Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease
Abstract
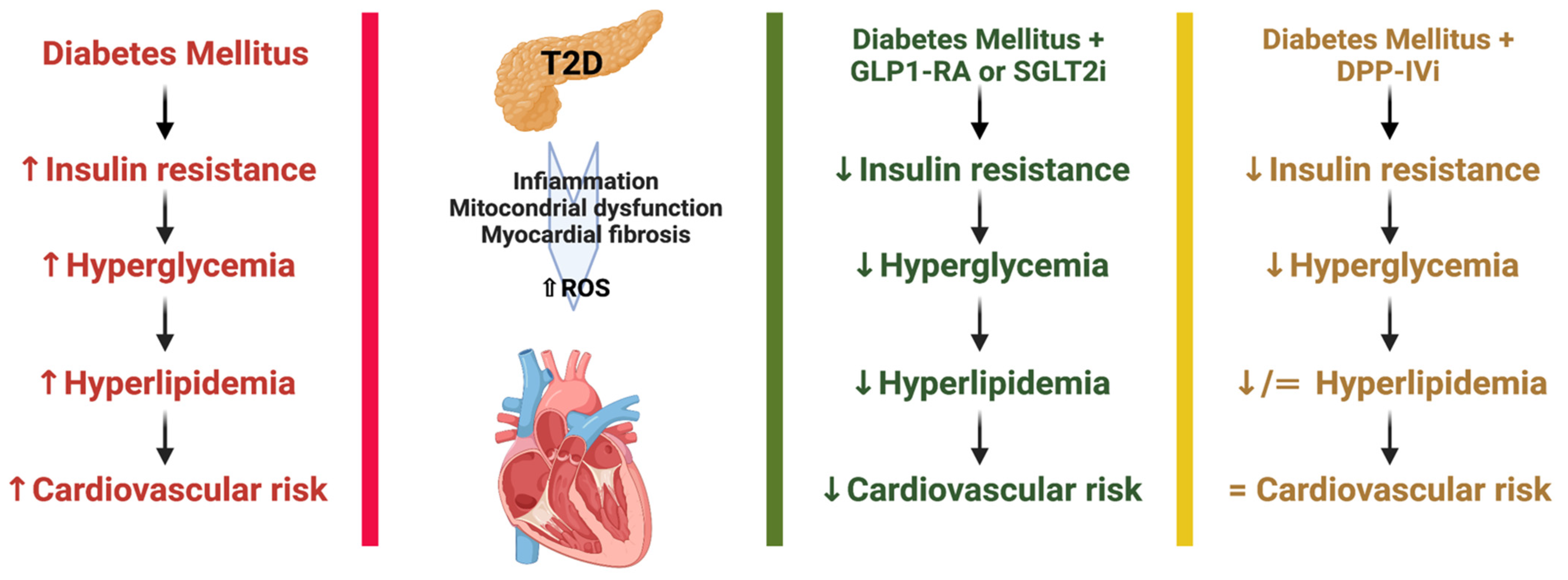
1. Introduction
2. Oxidative Stress
3. Pharmacological Treatment
3.1. Dipeptydilpeptidase-IV Inhibitor (DPP-IVi)
3.2. Glucagon-like-1 Receptor Agonists (GLP-1RAs)
3.3. Sodium-Glucose Cotransporter-2 Inhibitors (SGLT-2i)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Hunter, D.J.; Reddy, K.S. Noncommunicable Diseases. N. Engl. J. Med. 2013, 369, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, Regional, and National Comparative Risk Assessment of 79 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Rogliani, P.; Matera, M.G.; Calzetta, L.; Hanania, N.A.; Page, C.; Rossi, I.; Andreadi, A.; Galli, A.; Coppola, A.; Cazzola, M.; et al. Long-Term Observational Study on the Impact of GLP-1R Agonists on Lung Function in Diabetic Patients. Respir. Med. 2019, 154, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Chen, S. Spice Plant Allium Cepa: Dietary Supplement for Treatment of Type 2 Diabetes Mellitus. Nutrition 2014, 30, 1128–1137. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [CrossRef] [PubMed]
- Bellia, A.; Iellamo, F.; De Carli, E.; Andreadi, A.; Padua, E.; Lombardo, M.; Annino, G.; Campoli, F.; Tartaglione, S.; D’Ottavio, S.; et al. Exercise Individualized by TRIMPi Method Reduces Arterial Stiffness in Early Onset Type 2 Diabetic Patients: A Randomized Controlled Trial with Aerobic Interval Training. Int. J. Cardiol. 2017, 248, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, G.; Zheng, X.; Guo, Y. Contribution of Specific Diseases and Injuries to Changes in Health Adjusted Life Expectancy in 187 Countries from 1990 to 2013: Retrospective Observational Study. BMJ 2019, 364, l969. [Google Scholar] [CrossRef]
- Mollace, V.; Muscoli, C.; Dagostino, C.; Giancotti, L.A.; Gliozzi, M.; Sacco, I.; Visalli, V.; Gratteri, S.; Palma, E.; Malara, N.; et al. The Effect of Peroxynitrite Decomposition Catalyst MnTBAP on Aldehyde Dehydrogenase-2 Nitration by Organic Nitrates: Role in Nitrate Tolerance. Pharm. Res 2014, 89, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, S.; Barillà, F.; Tajmir, R.; Meloni, M.; Della Morte, D.; Bellia, A.; Di Daniele, N.; Lauro, D.; Andreadi, A. The New Role of SGLT2 Inhibitors in the Management of Heart Failure: Current Evidence and Future Perspective. Pharmaceutics 2022, 14, 1730. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Leahy, J.L. Islet Amyloid and Type 2 Diabetes: Overproduction or Inadequate Clearance and Detoxification? J. Clin. Invest. 2014, 124, 3292–3294. [Google Scholar] [CrossRef] [PubMed]
- Bellia, A.; Andreadi, A.; Giudice, L.; De Taddeo, S.; Maiorino, A.; D’Ippolito, I.; Giorgino, F.M.; Ruotolo, V.; Romano, M.; Magrini, A.; et al. Atherogenic Dyslipidemia on Admission Is Associated with Poorer Outcome in People with and without Diabetes Hospitalized for COVID-19. Diabetes Care 2021, 44, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Liao, Z.; Yao, B.; Chen, X.; Huang, Z.; Hu, G.; Weng, J. Induction of Long-Term Glycemic Control in Newly Diagnosed Type 2 Diabetic Patients Is Associated with Improvement of β-Cell Function. Diabetes Care 2004, 27, 2597–2602. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.-P.; et al. Normalizing Mitochondrial Superoxide Production Blocks Three Pathways of Hyperglycaemic Damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Horii, Y.; Nishino, T.; Shiiki, H.; Sakaguchi, Y.; Kagoshima, T.; Dohi, K.; Makita, Z.; Vlassara, H.; Bucala, R. Immunohistochemical Localization of Advanced Glycosylation End Products in Coronary Atheroma and Cardiac Tissue in Diabetes Mellitus. Am J Pathol 1993, 143, 1649–1656. [Google Scholar]
