J.W. Activation of human natural killer cells by lipopolysaccharide and generation of interleukin-1 alpha, beta, tumour necrosis factor and interleukin-6. Effect of IL-1 receptor antagonist. Immunology 1991, 73, 450. [Google Scholar] [PubMed]
- Carnevale, R.; Nocella, C.; Petrozza, V.; Cammisotto, V.; Pacini, L.; Sorrentino, V.; Martinelli, O.; Irace, L.; Sciarretta, S.; Frati, G.; et al. Localization of lipopolysaccharide from Escherichia Coli into human atherosclerotic plaque. Sci. Rep. 2018, 8, 3598. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Natural killer T cells in atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, K.; Hermodsson, S. Role of serotonin in the regulation of human natural killer cell cytotoxicity. J. Immunol. 1987, 139, 869. [Google Scholar] [CrossRef]
- Hellstrand, K.; Hermodsson, S. Serotonergic 5-HT1a Receptors Regulate a Cell Contact-Mediated Interaction between Natural Killer Cells and Monocytes. Scand. J. Immunol. 1993, 37, 7–18. [Google Scholar] [CrossRef]
- Hellstrand, K.; Czerkinsky, C.; Ricksten, A.; Jansson, B.; Asea, A.; Kylefjord, H.; Hermodsson, S.; Asea, A.; Asea, A. Role of Serotonin in the Regulation of Interferon-γ Production by Human Natural Killer Cells. J. Interf. Res. 1993, 13, 33–38. [Google Scholar] [CrossRef]
- Evans, D.L.; Lynch, K.G.; Benton, T.; Dubé, B.; Gettes, D.R.; Tustin, N.B.; Lai, J.P.; Metzger, D.; Douglas, S.D. Selective Serotonin Reuptake Inhibitor and Substance P Antagonist Enhancement of Natural Killer Cell Innate Immunity in Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome. Biol. Psychiatry 2008, 63, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Maclean, J.A.; Schoenwaelder, S.M. Serotonin in Platelets. In Serotonin; Academic Press: Cambridge, MA, USA, 2018; pp. 91–119. [Google Scholar] [CrossRef]
- Lindemann, S.; Krämer, B.; Seizer, P.; Gawaz, M. Platelets, inflammation and atherosclerosis. J. Thromb. Haemost. 2007, 5, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Vikenes, K.; Farstad, M.; Nordrehaug, J.E. Serotonin Is Associated with Coronary Artery Disease and Cardiac Events. Circulation 1999, 100, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Peter, J.-U.; Winter, S.; Höltje, M.; Paulmann, N.; Grohmann, M.; Vowinckel, J.; Alamo-Bethencourt, V.; Wilhelm, C.S.; Ahnert-Hilger, G.; et al. Serotonylation of Small GTPases Is a Signal Transduction Pathway that Triggers Platelet α-Granule Release. Cell 2003, 115, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Fraer, M.; Kilic, F. Serotonin. Hypertension 2015, 65, 942–948. [Google Scholar] [CrossRef]
- Stratz, C.; Trenk, D.; Bhatia, H.S.; Valina, C.; Neumann, F.J.; Fiebich, B.L. Identification of 5-HT3 receptors on human platelets: Increased surface immunoreactivity after activation with adenosine diphosphate (ADP) and thrombin re-ceptor-activating peptide (TRAP). Thromb. Haemost. 2008, 99, 784–786. [Google Scholar] [PubMed]
- Dürk, T.; Duerschmied, D.; Müller, T.; Grimm, M.; Reuter, S.; Vieira, R.P.; Ayata, K.; Cicko, S.; Sorichter, S.; Walther, D.J.; et al. Production of Serotonin by Tryptophan Hydroxylase 1 and Release via Platelets Contribute to Allergic Airway Inflammation. Am. J. Respir. Crit. Care Med. 2013, 187, 476–485. [Google Scholar] [CrossRef]
- Endresen, G.K.M. Evidence for activation of platelets in the synovial fluid from patients with rheumatoid arthritis. Rheumatol. Int. 1989, 9, 19–24. [Google Scholar] [CrossRef]
