Skin Cancer Metabolic Profile Assessed by Different Analytical Platforms
Abstract
1. Introduction
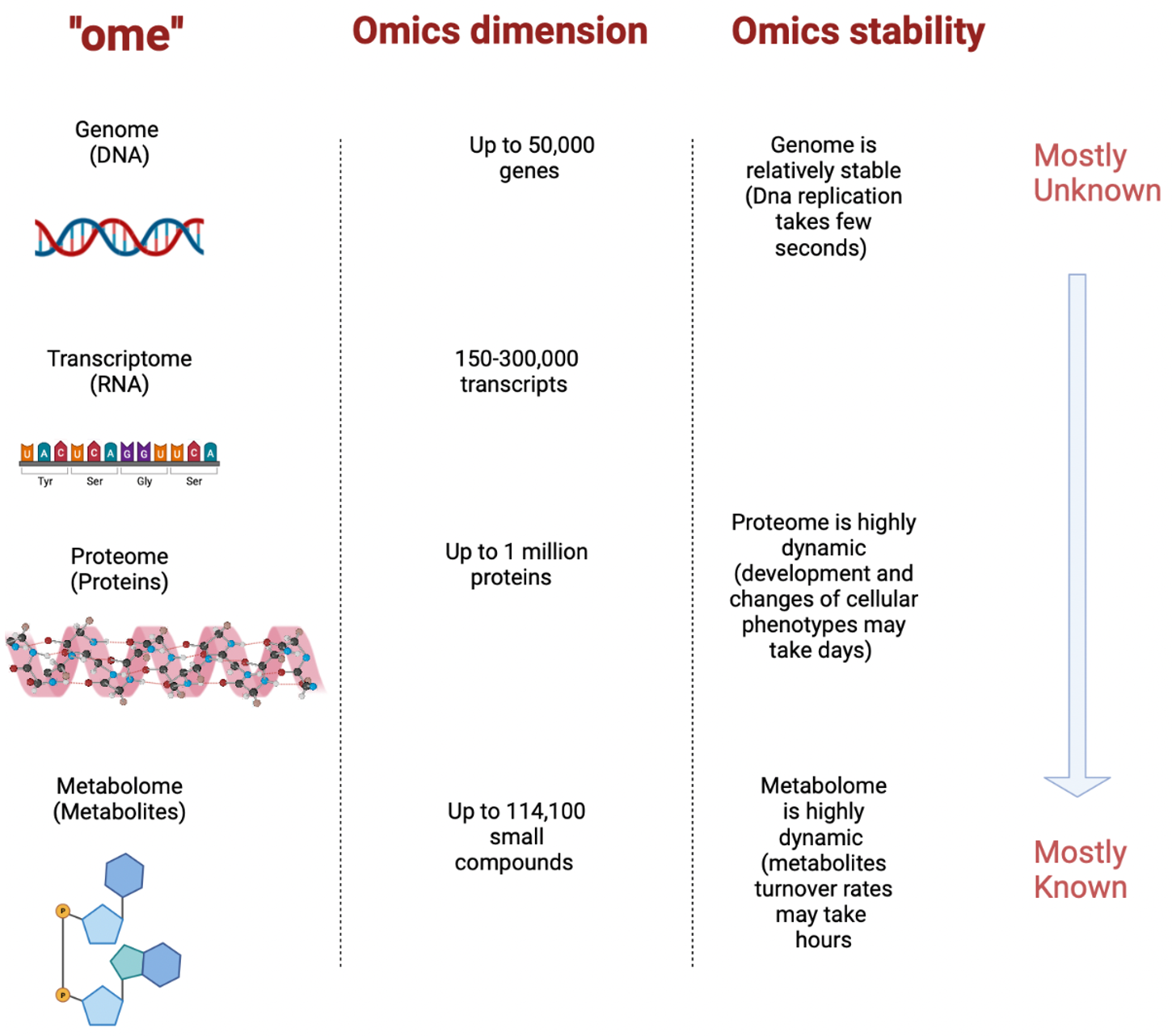
2. Omics Technologies
3. Skin Metabolites
3.1. Skin Metabolites Sources
3.2. The Emerging Role of Microbiota and Adipocyte-Derived Lipids
4. Skin or Blood Metabolomic Profiling for Diagnosing and Treating Skin Conditions
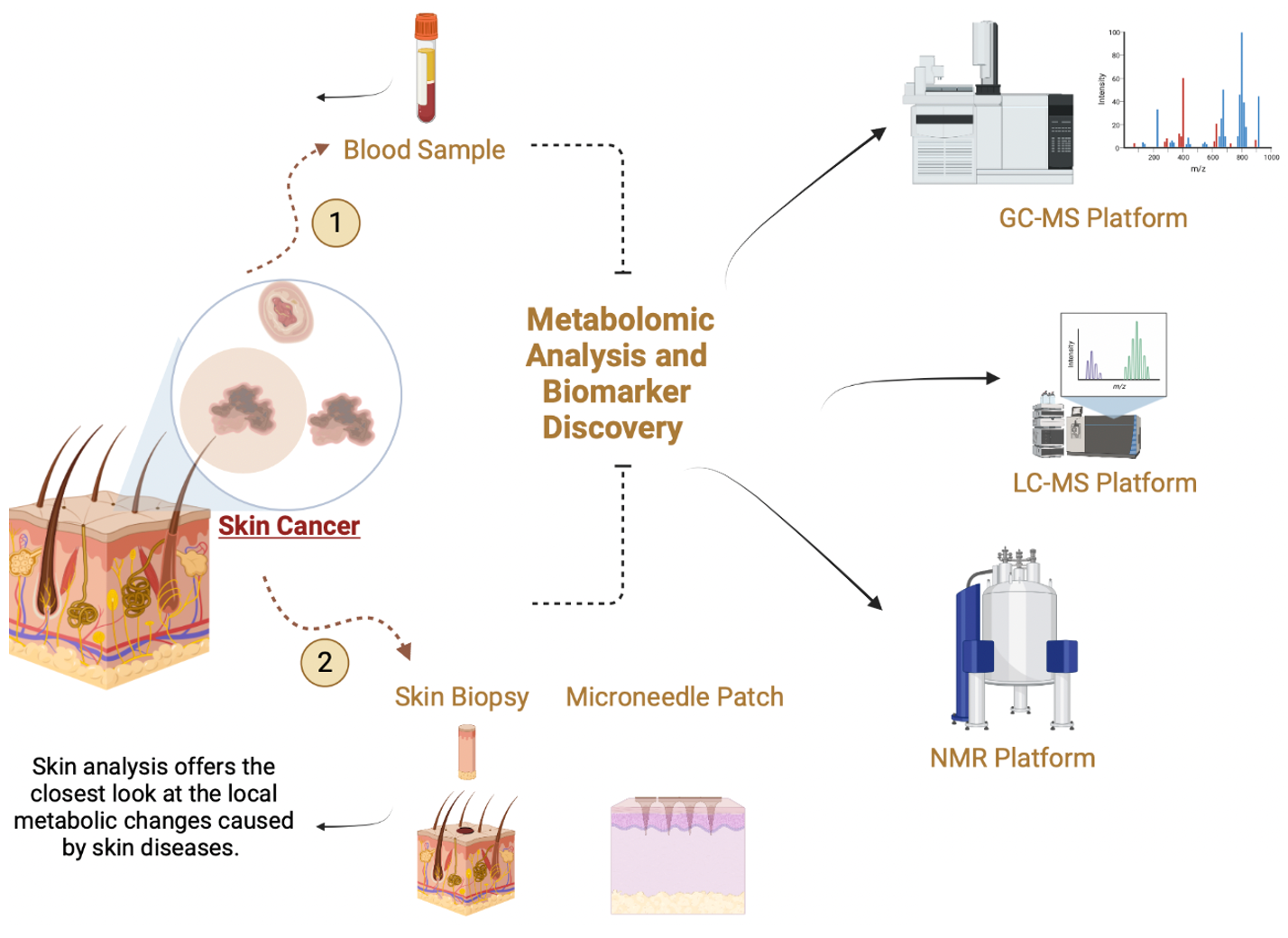
5. Analytical Platform in Metabolomics Workflow
5.1. Mass Spectrometry-Based Metabolomics
5.2. NMR-Based Metabolomics
6. Skin Biomarkers Discovery through Metabolomics Applications
6.1. Skin Disease Investigations
6.2. Sampling Procedures and Analysis of Skin Metabolites
7. Skin Cancer Metabolomics Using Different Analytical Platforms
7.1. GC-MS Based Skin Cancer Metabolomics
| Skin vs. Air Samples | Non-Melanoma vs. Matching Skin | Melanoma vs. Matching Skin |
|---|---|---|
|
|
|
7.2. LC-MS Based Skin Cancer Metabolomics
7.3. NMR Based Skin Cancer Metabolomics
8. Clinical Implications of the Metabolic Profile of Skin Cancer
9. Limitations
10. Conclusion and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Saba, T. Computer vision for microscopic skin cancer diagnosis using handcrafted and non-handcrafted features. Microsc. Res. Tech. 2021, 84, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Toğaçar, M.; Cömert, Z.; Ergen, B. Intelligent skin cancer detection applying autoencoder, MobileNetV2 and spiking neural networks. Chaos Solitons Fractals 2021, 144, 110714. [Google Scholar] [CrossRef]
- Haggenmüller, S.; Maron, R.C.; Hekler, A.; Utikal, J.S.; Barata, C.; Barnhill, R.L.; Beltraminelli, H.; Berking, C.; Betz-Stablein, B.; Blum, A.; et al. Skin cancer classification via convolutional neural networks: Systematic review of studies involving human experts. Eur. J. Cancer 2021, 156, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.R. The influence of climate change on skin cancer incidence—A review of the evidence. Int. J. Women’s Dermatol. 2021, 7, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.R.; Cho, S.H.; Lee, J.D.; Kim, H.S. The Human Microbiota and Skin Cancer. Int. J. Mol. Sci. 2022, 23, 1813. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Emerick, K.S.; Kaufman, H.L.; Miller, D.M. Immunotherapy for Non-melanoma Skin Cancer. Curr. Oncol. Rep. 2021, 23, 125. [Google Scholar] [CrossRef]
