Cytoplasmic Expression of TP53INP2 Modulated by Demethylase FTO and Mutant NPM1 Promotes Autophagy in Leukemia Cells
Abstract
1. Introduction
2. Results
2.1. Identification of the Autophagy-Related Genes in NPM1-Mutated AML
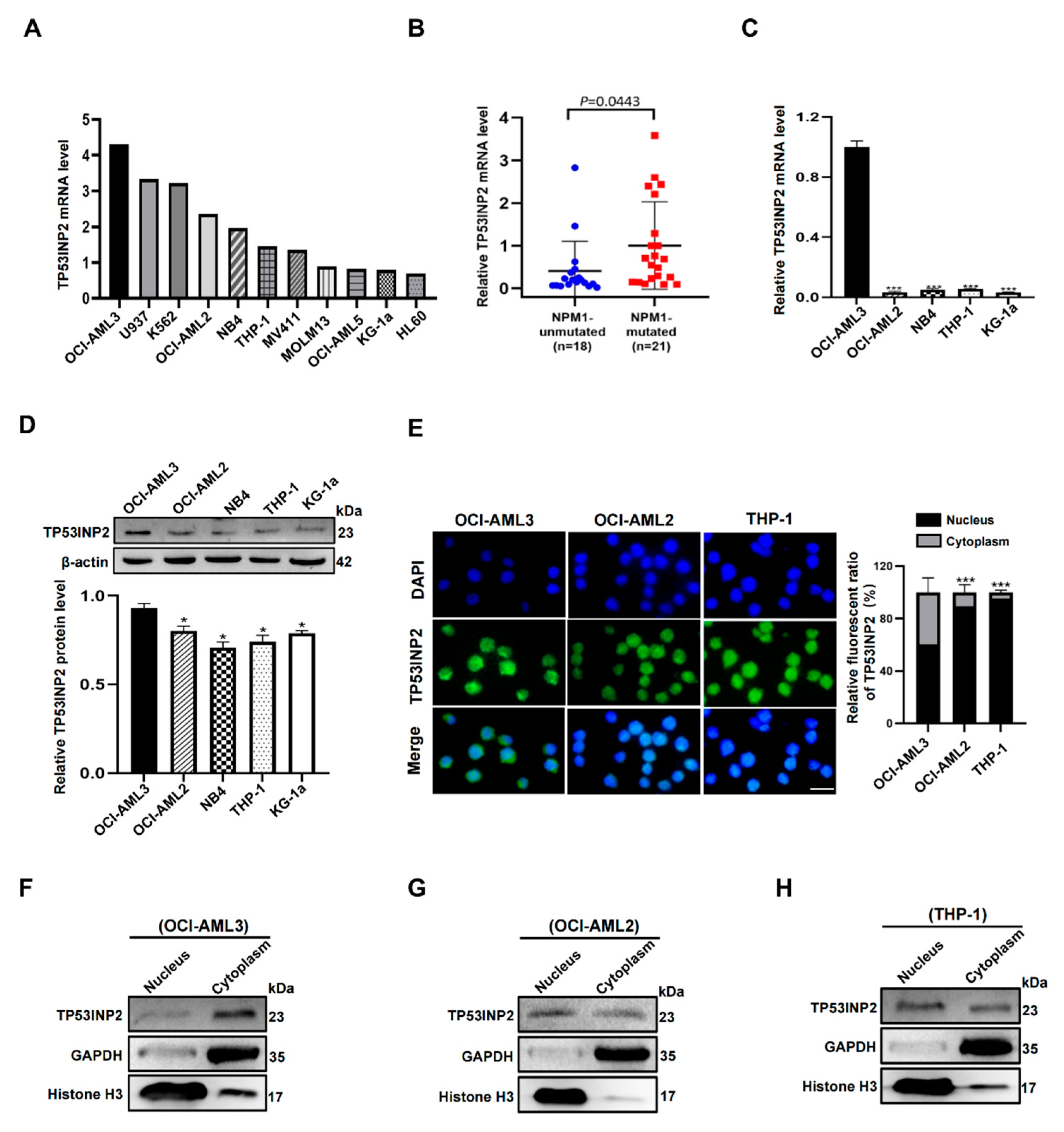
2.2. TP53INP2 Is Aberrantly Expressed in NPM1-Mutated Leukemia Cells
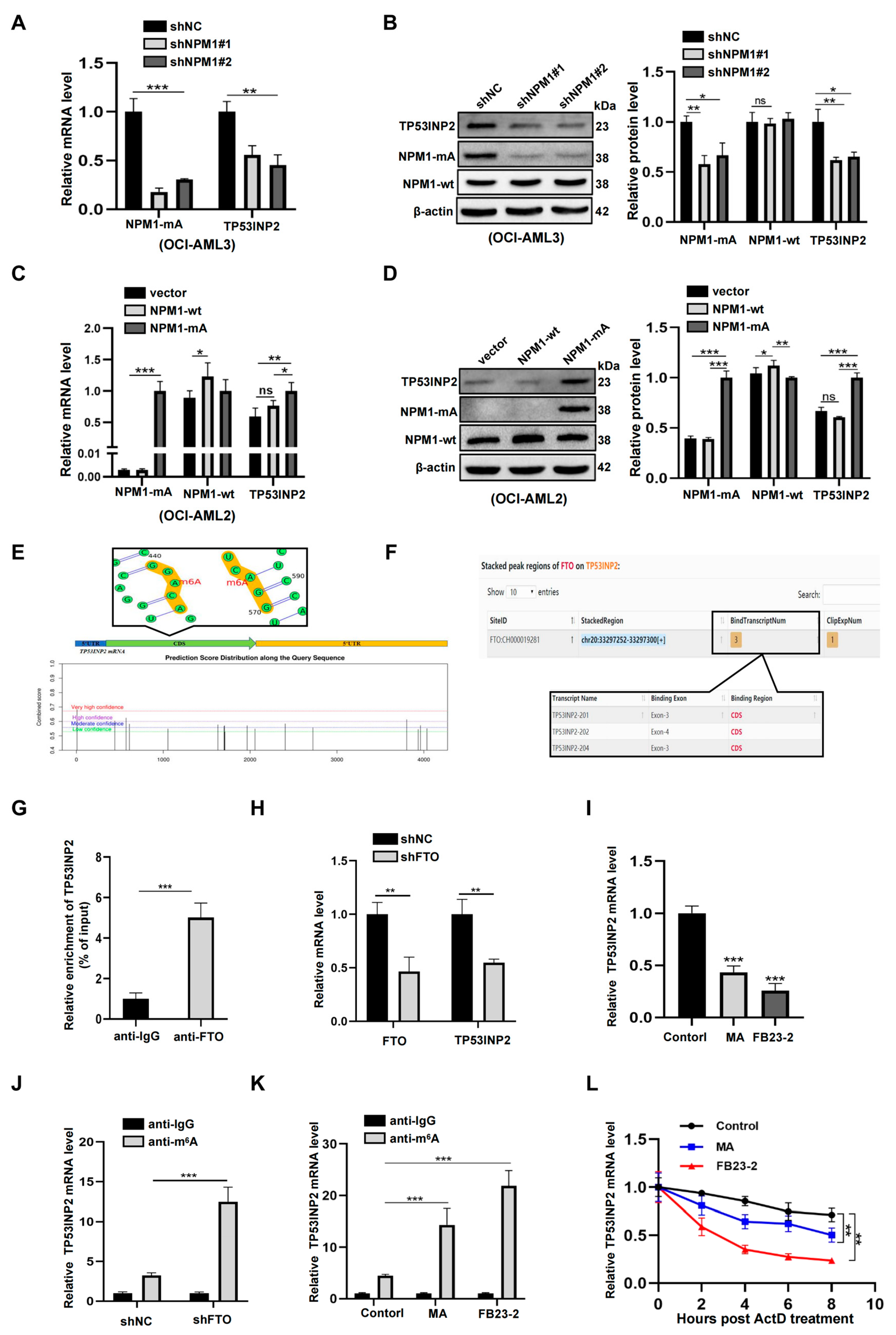
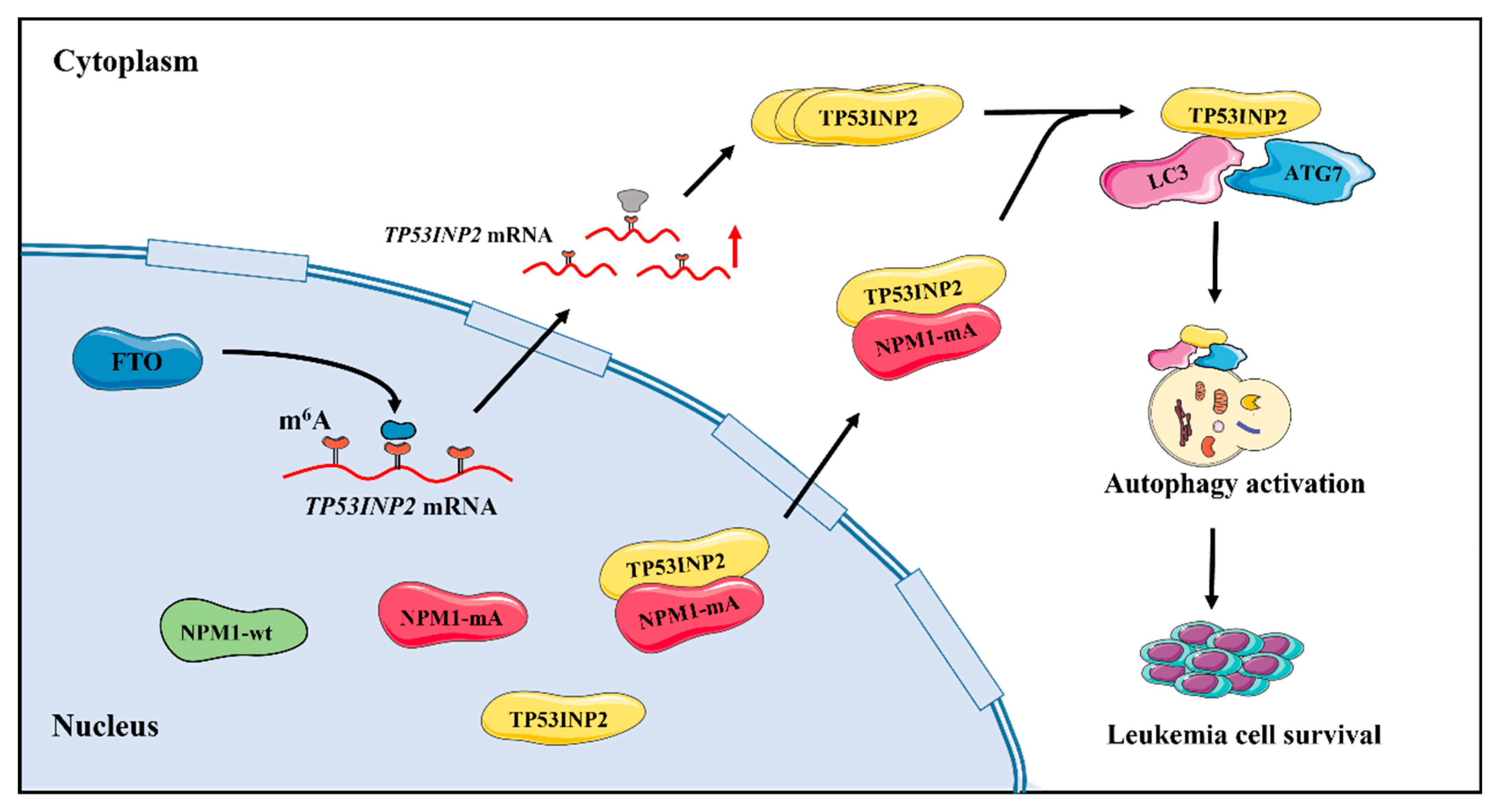
2.3. TP53INP2 Is Upregulated by FTO-Mediated m6A Modification
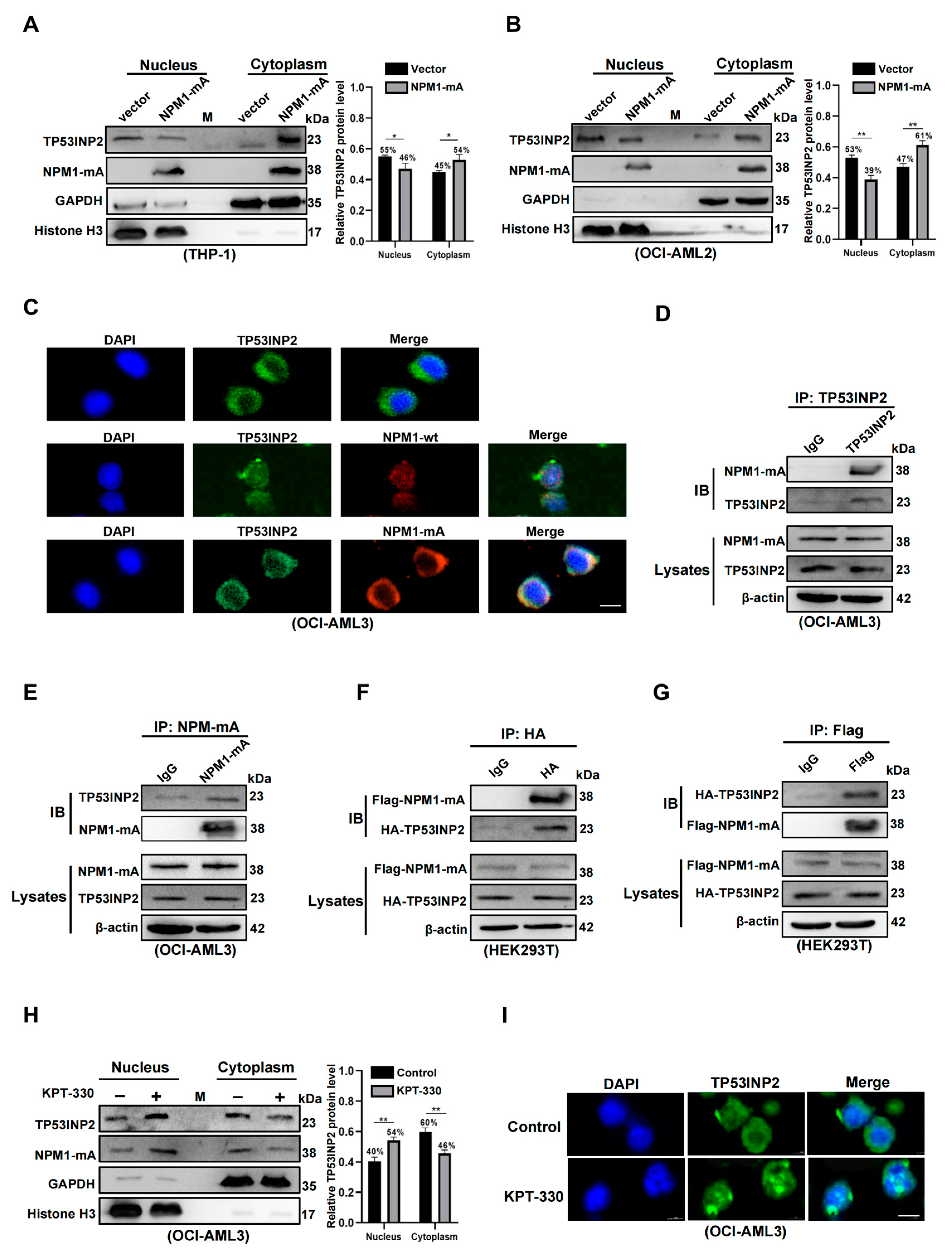
2.4. TP53INP2 Is Delocalized into the Cytoplasm by Interacting with NPM1-mA
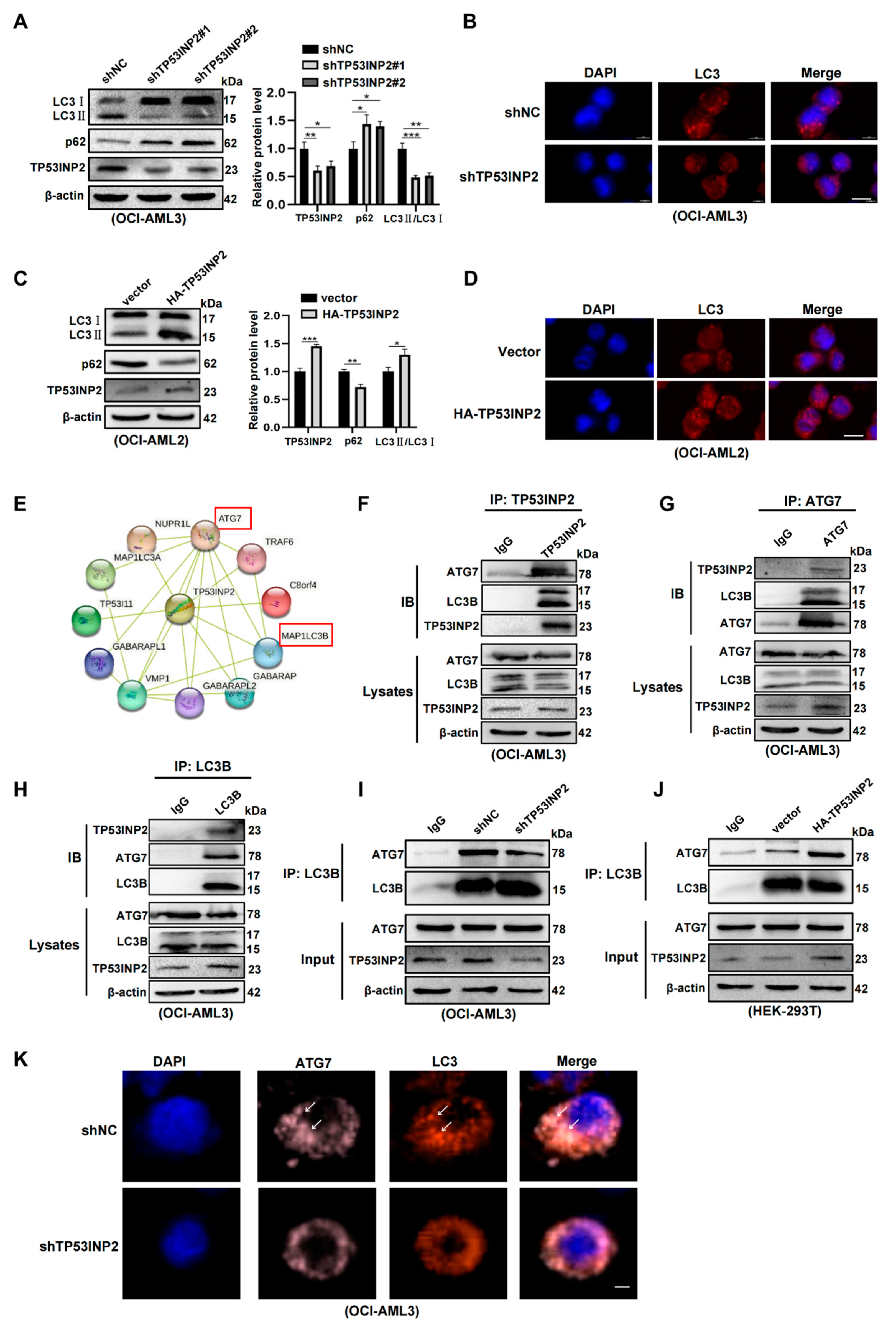
2.5. TP53INP2 Facilitates Autophagy Activity by Promoting the Interaction of LC3-ATG7
2.6. TP53INP2-Mediated Autophagy Is Vital for Leukemia Cell Survival
3. Discussion
4. Materials and Methods
4.1. Data Analysis of TCGA, GEO, Beat-AML, CCLE, and HADb Databases
4.2. Cell Lines and Culture
4.3. Clinical Samples
4.4. Lentiviral Vectors and Cell Infection
4.5. Plasmid Constructs and Cell Transfection
4.6. Quantitative Real-Time PCR (qRT-PCR)
4.7. Western Blot Analysis
4.8. Immunofluorescence
4.9. Reagents Treatment
4.10. RNA-Binding Protein Immunoprecipitation (RIP) Assays
4.11. mRNA Stability Assays
4.12. Nucleus and Cytoplasm Fraction Isolation Assays
4.13. Co-Immunoprecipitation Assays
4.14. Cell Counting Kit-8 (CCK-8) Assays
4.15. 5-Ethynyl-2′-Deoxyuridine (EdU) Assays
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khwaja, A.; Bjorkholm, M.; Gale, R.E.; Levine, R.L.; Jordan, C.T.; Ehninger, G.; Bloomfield, C.D.; Estey, E.; Burnett, A.; Cornelissen, J.J.; et al. Acute myeloid leukaemia. Nat. Rev Dis. Primers 2016, 2, 16010. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Sportoletti, P.; Martelli, M.P. NPM1-mutated acute myeloid leukemia: From bench to bedside. Blood 2020, 136, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.P.; Rossi, R.; Venanzi, A.; Meggendorfer, M.; Perriello, V.M.; Martino, G.; Spinelli, O.; Ciurnelli, R.; Varasano, E.; Brunetti, L.; et