- Schmidt, A.M.; Hori, O.; Chen, J.X.; Li, J.F.; Crandall, J.; Zhang, J.; Cao, R.; Yan, S.D.; Brett, J.; Stern, D. Advanced Glycation Endproducts Interacting with Their Endothelial Receptor Induce Expression of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Cultured Human Endothelial Cells and in Mice. A Potential Mechanism for the Accelerated Vasculopathy of Diabetes. J. Clin. Invest. 1995, 96, 1395–1403. [Google Scholar] [CrossRef]
- Sharma, R.B.; Alonso, L.C. Lipotoxicity in the Pancreatic Beta Cell: Not Just Survival and Function, but Proliferation as Well? Curr. Diab. Rep. 2014, 14, 492. [Google Scholar] [CrossRef]
- Ehses, J.A.; Perren, A.; Eppler, E.; Ribaux, P.; Pospisilik, J.A.; Maor-Cahn, R.; Gueripel, X.; Ellingsgaard, H.; Schneider, M.K.J.; Biollaz, G.; et al. Increased Number of Islet-Associated Macrophages in Type 2 Diabetes. Diabetes 2007, 56, 2356–2370. [Google Scholar] [CrossRef]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The Molecular Link between Oxidative Stress, Insulin Resistance, and Type 2 Diabetes: A Target for New Therapies against Cardiovascular Diseases. Curr. Opin. Pharm. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive Oxygen Species Have a Causal Role in Multiple Forms of Insulin Resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, C.; Durand, A.; Peyrol, S.; Chanseaume, E.; Chauvin, M.-A.; Morio, B.; Vidal, H.; Rieusset, J. Mitochondrial Dysfunction Results from Oxidative Stress in the Skeletal Muscle of Diet-Induced Insulin-Resistant Mice. J. Clin. Invest. 2008, 118, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Stockklauser-Färber, K.; Rösen, P. Generation of Reactive Oxygen Intermediates, Activation of NF-ΚB, and Induction of Apoptosis in Human Endothelial Cells by Glucose: Role of Nitric Oxide Synthase? Free Radic. Biol. Med. 1999, 27, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chough, E.; Daley, J.; Oates, P.; Tornheim, K.; Ruderman, N.B.; Keaney, J.F. Hyperglycemia Increases Endothelial Superoxide That Impairs Smooth Muscle Cell Na+ -K+ -ATPase Activity. Am. J. Physiol. -Cell Physiol. 2002, 282, C560–C566. [Google Scholar] [CrossRef]
- Martens, G.A.; Cai, Y.; Hinke, S.; Stangé, G.; Van de Casteele, M.; Pipeleers, D. Glucose Suppresses Superoxide Generation in Metabolically Responsive Pancreatic β Cells. J. Biol. Chem. 2005, 280, 20389–20396. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Apostolova, N.; Bañuls, C.; Muntané, J.; Rocha, M.; Victor, V.M. Mitochondria, the NLRP3 Inflammasome, and Sirtuins in Type 2 Diabetes: New Therapeutic Targets. Antioxid. Redox Signal. 2018, 29, 749–791. [Google Scholar] [CrossRef]
- Van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of Mitochondrial Fission and Fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Kiritoshi, S.; Nishikawa, T.; Sonoda, K.; Kukidome, D.; Senokuchi, T.; Matsuo, T.; Matsumura, T.; Tokunaga, H.; Brownlee, M.; Araki, E. Reactive Oxygen Species from Mitochondria Induce Cyclooxygenase-2 Gene Expression in Human Mesangial Cells: Potential Role in Diabetic Nephropathy. Diabetes 2003, 52, 2570–2577. [Google Scholar] [CrossRef]
- Leloup, C.; Tourrel-Cuzin, C.; Magnan, C.; Karaca, M.; Castel, J.; Carneiro, L.; Colombani, A.-L.; Ktorza, A.; Casteilla, L.; Pénicaud, L. Mitochondrial Reactive Oxygen Species Are Obligatory Signals for Glucose-Induced Insulin Secretion. Diabetes 2009, 58, 673–681. [Google Scholar] [CrossRef]
