Evaluation of Arterial Histopathology and microRNA Expression That Underlie Ultrasonography Findings in Temporal Arteries of Patients with Giant Cell Arteritis
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Differences in US Examination Parameters between Patient Groups
2.3. Differences in US-Measured Intima–Media Thickness between Patients with Different Clinical Characteristics and TAB-Derived Histopathological Features
2.4. miRNA Expression in TABs from GCA and Non-GCA Patients, Classified According to US-Measured Intima–Media TA Thickness
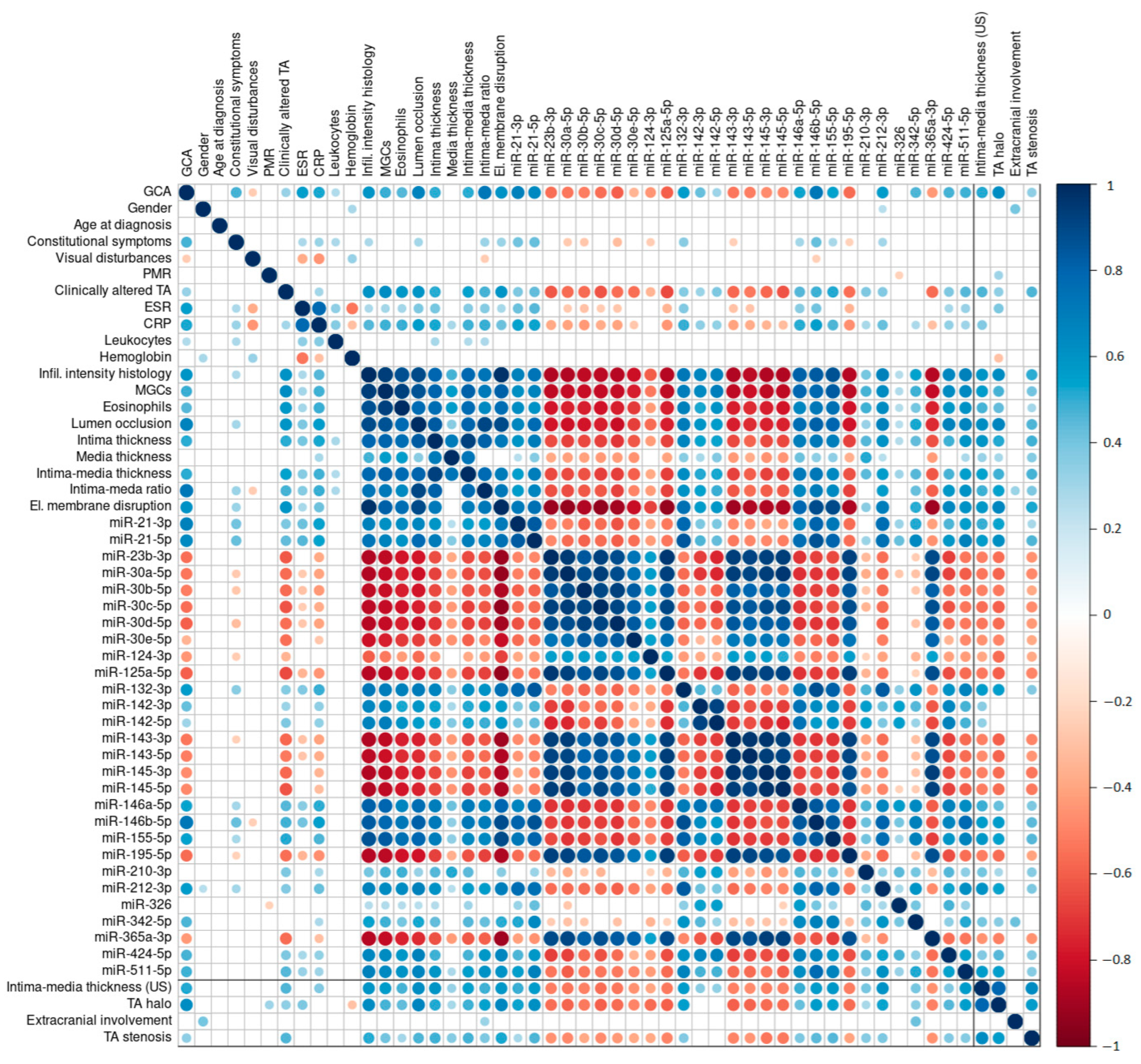
2.5. Association between GCA Diagnosis and US Examination Parameters, Histological Parameters, and miRNA Expression Profiles
2.5.1. US-Measured Intima–Media Thickness
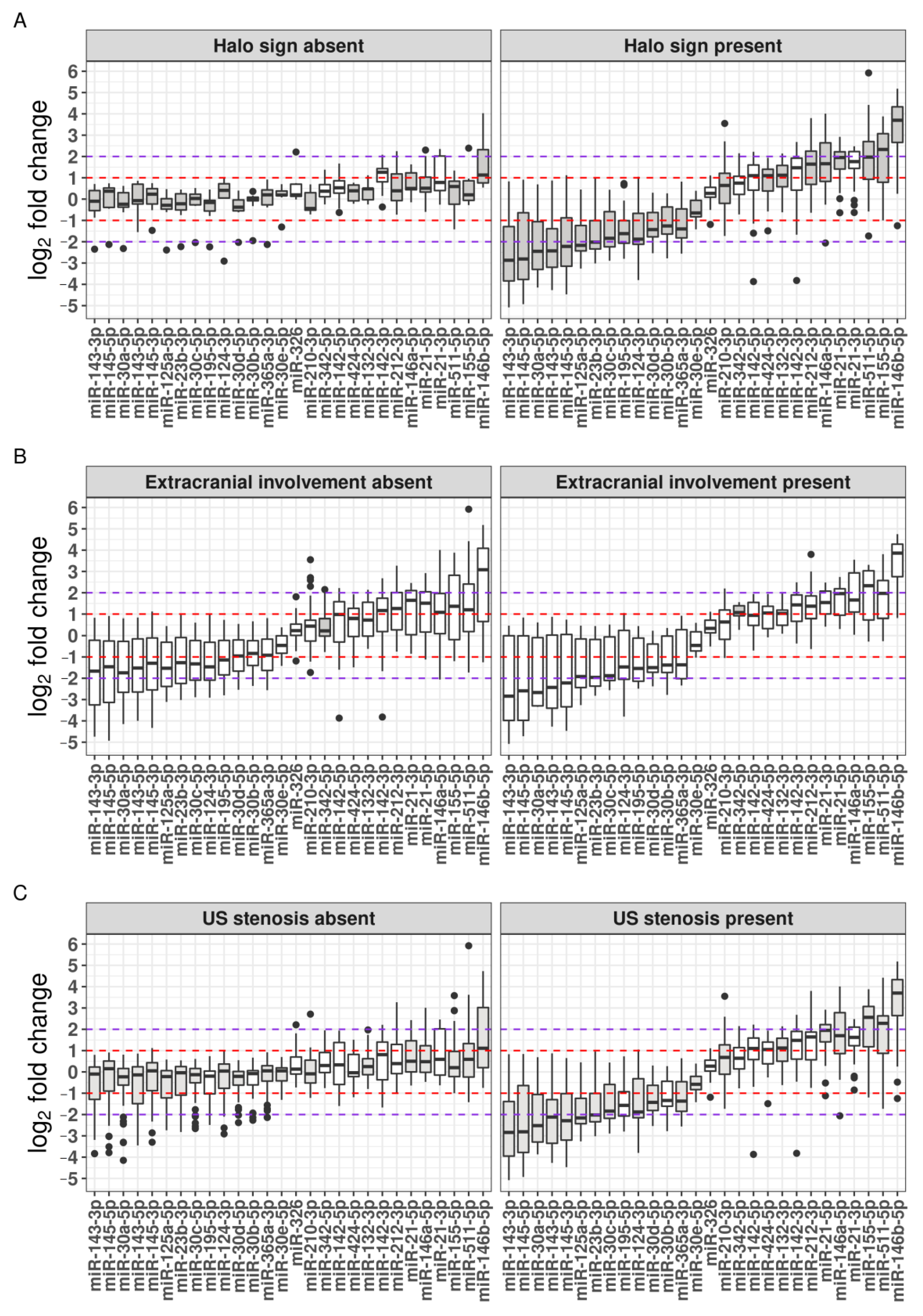
2.5.2. The TA Halo Sign Presence
2.5.3. Extracranial Involvement
2.5.4. Presence of TA Luminal Stenosis
2.6. Clinical Characteristics, TAB-Based Histopathological Features, and Differential miRNA Expression in Relation to Underlying US Examination Parameters
2.7. Discrepancies between GCA Diagnosis Based on TAB and TA US
2.8. Diagnostic Performance of US and Altered miRNAs in GCA Patients
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Clinical Parameters and US Examination Procedure
4.3. TAB Histopathological Assessment and miRNA Expression Analysis
- (a)
- Nine quantitatively assessed histopathological parameters, including inflammatory infiltrate intensity; extent of luminal stenosis; intima, media, and intima–media thickness; ratio between the intima and media thickness; internal elastic lamina disruption; and densities of MGCs and eosinophils per mm2.