al. Novel NPM1 exon 5 mutations and gene fusions leading to aberrant cytoplasmic nucleophosmin in AML. Blood 2021, 138, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Sportoletti, P.; Ito, K.; Clohessy, J.G.; Teruya-Feldstein, J.; Kutok, J.L.; Pandolfi, P.P. The cytoplasmic NPM mutant induces myeloproliferation in a transgenic mouse model. Blood 2010, 115, 3341–3345. [Google Scholar] [CrossRef] [PubMed]
- Grisendi, S.; Bernardi, R.; Rossi, M.; Cheng, K.; Khandker, L.; Manova, K.; Pandolfi, P.P. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 2005, 437, 147–153. [Google Scholar] [CrossRef]
- Sportoletti, P.; Varasano, E.; Rossi, R.; Bereshchenko, O.; Cecchini, D.; Gionfriddo, I.; Bolli, N.; Tiacci, E.; Intermesoli, T.; Zanghì, P.; et al. The human NPM1 mutation A perturbs megakaryopoiesis in a conditional mouse model. Blood 2013, 121, 3447–3458. [Google Scholar] [CrossRef]
- Mortensen, M.; Soilleux, E.J.; Djordjevic, G.; Tripp, R.; Lutteropp, M.; Sadighi-Akha, E.; Stranks, A.; Glanville, J.; Knight, S.; Jacobsen, S.E.W.; et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J. Exp. Med. 2011, 208, 455–467. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, M.-S. Autophagy—A key player in cellular and body metabolism. Nat. Rev. Endocrinol. 2014, 10, 322–337. [Google Scholar] [CrossRef]
- Auberger, P.; Puissant, A. Autophagy, a key mechanism of oncogenesis and resistance in leukemia. Blood 2017, 129, 547–552. [Google Scholar] [CrossRef]
- Seo, W.; Silwal, P.; Song, I.-C.; Jo, E.-K. The dual role of autophagy in acute myeloid leukemia. J. Hematol. Oncol. 2022, 15, 1–25. [Google Scholar] [CrossRef]
- Piya, S.; Andreeff, M.; Borthakur, G. Targeting autophagy to overcome chemoresistance in acute myleogenous leukemia. Autophagy 2016, 13, 214–215. [Google Scholar] [CrossRef]