- Talchai, C.; Xuan, S.; Lin, H.V.; Sussel, L.; Accili, D. Pancreatic β Cell Dedifferentiation as a Mechanism of Diabetic β Cell Failure. Cell 2012, 150, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Merry, T.L.; McConell, G.K. Skeletal Muscle Glucose Uptake during Exercise: A Focus on Reactive Oxygen Species and Nitric Oxide Signaling. IUBMB Life 2009, 61, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Elsner, M.; Gehrmann, W.; Lenzen, S. Peroxisome-Generated Hydrogen Peroxide as Important Mediator of Lipotoxicity in Insulin-Producing Cells. Diabetes 2011, 60, 200–208. [Google Scholar] [CrossRef]
- Poitout, V.; Robertson, R.P. Glucolipotoxicity: Fuel Excess and β-Cell Dysfunction. Endocr. Rev. 2008, 29, 351–366. [Google Scholar] [CrossRef]
- Morgan, D.; Oliveira-Emilio, H.R.; Keane, D.; Hirata, A.E.; Santos da Rocha, M.; Bordin, S.; Curi, R.; Newsholme, P.; Carpinelli, A.R. Glucose, Palmitate and pro-Inflammatory Cytokines Modulate Production and Activity of a Phagocyte-like NADPH Oxidase in Rat Pancreatic Islets and a Clonal Beta Cell Line. Diabetologia 2007, 50, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Lytrivi, M.; Castell, A.-L.; Poitout, V.; Cnop, M. Recent Insights into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef]
- Astiarraga, B.; Chueire, V.B.; Souza, A.L.; Pereira-Moreira, R.; Monte Alegre, S.; Natali, A.; Tura, A.; Mari, A.; Ferrannini, E.; Muscelli, E. Effects of Acute NEFA Manipulation on Incretin-Induced Insulin Secretion in Participants with and without Type 2 Diabetes. Diabetologia 2018, 61, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Goldberg, R.; Temprosa, M.; Otvos, J.; Brunzell, J.; Marcovina, S.; Mather, K.; Arakaki, R.; Watson, K.; Horton, E.; Barrett-Connor, E. Lifestyle and Metformin Treatment Favorably Influence Lipoprotein Subfraction Distribution in the Diabetes Prevention Program. J. Clin. Endocrinol. Metab. 2013, 98, 3989–3998. [Google Scholar] [CrossRef]
- Muscoli, S.; Ifrim, M.; Russo, M.; Candido, F.; Sanseviero, A.; Milite, M.; Di Luozzo, M.; Marchei, M.; Sangiorgi, G.M. Current Options and Future Perspectives in the Treatment of Dyslipidemia. J. Clin. Med. 2022, 11, 4716. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Litvinova, L.; Poggio, P.; Moschetta, D.; Sukhorukov, V.N.; Orekhov, A.N. From Diabetes to Atherosclerosis: Potential of Metformin for Management of Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 9738. [Google Scholar] [CrossRef]
- Javadipour, M.; Rezaei, M.; Keshtzar, E.; Khodayar, M.J. Metformin in Contrast to Berberine Reversed Arsenic-induced Oxidative Stress in Mitochondria from Rat Pancreas Probably via Sirt3-dependent Pathway. J Biochem Mol Toxicol 2019, 33, e22368. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence That Metformin Exerts Its Anti-Diabetic Effects through Inhibition of Complex 1 of the Mitochondrial Respiratory Chain. Biochem J 2000, 348, 607–614. [Google Scholar] [CrossRef]
- Cen, J.; Sargsyan, E.; Forslund, A.; Bergsten, P. Mechanisms of Beneficial Effects of Metformin on Fatty Acid-Treated Human Islets. J. Mol. Endocrinol. 2018, 61, 91–99. [Google Scholar] [CrossRef]
- Roxo, D.F.; Arcaro, C.A.; Gutierres, V.O.; Costa, M.C.; Oliveira, J.O.; Lima, T.F.O.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Curcumin Combined with Metformin Decreases Glycemia and Dyslipidemia, and Increases Paraoxonase Activity in Diabetic Rats. Diabetol. Metab. Syndr. 2019, 11, 33. [Google Scholar] [CrossRef]
- Maegawa, H.; Nishio, Y.; Nakao, K.; Ugi, S.; Maeda, K.; Uzu, T.; Kashiwagi, A. Short-Term Low-Dosage Pioglitazone Treatment Improves Vascular Dysfunction in Patients with Type 2 Diabetes. Endocr. J. 2007, 54, 613–618. [Google Scholar] [CrossRef]
- Surapaneni, K.M.; Jainu, M. Comparative Effect of Pioglitazone, Quercetin and Hydroxy Citric Acid on the Status of Lipid Peroxidation and Antioxidants in Experimental Non-Alcoholic Steatohepatitis. J. Physiol. Pharm. 2014, 65, 67–74. [Google Scholar]