- (b)
- Expression levels of 28 miRNAs, specifically, miR-21-3p, miR-21-5p, miR-23b-3p, miR-30a-5p, miR-30b-5p, miR-30c-5p, miR-30d-5p, miR-30e-5p, miR-124-3p, miR-125a-5p, miR-132-3p, miR-142-3p, miR-142-5p, miR-143-3p, miR-143-5p, miR-145-3p, miR-145-5p, miR-146a-5p, miR-146b-5p, miR-155-5p, miR-195-5p, miR-210-3p, miR-212-3p, miR-326, miR-342-5p, miR-365a-3p, miR-424-5p, and miR-511-5p.
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Ward, T.N. Horton’s Disease: Past and Present. Curr. Pain Headache Rep. 2005, 9, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Gershwin, M.E. Giant Cell Arteritis: A Review of Classification, Pathophysiology, Geoepidemiology and Treatment. Autoimmun. Rev. 2012, 11, A544–A554. [Google Scholar] [CrossRef]
- Van Der Geest, K.S.M.; Sandovici, M.; Brouwer, E.; Mackie, S.L.; Van Der Geest, M. Diagnostic Accuracy of Symptoms, Physical Signs, and Laboratory Tests for Giant Cell Arteritis A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2020, 180, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.; Maldini, C.; Gonzalez-Chiappe, S.; Chevret, S.; Mahr, A. Sensitivity of Temporal Artery Biopsy in the Diagnosis of Giant Cell Arteritis: A Systematic Literature Review and Meta-Analysis. Rheumatology 2020, 59, 1011–1020. [Google Scholar] [CrossRef]
- Monti, S.; Floris, A.; Ponte, C.; Schmidt, W.A.; Diamantopoulos, A.P.; Pereira, C.; Piper, J.; Luqmani, R. The Use of Ultrasound to Assess Giant Cell Arteritis: Review of the Current Evidence and Practical Guide for the Rheumatologist. Rheumatology 2018, 57, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojczal, J.; Kozera, G.; Luchowski, P.; Neubauer-Geryk, J. Advantages in Diagnosis of Giant Cell Arteritis by Ultrasound. Postep. Dermatol. I Alergol. 2019, 36, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR Recommendations for the Use of Imaging in Large Vessel Vasculitis in Clinical Practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef]
- Schmidt, W.A. Ultrasound in the Diagnosis and Management of Giant Cell Arteritis. Rheumatology 2018, 57, ii22–ii31. [Google Scholar] [CrossRef] [Green Version]
- Salvarani, C.; Pipitone, N.; Versari, A.; Hunder, G.G. Clinical Features of Polymyalgia Rheumatica and Giant Cell Arteritis. Nat. Rev. Rheumatol. 2012, 8, 509–521. [Google Scholar] [CrossRef]
- Schäfer, V.S.; Chrysidis, S.; Dejaco, C.; Duftner, C.; Iagnocco, A.; Bruyn, G.A.; Carrara, G.; D’Agostino, M.A.; De Miguel, E.; Diamantopoulos, A.P.; et al. Assessing Vasculitis in Giant Cell Arteritis by Ultrasound: Results of OMERACT Patient-Based Reliability Exercises. J. Rheumatol. 2018, 45, 1289–1295. [Google Scholar] [CrossRef]