- Zou, Q.; Tan, S.; Yang, Z.; Zhan, Q.; Jin, H.; Xian, J.; Zhang, S.; Yang, L.; Wang, L.; Zhang, L. NPM1 Mutant Mediated PML Delocalization and Stabilization Enhances Autophagy and Cell Survival in Leukemic Cells. Theranostics 2017, 7, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Yang, Z.; Tang, Y.; Tao, Y.; Zhan, Q.; Lei, L.; Jing, Y.; Jiang, X.; Jin, H.; et al. Glycolytic Enzyme PKM2 Mediates Autophagic Activation to Promote Cell Survival in NPM1-Mutated Leukemia. Int. J. Biol. Sci. 2019, 15, 882–894. [Google Scholar] [CrossRef]
- Tang, Y.; Tao, Y.; Wang, L.; Yang, L.; Jing, Y.; Jiang, X.; Lei, L.; Yang, Z.; Wang, X.; Peng, M.; et al. NPM1 mutant maintains ULK1 protein stability via TRAF6-dependent ubiquitination to promote autophagic cell survival in leukemia. FASEB J. 2020, 35, e21192. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Y. Other Molecular Mechanisms Regulating Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Hu, D.; Wang, Y.; Shao, W.; Zhong, J.; Yang, S.; Liu, J.; Zhang, J. Insights into N6-methyladenosine and programmed cell death in cancer. Mol. Cancer 2022, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wei, J.; Gu, X.; Yao, X.; Zhang, D.; Li, F.; Sun, Y. Dysregulation of LINC00470 and METTL3 promotes chemoresistance and suppresses autophagy of chronic myelocytic leukaemia cells. J. Cell. Mol. Med. 2021, 25, 4248–4259. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, W. The bifunctional role of TP53INP2 in transcription and autophagy. Autophagy 2020, 16, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.; Depetris, D.; Iovanna, J.-L.; Mattei, M.-G.; Pébusque, M.-J. Assignment of the tumor protein p53 induced nuclear protein 2 (TP53INP2) gene to human chromosome band 20q11.2 by in situ hybridization. Cytogenet. Genome Res. 2004, 108, 362B. [Google Scholar] [CrossRef]
- Okamura, S.; Arakawa, H.; Tanaka, T.; Nakanishi, H.; Ng, C.C.; Taya, Y.; Monden, M.; Nakamura, Y. p53DINP1, a p53-Inducible Gene, Regulates p53-Dependent Apoptosis. Mol. Cell 2001, 8, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Cano, C.E.; Gommeaux, J.; Pietri, S.; Culcasi, M.; Garcia, S.; Seux, M.; Barelier, S.; Vasseur, S.; Spoto, R.P.; Pébusque, M.-J.; et al. Tumor Protein 53–Induced Nuclear Protein 1 Is a Major Mediator of p53 Antioxidant Function. Cancer Res 2008, 69, 219–226. [Google Scholar] [CrossRef]