- Schumacher, H.R., Jr. Synovial membrane and fluid morphologic alterations in early rheumatoid arthritis: Micro-vascular injury and virus-like particles. Ann. N. Y. Acad. Sci. 1975, 256, 39–64. [Google Scholar] [CrossRef]
- Ehlers, R.; Ustinov, V.; Chen, Z.; Zhang, X.; Rao, R.; Luscinskas, F.W.; Lopez, J.; Plow, E.; Simon, D.I. Targeting platelet–leukocyte interactions: Identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibα. J. Exp. Med. 2003, 198, 1077–1088. [Google Scholar] [CrossRef]
- Zeller, J.; Weissbarth, E.; Baruth, B.; Mielke, H.; Deicher, H. Serotonin content of platelets in inflammatory rheumatic diseases. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1983, 26, 532–540. [Google Scholar] [CrossRef]
- Cloutier, N.; Paré, A.; Farndale, R.W.; Schumacher, H.R.; Nigrovic, P.A.; Lacroix, S.; Boilard, E. Platelets can enhance vascular permeability. Blood J. Am. Soc. Hematol. 2012, 120, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.; Novotny, J.; Salbeck, D.; Zellner, K.R.; Nicolai, L.; Pekayvaz, K.; Kilani, B.; Stockhausen, S.; Bürgener, N.; Kupka, D.; et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood 2019, 134, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Boehme, S.A.; Lio, F.M.; Sikora, L.; Pandit, T.S.; Lavrador, K.; Rao, S.P.; Sriramarao, P. Cutting Edge: Serotonin Is a Chemotactic Factor for Eosinophils and Functions Additively with Eotaxin. J. Immunol. 2004, 173, 3599–3603. [Google Scholar] [CrossRef]
- Kang, B.N.; Ha, S.G.; Bahaie, N.S.; Hosseinkhani, M.R.; Ge, X.N.; Blumenthal, M.N.; Rao, S.P.; Sriramarao, P. Regulation of serotonin-induced trafficking and migration of eosinophils. PLoS ONE 2013, 8, e54840. [Google Scholar] [CrossRef] [PubMed]
- De Bie, J.J.; Henricks, P.A.J.; Cruikshank, W.W.; Hofman, G.; Jonker, E.H.; Nijkamp, F.P.; Van Oosterhout, A.J.M. Modulation of airway hyperresponsiveness and eosinophilia by selective histamine and 5-HT receptor antagonists in a mouse model of allergic asthma. J. Cereb. Blood Flow Metab. 1998, 124, 857–864. [Google Scholar] [CrossRef]
- Jickling, G.C.; Liu, D.; Ander, B.P.; Stamova, B.; Zhan, X.; Sharp, F.R. Targeting Neutrophils in Ischemic Stroke: Translational Insights from Experimental Studies. J. Cereb. Blood Flow Metab. 2015, 35, 888–901. [Google Scholar] [CrossRef]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875–888. [Google Scholar] [CrossRef]
- Mauler, M.; Herr, N.; Schoenichen, C.; Witsch, T.; Marchini, T.; Härdtner, C.; Koentges, C.; Kienle, K.; Ollivier, V.; Schell, M.; et al. Platelet Serotonin Aggravates Myocardial Ischemia/Reperfusion Injury via Neutrophil Degranulation. Circulation 2019, 139, 918–931. [Google Scholar] [CrossRef]
- Kaartinen, M.; Penttilä, A.; Kovanen, P.T. Extracellular Mast Cell Granules Carry Apolipoprotein B-100–Containing Lipoproteins into Phagocytes in Human Arterial Intima: Functional Coupling of Exocytosis and Phagocytosis in Neighboring Cells. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, J.O.; Kovanen, P.T. Stimulation of mast cells leads to cholesterol accumulation in macrophages in vitro by a mast cell granule-mediated uptake of low-density lipoprotein. Proc. Natl. Acad. Sci. USA 1987, 84, 2287–2291. [Google Scholar] [CrossRef]
