Distinguishing Plasmin-Generating Microvesicles: Tiny Messengers Involved in Fibrinolysis and Proteolysis
Abstract
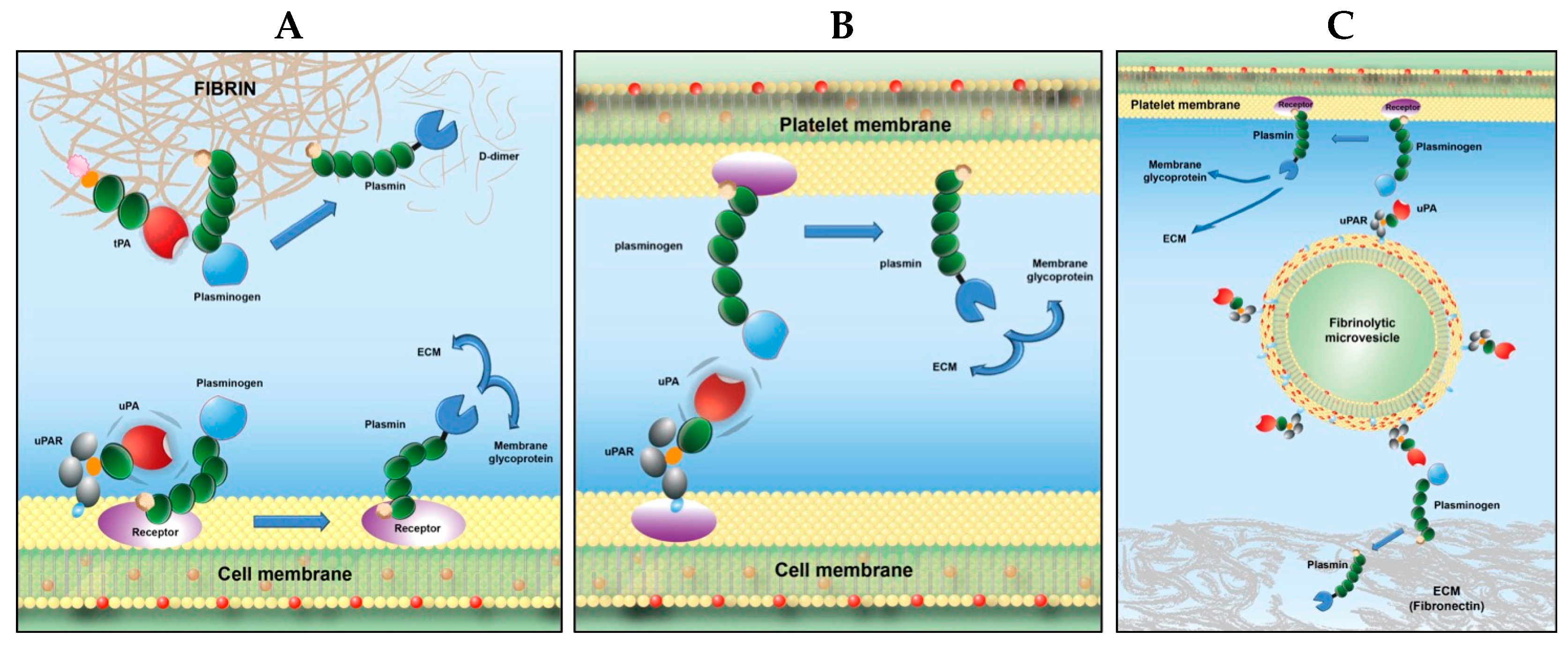
1. Cell Activation: A Scenario for Fibrinolytic and/or Proteolytic Development
2. Cell Activation: Membrane Blebbing and Microvesicle Release
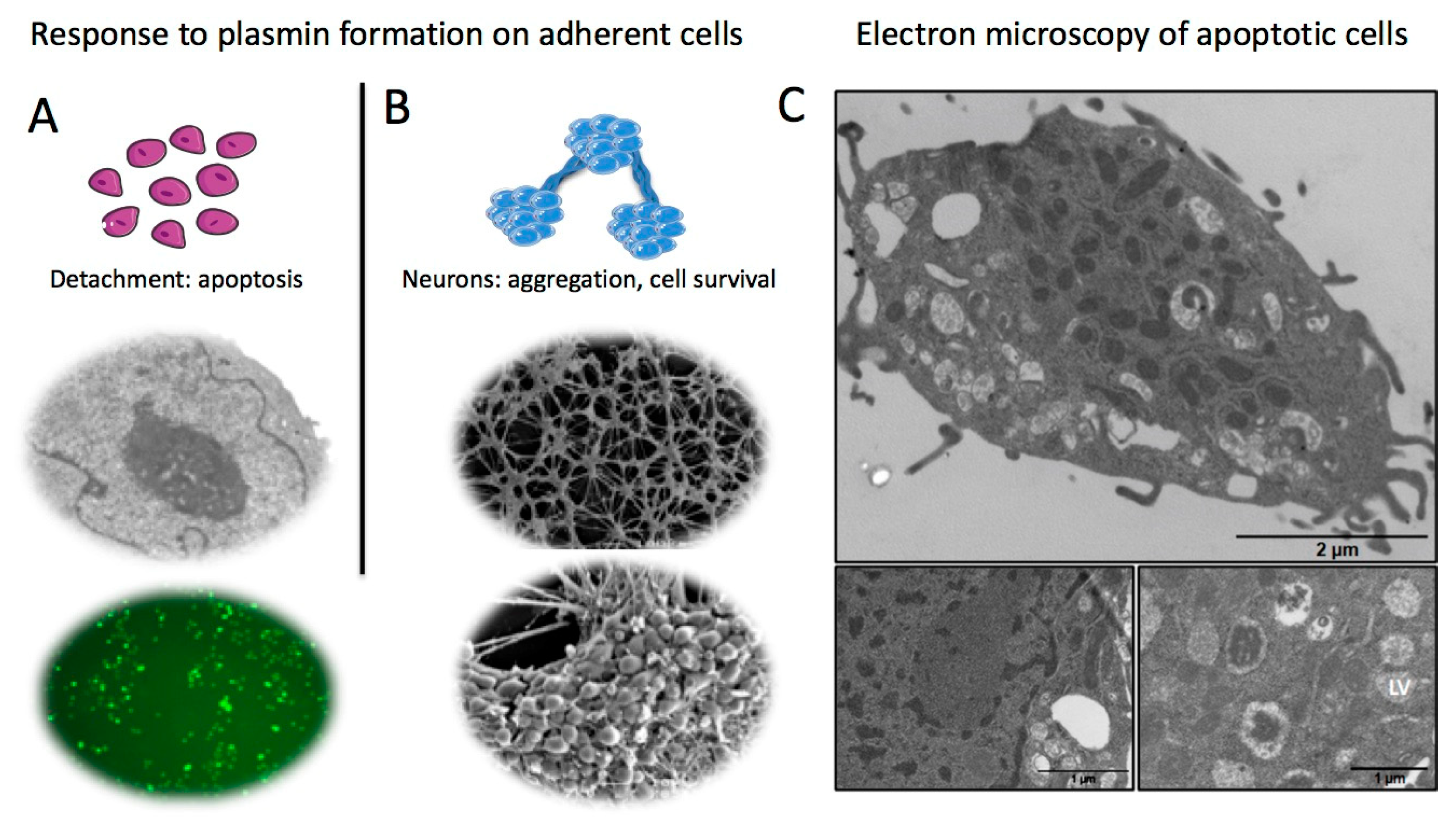
3. Receptor-Bound Plasmin Induces Cell Detachment and Apoptosis
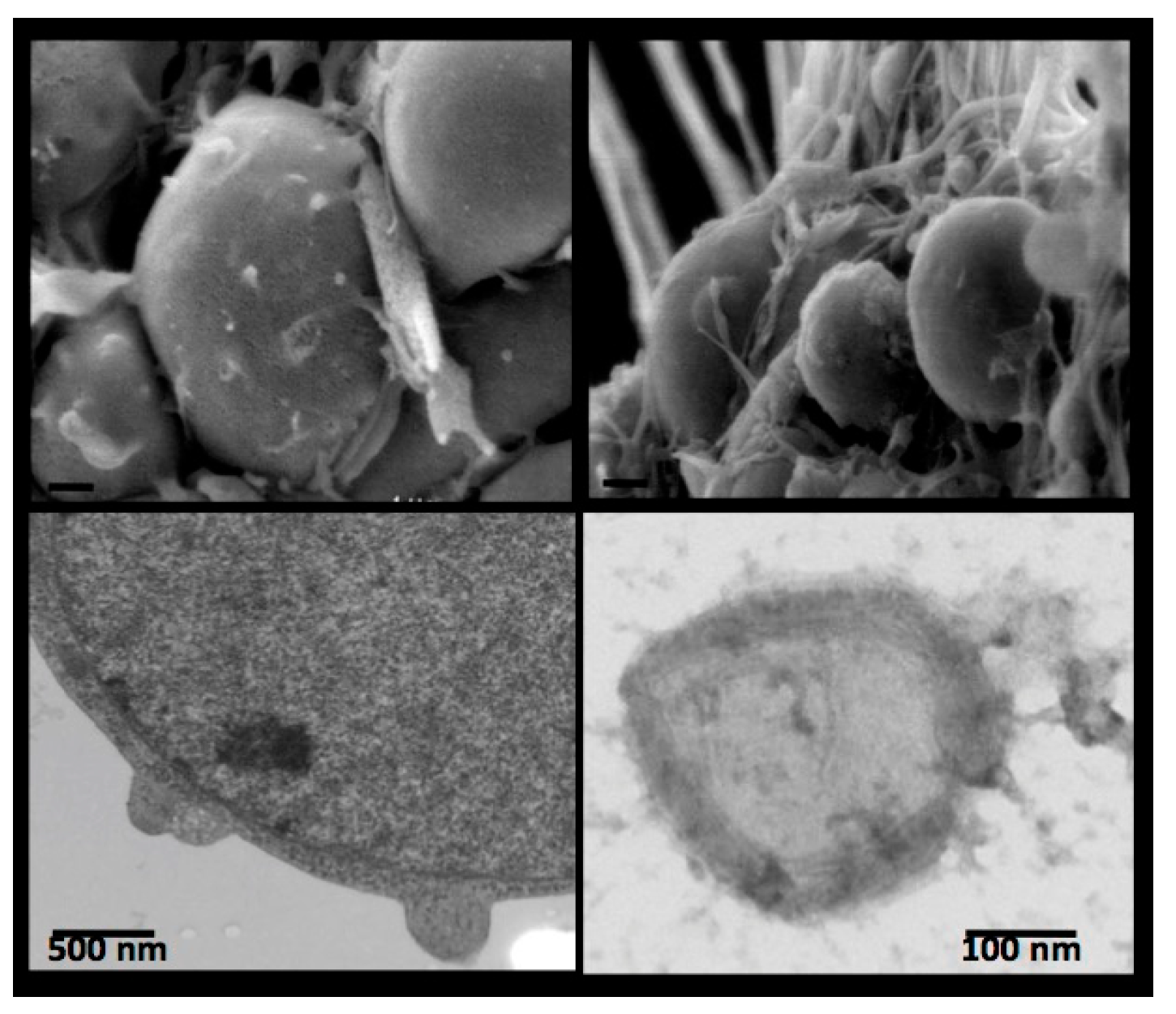
4. Receptor-Bound Plasmin Induces Membrane Blebbing and Release of Microvesicles
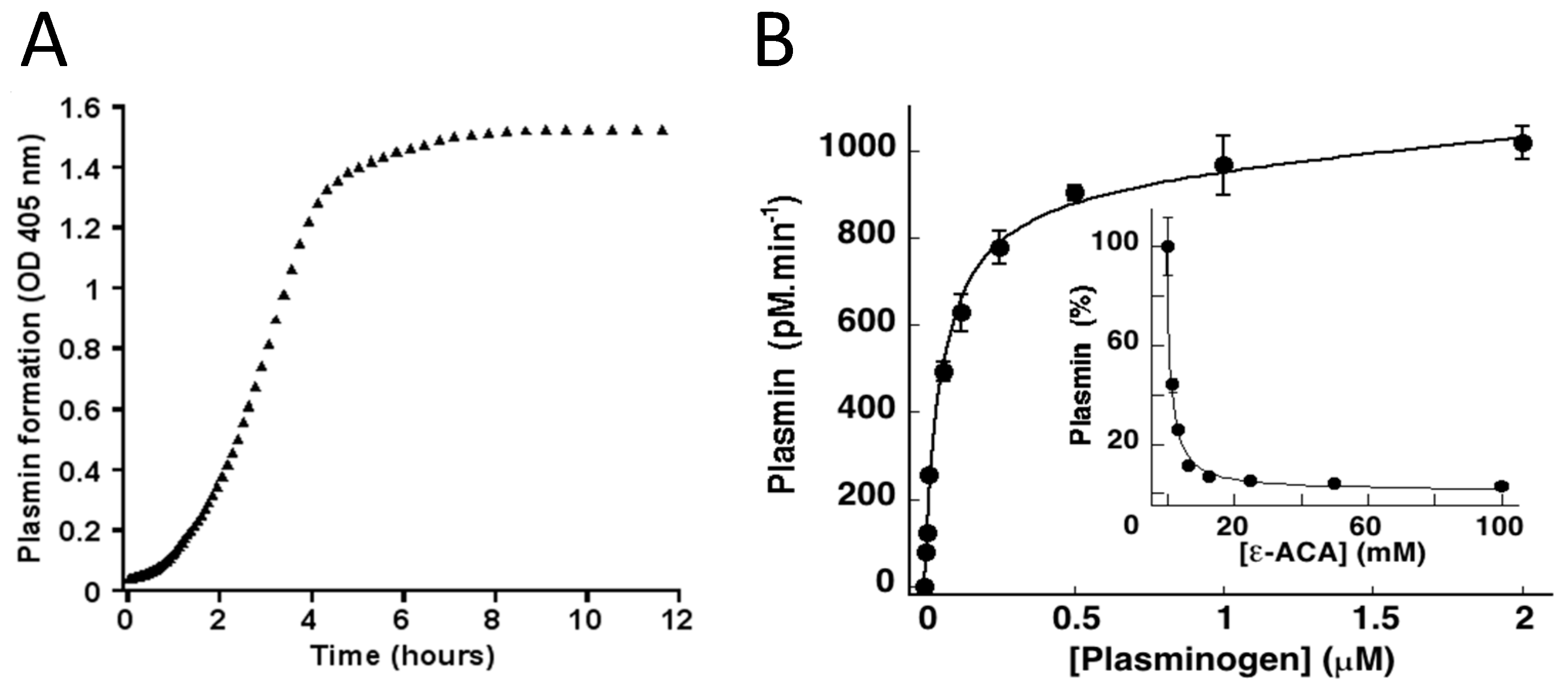
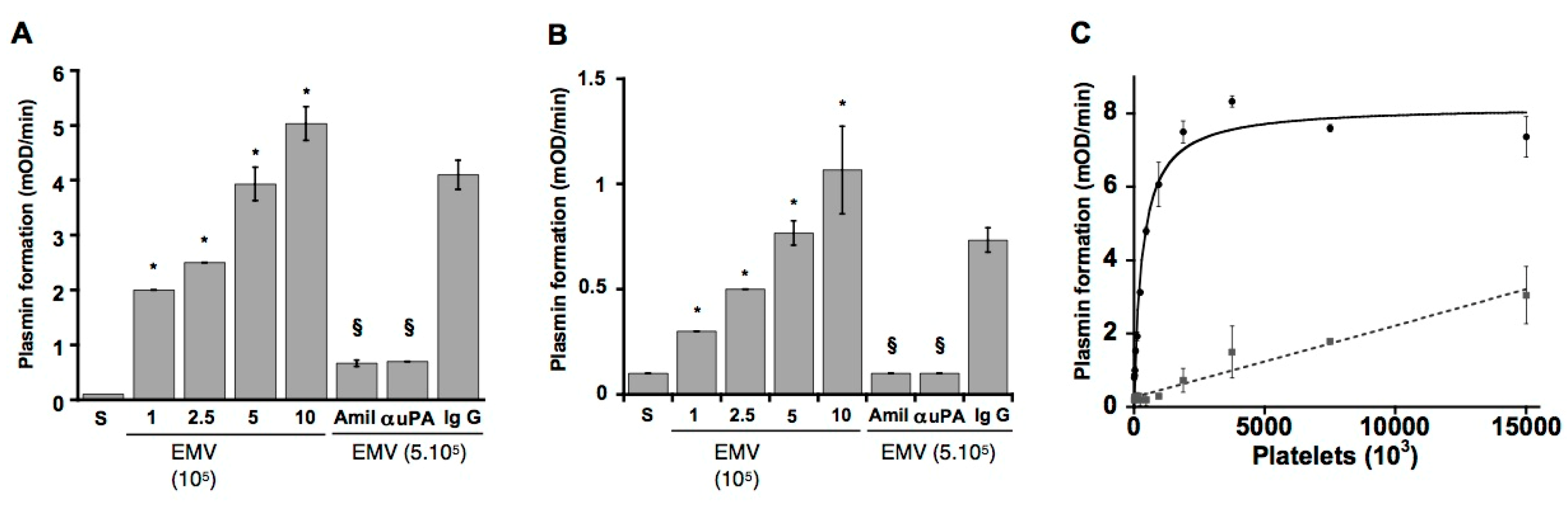
5. Beyond Coagulation, Microvesicles Are Dynamic Fibrinolytic Vectors
6. Microvesicles Bearing uPA Induce a Fibrinolytic Cross-Talk
7. Recent Developments
8. Concluding Remark
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenny, E.F.; Herzig, A.; Kruger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 2017, 6, e24437. [Google Scholar] [CrossRef]
- Huovila, A.P.; Turner, A.J.; Pelto-Huikko, M.; Karkkainen, I.; Ortiz, R.M. Shedding light on ADAM metalloproteinases. Trends Biochem. Sci. 2005, 30, 413–422. [Google Scholar] [CrossRef]
- Rijken, D.C.; Lijnen, H.R. New insights into the molecular mechanisms of the fibrinolytic system. J. Thromb. Haemost. 2009, 7, 4–13. [Google Scholar] [CrossRef]
- Blasi, F.; Sidenius, N. The urokinase receptor: Focused cell surface proteolysis, cell adhesion and signaling. FEBS Lett. 2010, 584, 1923–1930. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yasui, H.; Brzoska, T.; Mogami, H.; Urano, T. Surface-retained tPA is essential for effective fibrinolysis on vascular endothelial cells. Blood 2011, 118, 3182–3185. [Google Scholar] [CrossRef]
- Fleury, V.; Lijnen, H.R.; Angles-Cano, E. Mechanism of the enhanced intrinsic activity of single-chain urokinase-type plasminogen activator during ongoing fibrinolysis. J. Biol. Chem. 1993, 268, 18554–18559. [Google Scholar] [CrossRef]
- Ustach, C.V.; Kim, H.R. Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Mol. Cell. Biol. 2005, 25, 6279–6288. [Google Scholar] [CrossRef]
- Nicole, O.; Docagne, F.; Ali, C.; Margaill, I.; Carmeliet, P.; MacKenzie, E.T.; Vivien, D.; Buisson, A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001, 7, 59–64. [Google Scholar] [CrossRef]
- Fredriksson, L.; Li, H.; Fieber, C.; Li, X.; Eriksson, U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004, 23, 3793–3802. [Google Scholar] [CrossRef]
- Zwaal, R.F.; Comfurius, P.; Bevers, E.M. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 2005, 62, 971–988. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; Dekkers, D.W.; Zwaal, R.F. Lipid translocation across the plasma membrane of mammalian cells. Biochim. Biophys. Acta 1999, 1439, 317–330. [Google Scholar] [CrossRef]
- Daleke, D.L. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 2003, 44, 233–242. [Google Scholar] [CrossRef]
- Lhermusier, T.; Chap, H.; Payrastre, B. Platelet membrane phospholipid asymmetry: From the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J. Thromb. Haemost. 2011, 9, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Falzone, M.E.; Malvezzi, M.; Lee, B.C.; Accardi, A. Known structures and unknown mechanisms of TMEM16 scramblases and channels. J. Gen. Physiol. 2018, 150, 933–947. [Google Scholar] [CrossRef]