- Monti, S.; Floris, A.; Ponte, C.B.; Schmidt, W.A.; Diamantopoulos, A.P.; Pereira, C.; Vaggers, S.; Luqmani, R.A. The Proposed Role of Ultrasound in the Management of Giant Cell Arteritis in Routine Clinical Practice. Rheumatology 2018, 57, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Geest, K.S.M.; Borg, F.; Kayani, A.; Paap, D.; Gondo, P.; Schmidt, W.; Luqmani, R.A.; Dasgupta, B. Novel Ultrasonographic Halo Score for Giant Cell Arteritis: Assessment of Diagnostic Accuracy and Association with Ocular Ischaemia. Ann. Rheum. Dis. 2019, 79, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Geest, K.S.M.; Wolfe, K.; Borg, F.; Sebastian, A.; Kayani, A.; Tomelleri, A.; Gondo, P.; Schmidt, W.A.; Luqmani, R.; Dasgupta, B. Ultrasonographic Halo Score in Giant Cell Arteritis: Association with Intimal Hyperplasia and Ischaemic Sight Loss. Rheumatology 2021, 60, 4361–4366. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, A.; Muratore, F.; Boiardi, L.; Restuccia, G.; Pipitone, N.; Pazzola, G.; Tagliavini, E.; Ragazzi, M.; Rossi, G.; Salvarani, C. Inflamed Temporal Artery: Histologic Findings in 354 Biopsies, with Clinical Correlations. Am. J. Surg. Pathol. 2014, 38, 1360–1370. [Google Scholar] [CrossRef]
- Gunawardene, A.; Chant, H. Facial Nerve Injury during Temporal Artery Biopsy. Ann. R. Coll. Surg. Engl. 2014, 96, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Luqmani, R.; Lee, E.; Singh, S.; Gillett, M.; Schmidt, W.A.; Bradburn, M.; Dasgupta, B.; Diamantopoulos, A.P.; Forrester-Barker, W.; Hamilton, W.; et al. The Role of Ultrasound Compared to Biopsy of Temporal Arteries in the Diagnosis and Treatment of Giant Cell Arteritis (TABUL): A Diagnostic Accuracy and Cost-Effectiveness Study. Health Technol. Assess. 2016, 20, 1–238. [Google Scholar] [CrossRef] [Green Version]
- Keser, G.; Aksu, K. Diagnosis and Differential Diagnosis of Large-Vessel Vasculitides. Rheumatol. Int. 2019, 39, 169–185. [Google Scholar] [CrossRef]
- Pugh, D.; Karabayas, M.; Basu, N.; Cid, M.C.; Goel, R.; Goodyear, C.S.; Grayson, P.C.; McAdoo, S.P.; Mason, J.C.; Owen, C.; et al. Large-Vessel Vasculitis. Nat. Rev. Dis. Prim. 2022, 7, 93. [Google Scholar] [CrossRef]
- Prieto-Peña, D.; Castañeda, S.; Martínez-Rodríguez, I.; Atienza-Mateo, B.; Blanco, R.; González-Gay, M.A. Imaging Tests in the Early Diagnosis of Giant Cell Arteritis. J. Clin. Med. 2021, 10, 3704. [Google Scholar] [CrossRef]
- Laurent, C.; Ricard, L.; Fain, O.; Buvat, I.; Adedjouma, A.; Soussan, M.; Mekinian, A. PET/MRI in Large-Vessel Vasculitis: Clinical Value for Diagnosis and Assessment of Disease Activity. Sci. Rep. 2019, 9, 12388. [Google Scholar] [CrossRef] [Green Version]
- Robinette, M.L.; Rao, D.A.; Monach, P.A. The Immunopathology of Giant Cell Arteritis Across Disease Spectra. Front. Immunol. 2021, 12, 623716. [Google Scholar] [CrossRef]
- Salvarani, C.; Cantini, F.; Hunder, G.G. Polymyalgia Rheumatica and Giant-Cell Arteritis. Lancet 2008, 372, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Maleszewski, J.J.; Younge, B.R.; Fritzlen, J.T.; Hunder, G.G.; Goronzy, J.J.; Warrington, K.J.; Weyand, C.M. Clinical and Pathological Evolution of Giant Cell Arteritis: A Prospective Study of Follow-up Temporal Artery Biopsies in 40 Treated Patients. Mod. Pathol. 2017, 30, 788–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolha, L.; Hočevar, A.; Suljič, A.; Jurčić, V. Inflammatory Cell Composition and Immune-Related MicroRNA Signature of Temporal Artery Biopsies From Patients With Giant Cell Arteritis. Front. Immunol. 2021, 12, 791099. [Google Scholar] [CrossRef]
