Pseudomonas aeruginosa H3-T6SS Combats H2O2 Stress by Diminishing the Amount of Intracellular Unincorporated Iron in a Dps-Dependent Manner and Inhibiting the Synthesis of PQS
Abstract
1. Introduction
2. Results
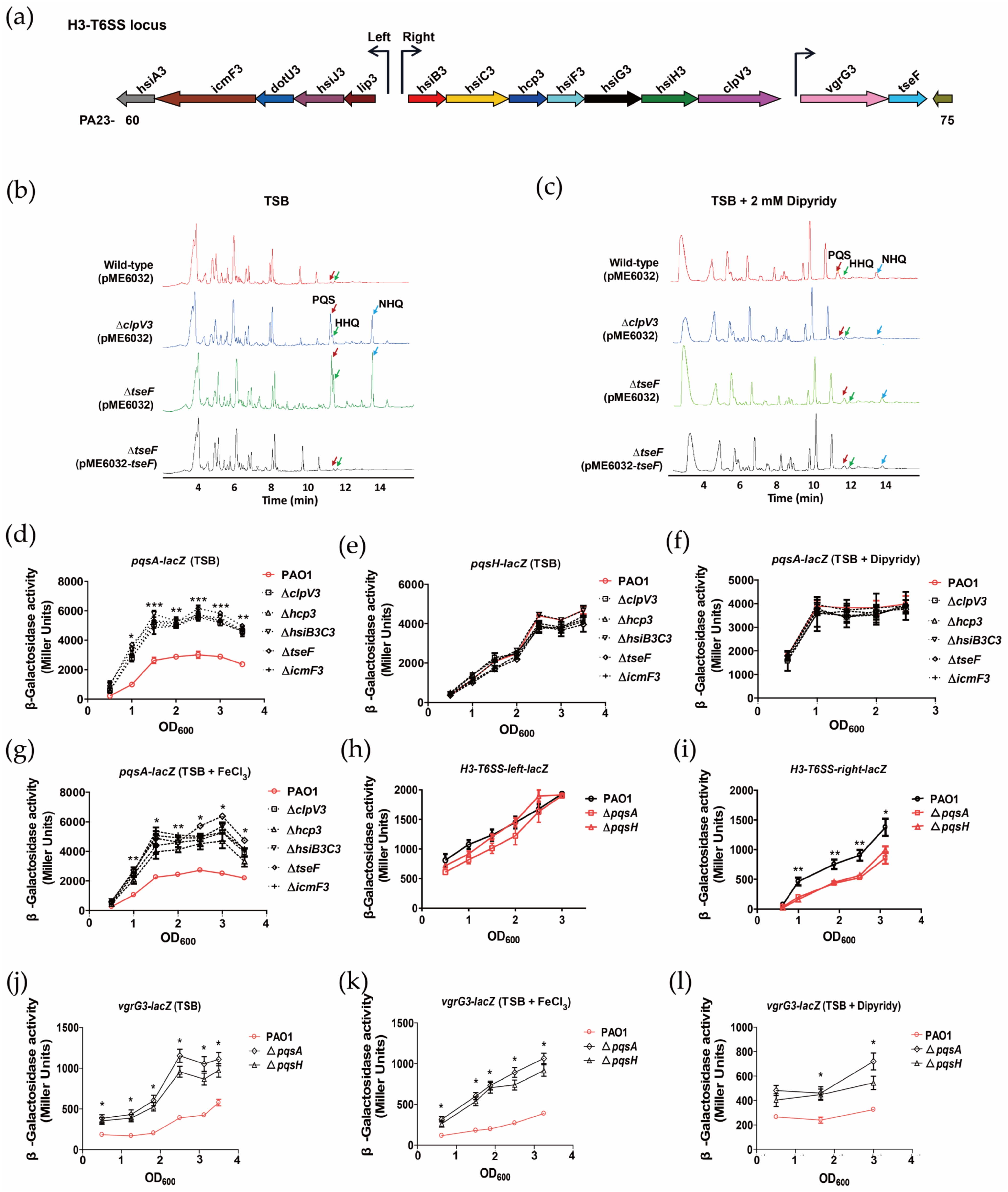
2.1. H3-T6SS Inhibits the Synthesis of PQS
2.2. PQS Differentially Regulates the Expression of H3-T6SS Structural Genes and tseF
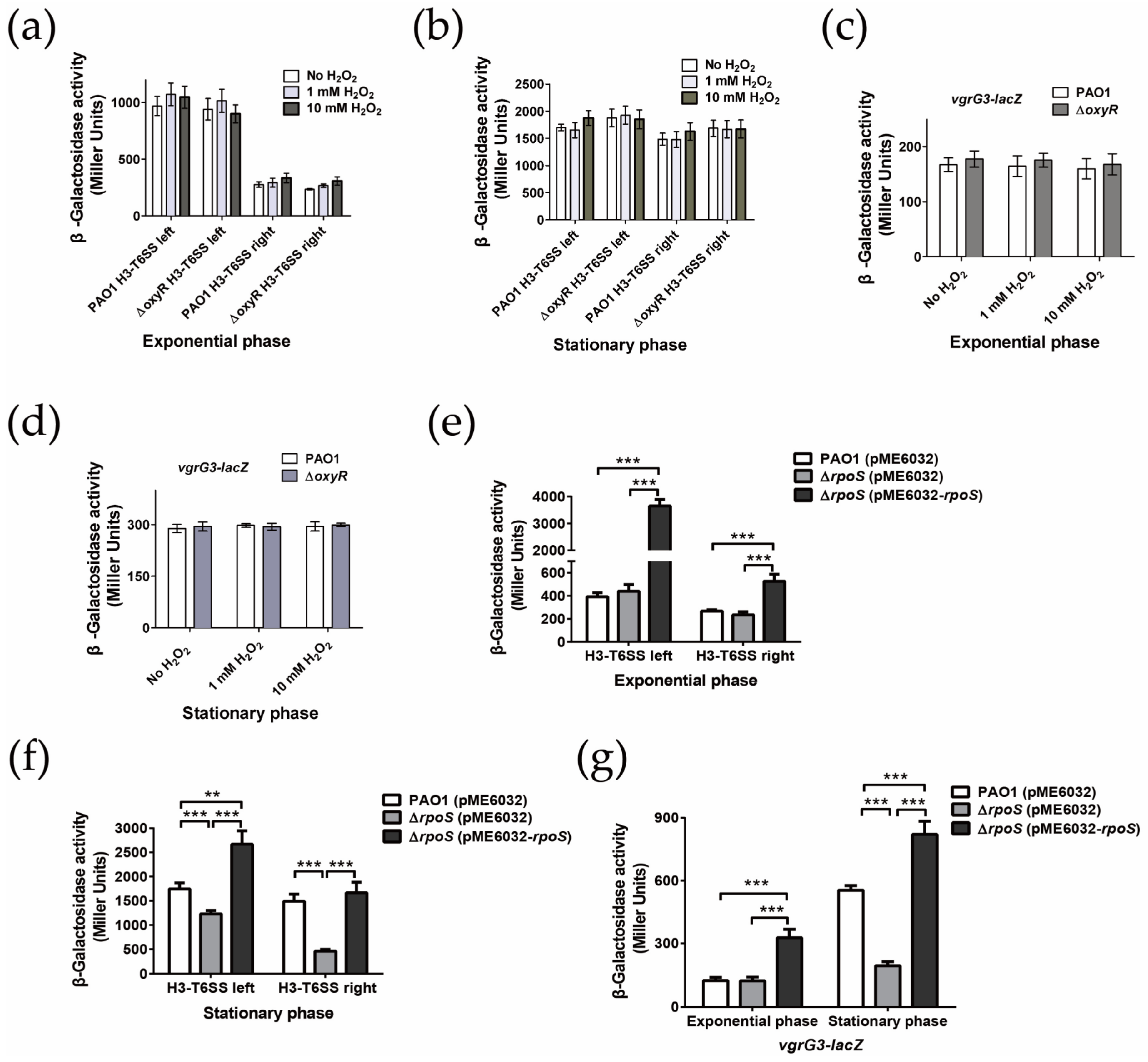
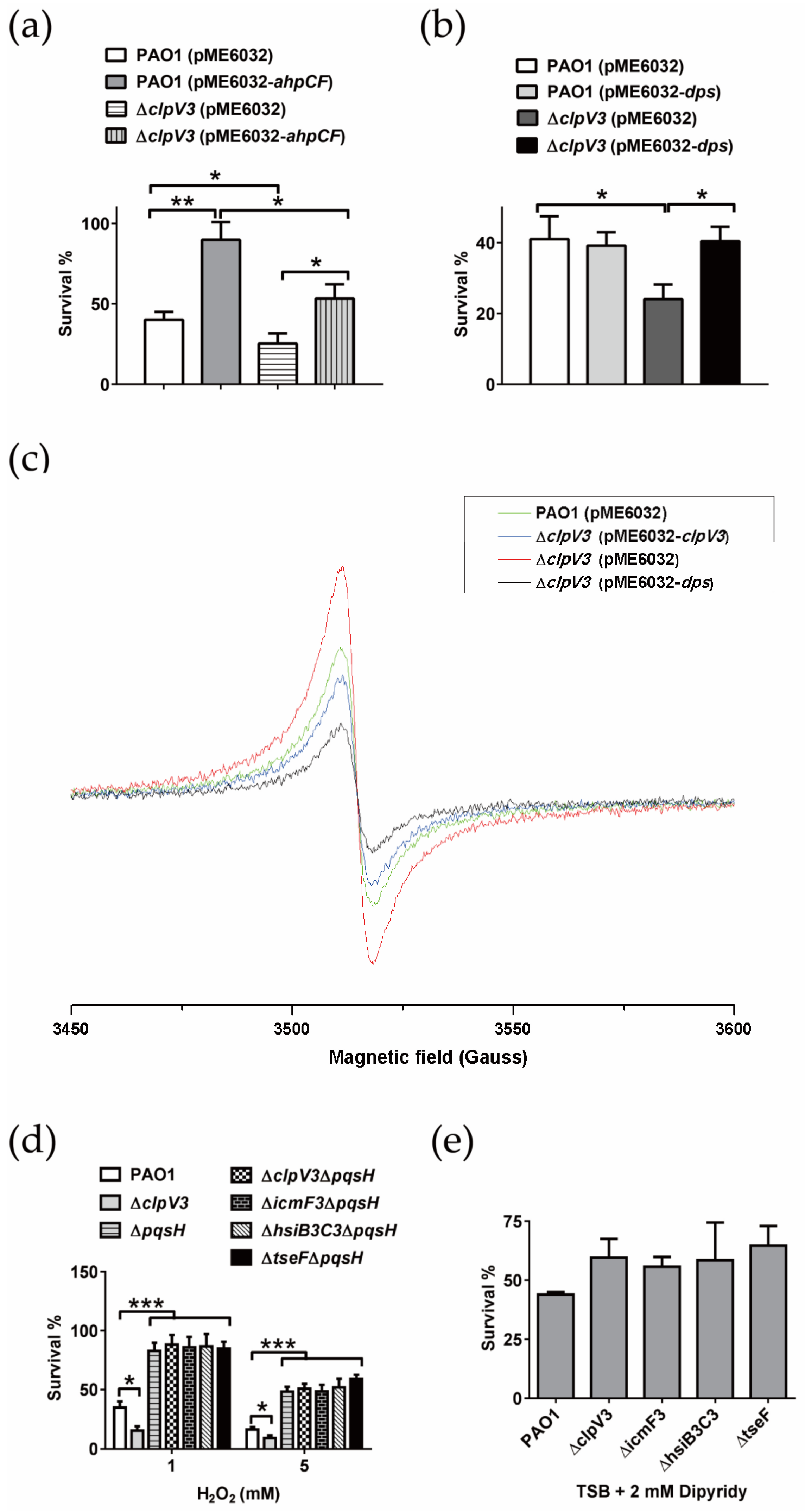
2.3. H3-T6SS Plays an Important Role in Combating Oxidative Stress Caused by H2O2
2.4. H3-T6SS Is Positively Regulated by RpoS
2.5. H3-T6SS Affects the Expression of Various Proteins Related to Oxidative Stress
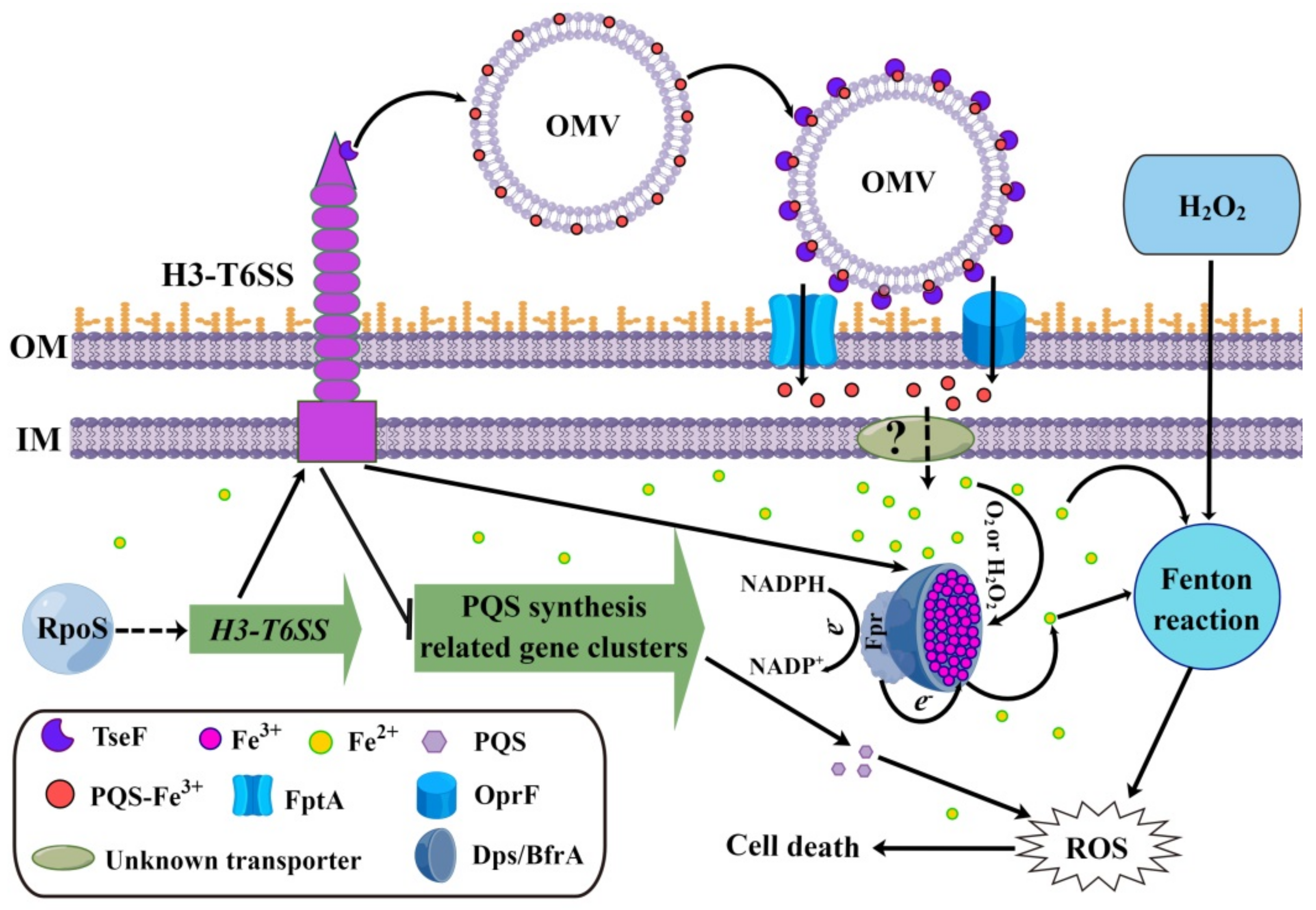
2.6. P. aeruginosa H3-T6SS Combats H2O2 Stress in Two Ways
2.6.1. H3-T6SS Combats H2O2 Stress by Reducing Intracellular Free Fe2+
2.6.2. H3-T6SS Combats H2O2 Stress by Inhibiting the Synthesis of PQS
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Plasmid Construction
4.3. Construction of Chromosomal Fusion Reporter Strains
4.4. β-Galactosidase Assays
4.5. Liquid Chromatography-Mass Spectrometry Analysis
4.6. Sensitivity Assays
4.7. Intracellular ROS Detection
4.8. Proteomic Analysis
4.9. Electron Paramagnetic Resonance Spectroscopy Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mielko, K.A.; Jabłoński, S.J.; Milczewska, J.; Sands, D.; Łukaszewicz, M.; Młynarz, P. Metabolomic studies of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2019, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hoggarth, A.; Weaver, A.; Pu, Q.; Huang, T.; Schettler, J.; Chen, F.; Yuan, X.; Wu, M. Mechanistic research holds promise for bacterial vaccines and phage therapies for Pseudomonas aeruginosa. Drug Des. Dev. Ther. 2019, 13, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Oh, E.; Kim, J.; Jeon, B. Regulation of oxidative stress resistance in Campylobacter jejuni, a microaerophilic foodborne pathogen. Front. Microbiol. 2015, 6, 751. [Google Scholar] [CrossRef] [PubMed]
- Green, E.R.; Clark, S.; Crimmins, G.T.; Mack, M.; Kumamoto, C.A.; Mecsas, J. Fis is essential for Yersinia pseudotuberculosis virulence and protects against reactive oxygen species produced by phagocytic cells during infection. PLoS Pathog. 2016, 12, e1005898. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, P.; Wei, Q.; Andrews, S.C.; Vinckx, T. Iron homeostasis and management of oxidative stress response in bacteria. Metallomics 2011, 3, 540–549. [Google Scholar] [CrossRef]