- Fijałkowska, M.; Koziej, M.; Antoszewski, B. Detailed head localization and incidence of skin cancers. Sci. Rep. 2021, 11, 12391. [Google Scholar] [CrossRef]
- Lay, J.O.; Liyanage, R.; Borgmann, S.; Wilkins, C.L. Problems with the “omics”. TrAC Trends Anal. Chem. 2006, 25, 1046–1056. [Google Scholar] [CrossRef]
- Stinkens, K.; Vanhove, K.; Thomeer, M. Metabolomics a novel biomarker in lung cancer. J. Thorac. Oncol. 2015, 10, e46. [Google Scholar] [CrossRef] [PubMed]
- Azimi, A.; Yang, P.; Ali, M.; Howard, V.; Mann, G.J.; Kaufman, K.L.; Fernandez-Penas, P. Data Independent Acquisition Proteomic Analysis Can Discriminate between Actinic Keratosis, Bowen’s Disease, and Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2020, 140, 212–222.e11. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Banerjee, S.; Suman; Kumar, R.; Buragohain, L.; Ghosh, M. Proteomics and Metabolomics in Cancer Diagnosis and Therapy. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Chakraborti, S., Ray, B.K., Roychowdhury, S., Eds.; Springer: Singapore, 2020; pp. 1–31. ISBN 978-981-15-4501-6. [Google Scholar]
- Qi, S.-a.; Wu, Q.; Chen, Z.; Zhang, W.; Zhou, Y.; Mao, K.; Li, J.; Li, Y.; Chen, J.; Huang, Y.; et al. High-resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Sci. Rep. 2021, 11, 11805. [Google Scholar] [CrossRef] [PubMed]
- Beger, R. A Review of Applications of Metabolomics in Cancer. Metabolites 2013, 3, 552–574. [Google Scholar] [CrossRef]
- Yu, L.; Li, K.; Zhang, X. Next-generation metabolomics in lung cancer diagnosis, treatment and precision medicine: Mini review. Oncotarget 2017, 8, 115774–115786. [Google Scholar] [CrossRef]
- Gu, X.; Ke, S.; Wang, Q.; Zhuang, T.; Xia, C.; Xu, Y.; Yang, L.; Zhou, M. Energy metabolism in major depressive disorder: Recent advances from omics technologies and imaging. Biomed. Pharmacother. 2021, 141, 111869. [Google Scholar] [CrossRef]
- Zhou, M.; Kong, Y.; Wang, X.; Li, W.; Chen, S.; Wang, L.; Wang, C.; Zhang, Q. LC-MS/MS-Based Quantitative Proteomics Analysis of Different Stages of Non-Small-Cell Lung Cancer. Biomed. Res. Int. 2021, 2021, 5561569. [Google Scholar] [CrossRef]
- Ma, L.; Muscat, J.E.; Sinha, R.; Sun, D.; Xiu, G. Proteomics of exhaled breath condensate in lung cancer and controls using data-independent acquisition (DIA): A pilot study. J. Breath Res. 2021, 15, 026002. [Google Scholar] [CrossRef]