- Bolha, L.; Pizem, J.; Frank-Bertoncelj, M.; Hocevar, A.; Tomsic, M.; Jurcic, V. Identification of MicroRNAs and Their Target Gene Networks Implicated in Arterial Wall Remodelling in Giant Cell Arteritis. Rheumatology 2020, 59, 3540–3552. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Wang, X.; Chen, Y.; Wang, L.; Zhao, M.; Lu, Q. Dysregulation of MicroRNAs in Autoimmune Diseases: Pathogenesis, Biomarkers and Potential Therapeutic Targets. Cancer Lett. 2018, 428, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Ahmed, S.A. MicroRNA, a New Paradigm for Understanding Immunoregulation, Inflammation, and Autoimmune Diseases. Transl. Res. 2011, 157, 163–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croci, S.; Zerbini, A.; Boiardi, L.; Muratore, F.; Bisagni, A.; Nicoli, D.; Farnetti, E.; Pazzola, G.; Cimino, L.; Moramarco, A.; et al. MicroRNA Markers of Inflammation and Remodelling in Temporal Arteries from Patients with Giant Cell Arteritis. Ann. Rheum. Dis. 2016, 75, 1527–1533. [Google Scholar] [CrossRef]
- Kuret, T.; Burja, B.; Feichtinger, J.; Thallinger, G.G.; Frank-Bertoncelj, M.; Lakota, K.; Žigon, P.; Sodin-Semrl, S.; Čučnik, S.; Tomšič, M.; et al. Gene and MiRNA Expression in Giant Cell Arteritis—A Concise Systematic Review of Significantly Modified Studies. Clin. Rheumatol. 2019, 38, 307–316. [Google Scholar] [CrossRef]
- Sebastian, A.; Coath, F.; Innes, S.; Jackson, J.; van der Geest, K.S.M.; Dasgupta, B. Role of the Halo Sign in the Assessment of Giant Cell Arteritis: A Systematic Review and Meta-Analysis. Rheumatol. Adv. Pract. 2021, 5, rkab059. [Google Scholar] [CrossRef]
- Ješe, R.; Rotar, Z.; Tomšič, M.; Hočevar, A. The Cut-off Values for the Intima-Media Complex Thickness Assessed by Colour Doppler Sonography in Seven Cranial and Aortic Arch Arteries. Rheumatology 2021, 60, 1346–1352. [Google Scholar] [CrossRef]
- Monti, S.; Ponte, C.; Pereira, C.; Manzoni, F.; Klersy, C.; Rumi, F.; Carrara, G.; Hutchings, A.; Schmidt, W.A.; Dasgupta, B.; et al. The Impact of Disease Extent and Severity Detected by Quantitative Ultrasound Analysis in the Diagnosis and Outcome of Giant Cell Arteritis. Rheumatology 2020, 59, 2299–2307. [Google Scholar] [CrossRef]
- Poller, D.N.; Van Wyk, Q.; Jevrey, M.J. The Importance of Skip Lesions in Temporal Arteritis. J. Clin. Pathol. 2000, 53, 137–139. [Google Scholar] [CrossRef] [Green Version]
- Dua, A.B.; Husainat, N.M.; Kalot, M.A.; Byram, K.; Springer, J.M.; James, K.E.; Chang Lin, Y.; Turgunbaev, M.; Villa-Forte, A.; Abril, A.; et al. Giant Cell Arteritis: A Systematic Review and Meta-Analysis of Test Accuracy and Benefits and Harms of Common Treatments. ACR Open Rheumatol. 2021, 3, 429–441. [Google Scholar] [CrossRef]