- Lewis, J.; Soto, E. Gene expression of putative type VI secretion system (T6SS) genes in the emergent fish pathogen Francisella noatunensis subsp. orientalis in different physiochemical conditions. BMC Microbiol. 2019, 19, 21. [Google Scholar] [CrossRef]
- Wan, B.; Zhang, Q.; Ni, J.; Li, S.; Wen, D.; Li, J.; Xiao, H.; He, P.; Ou, H.Y.; Tao, J.; et al. Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog. 2017, 13, e1006246. [Google Scholar] [CrossRef]
- Liaw, J.; Hong, G.; Davies, C.; Elmi, A.; Sima, F.; Stratakos, A.; Stef, L.; Pet, I.; Hachani, A.; Corcionivoschi, N.; et al. The Campylobacter jejuni type VI secretion system enhances the oxidative stress response and host colonization. Front. Microbiol. 2019, 10, 2864. [Google Scholar] [CrossRef]
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 2011, 475, 343–347. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wang, B.; Chen, L.; Zhao, Z.; Waterfield, N.R.; Yang, G.; Jin, Q. The Pseudomonas aeruginosa type VI secretion PGAP1-like effector induces host autophagy by activating endoplasmic reticulum stress. Cell Rep. 2016, 16, 1502–1509. [Google Scholar] [CrossRef]
- DeShazer, D. A novel contact-independent T6SS that maintains redox homeostasis via Zn2+ and Mn2+ acquisition is conserved in the Burkholderia pseudomallei complex. Microbiol. Res. 2019, 226, 48–54. [Google Scholar] [CrossRef]
- Chen, L.; Zou, Y.; Kronfl, A.A.; Wu, Y. Type VI secretion system of Pseudomonas aeruginosa is associated with biofilm formation but not environmental adaptation. Microbiologyopen 2020, 9, e991. [Google Scholar] [CrossRef]
- Si, M.; Zhao, C.; Burkinshaw, B.; Zhang, B.; Wei, D.; Wang, Y.; Dong, T.G.; Shen, X. Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl. Acad. Sci. USA 2017, 114, E2233–E2242. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Chen, G.; Pu, Q.; Liu, Q.; Zhang, Y.; Xu, L.; Wu, M.; Liang, H. A Pseudomonas aeruginosa type VI secretion system regulated by CueR facilitates copper acquisition. PLoS Pathog. 2019, 15, e1008198. [Google Scholar] [CrossRef]
- Wood, T.E.; Howard, S.A.; Forster, A.; Nolan, L.M.; Manoli, E.; Bullen, N.P.; Yau, H.C.L.; Hachani, A.; Hayward, R.D.; Whitney, J.C.; et al. The Pseudomonas aeruginosa T6SS delivers a periplasmic toxin that disrupts bacterial cell morphology. Cell Rep. 2019, 29, 187–201.e7. [Google Scholar] [CrossRef]
- Zhang, L.; Hinz, A.J.; Nadeau, J.P.; Mah, T.F. Pseudomonas aeruginosa tssC1 links type VI secretion and biofilm-specific antibiotic resistance. J. Bacteriol. 2011, 193, 5510–5513. [Google Scholar] [CrossRef]