- Nagata, S.; Sakuragi, T.; Segawa, K. Flippase and scramblase for phosphatidylserine exposure. Curr. Opin. Immunol. 2020, 62, 31–38. [Google Scholar] [CrossRef]
- Sun, J.; Nanjundan, M.; Pike, L.J.; Wiedmer, T.; Sims, P.J. Plasma membrane phospholipid scramblase 1 is enriched in lipid rafts and interacts with the epidermal growth factor receptor. Biochemistry 2002, 41, 6338–6345. [Google Scholar] [CrossRef]
- Kunzelmann-Marche, C.; Freyssinet, J.M.; Martinez, M.C. Loss of plasma membrane phospholipid asymmetry requires raft integrity. Role of transient receptor potential channels and ERK pathway. J. Biol. Chem. 2002, 277, 19876–19881. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.A.; del Conde, I.; Shrimpton, C.N. Receptors, rafts, and microvesicles in thrombosis and inflammation. J. Thromb. Haemost. 2005, 3, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan-Chari, V.; Clancy, J.W.; Sedgwick, A.; D’Souza-Schorey, C. Microvesicles: Mediators of extracellular communication during cancer progression. J. Cell Sci. 2010, 123, 1603–1611. [Google Scholar] [CrossRef]
- Pollet, H.; Conrard, L.; Cloos, A.S.; Tyteca, D. Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding? Biomolecules 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Ridger, V.C.; Boulanger, C.M.; Angelillo-Scherrer, A.; Badimon, L.; Blanc-Brude, O.; Bochaton-Piallat, M.L.; Boilard, E.; Buzas, E.I.; Caporali, A.; Dignat-George, F.; et al. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb. Haemost. 2017, 117, 1296–1316. [Google Scholar]
- Benedikter, B.J.; Weseler, A.R.; Wouters, E.F.M.; Savelkoul, P.H.M.; Rohde, G.G.U.; Stassen, F.R.M. Redox-dependent thiol modifications: Implications for the release of extracellular vesicles. Cell. Mol. Life Sci. 2018, 75, 2321–2337. [Google Scholar] [CrossRef]
- Pasquet, J.M.; Dachary-Prigent, J.; Nurden, A.T. Calcium influx is a determining factor of calpain activation and microparticle formation in platelets. Eur. J. Biochem. 1996, 239, 647–654. [Google Scholar] [CrossRef]
- Das, K.; Prasad, R.; Singh, A.; Bhattacharya, A.; Roy, A.; Mallik, S.; Mukherjee, A.; Sen, P. Protease-activated receptor 2 promotes actomyosin dependent transforming microvesicles generation from human breast cancer. Mol. Carcinog. 2018, 57, 1707–1722. [Google Scholar] [CrossRef] [PubMed]
- Key, N.S. Analysis of tissue factor positive microparticles. Thromb. Res. 2010, 125 (Suppl. 1), S42–S45. [Google Scholar] [CrossRef] [PubMed]
- Bach, R.R. Tissue factor encryption. Arter. Thromb. Vasc. Biol. 2006, 26, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Owens, A.P., 3rd; Mackman, N. Microparticles in hemostasis and thrombosis. Circ. Res. 2011, 108, 1284–1297. [Google Scholar] [CrossRef]
- Lacroix, R.; Sabatier, F.; Mialhe, A.; Basire, A.; Pannell, R.; Borghi, H.; Robert, S.; Lamy, E.; Plawinski, L.; Camoin-Jau, L.; et al. Activation of plasminogen into plasmin at the surface of endothelial microparticles: A mechanism that modulates angiogenic properties of endothelial progenitor cells in vitro. Blood 2007, 110, 2432–2439. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Malinowski, K.; Golightly, M.; Jesty, J.; Goligorsky, M.S. Plasminogen activator inhibitor-1 promotes formation of endothelial microparticles with procoagulant potential. Circulation 2002, 106, 2372–2378. [Google Scholar] [CrossRef]