- Kurg, K.; Planken, A.; Kurg, R. Proteomic and Biochemical Analysis of Extracellular Vesicles Isolated from Blood Serum of Patients with Melanoma. Separations 2022, 9, 86. [Google Scholar] [CrossRef]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef]
- Dopazo, J. Genomics and transcriptomics in drug discovery. Drug Discov. Today 2014, 19, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Del Giacco, L.; Cattaneo, C. Introduction to Genomics. In Molecular Profiling: Methods and Protocols; Espina, V., Liotta, L.A., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 79–88. ISBN 978-1-60327-216-2. [Google Scholar]
- Dong, Z.C.; Chen, Y. Transcriptomics: Advances and approaches. Sci. China Life Sci. 2013, 56, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Lovino, M.; Randazzo, V.; Ciravegna, G.; Barbiero, P.; Ficarra, E.; Cirrincione, G. A survey on data integration for multi-omics sample clustering. Neurocomputing 2022, 488, 494–508. [Google Scholar] [CrossRef]
- Elpa, D.P.; Chiu, H.-Y.; Wu, S.-P.; Urban, P.L. Skin Metabolomics. Trends Endocrinol. Metab. 2021, 32, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, E.P.; Hsieh, K.T.; Urban, P.L.; Chiu, H.Y. Temporal Correlations of Skin and Blood Metabolites with Clinical Outcomes of Biologic Therapy in Psoriasis. J. Appl. Lab. Med. 2020, 5, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.; Choi, Y.; Frankel, A.E.; Koh, A.Y. Unbiased Microbiome and Metabolomic Profiling of Fecal Samples from Patients with Melanoma. In Melanoma: Methods and Protocols; Hargadon, K.M., Ed.; Springer US: New York, NY, USA, 2021; pp. 461–474. ISBN 978-1-0716-1205-7. [Google Scholar]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, H.; Kim, S.H.; Jin, H.; Bae, J.; Choi, H.K. Discovery of potential biomarkers in human melanoma cells with different metastatic potential by metabolic and lipidomic profiling. Sci. Rep. 2017, 7, 8864. [Google Scholar] [CrossRef]
- Zhang, M.; Di Martino, J.S.; Bowman, R.L.; Campbell, N.R.; Baksh, S.C.; Simon-Vermot, T.; Kim, I.S.; Haldeman, P.; Mondal, C.; Yong-Gonzales, V.; et al. Adipocyte-Derived Lipids Mediate Melanoma Progression via FATP Proteins. Cancer Discov. 2018, 8, 1006–1025. [Google Scholar] [CrossRef]