- Sancho, A.; Duran, J.; García-España, A.; Mauvezin, C.; Alemu, E.A.; Lamark, T.; Macias, M.J.; DeSalle, R.; Royo, M.; Sala, D.; et al. DOR/Tp53inp2 and Tp53inp1 constitute a metazoan gene family encoding dual regulators of autophagy and transcription. PLoS ONE 2012, 7, e34034. [Google Scholar] [CrossRef]
- Baumgartner, B.G.; Orpinell, M.; Duran, J.; Ribas, V.; Burghardt, H.E.; Bach, D.; Villar, A.; Paz, J.C.; González, D.B.; Camps, M.; et al. Identification of a Novel Modulator of Thyroid Hormone Receptor-Mediated Action. PLoS ONE 2007, 2, e1183. [Google Scholar] [CrossRef]
- Mauvezin, C.; Orpinell, M.; Francis, V.A.; Mansilla, F.; Duran, J.; Ribas, V.; Palacín, M.; Boya, P.; Teleman, A.A.; Zorzano, A. The nuclear cofactor DOR regulates autophagy in mammalian and Drosophila cells. EMBO Rep. 2009, 11, 37–44. [Google Scholar] [CrossRef]
- Sabaté-Pérez, A.; Romero, M.; Sànchez-Fernàndez-De-Landa, P.; Carobbio, S.; Mouratidis, M.; Sala, D.; Engel, P.; Villena, J.A.; Virtue, S.; Vidal-Puig, A.; et al. Autophagy-mediated NCOR1 degradation is required for brown fat maturation and thermogenesis. Autophagy 2022, 25, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sala, D.; Ivanova, S.; Plana, N.; Ribas, V.; Duran, J.; Bach, D.; Turkseven, S.; Laville, M.; Vidal, H.; Karczewska-Kupczewska, M.; et al. Autophagy-regulating TP53INP2 mediates muscle wasting and is repressed in diabetes. J. Clin. Investig. 2014, 124, 1914–1927. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Cheng, C.; Xu, J.; Gao, P.; Wang, J.; Chen, L. miR-142-3p Regulates Tumor Cell Autophagy and Promotes Colon Cancer Progression by Targeting TP53INP2. Chemotherapy 2021, 67, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Mecucci, C.; Tiacci, E.; Alcalay, M.; Rosati, R.; Pasqualucci, L.; La Starza, R.; Diverio, D.; Colombo, E.; Santucci, A.; et al. Cytoplasmic