- Ffrench-Constant, C.; Colognato, H. Integrins: Versatile integrators of extracellular signals. Trends Cell Biol. 2004, 14, 678–686. [Google Scholar] [CrossRef]
- Meredith, J.E., Jr.; Fazeli, B.; Schwartz, M.A. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 1993, 4, 953–961. [Google Scholar] [CrossRef]
- Meilhac, O.; Ho-Tin-Noe, B.; Houard, X.; Philippe, M.; Michel, J.B.; Angles-Cano, E. Pericellular plasmin induces smooth muscle cell anoikis. FASEB J. 2003, 17, 1301–1303. [Google Scholar] [CrossRef]
- Rossignol, P.; Ho-Tin-Noe, B.; Vranckx, R.; Bouton, M.C.; Meilhac, O.; Lijnen, H.R.; Guillin, M.C.; Michel, J.B.; Angles-Cano, E. Protease nexin-1 inhibits plasminogen activation-induced apoptosis of adherent cells. J. Biol. Chem. 2004, 279, 10346–10356. [Google Scholar] [CrossRef]
- Zhang, X.; Chaudhry, A.; Chintala, S.K. Inhibition of plasminogen activation protects against ganglion cell loss in a mouse model of retinal damage. Mol. Vis. 2003, 9, 238–248. [Google Scholar]
- Rossignol, P.; Luttun, A.; Martin-Ventura, J.L.; Lupu, F.; Carmeliet, P.; Collen, D.; Angles-Cano, E.; Lijnen, H.R. Plasminogen activation: A mediator of vascular smooth muscle cell apoptosis in atherosclerotic plaques. J. Thromb. Haemost. 2006, 4, 664–670. [Google Scholar] [CrossRef]
- Mali, R.S.; Cheng, M.; Chintala, S.K. Plasminogen activators promote excitotoxicity-induced retinal damage. FASEB J. 2005, 19, 1280–1289. [Google Scholar] [CrossRef]
- Ho-Tin-Noe, B.; Meilhac, O.; Rossignol, P.; Lijnen, H.R.; Angles-Cano, E. Dual effect of apolipoprotein(a) on plasmin(ogen)-induced apoptosis through modulation of cell detachment of adherent cells. Thromb. Haemost. 2006, 95, 142–150. [Google Scholar] [CrossRef]
- Reijerkerk, A.; Mosnier, L.O.; Kranenburg, O.; Bouma, B.N.; Carmeliet, P.; Drixler, T.; Meijers, J.C.; Voest, E.E.; Gebbink, M.F. Amyloid endostatin induces endothelial cell detachment by stimulation of the plasminogen activation system. Mol. Cancer Res. 2003, 1, 561–568. [Google Scholar]
- Horowitz, J.C.; Rogers, D.S.; Simon, R.H.; Sisson, T.H.; Thannickal, V.J. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am. J. Respir. Cell Mol. Biol. 2008, 38, 78–87. [Google Scholar] [CrossRef]
- Kochtebane, N.; Choqueux, C.; Passefort, S.; Nataf, P.; Messika-Zeitoun, D.; Bartagi, A.; Michel, J.B.; Angles-Cano, E.; Jacob, M.P. Plasmin induces apoptosis of aortic valvular myofibroblasts. J. Pathol. 2010, 221, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Doeuvre, L.; Plawinski, L.; Goux, D.; Vivien, D.; Angles-Cano, E. Plasmin on adherent cells: From microvesiculation to apoptosis. Biochem. J. 2010, 432, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Redlitz, A.; Fowler, B.J.; Plow, E.F.; Miles, L.A. The role of an enolase-related molecule in plasminogen binding to cells. Eur. J. Biochem. 1995, 227, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Burke, T.; Plow, E.F. Histone H2B as a functionally important plasminogen receptor on macrophages. Blood 2007, 110, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Flood, E.C.; Hajjar, K.A. The annexin A2 system and vascular homeostasis. Vasc. Pharmacol. 2011, 54, 59–67. [Google Scholar] [CrossRef]