- Bot, I.; Shi, G.-P.; Kovanen, P.T. Mast Cells as Effectors in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2015, 35, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Crompton, R.; Clifton, V.L.; Bisits, A.T.; Read, M.A.; Smith, R.; Wright, I.M.R. Corticotropin-releasing hormone causes vasodilation in human skin via mast cell-dependent pathways. J. Clin. Endocrinol. Metab. 2003, 88, 5427–5432. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Et Biophys. Acta Mol. Basis Dis. 2012, 1822, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.; Stavenhagen, K.; Kolarich, D.; Sommerhoff, C.P.; Maurer, M.; Metz, M. Human Mast Cell Tryptase Is a Potential Treatment for Snakebite Envenoming Across Multiple Snake Species. Front. Immunol. 2018, 9, 1532. [Google Scholar] [CrossRef]
- Starkl, P.; Gaudenzio, N.; Marichal, T.; Reber, L.L.; Sibilano, R.; Watzenboeck, M.L.; Fontaine, F.; Mueller, A.C.; Tsai, M.; Knapp, S.; et al. IgE antibodies increase honeybee venom responsiveness and detoxifica-tion efficiency of mast cells. Allergy 2022, 77, 499–512. [Google Scholar] [CrossRef]
- Carroll, N.G.; Mutavdzic, S.; James, A.L. Distribution and degranulation of airway mast cells in normal and asth-matic subjects. Eur. Respir. J. 2002, 19, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Supajatura, V.; Ushio, H.; Nakao, A.; Akira, S.; Okumura, K.; Ra, C.; Ogawa, H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Investig. 2002, 109, 1351–1359. [Google Scholar] [CrossRef]
- Sheffer, A.L.; Tong, A.K.F.; Murphy, G.F.; Lewis, R.A.; McFadden, E.R., Jr.; Austen, K.F. Exercise-induced anaphylaxis: A serious form of physical allergy associated with mast cell degranulation. J. Allergy Clin. Immunol. 1985, 75, 479–484. [Google Scholar] [CrossRef]
- Laine, P.; Kaartinen, M.; Penttilä, A.; Panula, P.; Paavonen, T.; Kovanen, P.T. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation 1999, 99, 361–369. [Google Scholar] [CrossRef]
- Levick, S.P.; Meléndez, G.C.; Plante, E.; McLarty, J.L.; Brower, G.L.; Janicki, J.S. Cardiac mast cells: The centrepiece in adverse myocardial remodelling. Cardiovasc. Res. 2010, 89, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.B.; Stephen, S.; Borum, M.; Voltaggio, L.; Doman, D.B. Mast cells in gastrointestinal disease. Gastroenterol. Hepatol. 2010, 6, 772–777. [Google Scholar]
- Bischoff, S.; Wedemeyer, J.; Herrmann, A.; Meier, P.; Trautwein, C.; Cetin, Y.; Maschek, H.; Stolte, M.; Gebel, M.; Manns, M. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology 1996, 28, 1–13. [Google Scholar] [CrossRef]
- Kushnir-Sukhov, N.M.; Brown, J.M.; Wu, Y.; Kirshenbaum, A.; Metcalfe, D.D. Human mast cells are capable of serotonin synthesis and release. J. Allergy Clin. Immunol. 2007, 119, 498–499. [Google Scholar] [CrossRef]
- Kushnir-Sukhov, N.M.; Gilfillan, A.M.; Coleman, J.W.; Brown, J.M.; Bruening, S.; Toth, M.; Metcalfe, D.D. 5-Hydroxytryptamine Induces Mast Cell Adhesion and Migration. J. Immunol. 2006, 177, 6422–6432. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Desjardins, E.M.; Chan, E.J.; Day, E.A.; Leroux, J.M.; Wang, B.; Crane, E.D.; Wong, W.; Morrison, K.M.; Crane, J.D.; et al. Genetic deletion of mast cell serotonin synthesis prevents the development of obesity and insulin resistance. Nat. Commun. 2020, 11, 463. [Google Scholar] [CrossRef]
- Dürk, T.; Panther, E.; Müller, T.; Sorichter, S.; Ferrari, D.; Pizzirani, C.; Di Virgilio, F.; Myrtek, D.; Norgauer, J.; Idzko, M. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int. Immunol. 2005, 17, 599–606. [Google Scholar] [CrossRef]