- Wang, T.; Du, X.; Ji, L.; Han, Y.; Dang, J.; Wen, J.; Wang, Y.; Pu, Q.; Wu, M.; Liang, H. Pseudomonas aeruginosa T6SS-mediated molybdate transport contributes to bacterial competition during anaerobiosis. Cell Rep. 2021, 35, 108957. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Cui, R.; Zhang, Z.; Chen, K.; Li, M.; Hua, Y.; Gu, H.; Xu, L.; Wang, Y.; et al. HpaR, the repressor of aromatic compound metabolism, positively regulates the expression of T6SS4 to resist oxidative stress in Yersinia pseudotuberculosis. Front. Microbiol. 2020, 11, 705. [Google Scholar] [CrossRef]
- Weber, B.; Hasic, M.; Chen, C.; Wai, S.N.; Milton, D.L. Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ. Microbiol. 2009, 11, 3018–3028. [Google Scholar] [CrossRef]
- Li, J.; Xie, L.; Qian, S.; Tang, Y.; Shen, M.; Li, S.; Wang, J.; Xiong, L.; Lu, J.; Zhong, W. Type VI secretion system facilitates fitness, homeostasis, and competitive advantages for environmental adaptability and efficient nicotine biodegradation. Appl. Environ. Microbiol. 2021, 87, e03113–e03120. [Google Scholar] [CrossRef]
- Qin, L.; Wang, X.; Gao, Y.; Bi, K.; Wang, W. Roles of EvpP in Edwardsiella piscicida-macrophage interactions. Front. Cell. Infect. Microbiol. 2020, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Si, M.; Song, Y.; Zhu, W.; Gao, F.; Wang, Y.; Zhang, L.; Zhang, W.; Wei, G.; Luo, Z.Q.; et al. Type VI secretion system transports Zn2+ to combat multiple stresses and host immunity. PLoS Pathog. 2015, 11, e1005020. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Wang, Y.; Zhang, B.; Zhao, C.; Kang, Y.; Bai, H.; Wei, D.; Zhu, L.; Zhang, L.; Dong, T.G.; et al. The type VI secretion system engages a redox-regulated dual-functional heme transporter for zinc acquisition. Cell Rep. 2017, 20, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordonez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.G.; Berni, B.; Bleves, S. The T6SSs of Pseudomonas aeruginosa strain PAO1 and their effectors: Beyond bacterial-cell targeting. Front. Cell. Infect. Microbiol. 2016, 6, 61. [Google Scholar] [CrossRef]
- Nolan, L.M.; Cain, A.K.; Manoli, E.; Sainz-Polo, M.A.; Dougan, G.; Mavridou, D.A.I.; Albesa-Jové, D.; Parkhill, J.; Filloux, A. Discovery of a Pseudomonas aeruginosa type VI secretion system toxin targeting bacterial protein synthesis using a global genomics approach. bioRxiv 2019. [Google Scholar] [CrossRef]
- Yang, X.Y.; Li, Z.Q.; Gao, Z.Q.; Wang, W.J.; Geng, Z.; Xu, J.H.; She, Z.; Dong, Y.H. Structural and SAXS analysis of Tle5-Tli5 complex reveals a novel inhibition mechanism of H2-T6SS in Pseudomonas aeruginosa. Protein Sci. 2017, 26, 2083–2091. [Google Scholar] [CrossRef]