- Gowda, G.A.N.; Raftery, D. NMR-Based Metabolomics. Adv. Exp. Med. Biol. 2021, 1280, 19–37. [Google Scholar] [CrossRef]
- Mann, M.; Hendrickson, R.C.; Pandey, A. Analysis of Proteins and Proteomes by Mass Spectrometry. Annu. Rev. Biochem. 2001, 70, 437–473. [Google Scholar] [CrossRef] [PubMed]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. Nmr spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Bell, J.A.; Ferreira, D.L.S.; Fraser, A.; Soares, A.L.G.; Howe, L.D.; Lawlor, D.A.; Carslake, D.; Smith, G.D.; O’Keeffe, L.M. Sex differences in systemic metabolites at four life stages: Cohort study with repeated metabolomics. BMC Med. 2021, 19, 58. [Google Scholar] [CrossRef]
- Li, J.; Xu, W.; Liang, Y.; Wang, H. The application of skin metabolomics in the context of transdermal drug delivery. Pharmacol. Rep. 2017, 69, 252–259. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Xing, X.; Xing, E.; Wasikowski, R.; Shao, S.; Zeng, C.; Plazyo, O.; Kirma, J.; Jiang, Y.; Billi, A.C.; et al. Noninvasive Tape-Stripping with High-Resolution RNA Profiling Effectively Captures a Preinflammatory State in Nonlesional Psoriatic Skin. J. Investig. Dermatol. 2022, 142, 1587–1596.e2. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Björklund, S.; Jankovskaja, S.; Moore, K.; Delgado-Charro, M.B.; Ruzgas, T.; Guy, R.H.; Engblom, J. Reverse Iontophoretic Extraction of Skin Cancer-Related Biomarkers. Pharmaceutics 2022, 14, 79. [Google Scholar] [CrossRef]
- Lunte, S.M.; Lunte, C.E. Microdialysis sampling for pharmacological studies: HPLC and CE analysis. Adv. Chromatogr. 1996, 36, 383–432. [Google Scholar]
- van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine-needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2020, 53, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Kashaninejad, N.; Munaz, A.; Moghadas, H.; Yadav, S.; Umer, M.; Nguyen, N.T. Microneedle arrays for sampling and sensing skin interstitial fluid. Chemosensors 2021, 9, 83. [Google Scholar] [CrossRef]
- Adebo, O.A.; Oyeyinka, S.A.; Adebiyi, J.A.; Feng, X.; Wilkin, J.D.; Kewuyemi, Y.O.; Abrahams, A.M.; Tugizimana, F. Application of gas chromatography–mass spectrometry (GC-MS)-based metabolomics for the study of fermented cereal and legume foods: A review. Int. J. Food Sci. Technol. 2021, 56, 1514–1534. [Google Scholar] [CrossRef]