- Ahsan, F.; Rivas, I.P.; Khan, M.A.; Torres Suárez, A.I. Targeting to macrophages: Role of physicochemical properties of particulate carriers—Liposomes and microspheres—On the phagocytosis by macrophages. J. Control. Release 2002, 79, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arter. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, E.M.; Wedner, H.J.; Leung, M.K.; Parker, C.W. Effect of serotonin (5-HT) and other monoamines on murine macrophages: Modulation of interferon-gamma induced phagocytosis. J. Immunol. 1987, 138, 4360. [Google Scholar] [CrossRef] [PubMed]
- Freire-Garabal, M.; Núñez, M.J.; Balboa, J.; López-Delgado, P.; Gallego, R.; García-Caballero, T.; Fernández-Roel, M.D.; Brenlla, J.; Rey-Méndez, M. Serotonin upregulates the activity of phagocytosis through 5-HT1Areceptors. Br. J. Pharmacol. 2003, 139, 457–463. [Google Scholar] [CrossRef] [PubMed]
- de las Casas-Engel, M.; Domínguez-Soto, A.; Sierra-Filardi, E.; Bragado, R.; Nieto, C.; Puig-Kroger, A.; Samaniego, R.; Loza, M.; Corcuera, M.T.; Gómez-Aguado, F.; et al. Serotonin Skews Human Macrophage Polarization through HTR2B and HTR7. J. Immunol. 2013, 190, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, Z.; Zasłona, Z.; Cakarova, L.; Hartmann, P.; Wilhelm, J.; Tecott, L.H.; Lohmeyer, J.; Kummer, W. Serotonin activates murine alveolar macrophages through 5-HT2Creceptors. Am. J. Physiol. Cell. Mol. Physiol. 2010, 299, L272–L280. [Google Scholar] [CrossRef]
- Winter, C.; Silvestre-Roig, C.; Ortega-Gomez, A.; Lemnitzer, P.; Poelman, H.; Schumski, A.; Winter, J.; Drechsler, M.; de Jong, R.; Immler, R.; et al. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 2018, 28, 175–182.e5. [Google Scholar] [CrossRef]
- Zernecke, A. Dendritic Cells in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2015, 35, 763–770. [Google Scholar] [CrossRef]
- Katoh, N.; Soga, F.; Nara, T.; Tamagawa-Mineoka, R.; Nin, M.; Kotani, H.; Masuda, K.; Kishimoto, S. Effect of serotonin on the differentiation of human monocytes into dendritic cells. Clin. Exp. Immunol. 2006, 146, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Szabo, A.; Gogolak, P.; Koncz, G.; Foldvari, Z.; Pazmandi, K.; Miltner, N.; Poliska, S.; Bacsi, A.; Djurovic, S.; Rajnavolgyi, E. Immunomodulatory capacity of the serotonin receptor 5-HT2B in a subset of human dendritic cells. Sci. Rep. 2018, 8, 1765. [Google Scholar] [CrossRef]
- Idzko, M.; Panther, E.; Stratz, C.; Müller, T.; Bayer, H.; Zissel, G.; Dürk, T.; Sorichter, S.; Di Virgilio, F.; Geissler, M.; et al. The Serotoninergic Receptors of Human Dendritic Cells: Identification and Coupling to Cytokine Release. J. Immunol. 2004, 172, 6011–6019. [Google Scholar] [CrossRef] [PubMed]
- Bachus, H.; Kaur, K.; Papillion, A.M.; Marquez-Lago, T.T.; Yu, Z.; Ballesteros-Tato, A.; Matalon, S.; León, B. Impaired Tumor-Necrosis-Factor-α-driven Dendritic Cell Activation Limits Lipopolysaccharide-Induced Protection from Allergic Inflammation in Infants. Immunity 2019, 50, 225–240.e4. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Wang, X.; Leon-Ponte, M.; Griffiths, C.; Pingle, S.C.; Ahern, G.P. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 2006, 107, 1010–1017. [Google Scholar] [CrossRef]
- Hare, D.L.; Toukhsati, S.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2013, 35, 1365–1372. [Google Scholar] [CrossRef]