Nucleophosmin in Acute Myelogenous Leukemia with a Normal Karyotype. N. Engl. J. Med. 2005, 352, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.M.; Chan, S.M.; Minden, M.D.; Murphy, T.; Shlush, L.I.; Schimmer, A.D. Biological and clinical consequences of NPM1 mutations in AML. Leukemia 2017, 31, 798–807. [Google Scholar] [CrossRef]
- Xiao, Q.; Lei, L.; Ren, J.; Peng, M.; Jing, Y.; Jiang, X.; Huang, J.; Tao, Y.; Lin, C.; Yang, J.; et al. Mutant NPM1-Regulated FTO-Mediated m6A Demethylation Promotes Leukemic Cell Survival via PDGFRB/ERK Signaling Axis. Front. Oncol. 2022, 12, 817584. [Google Scholar] [CrossRef]
- Falini, B.; Bolli, N.; Liso, A.; Martelli, M.P.; Mannucci, R.; Pileri, S.; Nicoletti, I. Altered nucleophosmin transport in acute myeloid leukaemia with mutated NPM1: Molecular basis and clinical implications. Leukemia 2009, 23, 1731–1743. [Google Scholar] [CrossRef]
- Chang, C.; Jensen, L.E.; Hurley, J.H. Autophagosome biogenesis comes out of the black box. Nature 2021, 23, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Gundry, M.C.; Sorcini, D.; Guzman, A.G.; Huang, Y.-H.; Ramabadran, R.; Gionfriddo, I.; Mezzasoma, F.; Milano, F.; Nabet, B.; et al. Mutant NPM1 Maintains the Leukemic State through HOX Expression. Cancer Cell 2018, 34, 499–512.e9. [Google Scholar] [CrossRef]
- Chen, L.-S.; Zhang, M.; Chen, P.; Xiong, X.-F.; Liu, P.-Q.; Wang, H.-B.; Wang, J.-J.; Shen, J. The m6A demethylase FTO promotes the osteogenesis of mesenchymal stem cells by downregulating PPARG. Acta Pharmacol. Sin. 2021, 43, 1311–1323. [Google Scholar] [CrossRef]
- Li, Z.; Weng, H.; Su, R.; Weng, X.; Zuo, Z.; Li, C.; Huang, H.; Nachtergaele, S.; Dong, L.; Hu, C.; et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N 6 -Methyladenosine RNA Demethylase. Cancer Cell 2016, 31, 127–141. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Wu, A.; Peng, Y.; Shu, G.; Yin, G. Functions of N6-methyladenosine and its role in cancer. Mol. Cancer 2019, 18, 1–15. [Google Scholar] [CrossRef]