- Abaffy, T.; Möller, M.G.; Riemer, D.D.; Milikowski, C.; DeFazio, R.A. Comparative Analysis of Volatile Metabolomics Signals from Melanoma and Benign Skin: A Pilot Study. Metabolomics 2013, 9, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Abaffy, T.; Möller, M.; Riemer, D.D.; Milikowski, C.; Defazio, R.A. A case report—Volatile metabolomic signature of malignant melanoma using matching skin as a control. J. Cancer Sci. Ther. 2011, 3, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Millanta, F.; Parisi, F.; Poli, A.; Sorelli, V.; Abramo, F. Auricular Non-Epithelial Tumors with Solar Elastosis in Cats: A Possible UV-Induced Pathogenesis. Vet. Sci. 2022, 9, 34. [Google Scholar] [CrossRef]
- Abaffy, T.; Duncan, R.; Riemer, D.D.; Tietje, O.; Elgart, G.; Milikowski, C.; DeFazio, R.A. Differential volatile signatures from skin, naevi and melanoma: A novel approach to detect a pathological process. PLoS ONE 2010, 5, e13813. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, M.; Jezierski, T.; Broffman, M.; Hubbard, A.; Turner, K.; Janecki, T. Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integr. Cancer Ther. 2006, 5, 30–39. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. Biosyst. 2012, 8, 470–481. [Google Scholar] [CrossRef]
- Guillarme, D.; Nguyen, D.T.-T.; Rudaz, S.; Veuthey, J.-L. Recent developments in liquid chromatography—Impact on qualitative and quantitative performance. J. Chromatogr. A 2007, 1149, 20–29. [Google Scholar] [CrossRef]
- Taylor, N.J.; Gaynanova, I.; Eschrich, S.A.; Welsh, E.A.; Garrett, T.J.; Beecher, C.; Sharma, R.; Koomen, J.M.; Smalley, K.S.M.; Messina, J.L.; et al. Metabolomics of primary cutaneous melanoma and matched adjacent extratumoral microenvironment. PLoS ONE 2020, 15, e0240849. [Google Scholar] [CrossRef]
- Gunturi, A.; McDermott, D.F. Nivolumab for the treatment of cancer. Expert Opin. Investig. Drugs 2015, 24, 253–260. [Google Scholar] [CrossRef]
- Giannakis, M.; Li, H.; Jin, C.; Gopal, S.; Desai, K.; Horak, C.; Wind-Rotolo, M.; Van Allen, E.M.; Clish, C.; Hodi, F.S.; et al. Metabolomic correlates of response in nivolumab-treated renal cell carcinoma and melanoma patients. J. Clin. Oncol. 2017, 35, 3036. [Google Scholar] [CrossRef]