- Rami, M.; Guillamat-Prats, R.; Rinne, P.; Salvermoser, M.; Ring, L.; Bianchini, M.; Blanchet, X.; Megens, R.T.; Döring, Y.; Walzog, B.; et al. Chronic Intake of the Selective Serotonin Reuptake Inhibitor Fluoxetine Enhances Atherosclerosis. Arter. Thromb. Vasc. Biol. 2018, 38, 1007–1019. [Google Scholar] [CrossRef]
- Shively, C.A.; Register, T.C.; Appt, S.E.; Clarkson, T.B. Effects of Long-Term Sertraline Treatment and Depression on Coronary Artery Atherosclerosis in Premenopausal Female Primates. Psychosom. Med. 2015, 77, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Silverstein-Metzler, M.G.; Justice, J.N.; Appt, S.E.; Groban, L.; Kitzman, D.W.; Carr, J.J.; Register, T.C.; Shively, C.A. Long-term sertraline treatment and depression effects on carotid artery atherosclerosis in premenopausal female primates. Menopause 2017, 24, 1175. [Google Scholar] [CrossRef]
- Camacho, Á.; McClelland, R.L.; Delaney, J.A.; Allison, M.A.; Psaty, B.M.; Rifkin, D.E.; Rapp, S.R.; Szklo, M.; Stein, M.B.; Criqui, M.H. Antidepressant use and subclinical measures of atherosclerosis: The multi-ethnic study of atherosclerosis (mesa). J. Clin. Psychopharmacol. 2016, 36, 340. [Google Scholar] [CrossRef] [PubMed]
- Tynan, R.J.; Weidenhofer, J.; Hinwood, M.; Cairns, M.J.; Day, T.A.; Walker, F.R. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain, Behav. Immun. 2012, 26, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Ye, G.; Liu, Y.; Chen, X.; Pan, M.; Zhu, F.; Fu, J.; Fu, T.; Liu, Q.; Gao, Z.; et al. Effects of SSRIs on peripheral inflammatory cytokines in patients with Generalized Anxiety Disorder. Brain, Behav. Immun. 2019, 81, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, R.; Liu, L.; Qiao, D.; Baldwin, D.S.; Hou, R. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: A systematic review and meta-analysis. Brain, Behav. Immun. 2019, 79, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Coupland, C.; Hill, T.; Morriss, R.; Moore, M.; Arthur, A.; Hippisley-Cox, J. Antidepressant use and risk of cardiovascular outcomes in people aged 20 to 64: Cohort study using primary care database. BMJ 2016, 352, i1350. [Google Scholar] [CrossRef]
- Bansal, N.; Hudda, M.; Payne, R.A.; Smith, D.J.; Kessler, D.; Wiles, N. Antidepressant use and risk of adverse outcomes: Population-based cohort study. BJPsych Open 2022, 8, e164. [Google Scholar] [CrossRef] [PubMed]
- Smoller, J.W.; Allison, M.; Cochrane, B.B.; Curb, J.D.; Perlis, R.H.; Robinson, J.G.; Rosal, M.C.; Wenger, N.K.; Wassertheil-Smoller, S. Antidepressant Use and Risk of Incident Cardiovascular Morbidity and Mortality Among Postmenopausal Women in the Women’s Health Initiative Study. Arch. Intern. Med. 2009, 169, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, Y.S.; Kim, M.G.; Song, Y.K.; Kim, Y.; Jang, H.; Kim, J.H.; Han, N.; Ji, E.; Kim, I.W.; et al. The effect of selective serotonin reuptake inhibitors on major adverse cardiovascular events: A me-ta-analysis of randomized-controlled studies in depression. Int. Clin. Psychopharmacol. 2019, 34, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, C.; Rutjes, A.W.S.; Costa, G.M.; Fontana, F.; Mezzetti, A.; Manzoli, L. Meta-Analysis of Selective Serotonin Reuptake Inhibitors in Patients with Depression and Coronary Heart Disease. Am. J. Cardiol. 2011, 107, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.; Prada, L.; Rosa, M.M.; Ferreira, J.J.; Costa, J.; Pinto, F.J.; Caldeira, D. The impact of SSRIs on mortality and cardiovascular events in patients with coronary artery disease and depression: Systematic review and meta-analysis. Clin. Res. Cardiol. 2020, 110, 183–193. [Google Scholar] [CrossRef]