- Surette, A.P.; Madureira, P.A.; Phipps, K.D.; Miller, V.A.; Svenningsson, P.; Waisman, D.M. Regulation of fibrinolysis by S100A10 in vivo. Blood 2011, 118, 3172–3181. [Google Scholar] [CrossRef]
- Andronicos, N.M.; Chen, E.I.; Baik, N.; Bai, H.; Parmer, C.M.; Kiosses, W.B.; Kamps, M.P.; Yates, J.R., 3rd; Parmer, R.J.; Miles, L.A. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood 2010, 115, 1319–1330. [Google Scholar] [CrossRef]
- Plow, E.F.; Doeuvre, L.; Das, R. So many plasminogen receptors: Why? J. Biomed. Biotechnol. 2012, 2012, 141806. [Google Scholar] [CrossRef]
- Ho-Tin-Noe, B.; Rojas, G.; Vranckx, R.; Lijnen, H.R.; Angles-Cano, E. Functional hierarchy of plasminogen kringles 1 and 4 in fibrinolysis and plasmin-induced cell detachment and apoptosis. FEBS J. 2005, 272, 3387–3400. [Google Scholar] [CrossRef]
- Ho-Tin-Noe, B.; Enslen, H.; Doeuvre, L.; Corsi, J.M.; Lijnen, H.R.; Angles-Cano, E. Role of plasminogen activation in neuronal organization and survival. Mol. Cell. Neurosci. 2009, 42, 288–295. [Google Scholar] [CrossRef]
- Miles, L.A.; Plow, E.F. Binding and activation of plasminogen on the platelet surface. J. Biol. Chem. 1985, 260, 4303–4311. [Google Scholar] [CrossRef] [PubMed]
- Loef, E.J.; Sheppard, H.M.; Birch, N.P.; Dunbar, P.R. Plasminogen and plasmin can bind to human T cells and generate truncated CCL21 that increases dendritic cell chemotactic responses. J. Biol. Chem. 2022, 298, 102112. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.A.; Krajewski, S.; Baik, N.; Parmer, R.J.; Mueller, B.M. Plg-R(KT) Expression in Human Breast Cancer Tissues. Biomolecules 2022, 12, 503. [Google Scholar] [CrossRef]
- Declerck, P.J. Thrombin activatable fibrinolysis inhibitor. Hamostaseologie 2011, 31, 165–166. [Google Scholar] [CrossRef]
- Tarui, T.; Majumdar, M.; Miles, L.A.; Ruf, W.; Takada, Y. Plasmin-induced migration of endothelial cells. A potential target for the anti-angiogenic action of angiostatin. J. Biol. Chem. 2002, 277, 33564–33570. [Google Scholar] [CrossRef]
- O’Connell, P.A.; Surette, A.P.; Liwski, R.S.; Svenningsson, P.; Waisman, D.M. S100A10 regulates plasminogen-dependent macrophage invasion. Blood 2010, 116, 1136–1146. [Google Scholar] [CrossRef]
- Horowitz, J.C.; Rogers, D.S.; Sharma, V.; Vittal, R.; White, E.S.; Cui, Z.; Thannickal, V.J. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell. Signal. 2007, 19, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Wagner, M.R.; Zhang, W.; Xu, F.; Van Nostrand, W.E. Amyloid beta-protein stimulates the expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) in human cerebrovascular smooth muscle cells. J. Biol. Chem. 2003, 278, 19054–19061. [Google Scholar] [CrossRef]
- Griffin, M.O.; Jinno, M.; Miles, L.A.; Villarreal, F.J. Reduction of myocardial infarct size by doxycycline: A role for plasmin inhibition. Mol. Cell. Biochem. 2005, 270, 1–11. [Google Scholar] [CrossRef]