- Shaaban, H.H.; Alzaim, I.; El-Mallah, A.; Aly, R.G.; El-Yazbi, A.F.; Wahid, A. Metformin, Pioglitazone, Dapagliflozin and Their Combinations Ameliorate Manifestations Associated with NAFLD in Rats via Anti-Inflammatory, Anti-Fibrotic, Anti-Oxidant and Anti-Apoptotic Mechanisms. Life Sci. 2022, 308, 120956. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, M.; Suraamornkul, S.; Piper, P.; Hardies, L.J.; Glass, L.; Cersosimo, E.; Pratipanawatr, T.; Miyazaki, Y.; DeFronzo, R.A. Decreased Plasma Adiponectin Concentrations Are Closely Related to Hepatic Fat Content and Hepatic Insulin Resistance in Pioglitazone-Treated Type 2 Diabetic Patients. J. Clin. Endocrinol. Metab. 2004, 89, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Viscoli, C.M.; Furie, K.L.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Guarino, P.D.; Lovejoy, A.M.; Peduzzi, P.N.; Conwit, R.; et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2016, 374, 1321–1331. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary Prevention of Macrovascular Events in Patients with Type 2 Diabetes in the PROactive Study (PROspective PioglitAzone Clinical Trial in MacroVascular Events): A Randomised Controlled Trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Guillausseau, P.-J. PROactive Study. Lancet 2006, 367, 23. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.-K.; Yadav, D.; Sharma, N.; Jin, J.-O. Dipeptidyl Peptidase (DPP)-IV Inhibitors with Antioxidant Potential Isolated from Natural Sources: A Novel Approach for the Management of Diabetes. Pharmaceuticals 2021, 14, 586. [Google Scholar] [CrossRef]
- Yin, R.; Xu, Y.; Wang, X.; Yang, L.; Zhao, D. Role of Dipeptidyl Peptidase 4 Inhibitors in Antidiabetic Treatment. Molecules 2022, 27, 3055. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Abdalla, M.A.; Sathyapalan, T.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. The Effects of Glucagon-Like Peptide-1 Receptor Agonists and Dipeptydilpeptidase-4 Inhibitors on Blood Pressure and Cardiovascular Complications in Diabetes. J. Diabetes Res. 2021, 2021, 6518221. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Greenwell, A.A.; Nguyen, M.-A.; Mulvihill, E.E. Cardiovascular Effects of Incretin-Based Therapies: Integrating Mechanisms with Cardiovascular Outcome Trials. Diabetes 2022, 71, 173–183. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium–Glucose Cotransporter Inhibitors and Oxidative Stress: An Update. J. Cell. Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and Hypoglycaemic Activity of Bergamot Polyphenols: From Animal Models to Human Studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef]
- Fehse, F.; Trautmann, M.; Holst, J.J.; Halseth, A.E.; Nanayakkara, N.; Nielsen, L.L.; Fineman, M.S.; Kim, D.D.; Nauck, M.A. Exenatide Augments First- and Second-Phase Insulin Secretion in Response to Intravenous Glucose in Subjects with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2005, 90, 5991–5997. [Google Scholar] [CrossRef] [PubMed]
- Ottney, A. Glucagon-like Peptide-1 Receptor Agonists for Weight Loss in Adult Patients without Diabetes. Am. J. Health-Syst. Pharm. 2013, 70, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol Metab 2021, 46, 101102. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease (Harmony Outcomes): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Sélley, E.; Kun, S.; Szijártó, I.A.; Kertész, M.; Wittmann, I.; Molnár, G.A. Vasodilator Effect of Glucagon: Receptorial Crosstalk Among Glucagon, GLP-1, and Receptor for Glucagon and GLP-1. Horm. Metab. Res. 2016, 48, 476–483. [Google Scholar] [CrossRef]