- Kuret, T.; Lakota, K.; Čučnik, S.; Jurčič, V.; Distler, O.; Rotar, Ž.; Hočevar, A.; Sodin-šemrl, S.; Frank-Bertoncelj, M. Dysregulated Expression of Arterial Micrornas and Their Target Gene Networks in Temporal Arteries of Treatment-Naïve Patients with Giant Cell Arteritis. Int. J. Mol. Sci. 2021, 22, 6520. [Google Scholar] [CrossRef]
- Davis-Dusenbery, B.N.; Chan, M.C.; Reno, K.E.; Weisman, A.S.; Layne, M.D.; Lagna, G.; Hata, A. Down-Regulation of Krüppel-like Factor-4 (KLF4) by MicroRNA-143/145 Is Critical for Modulation of Vascular Smooth Muscle Cell Phenotype by Transforming Growth Factor-β and Bone Morphogenetic Protein 4. J. Biol. Chem. 2011, 286, 28097–28110. [Google Scholar] [CrossRef] [Green Version]
- Cordes, K.R.; Sheehy, N.T.; White, M.P.; Berry, E.C.; Morton, S.U.; Muth, A.N.; Lee, T.; Miano, J.M.; Ivey, K.N.; Srivastava, D. MiR-145 and MiR-143 Regulate Smooth Muscle Cell Fate and Plasticity. Nature 2009, 460, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Quintavalle, M.; Elia, L.; Condorelli, G.; Courtneidge, S.A. MicroRNA Control of Podosome Formation in Vascular Smooth Muscle Cells in Vivo and in Vitro. J. Cell Biol. 2010, 189, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Liu, X.; Yang, J.; Lin, Y.; Xu, D.Z.; Lu, Q.; Deitch, E.A.; Huo, Y.; Delphin, E.S.; Zhang, C. MicroRNA-145, a Novel Smooth Muscle Cell Phenotypic Marker and Modulator, Controls Vascular Neointimal Lesion Formation. Circ. Res. 2009, 105, 158–166. [Google Scholar] [CrossRef]
- Gareri, C.; Iaconetti, C.; Sorrentino, S.; Covello, C.; De Rosa, S.; Indolfi, C. MiR-125a-5p Modulates Phenotypic Switch of Vascular Smooth Muscle Cells by Targeting ETS-1. J. Mol. Biol. 2017, 429, 1817–1828. [Google Scholar] [CrossRef]
- Liu, Y.F.; Spinelli, A.; Sun, L.Y.; Jiang, M.; Singer, D.V.; Ginnan, R.; Saddouk, F.Z.; Van Riper, D.; Singer, H.A. MicroRNA-30 Inhibits Neointimal Hyperplasia by Targeting Ca 2+ /Calmodulin-Dependent Protein Kinase IIδ (CaMKIIδ). Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.; Cheng, Y.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNA Expression Signature and Antisense-Mediated Depletion Reveal an Essential Role of MicroRNA in Vascular Neointimal Lesion Formation. Circ. Res. 2007, 100, 1579–1588. [Google Scholar] [CrossRef]
- Sarkar, J.; Gou, D.; Turaka, P.; Viktorova, E.; Ramchandran, R.; Raj, J.U. MicroRNA-21 Plays a Role in Hypoxia-Mediated Pulmonary Artery Smooth Muscle Cell Proliferation and Migration. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jiang, M.; Xu, Z.; Huang, H.; Gong, P.; Zhu, H.; Ruan, C. MiR-146b-5p Promotes VSMC Proliferation and Migration. Int. J. Clin. Exp. Pathol. 2015, 8, 12901–12907. [Google Scholar]
- Samson, M.; Corbera-Bellalta, M.; Audia, S.; Planas-Rigol, E.; Martin, L.; Cid, M.C.; Bonnotte, B. Recent Advances in Our Understanding of Giant Cell Arteritis Pathogenesis. Autoimmun. Rev. 2017, 16, 833–844. [Google Scholar] [CrossRef]
- Terrades-Garcia, N.; Cid, M.C. Pathogenesis of Giant-Cell Arteritis: How Targeted Therapies Are Influencing Our Understanding of the Mechanisms Involved. Rheumatology 2018, 57, ii51–ii62. [Google Scholar] [CrossRef] [Green Version]