- Wang, T.; Kong, S.; Tao, M.; Ju, S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol. Cancer 2020, 19, 1–18. [Google Scholar] [CrossRef]
- Qing, Y.; Dong, L.; Gao, L.; Li, C.; Li, Y.; Han, L.; Prince, E.; Tan, B.; Deng, X.; Wetzel, C.; et al. R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m6A/PFKP/LDHB axis. Mol. Cell 2021, 81, 922–939.e9. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Martinelli, P.; Zamponi, R.; Shing, D.C.; Bonetti, P.; Luzi, L.; Volorio, S.; Bernard, L.; Pruneri, G.; Alcalay, M.; et al. Delocalization and Destabilization of the Arf Tumor Suppressor by the Leukemia-Associated NPM Mutant. Cancer Res 2006, 66, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Struthers, H.; Wilson, E.R.; Spencer, D.H. Contribution of CTCF binding to transcriptional activity at the HOXA locus in NPM1-mutant AML cells. Leukemia 2020, 35, 404–416. [Google Scholar] [CrossRef]
- Gurumurthy, M.; Tan, C.H.; Ng, R.; Zeiger, L.; Lau, J.; Lee, J.; Dey, A.; Philp, R.; Li, Q.; Lim, T.M.; et al. Nucleophosmin Interacts with HEXIM1 and Regulates RNA Polymerase II Transcription. J. Mol. Biol. 2008, 378, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Pianigiani, G.; Betti, C.; Bigerna, B.; Rossi, R.; Brunetti, L. PU. 1 subcellular localization in acute myeloid leukaemia with mutated NPM1. Br. J. Haematol. 2019, 188, 184–187. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Mao, W.-W.; Zhang, H.; Ni, B.; Jiang, L. Oxidative stress induces downregulation of TP53INP2 and suppresses osteogenic differentiation of BMSCs during osteoporosis through the autophagy degradation pathway. Free. Radic. Biol. Med. 2021, 166, 226–237. [Google Scholar] [CrossRef]
- You, Z.; Xu, Y.; Wan, W.; Zhou, L.; Li, J.; Zhou, T.; Shi, Y.; Liu, W. TP53INP2 contributes to autophagosome formation by promoting LC3-ATG7 interaction. Autophagy 2019, 15, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Klionsky, D.J. TP53INP2/DOR protein chaperones deacetylated nuclear LC3 to the cytoplasm to promote macroautophagy. Autophagy 2015, 11, 1441–1442. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wan, W. TP 53 INP 2 mediates autophagic degradation of ubiquitinated proteins through its ubiquitin-interacting motif. FEBS Lett. 2019, 593, 1974–1982. [Google Scholar] [CrossRef]