- Shi, L.; Ji, Y.; Jiang, X.; Zhou, L.; Xu, Y.; Li, Y.; Jiang, W.; Meng, P.; Liu, X. Liraglutide Attenuates High Glucose-Induced Abnormal Cell Migration, Proliferation, and Apoptosis of Vascular Smooth Muscle Cells by Activating the GLP-1 Receptor, and Inhibiting ERK1/2 and PI3K/Akt Signaling Pathways. Cardiovasc. Diabetol. 2015, 14, 18. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-analysis of Randomised Controlled Trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Jun, H.-S. Effects of Glucagon-Like Peptide-1 on Oxidative Stress and Nrf2 Signaling. Int. J. Mol. Sci. 2017, 19, 26. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The Emerging Role of Nrf2 in Mitochondrial Function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Esposito, K.; Testa, R.; Bonfigli, A.R.; Marra, M.; Giugliano, D. The Possible Protective Role of Glucagon-Like Peptide 1 on Endothelium During the Meal and Evidence for an “Endothelial Resistance” to Glucagon-Like Peptide 1 in Diabetes. Diabetes Care 2011, 34, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Abate, N.; Chandalia, M.; Rizvi, A.A.; Giglio, R.V.; Nikolic, D.; Marino Gammazza, A.; Barbagallo, I.; Isenovic, E.R.; Banach, M.; et al. Liraglutide Reduces Oxidative Stress and Restores Heme Oxygenase-1 and Ghrelin Levels in Patients with Type 2 Diabetes: A Prospective Pilot Study. J. Clin. Endocrinol. Metab. 2015, 100, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Bunck, M.C.; Cornér, A.; Eliasson, B.; Heine, R.J.; Shaginian, R.M.; Wu, Y.; Yan, P.; Smith, U.; Yki-Järvinen, H.; Diamant, M.; et al. One-Year Treatment with Exenatide vs. Insulin Glargine: Effects on Postprandial Glycemia, Lipid Profiles, and Oxidative Stress. Atherosclerosis 2010, 212, 223–229. [Google Scholar] [CrossRef]
- Ravassa, S.; Beaumont, J.; Huerta, A.; Barba, J.; Coma-Canella, I.; González, A.; López, B.; Díez, J. Association of Low GLP-1 with Oxidative Stress Is Related to Cardiac Disease and Outcome in Patients with Type 2 Diabetes Mellitus: A Pilot Study. Free Radic. Biol. Med. 2015, 81, 1–12. [Google Scholar] [CrossRef]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide in Mice With Experimental Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef]
- Tashiro, Y.; Sato, K.; Watanabe, T.; Nohtomi, K.; Terasaki, M.; Nagashima, M.; Hirano, T. A Glucagon-like Peptide-1 Analog Liraglutide Suppresses Macrophage Foam Cell Formation and Atherosclerosis. Peptides 2014, 54, 19–26. [Google Scholar] [CrossRef]
- Tanaka, M.; Matsuo, Y.; Yamakage, H.; Masuda, S.; Terada, Y.; Muranaka, K.; Wada, H.; Hasegawa, K.; Shimatsu, A.; Satoh-Asahara, N. Differential Effects of GLP-1 Receptor Agonist on Foam Cell Formation in Monocytes between Non-Obese and Obese Subjects. Metabolism 2016, 65, 1–11. [Google Scholar] [CrossRef]
- Dai, Y.; Dai, D.; Wang, X.; Ding, Z.; Li, C.; Mehta, J.L. GLP-1 Agonists Inhibit Ox-LDL Uptake in Macrophages by Activating Protein Kinase A. J. Cardiovasc. Pharmacol. 2014, 64, 47–52. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Yang, T.-L. Liraglutide Reduces Oxidized LDL-Induced Oxidative Stress and Fatty Degeneration in Raw 264.7 Cells Involving the AMPK/SREBP1 Pathway. J Geriatr Cardiol 2015, 12, 410–416. [Google Scholar] [CrossRef]