- Sana, T.G.; Baumann, C.; Merdes, A.; Soscia, C.; Rattei, T.; Hachani, A.; Jones, C.; Bennett, K.L.; Filloux, A.; Superti-Furga, G.; et al. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. mBio 2015, 6, e00712. [Google Scholar] [CrossRef]
- Sana, T.G.; Hachani, A.; Bucior, I.; Soscia, C.; Garvis, S.; Termine, E.; Engel, J.; Filloux, A.; Bleves, S. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and Fur and modulates internalization in epithelial cells. J. Biol. Chem. 2012, 287, 27095–27105. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, W.; Cheng, J.; Yang, X.; Zhu, K.; Wang, Y.; Wei, G.; Qian, P.Y.; Luo, Z.Q.; Shen, X. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 2017, 8, 14888. [Google Scholar] [CrossRef]
- Sams, T.; Baker, Y.; Hodgkinson, J.; Gross, J.; Spring, D.; Welch, M. The Pseudomonas Quinolone Signal (PQS). Isr. J. Chem. 2016, 56, 282–294. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, J.; Wang, Y.; Shen, X. The Pseudomonas Quinolone Signal (PQS): Not just for quorum sensing anymore. Front. Cell. Infect. Microbiol. 2018, 8, 230. [Google Scholar] [CrossRef]
- Fito-Boncompte, L.; Chapalain, A.; Bouffartigues, E.; Chaker, H.; Lesouhaitier, O.; Gicquel, G.; Bazire, A.; Madi, A.; Connil, N.; Veron, W.; et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect. Immun. 2011, 79, 1176–1186. [Google Scholar] [CrossRef]
- Lesic, B.; Starkey, M.; He, J.; Hazan, R.; Rahme, L.G. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology 2009, 155, 2845–2855. [Google Scholar] [CrossRef]
- Déziel, E.; Lépine, F.; Milot, S.; He, J.; Mindrinos, M.N.; Tompkins, R.G.; Rahme, L.G. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2004, 101, 1339–1344. [Google Scholar] [CrossRef]
- Bredenbruch, F.; Geffers, R.; Nimtz, M.; Buer, J.; Haussler, S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ. Microbiol. 2006, 8, 1318–1329. [Google Scholar] [CrossRef]
- Karlsson, M.; Kurz, T.; Brunk, U.T.; Nilsson, S.E.; Frennesson, C.I. What does the commonly used DCF test for oxidative stress really show? Biochem. J. 2010, 428, 183–190. [Google Scholar] [CrossRef]
- Grant, S.S.; Kaufmann, B.B.; Chand, N.S.; Haseley, N.; Hung, D.T. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc. Natl. Acad. Sci. USA 2012, 109, 12147–12152. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Wei, Q.; Minh, P.N.; Dotsch, A.; Hildebrand, F.; Panmanee, W.; Elfarash, A.; Schulz, S.; Plaisance, S.; Charlier, D.; Hassett, D.; et al. Global regulation of gene expression by OxyR in an important human opportunistic pathogen. Nucleic Acids Res. 2012, 40, 4320–4333. [Google Scholar] [CrossRef]