- Nowak, J.; Iovanna, J.L. TP53INP2 is the new guest at the table of self-eating. Autophagy 2009, 5, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Klionsky, D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Pan, R.; Zhao, Y.; Li, H.; Zhu, H.; Wang, S.; Khan, A.A.; Wang, J.; Liu, X. L3MBTL2-mediated CGA transcriptional suppression promotes pancreatic cancer progression through modulating autophagy. Iscience 2022, 25, 104249. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Xue, W.; Pang, J.; Meng, Y.; Shen, Y.; Xu, Q. TP53INP2-related basal autophagy is involved in the growth and malignant progression in human liposarcoma cells. Biomed. Pharmacother. 2017, 88, 562–568. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Li, Y.; Li, J.; Deng, W.; Zhong, J.; Chen, L.; Li, Y.; Zeng, X.; Wang, G.; et al. TP53INP2 Modulates Epithelial-to-Mesenchymal Transition via the GSK-3β/β-Catenin/Snail1 Pathway in Bladder Cancer Cells. OncoTargets Ther. 2020, 13, 9587–9597. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.; Polajnar, M.; Narbona-Perez, A.J.; Hernandez-Alvarez, M.I.; Frager, P.; Slobodnyuk, K.; Plana, N.; Nebreda, A.R.; Palacin, M.; Gomis, R.R.; et al. Regulation of death receptor signaling by the autophagy protein TP 53 INP 2. EMBO J. 2019, 38, e99300. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Median (Range) | No. of Cases |
|---|---|---|
| Gender | ||
| Female | 20 | |
| Male | 19 | |
| Total | 39 | |
| Median age (years) | 51.0 (16.0–82.0) | |
| Younger than 40 y | 12 | |
| 40–60 y | 19 | |
| Older than 60 y | 8 | |
| Median WBC counts (×109) | 51.1 (0.7–260.8) | |
| Median platelets (×109) | 40.0 (3.0–176.0) | |
| AML FAB subtype | ||
| M1 | 2 | |
| M2 | 7 | |
| M3 | 7 | |
| M4 | 6 | |
| M5 | 11 | |