- Piao, L.; Zhao, G.; Zhu, E.; Inoue, A.; Shibata, R.; Lei, Y.; Hu, L.; Yu, C.; Yang, G.; Wu, H.; et al. Chronic Psychological Stress Accelerates Vascular Senescence and Impairs Ischemia-Induced Neovascularization: The Role of Dipeptidyl Peptidase-4/Glucagon-Like Peptide-1-Adiponectin Axis. J. Am. Heart Assoc. 2017, 6, e006421. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; She, M.; Xu, M.; Chen, H.; Li, J.; Chen, X.; Zheng, D.; Liu, J.; Chen, S.; Zhu, J.; et al. GLP-1 Treatment Protects Endothelial Cells from Oxidative Stress-Induced Autophagy and Endothelial Dysfunction. Int. J. Biol. Sci. 2018, 14, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Schisano, B.; Harte, A.L.; Lois, K.; Saravanan, P.; Al-Daghri, N.; Al-Attas, O.; Knudsen, L.B.; McTernan, P.G.; Ceriello, A.; Tripathi, G. GLP-1 Analogue, Liraglutide Protects Human Umbilical Vein Endothelial Cells against High Glucose Induced Endoplasmic Reticulum Stress. Regul. Pept. 2012, 174, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Chao, E.C. SGLT-2 Inhibitors: A New Mechanism for Glycemic Control. Clin. Diabetes 2014, 32, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Kalra, S. Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes 2014, 5, 355–366. [Google Scholar] [CrossRef]
- Schork, A.; Saynisch, J.; Vosseler, A.; Jaghutriz, B.A.; Heyne, N.; Peter, A.; Häring, H.-U.; Stefan, N.; Fritsche, A.; Artunc, F. Effect of SGLT2 Inhibitors on Body Composition, Fluid Status and Renin–Angiotensin–Aldosterone System in Type 2 Diabetes: A Prospective Study Using Bioimpedance Spectroscopy. Cardiovasc. Diabetol. 2019, 18, 46. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Yamagishi, S. Tofogliflozin, A Highly Selective Inhibitor of SGLT2 Blocks Proinflammatory and Proapoptotic Effects of Glucose Overload on Proximal Tubular Cells Partly by Suppressing Oxidative Stress Generation. Horm. Metab. Res. 2015, 48, 191–195. [Google Scholar] [CrossRef]
- Sugizaki, T.; Zhu, S.; Guo, G.; Matsumoto, A.; Zhao, J.; Endo, M.; Horiguchi, H.; Morinaga, J.; Tian, Z.; Kadomatsu, T.; et al. Treatment of Diabetic Mice with the SGLT2 Inhibitor TA-1887 Antagonizes Diabetic Cachexia and Decreases Mortality. Npj Aging Mech. Dis. 2017, 3, 12. [Google Scholar] [CrossRef]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of SGLT2 Selective Inhibitor Ipragliflozin on Hyperglycemia, Hyperlipidemia, Hepatic Steatosis, Oxidative Stress, Inflammation, and Obesity in Type 2 Diabetic Mice. Eur. J. Pharmacol. 2013, 715, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-Term Treatment with the Sodium Glucose Cotransporter 2 Inhibitor, Dapagliflozin, Ameliorates Glucose Homeostasis and Diabetic Nephropathy in Db/Db Mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan Komala, M.; Gross, S.; Mudaliar, H.; Huang, C.; Pegg, K.; Mather, A.; Shen, S.; Pollock, C.A.; Panchapakesan, U. Inhibition of Kidney Proximal Tubular Glucose Reabsorption Does Not Prevent against Diabetic Nephropathy in Type 1 Diabetic ENOS Knockout Mice. PLoS ONE 2014, 9, e108994. [Google Scholar] [CrossRef] [PubMed]
- Kawanami, D.; Matoba, K.; Takeda, Y.; Nagai, Y.; Akamine, T.; Yokota, T.; Sango, K.; Utsunomiya, K. SGLT2 Inhibitors as a Therapeutic Option for Diabetic Nephropathy. Int. J. Mol. Sci. 2017, 18, 1083. [Google Scholar] [CrossRef] [PubMed]
- Sa-Nguanmoo, P.; Tanajak, P.; Kerdphoo, S.; Jaiwongkam, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. SGLT2-Inhibitor and DPP-4 Inhibitor Improve Brain Function via Attenuating Mitochondrial Dysfunction, Insulin Resistance, Inflammation, and Apoptosis in HFD-Induced Obese Rats. Toxicol. Appl. Pharm. 2017, 333, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Oelze, M.; Kröller-Schön, S.; Welschof, P.; Jansen, T.; Hausding, M.; Mikhed, Y.; Stamm, P.; Mader, M.; Zinßius, E.; Agdauletova, S.; et al. The Sodium-Glucose Co-Transporter 2 Inhibitor Empagliflozin Improves Diabetes-Induced Vascular Dysfunction in the Streptozotocin Diabetes Rat Model by Interfering with Oxidative Stress and Glucotoxicity. PLoS ONE 2014, 9, e112394. [Google Scholar] [CrossRef]