- Bharti, S.K.; Roy, R. Quantitative 1H NMR spectroscopy. TrAC Trends Anal. Chem. 2012, 35, 5–26. [Google Scholar] [CrossRef]
- Reo, N.V. NMR-BASED METABOLOMICS. Drug Chem. Toxicol. 2002, 25, 375–382. [Google Scholar] [CrossRef]
- Barding, G.A., Jr.; Béni, S.; Fukao, T.; Bailey-Serres, J.; Larive, C.K. Comparison of GC-MS and NMR for Metabolite Profiling of Rice Subjected to Submergence Stress. J. Proteome Res. 2013, 12, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Silva, D.B.; Silva, R.R.; Vêncio, R.Z.N.; Lopes, N.P. Mass spectrometry in plant metabolomics strategies: From analytical platforms to data acquisition and processing. Nat. Prod. Rep. 2014, 31, 784–806. [Google Scholar] [CrossRef] [PubMed]
- Metz, T.O.; Zhang, Q.; Page, J.S.; Shen, Y.; Callister, S.J.; Jacobs, J.M.; Smith, R.D. Future of liquid chromatography–mass spectrometry in metabolic profiling and metabolomic studies for biomarker discovery. Biomark. Med. 2007, 1, 159–185. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, B.; Bauddh, K.; Korstad, J. Bio-oil and biodiesel as biofuels derived from microalgal oil and their characterization by using instrumental techniques. In Algae and Environmental Sustainability; Singh, B., Bauddh, K., Bux, F., Eds.; Springer: New Delhi, India, 2015; pp. 87–96. ISBN 978-81-322-2641-3. [Google Scholar]
- Xiao, Y.; Bi, M.; Guo, H.; Li, M. Multi-omics approaches for biomarker discovery in early ovarian cancer diagnosis. eBioMedicine 2022, 79, 104001. [Google Scholar] [CrossRef]
- Kosmopoulou, M.; Giannopoulou, A.F.; Iliou, A.; Benaki, D.; Panagiotakis, A.; Velentzas, A.D.; Konstantakou, E.G.; Papassideri, I.S.; Mikros, E.; Stravopodis, D.J.; et al. Human melanoma-cell metabolic profiling: Identification of novel biomarkers indicating metastasis. Int. J. Mol. Sci. 2020, 21, 2436. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Griffin, J.L.; Shockcor, J.P. Metabolic profiles of cancer cells. Nat. Rev. Cancer 2004, 4, 551–561. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2010, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Ying, L.; Wang, H.; Xu, G.; Ye, X.; Yang, G. 1H NMR-based metabolomics of skin squamous cell carcinoma and peri-tumoral region tissues. J. Pharm. Biomed. Anal. 2022, 212, 114643. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Dang, C. V Glutaminolysis: Supplying carbon or nitrogen or both for cancer cells? Cell Cycle 2010, 9, 3884–3886. [Google Scholar] [CrossRef]