- Weyand, C.M.; Goronzy, J.J. Immune Mechanisms in Medium and Large-Vessel Vasculitis. Nat. Rev. Rheumatol. 2013, 9, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Welten, S.M.J.; Goossens, E.A.C.; Quax, P.H.A.; Nossent, A.Y. The Multifactorial Nature of MicroRNAs in Vascular Remodelling. Cardiovasc. Res. 2016, 110, 6–22. [Google Scholar] [CrossRef] [Green Version]
- Gareri, C.; De Rosa, S.; Indolfi, C. MicroRNAs for Restenosis and Thrombosis after Vascular Injury. Circ. Res. 2016, 118, 1170–1184. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, V.S.; Juche, A.; Ramiro, S.; Krause, A.; Schmidt, W.A. Ultrasound Cut-off Values for Intima-Media Thickness of Temporal, Facial and Axillary Arteries in Giant Cell Arteritis. Rheumatology 2017, 56, 1479–1483. [Google Scholar] [CrossRef]
- Couronné, R.; Probst, P.; Boulesteix, A.L. Random Forest versus Logistic Regression: A Large-Scale Benchmark Experiment. BMC Bioinform. 2018, 19, 270. [Google Scholar] [CrossRef] [Green Version]
- Sheedy, F.J. Turning 21: Induction of MiR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 2015, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Hu, B.; Jadhav, R.R.; Jin, J.; Zhang, H.; Cavanagh, M.M.; Akondy, R.S.; Ahmed, R.; Weyand, C.M.; Goronzy, J.J. Activation of MiR-21-Regulated Pathways in Immune Aging Selects against Signatures Characteristic of Memory T Cells. Cell Rep. 2018, 25, 2148–2162.e5. [Google Scholar] [CrossRef] [Green Version]
- Hunder, G.G.; Bloch, D.A.; Michel, B.A.; Stevens, M.B.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Giant Cell Arteritis. Arthritis Rheum. 1990, 33, 1122–1128. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data. Version 3.1.3. Available online: https://github.com/talgalili/gplots (accessed on 26 September 2022).


| Non-GCA Patients [n = 22] a | GCA Patients [n = 46] b | |
|---|---|---|
| Demographics | ||
| Age at diagnosis (years); median (range) | 73 (58–87) | 73 (55–92) |
| Gender (female); n (%) | 18 (82) | 30 (65) |
| Symptoms and signs | ||
| Constitutional symptoms; n (%) | 5 (23) | 34 (74) *** |
| Visual disturbances; n (%) | 11 (52) | 12 (26) |
| PMR; n (%) | 3 (18) | 9 (20) |
| Clinically altered TA; n (%) | 6 (32) | 29 (63) * |
| Laboratory | ||
| ESR (mm/h); median (range) | 35 (11–113) | 81 (28–130) *** |
| CRP (mg/ml); median (range) | 5 (0–83) | 68 (7–218) *** |
| Leukocytes (108 cells); median (range) | 7.4 (5–16) | 9.5 (6–18) * |
| Hemoglobin (g/dl); median (range) | 122 (101–156) | 120 (99–140) |
| Histopathology | ||
| Infiltrate intensity (score); median (range) | 0 (0–0) | 3 (0–4) *** |
| MGCs (score); median (range) | 0 (0–0) | 1 (0–4) *** |
| Eosinophils (score); median (range) | 0 (0–0) | 1 (0–3) *** |
| Luminal stenosis (score); median (range) | 1 (1–1) | 3 (1–4) *** |
| Intima thickness (mm); median (range) | 0.1 (0.05–0.6) | 0.35 (0.06–1.2) *** |
| Media thickness (mm); median (range) | 0.12 (0.05–0.8) | 0.15 (0.05–0.35) |