- Chen, Z.L.; Indyk, J.A.; Bugge, T.H.; Kombrinck, K.W.; Degen, J.L.; Strickland, S. Neuronal death and blood-brain barrier breakdown after excitotoxic injury are independent processes. J. Neurosci. 1999, 19, 9813–9820. [Google Scholar] [CrossRef]
- Bauriedel, G.; Hutter, R.; Welsch, U.; Bach, R.; Sievert, H.; Luderitz, B. Role of smooth muscle cell death in advanced coronary primary lesions: Implications for plaque instability. Cardiovasc. Res. 1999, 41, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Moons, L.; Lijnen, R.; Baes, M.; Lemaitre, V.; Tipping, P.; Drew, A.; Eeckhout, Y.; Shapiro, S.; Lupu, F.; et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat. Genet. 1997, 17, 439–444. [Google Scholar] [CrossRef]
- Heymans, S.; Lupu, F.; Terclavers, S.; Vanwetswinkel, B.; Herbert, J.M.; Baker, A.; Collen, D.; Carmeliet, P.; Moons, L. Loss or inhibition of uPA or MMP-9 attenuates LV remodeling and dysfunction after acute pressure overload in mice. Am. J. Pathol. 2005, 166, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell. Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef]
- Liu, R.M. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid. Redox Signal. 2008, 10, 303–319. [Google Scholar] [CrossRef]
- Eddy, A.A. Serine proteases, inhibitors and receptors in renal fibrosis. Thromb. Haemost. 2009, 101, 656–664. [Google Scholar] [CrossRef]
- Matsuoka, H.; Sisson, T.H.; Nishiuma, T.; Simon, R.H. Plasminogen-mediated activation and release of hepatocyte growth factor from extracellular matrix. Am. J. Respir. Cell Mol. Biol. 2006, 35, 705–713. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rodriguez-Vita, J.; Sanchez-Lopez, E.; Carvajal, G.; Egido, J. TGF-beta signaling in vascular fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef]
- Morel, O.; Hugel, B.; Jesel, L.; Mallat, Z.; Lanza, F.; Douchet, M.P.; Zupan, M.; Chauvin, M.; Cazenave, J.P.; Tedgui, A.; et al. Circulating procoagulant microparticles and soluble GPV in myocardial infarction treated by primary percutaneous transluminal coronary angioplasty. A possible role for GPIIb-IIIa antagonists. J. Thromb. Haemost. 2004, 2, 1118–1126. [Google Scholar] [CrossRef]
- Simak, J.; Gelderman, M.P.; Yu, H.; Wright, V.; Baird, A.E. Circulating endothelial microparticles in acute ischemic stroke: A link to severity, lesion volume and outcome. J. Thromb. Haemost. 2006, 4, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Chironi, G.N.; Boulanger, C.M.; Simon, A.; Dignat-George, F.; Freyssinet, J.M.; Tedgui, A. Endothelial microparticles in diseases. Cell Tissue Res. 2009, 335, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Doeuvre, L.; Plawinski, L.; Toti, F.; Angles-Cano, E. Cell-derived microparticles: A new challenge in neuroscience. J. Neurochem. 2009, 110, 457–468. [Google Scholar] [CrossRef]
- Morel, O.; Toti, F.; Hugel, B.; Freyssinet, J.M. Cellular microparticles: A disseminated storage pool of bioactive vascular effectors. Curr. Opin. Hematol. 2004, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Satta, N.; Freyssinet, J.M.; Toti, F. The significance of human monocyte thrombomodulin during membrane vesiculation and after stimulation by lipopolysaccharide. Br. J. Haematol. 1997, 96, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Perez-Casal, M.; Downey, C.; Fukudome, K.; Marx, G.; Toh, C.H. Activated protein C induces the release of microparticle-associated endothelial protein C receptor. Blood 2005, 105, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; D’Ascenzo, S.; Borsotti, P.; Giavazzi, R.; Pavan, A.; Dolo, V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 2002, 160, 673–680. [Google Scholar] [CrossRef]