- Schuster, M.; Hawkins, A.C.; Harwood, C.S.; Greenberg, E.P. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol. Microbiol. 2004, 51, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Vattanaviboon, P.; Tanboon, W.; Mongkolsuk, S. Physiological and expression analyses of Agrobacterium tumefaciens trxA, encoding thioredoxin. J. Bacteriol. 2007, 189, 6477–6481. [Google Scholar] [CrossRef] [PubMed]
- Small, D.A.; Chang, W.; Toghrol, F.; Bentley, W.E. Comparative global transcription analysis of sodium hypochlorite, peracetic acid, and hydrogen peroxide on Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2007, 76, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, P.; Topfer, T.; Buer, J.; Tummler, B. Genome-wide transcriptional profiling of the steady-state response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 2005, 187, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Small, D.A.; Toghrol, F.; Bentley, W.E. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genom. 2005, 6, 115. [Google Scholar] [CrossRef]
- Palma, M.; DeLuca, D.; Worgall, S.; Quadri, L.E. Transcriptome analysis of the response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 2004, 186, 248–252. [Google Scholar] [CrossRef]
- Rae, J.L.; Cutfield, J.F.; Lamont, I.L. Sequences and expression of pyruvate dehydrogenase genes from Pseudomonas aeruginosa. J. Bacteriol. 1997, 179, 3561–3571. [Google Scholar] [CrossRef]
- Mongkolsuk, S.; Loprasert, S.; Whangsuk, W.; Fuangthong, M.; Atichartpongkun, S. Characterization of transcription organization and analysis of unique expression patterns of an alkyl hydroperoxide reductase C gene (ahpC) and the peroxide regulator operon ahpF-oxyR-orfX from Xanthomonas campestris pv. phaseoli. J. Bacteriol. 1997, 179, 3950–3955. [Google Scholar] [CrossRef]
- Velterop, J.S.; Sellink, E.; Meulenberg, J.J.; David, S.; Bulder, I.; Postma, P.W. Synthesis of pyrroloquinoline quinone in vivo and in vitro and detection of an intermediate in the biosynthetic pathway. J. Bacteriol. 1995, 177, 5088–5098. [Google Scholar] [CrossRef]
- Hare, N.J.; Scott, N.E.; Shin, E.H.; Connolly, A.M.; Larsen, M.R.; Palmisano, G.; Cordwell, S.J. Proteomics of the oxidative stress response induced by hydrogen peroxide and paraquat reveals a novel AhpC-like protein in Pseudomonas aeruginosa. Proteomics 2011, 11, 3056–3069. [Google Scholar] [CrossRef]
- Yao, H.; Jepkorir, G.; Lovell, S.; Nama, P.V.; Weeratunga, S.; Battaile, K.P.; Rivera, M. Two distinct ferritin-like molecules in Pseudomonas aeruginosa: The product of the bfrA gene is a bacterial ferritin (FtnA) and not a bacterioferritin (Bfr). Biochemistry 2011, 50, 5236–5248. [Google Scholar] [CrossRef]