| Intima–media thickness (mm); median (range) | 0.22 (0.1–0.6) | 0.48 (0.11–1.55) *** |
| Intima–media ratio; median (range) | 0.85 (0.5–1.5) | 2.5 (0.7–4) *** |
| El. Membrane disruption; n (%) | 0 | 29 (63) *** |
| Ultrasonography | ||
| TA halo; n (%) | 0 | 37 (80) *** |
| Extracranial involvement; n (%) | 0 | 15 (36) |
| TA stenosis n; (%) | 2 (17) | 27 (59) * |
| US Examination Parameter | GCA+/ TAB+ (n = 30) | GCA+/ TAB− (n = 16) a | GCA−/ TAB− (n = 22) b |
|---|---|---|---|
| TA halo sign; n (%) | 28 (93) | 9 (56) ** | 0 |
| TA stenosis; n (%) | 22 (73) | 5 (31) * | 2 (17) |
| Extracranial involvement; n (%) | 11 (37) | 4 (33) | 0 |
| Presence of the TA Halo Sign (n = 37) | Extracranial Involvement (n = 15) a | Presence of US Stenosis (n = 27) | |
|---|---|---|---|
| Demographics | |||
| Gender (female); n (%) | 22 (60) | 14 (93) ** | 17 (63) |
| Symptoms and signs | |||
| PMR; n (%) | 9 (24) | 3 (20) | 6 (22) |
| Clinically altered TA; n (%) | 28 (76) *** | 8 (53) | 22 (81) *** |
| Laboratory | |||
| ESR (mm/h); median (range) | 83 (28–130) | 89 (28–120) | 78 (28–120) |
| CRP (mg/ml); median (range) | 68 (10–218) | 76 (12–212) | 66 (12–212) |
| Hemoglobin (g/dl); median (range) | 119 (99–140) | 116 (99–135) | 120 (99–140) |
| Histopathology | |||
| Infiltrate intensity (score); median (range) | 3 (0–4) ** | 4 (0–4) | 4 (0–4) ** |
| MGCs (score); median (range) | 2 (0–4) *** | 4 (0–4) | 2 (0–4) *** |
| Eosinophils (score); median (range) | 1 (0–3) * | 1 (0–3) | 1 (0–3) |
| El. membrane disruption; n (%) | 28 (76) *** | 10 (75) | 2 (81) ** |
| Luminal stenosis (score); median (range) | 4 (1–4) *** | 4 (1–4) | 4 (1–4) * |
| Intima thickness (mm); median (range) | 0.4 (0.15–1.2) ** | 0.4 (0.09–0.8) | 0.41 (0.17–1.2) ** |
| Media thickness (mm); median (range) | 0.15 (0.0 –0.35) ** | 0.16 (0.06–0.25) | 0.2 (0.06–0.35) * |
| Intima–media thickness (mm); median (range) | 0.55 (0.2–1.55) ** | 0.55 (0.22–1.05) | 0.6 (0.23–1.55) ** |
| Intima–media ratio; median (range) | 2.5 (0.9–4) | 3.0 (0.7–4) | 2.5 (1.6–4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suljič, A.; Hočevar, A.; Jurčić, V.; Bolha, L. Evaluation of Arterial Histopathology and microRNA Expression That Underlie Ultrasonography Findings in Temporal Arteries of Patients with Giant Cell Arteritis. Int. J. Mol. Sci. 2023, 24, 1572. https://doi.org/10.3390/ijms24021572
Suljič A, Hočevar A, Jurčić V, Bolha L. Evaluation of Arterial Histopathology and microRNA Expression That Underlie Ultrasonography Findings in Temporal Arteries of Patients with Giant Cell Arteritis. International Journal of Molecular Sciences. 2023; 24(2):1572. https://doi.org/10.3390/ijms24021572
Chicago/Turabian StyleSuljič, Alen, Alojzija Hočevar, Vesna Jurčić, and Luka Bolha. 2023. "Evaluation of Arterial Histopathology and microRNA Expression That Underlie Ultrasonography Findings in Temporal Arteries of Patients with Giant Cell Arteritis" International Journal of Molecular Sciences 24, no. 2: 1572. https://doi.org/10.3390/ijms24021572