- Gassama, Y.; Favereaux, A. Emerging Roles of Extracellular Vesicles in the Central Nervous System: Physiology, Pathology, and Therapeutic Perspectives. Front. Cell. Neurosci. 2021, 15, 626043. [Google Scholar] [CrossRef]
- Canault, M.; Leroyer, A.S.; Peiretti, F.; Leseche, G.; Tedgui, A.; Bonardo, B.; Alessi, M.C.; Boulanger, C.M.; Nalbone, G. Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-alpha converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am. J. Pathol. 2007, 171, 1713–1723. [Google Scholar] [CrossRef]
- Ellis, V. Plasminogen activation at the cell surface. Curr. Top. Dev. Biol. 2003, 54, 263–312. [Google Scholar]
- Doeuvre, L.; Angles-Cano, E. Cell-derived microparticles unveil their fibrinolytic and proteolytic function. Med. Sci. 2009, 25, 37–44. [Google Scholar]
- Angelucci, A.; D’Ascenzo, S.; Festuccia, C.; Gravina, G.L.; Bologna, M.; Dolo, V.; Pavan, A. Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin. Exp. Metastasis 2000, 18, 163–170. [Google Scholar] [CrossRef]
- Lopez-Alemany, R.; Longstaff, C.; Hawley, S.; Mirshahi, M.; Fabregas, P.; Jardi, M.; Merton, E.; Miles, L.A.; Felez, J. Inhibition of cell surface mediated plasminogen activation by a monoclonal antibody against alpha-Enolase. Am. J. Hematol. 2003, 72, 234–242. [Google Scholar] [CrossRef]
- Das, R.; Plow, E.F. Phosphatidylserine as an anchor for plasminogen and its plasminogen receptor, histone H2B, to the macrophage surface. J. Thromb. Haemost. 2011, 9, 339–349. [Google Scholar] [CrossRef]
- Markus, G. Conformational changes in plasminogen, their effect on activation, and the agents that modulatie activation rates—A review. Fibrinolysis 1996, 10, 75–85. [Google Scholar] [CrossRef]
- Law, R.H.P.; Caradoc-Davies, T.; Cowieson, N.; Horvath, A.J.; Quek, A.J.; Amarante Encarnacao, J.; Steer, D.; Cowan, A.; Zhang, Q.; Lu, B.G.C.; et al. The X-rayCrystalStructure of Full-Length HumanPlasminogen. Cell Rep. 2012, 1, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Marshall, J.M.; Dawson, K.M.; Cederholm-Williams, S.A.; Ponting, C.P. Evidence that the conformation of unliganded human plasminogen is maintained via an intramolecular interaction between the lysine-binding site of kringle 5 and the N-terminal peptide. Biochem. J. 1998, 333, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Mangel, W.F.; Lin, B.H.; Ramakrishnan, V. Characterization of an extremely large, ligand-induced conformational change in plasminogen. Science 1990, 248, 69–73. [Google Scholar] [CrossRef]
- Plow, E.F.; Herren, T.; Redlitz, A.; Miles, L.A.; Hoover-Plow, J.L. The cell biology of the plasminogen system. FASEB J. 1995, 9, 939–945. [Google Scholar] [CrossRef]