- Eriksson, J.W.; Lundkvist, P.; Jansson, P.-A.; Johansson, L.; Kvarnström, M.; Moris, L.; Miliotis, T.; Forsberg, G.-B.; Risérus, U.; Lind, L.; et al. Effects of Dapagliflozin and N-3 Carboxylic Acids on Non-Alcoholic Fatty Liver Disease in People with Type 2 Diabetes: A Double-Blind Randomised Placebo-Controlled Study. Diabetologia 2018, 61, 1923–1934. [Google Scholar] [CrossRef]
- Nishimura, R.; Tanaka, Y.; Koiwai, K.; Inoue, K.; Hach, T.; Salsali, A.; Lund, S.S.; Broedl, U.C. Effect of Empagliflozin Monotherapy on Postprandial Glucose and 24-Hour Glucose Variability in Japanese Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled, 4-Week Study. Cardiovasc. Diabetol. 2015, 14, 11. [Google Scholar] [CrossRef]
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin Acutely Improves Endothelial Dysfunction, Reduces Aortic Stiffness and Renal Resistive Index in Type 2 Diabetic Patients: A Pilot Study. Cardiovasc. Diabetol. 2017, 16, 138. [Google Scholar] [CrossRef]
- Santamarina, M.; Carlson, C.J. Review of the Cardiovascular Safety of Dipeptidyl Peptidase-4 Inhibitors and the Clinical Relevance of the CAROLINA Trial. BMC Cardiovasc. Disord. 2019, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Kalra, S.; Nair, T. Modern Sulphonylureas and Cardiovascular Adverse Effects: Will CAROLINA Put an End to the Controversy? Indian Heart J. 2020, 72, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69. [Google Scholar] [CrossRef] [PubMed]
- White, W.B.; Cannon, C.P.; Heller, S.R.; Nissen, S.E.; Bergenstal, R.M.; Bakris, G.L.; Perez, A.T.; Fleck, P.R.; Mehta, C.R.; Kupfer, S.; et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N. Engl. J. Med. 2013, 369, 1327–1335. [Google Scholar] [CrossRef]
- Madievsky, R. Spotlight on Antidiabetic Agents with Cardiovascular or Renoprotective Benefits. Perm. J. 2018, 22, 18–034. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Køber, L.V.; Lawson, F.C.; Ping, L.; Wei, X.; Lewis, E.F.; et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med. 2015, 373, 2247–2257. [Google Scholar] [CrossRef]
- Holman, R.R.; Bethel, M.A.; Mentz, R.J.; Thompson, V.P.; Lokhnygina, Y.; Buse, J.B.; Chan, J.C.; Choi, J.; Gustavson, S.M.; Iqbal, N.; et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 1228–1239. [Google Scholar] [CrossRef]
- Jonik, S.; Marchel, M.; Grabowski, M.; Opolski, G.; Mazurek, T. Gastrointestinal Incretins—Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) beyond Pleiotropic Physiological Effects Are Involved in Pathophysiology of Atherosclerosis and Coronary Artery Disease—State of the Art. Biology 2022, 11, 288. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R. Cardiovascular Safety and Benefits of Semaglutide in Patients with Type 2 Diabetes: Findings From SUSTAIN 6 and PIONEER 6. Front. Endocrinol. 2021, 12, 645566. [Google Scholar] [CrossRef]
- Sattar, N.; McGuire, D.K.; Pavo, I.; Weerakkody, G.J.; Nishiyama, H.; Wiese, R.J.; Zoungas, S. Tirzepatide Cardiovascular Event Risk Assessment: A Pre-Specified Meta-Analysis. Nat. Med. 2022, 28, 591–598. [Google Scholar] [CrossRef]
- Kaneko, S. Division of Diabetes/Endocrinology/Lifestyle-Related Disease, Takatsuki Red Cross Hospital, Takatsuki, Japan Tirzepatide: A Novel, Once-Weekly Dual GIP and GLP-1 Receptor Agonist for the Treatment of Type 2 Diabetes. Endocrinology 2022, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Kuwahara, K. Sodium-Glucose Cotransporter-2 Inhibitors Are Potential Therapeutic Agents for Treatment of Non-Diabetic Heart Failure Patients. J. Cardiol. 2020, 76, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, S.H.; Lee, C.J.; Kang, S.M. Sodium-Glucose Co-Transporter 2 Inhibitors: A New Path for Heart Failure Treatment. Korean Circ. J. 2021, 51, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kluger, A.Y.; Tecson, K.M.; Lee, A.Y.; Lerma, E.V.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Class Effects of SGLT2 Inhibitors on Cardiorenal Outcomes. Cardiovasc. Diabetol. 2019, 18, 99. [Google Scholar] [CrossRef]