- Haussler, S.; Becker, T. The pseudomonas quinolone signal (PQS) balances life and death in Pseudomonas aeruginosa populations. PLoS Pathog. 2008, 4, e1000166. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Wang, D.; Wei, Z.; Hao, X.; Wang, Z.; Li, T.; Zhang, L.; Lu, Z.; Long, M.; et al. T6SS secretes an LPS-binding effector to recruit OMVs for exploitative competition and horizontal gene transfer. ISME J. 2022, 16, 500–510. [Google Scholar] [CrossRef]
- Ho, B.T.; Fu, Y.; Dong, T.G.; Mekalanos, J.J. Vibrio cholerae type 6 secretion system effector trafficking in target bacterial cells. Proc. Natl. Acad. Sci. USA 2017, 114, 9427–9432. [Google Scholar] [CrossRef]
- Lin, J.; Xu, L.; Yang, J.; Wang, Z.; Shen, X. Beyond dueling: Roles of the type VI secretion system in microbiome modulation, pathogenesis and stress resistance. Stress Biol. 2021, 1, 11. [Google Scholar] [CrossRef]
- Hu, L.; Yu, F.; Liu, M.; Chen, J.; Zong, B.; Zhang, Y.; Chen, T.; Wang, C.; Zhang, T.; Zhang, J.; et al. RcsB-dependent regulation of type VI secretion system in porcine extra-intestinal pathogenic Escherichia coli. Gene 2021, 768, 145289. [Google Scholar] [CrossRef]
- Ishikawa, T.; Mizunoe, Y.; Kawabata, S.; Takade, A.; Harada, M.; Wai, S.N.; Yoshida, S. The iron-binding protein Dps confers hydrogen peroxide stress resistance to Campylobacter jejuni. J. Bacteriol. 2003, 185, 1010–1017. [Google Scholar] [CrossRef]
- Ochsner, U.A.; Wilderman, P.J.; Vasil, A.I.; Vasil, M.L. GeneChip expression analysis of the iron starvation response in Pseudomonas aeruginosa: Identification of novel pyoverdine biosynthesis genes. Mol. Microbiol. 2002, 45, 1277–1287. [Google Scholar] [CrossRef]
- Palma, M.; Worgall, S.; Quadri, L.E. Transcriptome analysis of the Pseudomonas aeruginosa response to iron. Arch. Microbiol. 2003, 180, 374–379. [Google Scholar] [CrossRef]
- Wilderman, P.J.; Sowa, N.A.; FitzGerald, D.J.; FitzGerald, P.C.; Gottesman, S.; Ochsner, U.A.; Vasil, M.L. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc. Natl. Acad. Sci. USA 2004, 101, 9792–9797. [Google Scholar] [CrossRef]
- Reindel, S.; Schmidt, C.L.; Anemuller, S.; Matzanke, B.F. Expression and regulation pattern of ferritin-like DpsA in the archaeon Halobacterium salinarum. Biometals 2006, 19, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Cheng, J.; Chen, K.; Guo, C.; Zhang, W.; Yang, X.; Ding, W.; Ma, L.; Wang, Y.; Shen, X. The icmF3 locus is involved in multiple adaptation- and virulence-related characteristics in Pseudomonas aeruginosa PAO1. Front. Cell. Infect. Microbiol. 2015, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Becher, A.; Schweizer, H.P. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques 2000, 29, 948–950, 952. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.T.; Kutchma, A.J.; Becher, A.; Schweizer, H.P. Integration-proficient plasmids for Pseudomonas aeruginosa: Site-specific integration and use for engineering of reporter and expression strains. Plasmid 2000, 43, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, A.; Roche, B.; Schalk, I.J. Iron acquisition in Pseudomonas aeruginosa by the siderophore pyoverdine: An intricate interacting network including periplasmic and membrane proteins. Sci. Rep. 2020, 10, 120. [Google Scholar] [CrossRef]
- Khakimova, M.; Ahlgren, H.G.; Harrison, J.J.; English, A.M.; Nguyen, D. The stringent response controls catalases in Pseudomonas aeruginosa and is required for hydrogen peroxide and antibiotic tolerance. J. Bacteriol. 2013, 195, 2011–2020. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Woodmansee, A.N.; Imlay, J.A. Quatitation of intracellular free iron by electron paramagnetic resonance spectroscopy. Methods Enzymol. 2002, 349, 3–9. [Google Scholar]
- Macomber, L.; Rensing, C.; Imlay, J.A. Intracellular copper does not catalyze the formation of oxidative DNA damage in Escherichia coli. J. Bacteriol. 2007, 189, 1616–1626. [Google Scholar] [CrossRef]
- Dennis, J.J.; Zylstra, G.J. Plasposons: Modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 1998, 64, 2710–2715. [Google Scholar] [CrossRef]
- Schafer, A.; Tauch, A.; Jager, W.; Kalinowski, J.; Thierbach, G.; Puhler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Heeb, S.; Blumer, C.; Haas, D. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 2002, 184, 1046–1056. [Google Scholar] [CrossRef]
- Hoang, T.T.; Karkhoff-Schweizer, R.R.; Kutchma, A.J.; Schweizer, H.P. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: Application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 1998, 212, 77–86. [Google Scholar] [CrossRef]

| PA No. a | Gene | Fold Change b | p-Values | Protein Description c |
|---|---|---|---|---|
| PQS biosynthesis | ||||
| PA0999 | pqsD | 29.6 | 2.23 × 10−7 | 3-oxoacyl-ACP synthase |
| PA2080 | kynU | 10.9 | 7.36 × 10−3 | Kynureninase KynU |
| Oxidative processes and oxidative stress | ||||
| PA0140 | ahpF | 10.9 | 7.36 × 10−3 | Alkyl hydroperoxide reductase |
| PA3529 | 10.9 | 7.36 × 10−3 | Peroxidase | |
| PA5240 | trxA | 3.4 | 8.51 × 10−4 | Thioredoxin |
| General stress response | ||||
| PA4385 | groEL | 15.6 | 5.92 × 10−4 | Molecular chaperone GroEL |
| PA4386 | groES | 29.6 | 2.23 × 10−7 | Co-chaperonin GroES |