- Ho, C.; Argáez, C. Mohs Surgery for the Treatment of Skin Cancer: A Review of Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Mukunda, N.; Vallabhaneni, S.; Lefebvre, B.; Fradley, M.G. Cardiotoxicity of Systemic Melanoma Treatments. Curr. Treat. Options Oncol. 2022, 23, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy Resistance Mechanisms in Advanced Skin Cancer. Oncol. Rev. 2017, 11, 326. [Google Scholar] [CrossRef]
- Patrinely, J.R., Jr.; Dewan, A.K.; Johnson, D.B. The Role of Anti-PD-1/PD-L1 in the Treatment of Skin Cancer. BioDrugs 2020, 34, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.P. Role of Biomarkers in the Integrated Management of Melanoma. Dis. Markers 2021, 2021, 6238317. [Google Scholar] [CrossRef]
- Valenti, F.; Falcone, I.; Ungania, S.; Desiderio, F.; Giacomini, P.; Bazzichetto, C.; Conciatori, F.; Gallo, E.; Cognetti, F.; Ciliberto, G.; et al. Precision medicine and melanoma: Multi-omics approaches to monitoring the immunotherapy response. Int. J. Mol. Sci. 2021, 22, 3837. [Google Scholar] [CrossRef]
- Matthews, H.; Hanison, J.; Nirmalan, N. “Omics”-informed drug and biomarker discovery: Opportunities, challenges and future perspectives. Proteomes 2016, 4, 28. [Google Scholar] [CrossRef]
- Ning, M.M.; Lo, E.H. Opportunities and challenges in omics. Transl. Stroke Res. 2010, 1, 233–237. [Google Scholar] [CrossRef] [PubMed]
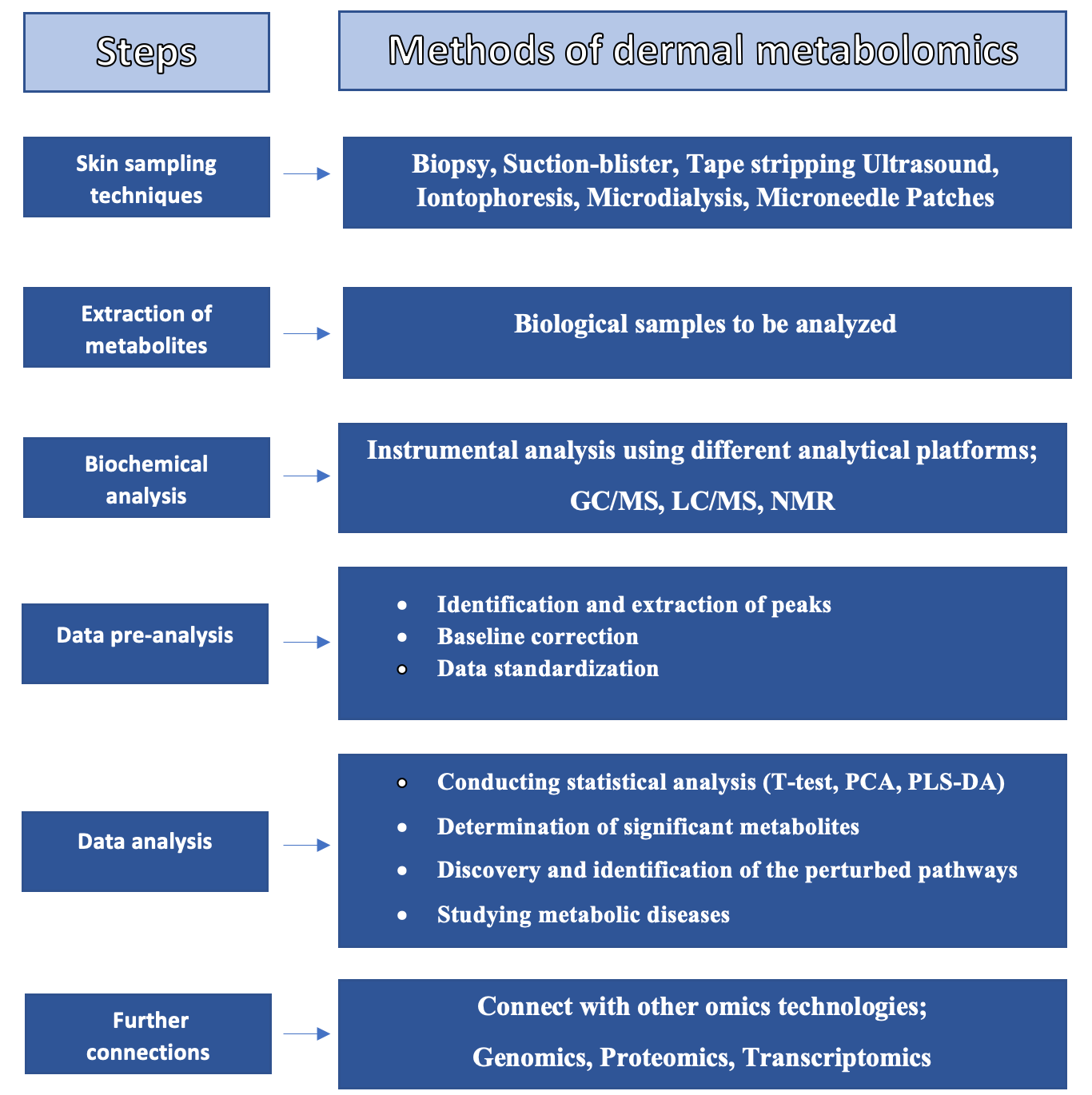
| Sampling Method | Advantages | Disadvantages |
|---|---|---|
| Biopsy [38] |
|
|
| Suction-blister [38] |
|
|
| Tape stripping [39] |
|
|
| Ultrasound [42] |
|
|
| Iontophoresis [40] |
|
|
| Microdialysis [41] |
|
|
| Microneedle Patches [43] |
|
|
| Volatile compounds | %TIC in Melanoma (M) | %TIC in Healthy Skin (S) | RATIO M/S |
|---|---|---|---|
| 1,2-Benzenedicarboxylic acid, diisooctyl ester | 1.03 | 0.61 | 1.68 |
| 1-Hexadecanol | 3.29 | 0.09 | 35.90 |
| 2-Ethylhexyl trans-4-methoxycinnamate | 1.02 | 0.78 | 1.31 |
| Benzene, 1,3,5-trimethyl | 0.12 | 0.03 | 3.41 |
| Dichlorodifluoromethane | 0.03 | 0.01 | 1.80 |
| Dodecane | 0.13 | 0.11 | 1.27 |
| N-Morpholinomethyl-isopropyl-sulfide | 0.32 | 0.23 | 1.37 |
| Nonanal | 0.30 | 0.11 | 2.79 |
| Undecane, 4,7-dimethyl | 0.17 | 0.11 | 1.54 |