| Other subtypes | 6 | |
| Karyotype | ||
| Normal | 21 | |
| t (8,21) | 3 | |
| t (15,17) | 7 | |
| inv (16) | 1 | |
| Unknown | 7 | |
| Gene mutations | ||
| FLT3-ITD | 12 | |
| NPM1 | 21 | |
| IDH1/IDH2 | 8 | |
| DNMT3A | 9 | |
| WT1 | 19 |
| Genes | Sequences (5′-3′) |
|---|---|
| TP53INP2 | F: 5′-CCTCCCCTTCTCCTCCAGTAAA-3′ R: 5′-AGCCCAAAATTCAGTCTCACCA-3′ |
| FTO | F: 5′-ACTTGGCTCCCTTATCTGACC-3′ R: 5′-TGTGCAGTGTGAGAAAGGCTT-3′ |
| NPM1-mA | F: 5′-TGGAGGTGGTAGCAAGGTTC-3′ R: 5′- CTTCCTCCACTGCCAGACAGA-3′ |
| NPM1-wt | F: 5′-ACGGTCAGTTTAGGGGCTG-3′ R: 5′-CTGTGGAACCTTGCTACCACC-3′ |
| β-actin | F: 5′-TAGTTGCGTTACACCCTTTCTTG-3′ R: 5′-TGCTGTCACCTTCACCGTTC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Sun, M.; Tao, Y.; Ren, J.; Peng, M.; Jing, Y.; Xiao, Q.; Yang, J.; Lin, C.; Lei, L.; et al. Cytoplasmic Expression of TP53INP2 Modulated by Demethylase FTO and Mutant NPM1 Promotes Autophagy in Leukemia Cells. Int. J. Mol. Sci. 2023, 24, 1624. https://doi.org/10.3390/ijms24021624
Huang J, Sun M, Tao Y, Ren J, Peng M, Jing Y, Xiao Q, Yang J, Lin C, Lei L, et al. Cytoplasmic Expression of TP53INP2 Modulated by Demethylase FTO and Mutant NPM1 Promotes Autophagy in Leukemia Cells. International Journal of Molecular Sciences. 2023; 24(2):1624. https://doi.org/10.3390/ijms24021624
Chicago/Turabian StyleHuang, Junpeng, Minghui Sun, Yonghong Tao, Jun Ren, Meixi Peng, Yipei Jing, Qiaoling Xiao, Jing Yang, Can Lin, Li Lei, and et al. 2023. "Cytoplasmic Expression of TP53INP2 Modulated by Demethylase FTO and Mutant NPM1 Promotes Autophagy in Leukemia Cells" International Journal of Molecular Sciences 24, no. 2: 1624. https://doi.org/10.3390/ijms24021624
APA StyleHuang, J., Sun, M., Tao, Y., Ren, J., Peng, M., Jing, Y., Xiao, Q., Yang, J., Lin, C., Lei, L., Yang, Z., & Zhang, L. (2023). Cytoplasmic Expression of TP53INP2 Modulated by Demethylase FTO and Mutant NPM1 Promotes Autophagy in Leukemia Cells. International Journal of Molecular Sciences, 24(2), 1624. https://doi.org/10.3390/ijms24021624