- Wiman, B.; Collen, D. Molecular mechanism of physiological fibrinolysis. Nature 1978, 272, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Violand, B.N.; Byrne, R.; Castellino, F.J. The effect of alpha-,omega-amino acids on human plasminogen structure and activation. J. Biol. Chem. 1978, 253, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- Walther, P.J.; Hill, R.L.; McKee, P.A. The importance of the preactivation peptide in the two-stage mechanism of human plasminogen activation. J. Biol. Chem. 1975, 250, 5926–5933. [Google Scholar] [CrossRef]
- Claeys, H.; Vermylen, J. Physico-chemical and proenzyme properties of NH2-terminal glutamic acid and NH2-terminal lysine human plasminogen. Influence of 6-aminohexanoic acid. Biochim. Biophys. Acta 1974, 342, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.; Mullertz, S. Rate of activation and electrophoretic mobility of unmodified and partially degraded plasminogen. Effects of 6-aminohexanoic acid and related compounds. Scand. J. Clin. Lab. Investig. 1974, 34, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Peltz, S.W.; Hardt, T.A.; Mangel, W.F. Positive regulation of activation of plasminogen by urokinase: Differences in Km for (glutamic acid)-plasminogen and lysine-plasminogen and effect of certain alpha, omega-amino acids. Biochemistry 1982, 21, 2798–2804. [Google Scholar] [CrossRef]
- Dejouvencel, T.; Doeuvre, L.; Lacroix, R.; Plawinski, L.; Dignat-George, F.; Lijnen, H.R.; Angles-Cano, E. Fibrinolytic cross-talk: A new mechanism for plasmin formation. Blood 2010, 115, 2048–2056. [Google Scholar] [CrossRef]
- Xue, Y.; Bodin, C.; Olsson, K. Crystal structure of the native plasminogen reveals an activation-resistant compact conformation. J. Thromb. Haemost. 2012, 10, 1385–1396. [Google Scholar] [CrossRef]
- Humphries, J.; Gossage, J.A.; Modarai, B.; Burnand, K.G.; Sisson, T.H.; Murdoch, C.; Smith, A. Monocyte urokinase-type plasminogen activator up-regulation reduces thrombus size in a model of venous thrombosis. J. Vasc. Surg. 2009, 50, 1127–1134. [Google Scholar] [CrossRef]
- Singh, I.; Burnand, K.G.; Collins, M.; Luttun, A.; Collen, D.; Boelhouwer, B.; Smith, A. Failure of thrombus to resolve in urokinase-type plasminogen activator gene-knockout mice: Rescue by normal bone marrow-derived cells. Circulation 2003, 107, 869–875. [Google Scholar] [CrossRef]
- Leroyer, A.S.; Isobe, H.; Leseche, G.; Castier, Y.; Wassef, M.; Mallat, Z.; Binder, B.R.; Tedgui, A.; Boulanger, C.M. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J. Am. Coll. Cardiol. 2007, 49, 772–777. [Google Scholar] [CrossRef]
- Lishko, V.K.; Yermolenko, I.S.; Ugarova, T.P. Plasminogen on the surfaces of fibrin clots prevents adhesion of leukocytes and platelets. J. Thromb. Haemost. 2010, 8, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Gaussem, P.; Plawinski, L.; Angles-Cano, E. Physiologie et exploration de la fibrinolyse. EMC-Hématologie 2021, 32, 1–12. [Google Scholar]
- Miles, L.A.; Ginsberg, M.H.; White, J.G.; Plow, E.F. Plasminogen interacts with human platelets through two distinct mechanisms. J. Clin. Investig. 1986, 77, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Baeten, K.M.; Richard, M.C.; Kanse, S.M.; Mutch, N.J.; Degen, J.L.; Booth, N.A. Activation of single-chain urokinase-type plasminogen activator by platelet-associated plasminogen: A mechanism for stimulation of fibrinolysis by platelets. J. Thromb. Haemost. 2010, 8, 1313–1322. [Google Scholar] [CrossRef]