- Maslej, M.M.; Bolker, B.M.; Russell, M.J.; Eaton, K.; Durisko, Z.; Hollon, S.D.; Swanson, G.M., Jr.; Mulsant, B.H.; Andrews, P.W. The Mortality and Myocardial Effects of Antidepressants Are Moderated by Preexisting Cardiovascular Disease: A Meta-Analysis. Psychother. Psychosom. 2017, 86, 268–282. [Google Scholar] [CrossRef]
- Serebruany, V.L.; Glassman, A.H.; Malinin, A.I.; Nemeroff, C.B.; Musselman, D.L.; Van Zyl, L.T.; Finkel, M.S.; Krishnan, K.R.R.; Gaffney, M.; Harrison, W.; et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: The Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003, 108, 939–944. [Google Scholar] [CrossRef]
- Tseng, Y.-L.; Chiang, M.-L.; Huang, T.-F.; Su, K.-P.; Lane, H.-Y.; Lai, Y.-C. A selective serotonin reuptake inhibitor, citalopram, inhibits collagen-induced platelet aggregation and activation. Thromb. Res. 2010, 126, 517–523. [Google Scholar] [CrossRef]
- Nezafati, P.; Nezafati, M.H.; Eshraghi, A.; Vojdanparast, M.; Abtahi, S. Selective serotonin reuptake inhibitors and cardiovascular events: A systematic review. J. Res. Med. Sci. 2016, 21, 66. [Google Scholar] [CrossRef]
- Kogut, C.; Crouse, E.B.; Vieweg, W.V.R.; Hasnain, M.; Baranchuk, A.; Digby, G.C.; Koneru, J.N.; Fernandez, A.; Deshmukh, A.; Hancox, J.C.; et al. Selective serotonin reuptake inhibitors and torsade de pointes: New concepts and new directions de-rived from a systematic review of case reports. Ther. Adv. Drug Saf. 2013, 4, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ludman, E.J.; Katon, W.; Russo, J.; Von Korff, M.; Simon, G.; Ciechanowski, P.; Lin, E.; Bush, T.; Walker, E.; Young, B. Depression and diabetes symptom burden. Gen. Hosp. Psychiatry 2004, 26, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Perera, L.P.; Radigan, M.; Guilday, C.; Banerjee, I.; Eastwood, D.; Babygirija, R.; Massey, B.T. Presence of irritable bowel syndrome symptoms in quiescent inflamma-tory bowel disease is associated with high rate of anxiety and depression. Dig. Dis. Sci. 2019, 64, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Vallerand, I.A.; Patten, S.; Barnabe, C. Depression and the risk of rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 279–284. [Google Scholar] [CrossRef]
- Brunello, N.; Alboni, S.; Capone, G.; Benatti, C.; Blom, J.M.; Tascedda, F.; Kriwin, P.; Mendlewicz, J. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int. Clin. Psychopharmacol. 2006, 21, 219–225. [Google Scholar] [CrossRef]
- Taler, M.; Gil-Ad, I.; Lomnitski, L.; Korov, I.; Baharav, E.; Bar, M.; Zolokov, A.; Weizman, A. Immunomodulatory effect of selective serotonin reuptake inhibitors (SSRIs) on human T lymphocyte function and gene expression. Eur. Neuropsychopharmacol. 2007, 17, 774–780. [Google Scholar] [CrossRef]
- Yu, B.; Becnel, J.; Zerfaoui, M.; Rohatgi, R.; Boulares, A.H.; Nichols, C.D. Serotonin 5-Hydroxytryptamine2A Receptor Activation Suppresses Tumor Necrosis Factor-α-Induced Inflammation with Extraordinary Potency. J. Pharmacol. Exp. Ther. 2008, 327, 316–323. [Google Scholar] [CrossRef]
- Voog, U.; Alstergren, P.; Eliasson, S.; Leibur, E.; Kallikorm, R.; Kopp, S. Progression of radiographic changes in the temporomandibular joints of patients with rheumatoid arthritis in relation to inflammatory markers and mediators in the blood. Acta Odontol. Scand. 2004, 62, 7–13. [Google Scholar] [CrossRef]