- Provenzano, M.; Pelle, M.C.; Zaffina, I.; Tassone, B.; Pujia, R.; Ricchio, M.; Serra, R.; Sciacqua, A.; Michael, A.; Andreucci, M.; et al. Sodium-Glucose Co-Transporter-2 Inhibitors and Nephroprotection in Diabetic Patients: More Than a Challenge. Front. Med. 2021, 8, 654557. [Google Scholar] [CrossRef]
- Docherty, K.F.; McMurray, J.J.V. SOLOIST-WHF and updated meta-analysis: Sodium–glucose co-transporter 2 inhibitors should be initiated in patients hospitalized with worsening heart failure. Eur. J. Heart Fail. 2021, 23, 27–30. [Google Scholar] [CrossRef]
| Pharmacological Treatment | ||||
|---|---|---|---|---|
| Metformin | Thiazolidinediones | DPP-IVi | GLP1-RAs | SGLT2i |
|
|
|
|
|
| Class | Trial | Duration of the Study (In Years) | Diabetes | Primary Outcome in Drug vs. Control (%) |
|---|---|---|---|---|
| DPP-IVi | SAVOR/TIMI 53 (saxagliptin) | 2.1 | Yes | ↑ 7.3% vs. 7.2% |
| CARMELINA (Linagliptin) | 2.2 | Yes | ↑ 12.4% vs. 12.1% | |
| CAROLINA (linagliptin) | 6.3 | Yes | ↑ 11.8% vs. 12% | |
| TECOS (sitagliptin) | 3 | Yes | ↓ 11.4% vs. 11.6% | |
| EXAMINE (alogliptin) | 1.5 | Yes | ↓ 11.3% vs. 11.8% | |
| SGLT2i | EMPEROR-reduced (empagliflozin) | 1.4 | With/without | ↓ 15.8% vs. 21.0% |
| EMPA-REG (empagliflozin) | 3.1 | Yes | ↓ 37.4% vs. 43.9% | |
| Emperor-presrved (empagliflozin) | 2.4 | With/without | ↓ 13.8% vs. 17.1% | |
| Declare-TIMI (dapagliflozin) | 4.2 | Yes | ↓ 22.6% vs. 24.2% | |
| DAPA-HF (dapagliflozin) | 1.7 | With/without | ↓ 11.6% vs. 15.6% | |
| CANVAS (canagliflozin) | 3.6 | Yes | ↓ 26.9% vs. 31.5% | |
| CREDENCE (canagliflozin) | 2.6 | Yes | ↓ 11.1% vs. 15.5% | |
| VERTIS CV (ertuglifozin) | 3.5 | Yes | n 11.9% vs. 11.9% | |
| SOLOIST-WHF (sotagliflozin) | 0.9 | Yes | ↓ 51.0% vs. 76.3% | |
| SCORED (sotagliflozin) | 1.3 | Yes | ↓ 56% vs. 75% | |
| GLP1-RAs | LEADER (liraglutide) | 3.8 | Yes | ↓ 13% vs. 14.9% |
| REWIND (dulaglutide) | 5.4 | Yes | ↓ 12% vs. 13.4% | |
| HARMONY (albiglutide) | 1.6 | Yes | ↓ 7% vs. 9% | |
| SUSTAIN-6 (semaglutide) | 2.1 | Yes | ↓ 6.6% vs. 8.9% | |
| ELIXA (lixisenatide) | 2.1 | Yes | ↓ 13.4% vs. 13.2% | |
| EXSCEL (exenatide) | 3.2 | Yes | ↓ 11.4% vs. 12.2% | |
| GIP/GLP-1Ra | SUPRASS (terzipatide) | ongoing | Yes | ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreadi, A.; Muscoli, S.; Tajmir, R.; Meloni, M.; Muscoli, C.; Ilari, S.; Mollace, V.; Della Morte, D.; Bellia, A.; Di Daniele, N.; et al. Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1646. https://doi.org/10.3390/ijms24021646
Andreadi A, Muscoli S, Tajmir R, Meloni M, Muscoli C, Ilari S, Mollace V, Della Morte D, Bellia A, Di Daniele N, et al. Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. International Journal of Molecular Sciences. 2023; 24(2):1646. https://doi.org/10.3390/ijms24021646
Chicago/Turabian StyleAndreadi, Aikaterini, Saverio Muscoli, Rojin Tajmir, Marco Meloni, Carolina Muscoli, Sara Ilari, Vincenzo Mollace, David Della Morte, Alfonso Bellia, Nicola Di Daniele, and et al. 2023. "Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease" International Journal of Molecular Sciences 24, no. 2: 1646. https://doi.org/10.3390/ijms24021646
APA StyleAndreadi, A., Muscoli, S., Tajmir, R., Meloni, M., Muscoli, C., Ilari, S., Mollace, V., Della Morte, D., Bellia, A., Di Daniele, N., Tesauro, M., & Lauro, D. (2023). Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. International Journal of Molecular Sciences, 24(2), 1646. https://doi.org/10.3390/ijms24021646