- Farina, A.R.; Tiberio, A.; Tacconelli, A.; Cappabianca, L.; Gulino, A.; Mackay, A.R. Identification of plasminogen in Matrigel and its activation by reconstitution of this basement membrane extract. Biotechniques 1996, 21, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.P.; Ismail, N.; Zhang, X.; Aguda, B.D.; Lee, E.J.; Yu, L.; Xiao, T.; Schafer, J.; Lee, M.L.; Schmittgen, T.D.; et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE 2008, 3, e3694. [Google Scholar] [CrossRef]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef]
- Gomez, I.; Ward, B.; Souilhol, C.; Recarti, C.; Ariaans, M.; Johnston, J.; Burnett, A.; Mahmoud, M.; Luong, L.A.; West, L.; et al. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat. Commun. 2020, 11, 214. [Google Scholar] [CrossRef]
- Gyorgy, B.; Modos, K.; Pallinger, E.; Paloczi, K.; Pasztoi, M.; Misjak, P.; Deli, M.A.; Sipos, A.; Szalai, A.; Voszka, I.; et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 2011, 117, e39–e48. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Thery, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Van der Heyden, A.; Chanthavong, P.; Angles-Cano, E.; Bonnet, H.; Dejeu, J.; Cras, A.; Philouze, C.; Serratrice, G.; Zoubari El-Ghazouani, F.; Toti, F.; et al. Grafted dinuclear zinc complexes for selective recognition of phosphatidylserine: Application to the capture of extracellular membrane microvesicles. J. Inorg. Biochem. 2022, 239, 112065. [Google Scholar] [CrossRef]
- Ma, Y.H.; Li, B.; Yang, J.; Han, X.; Chen, Z.; Lu, X. Calcium-dependent and -independent annexin V binding: Distinct molecular behaviours at cell membrane interfaces. Chem. Commun. 2020, 56, 1653–1656. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plawinski, L.; Cras, A.; Hernández Lopez, J.R.; de la Peña, A.; Van der Heyden, A.; Belle, C.; Toti, F.; Anglés-Cano, E. Distinguishing Plasmin-Generating Microvesicles: Tiny Messengers Involved in Fibrinolysis and Proteolysis. Int. J. Mol. Sci. 2023, 24, 1571. https://doi.org/10.3390/ijms24021571
Plawinski L, Cras A, Hernández Lopez JR, de la Peña A, Van der Heyden A, Belle C, Toti F, Anglés-Cano E. Distinguishing Plasmin-Generating Microvesicles: Tiny Messengers Involved in Fibrinolysis and Proteolysis. International Journal of Molecular Sciences. 2023; 24(2):1571. https://doi.org/10.3390/ijms24021571
Chicago/Turabian StylePlawinski, Laurent, Audrey Cras, José Rubicel Hernández Lopez, Aurora de la Peña, Angéline Van der Heyden, Catherine Belle, Florence Toti, and Eduardo Anglés-Cano. 2023. "Distinguishing Plasmin-Generating Microvesicles: Tiny Messengers Involved in Fibrinolysis and Proteolysis" International Journal of Molecular Sciences 24, no. 2: 1571. https://doi.org/10.3390/ijms24021571
APA StylePlawinski, L., Cras, A., Hernández Lopez, J. R., de la Peña, A., Van der Heyden, A., Belle, C., Toti, F., & Anglés-Cano, E. (2023). Distinguishing Plasmin-Generating Microvesicles: Tiny Messengers Involved in Fibrinolysis and Proteolysis. International Journal of Molecular Sciences, 24(2), 1571. https://doi.org/10.3390/ijms24021571