- Lechin, F.; van der Dijs, B.; Orozco, B.; Lechin, M.; Lechin, E.A. Increased Levels of Free Serotonin in Plasma of Symptomatic Asthmatic Patients. Ann. Allergy Asthma Immunol. 1996, 77, 245–253. [Google Scholar] [CrossRef]
- Lechin, F.; Van Der Dijs, B.; Orozco, B.; Jara, H.; Rada, I.; Lechin, M.E.; Lechin, A.E. The Serotonin Uptake-Enhancing Drug Tianeptine Suppresses Asthmatic Symptoms in Children: A Double-Blind, Crossover, Placebo-Controlled Study. J. Clin. Pharmacol. 1998, 38, 918–925. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Chehrehgosha, M.; Sajjadi-Jazi, S.M.; Meftah, A.M. Tryptophan and serotonin levels as potent biomarkers in diabetes mellitus complications: A new approach of diagnostic role. J. Diabetes Metab. Disord. 2022, 21, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Young, R.L.; Lumsden, A.L.; Martin, A.M.; Schober, G.; Pezos, N.; Thazhath, S.S.; Isaacs, N.J.; Cvijanovic, N.; Sun, E.W.-L.; Wu, T.; et al. Augmented capacity for peripheral serotonin release in human obesity. Int. J. Obes. 2018, 42, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Manzella, C.R.; Jayawardena, D.; Pagani, W.; Li, Y.; Alrefai, W.A.; Bauer, J.; Jung, B.; Weber, C.R.; Gill, R.K. Serum Serotonin Differentiates Between Disease Activity States in Crohn’s Patients. Inflamm. Bowel Dis. 2020, 26, 1607–1618. [Google Scholar] [CrossRef] [PubMed]

| Receptor | Vasoreactivity | Location | Vessel Type | Reference |
|---|---|---|---|---|
| 5-HT1 | Constriction | Human superficial hand vein segments ex vivo | Venous | [57] |
| Contraction in both healthy and ischemic vessels | Epicardial coronary artery in vitro | Arterial | [59] | |
| Dilatation | Renal, mesenteric, and hindquarter vessels in rabbits | Unspecified | [62] | |
| 5-HT2 | Constriction | human superficial hand vein segments ex vivo | Venous | [57] |
| Contraction in healthy but not ischemic vessels | Epicardial coronary artery in vitro | Arterial | [59] | |
| Constriction | Renal artery in rabbits | Arterial | [62] | |
| Constriction | Iliac artery | Arterial | [61] | |
| 5-HT3 | Dilatation | Healthy individuals—venous flow in forearm vessels | Venous | [58] |
| No significant effect | human superficial hand vein segments ex vivo | Venous | [57] | |
| No significant effect | Epicardial coronary artery in vitro | Arterial | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamdin, A.; van der Vorst, E.P.C. Exploring the Role of Serotonin as an Immune Modulatory Component in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1549. https://doi.org/10.3390/ijms24021549
Imamdin A, van der Vorst EPC. Exploring the Role of Serotonin as an Immune Modulatory Component in Cardiovascular Diseases. International Journal of Molecular Sciences. 2023; 24(2):1549. https://doi.org/10.3390/ijms24021549
Chicago/Turabian StyleImamdin, Aqeela, and Emiel P. C. van der Vorst. 2023. "Exploring the Role of Serotonin as an Immune Modulatory Component in Cardiovascular Diseases" International Journal of Molecular Sciences 24, no. 2: 1549. https://doi.org/10.3390/ijms24021549
APA StyleImamdin, A., & van der Vorst, E. P. C. (2023). Exploring the Role of Serotonin as an Immune Modulatory Component in Cardiovascular Diseases. International Journal of Molecular Sciences, 24(2), 1549. https://doi.org/10.3390/ijms24021549