| Compounds Found Only in Melanoma |
|---|
| 1,3-Cyclopentadiene, 5-(1-methylethylidene)- |
| 1-Eicosanol |
| 1-Hexadecene |
| 2-Ethoxyethyl acrylate |
| 2-Isopropylamino-4-methylbenzonitrile |
| Adenosine, 5’-amino-5’-deoxy |
| Benzaldehyde, 4-methoxy |
| Bis(2-ethylhexyl) phthalate |
| Cyclohexane, ethyl |
| Decanal |
| Decane, 2-methyl |
| Decane, 4-methyl |
| Ethanol, 2-butoxy |
| Ethylene oxide |
| Formamide |
| Heptane, 2,4-dimethyl |
| Isopropyl Palmitate |
| Nonane, 1-iodo |
| Pentane, 2,3,4-trimethyl |
| Phthalic acid, isobutyl 4-octyl ester |
| Spiro [bicyclo [2.2.1] hept-5-ene-2,1’-cyclopropane] |
| Undecane |
| Analytical Platform | Advantages | Disadvantages | Detectable Molecules |
|---|---|---|---|
| GC-MS [57,58] |
|
|
|
| LC-MS [58,59] |
|
|
|
| NMR [43,60,61] |
|
|
|
| Groups | Metabolomics Signatures |
|---|---|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagyousif, Y.A.; Sharaf, B.M.; Zenati, R.A.; El-Huneidi, W.; Bustanji, Y.; Abu-Gharbieh, E.; Alqudah, M.A.Y.; Giddey, A.D.; Abuhelwa, A.Y.; Alzoubi, K.H.; et al. Skin Cancer Metabolic Profile Assessed by Different Analytical Platforms. Int. J. Mol. Sci. 2023, 24, 1604. https://doi.org/10.3390/ijms24021604
Hagyousif YA, Sharaf BM, Zenati RA, El-Huneidi W, Bustanji Y, Abu-Gharbieh E, Alqudah MAY, Giddey AD, Abuhelwa AY, Alzoubi KH, et al. Skin Cancer Metabolic Profile Assessed by Different Analytical Platforms. International Journal of Molecular Sciences. 2023; 24(2):1604. https://doi.org/10.3390/ijms24021604
Chicago/Turabian StyleHagyousif, Yousra A., Basma M. Sharaf, Ruba A. Zenati, Waseem El-Huneidi, Yasser Bustanji, Eman Abu-Gharbieh, Mohammad A. Y. Alqudah, Alexander D. Giddey, Ahmad Y. Abuhelwa, Karem H. Alzoubi, and et al. 2023. "Skin Cancer Metabolic Profile Assessed by Different Analytical Platforms" International Journal of Molecular Sciences 24, no. 2: 1604. https://doi.org/10.3390/ijms24021604
APA StyleHagyousif, Y. A., Sharaf, B. M., Zenati, R. A., El-Huneidi, W., Bustanji, Y., Abu-Gharbieh, E., Alqudah, M. A. Y., Giddey, A. D., Abuhelwa, A. Y., Alzoubi, K. H., Soares, N. C., & Semreen, M. H. (2023). Skin Cancer Metabolic Profile Assessed by Different Analytical Platforms. International Journal of Molecular Sciences, 24(2), 1604. https://doi.org/10.3390/ijms24021604