| PA4761 | dnaK | 2.3 | 1.68 × 10−3 | Molecular chaperone DnaK |
| PA0962 | dps | −21.8 | 8.05 × 10−7 | DNA-binding stress protein |
| Genes of the oxidative stress response d | ||||
| PA0102 | 15.6 | 5.92 × 10−4 | Carbonic anhydrase | |
| PA5015 | aceE | 3.0 | 5.12 × 10−5 | Pyruvate dehydrogenase subunit E1 |
| PA1973 | pqqF | 15.6 | 5.92 × 10−4 | Pyrroloquinoline quinone biosynthesis protein F |
| PA4131 | 8.6 | 2.44 × 10−6 | Iron-sulfur protein | |
| PA3820 | secF | 15.6 | 5.92 × 10−4 | Preprotein translocase subunit SecF |
| PA3821 | secD | 5.1 | 2.19 × 10−3 | Preprotein translocase subunit SecD |
| PA5128 | secB | 10.9 | 7.36 × 10−3 | Preprotein translocase subunit SecB |
| PA1583 | sdhA | −2.3 | 4.72 × 10−2 | Succinate dehydrogenase flavoprotein subunit |
| PA1880 | −2.6 | 2.32 × 10−2 | Oxidoreductase | |
| PA4694 | ilvC | −3.9 | 7.04 × 10−6 | Ketol-acid reductoisomerase |
| Iron sequestration | ||||
| PA3397 | fpr | 5.1 | 2.19 × 10−3 | Ferredoxin-NADP reductase |
| PA4235 | bfrA | −1.6 | 2.01 × 10−2 | Bacterioferritin |
| PA0962 | dps | −21.8 | 8.05 × 10−7 | DNA-binding stress protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Yang, J.; Cheng, J.; Zhang, W.; Yang, X.; Ding, W.; Zhang, H.; Wang, Y.; Shen, X. Pseudomonas aeruginosa H3-T6SS Combats H2O2 Stress by Diminishing the Amount of Intracellular Unincorporated Iron in a Dps-Dependent Manner and Inhibiting the Synthesis of PQS. Int. J. Mol. Sci. 2023, 24, 1614. https://doi.org/10.3390/ijms24021614
Lin J, Yang J, Cheng J, Zhang W, Yang X, Ding W, Zhang H, Wang Y, Shen X. Pseudomonas aeruginosa H3-T6SS Combats H2O2 Stress by Diminishing the Amount of Intracellular Unincorporated Iron in a Dps-Dependent Manner and Inhibiting the Synthesis of PQS. International Journal of Molecular Sciences. 2023; 24(2):1614. https://doi.org/10.3390/ijms24021614
Chicago/Turabian StyleLin, Jinshui, Jianshe Yang, Juanli Cheng, Weipeng Zhang, Xu Yang, Wei Ding, Heng Zhang, Yao Wang, and Xihui Shen. 2023. "Pseudomonas aeruginosa H3-T6SS Combats H2O2 Stress by Diminishing the Amount of Intracellular Unincorporated Iron in a Dps-Dependent Manner and Inhibiting the Synthesis of PQS" International Journal of Molecular Sciences 24, no. 2: 1614. https://doi.org/10.3390/ijms24021614
APA StyleLin, J., Yang, J., Cheng, J., Zhang, W., Yang, X., Ding, W., Zhang, H., Wang, Y., & Shen, X. (2023). Pseudomonas aeruginosa H3-T6SS Combats H2O2 Stress by Diminishing the Amount of Intracellular Unincorporated Iron in a Dps-Dependent Manner and Inhibiting the Synthesis of PQS. International Journal of Molecular Sciences, 24(2), 1614. https://doi.org/10.3390/ijms24021614






