Modulation of Cell Death Pathways for Cellular Protection and Anti-Tumoral Activity: The Role of Thymus spp. Extracts and Their Bioactive Molecules
Abstract
:1. Introduction
2. Signaling Pathways in Cell Survival and Cell Death
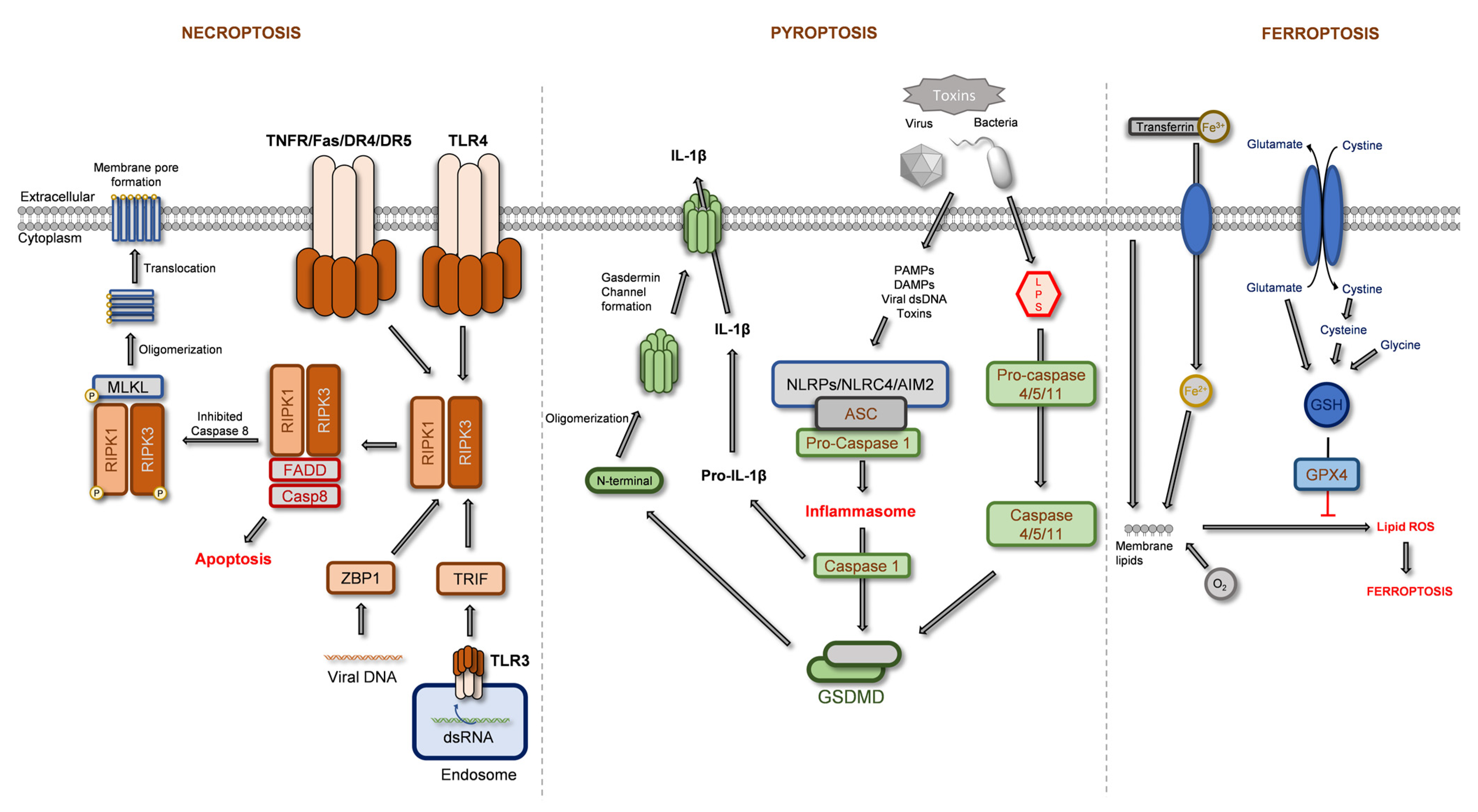
2.1. Regulated Necrosis
2.1.1. Necroptosis
2.1.2. Pyroptosis
2.1.3. Ferroptosis
2.2. Apoptosis
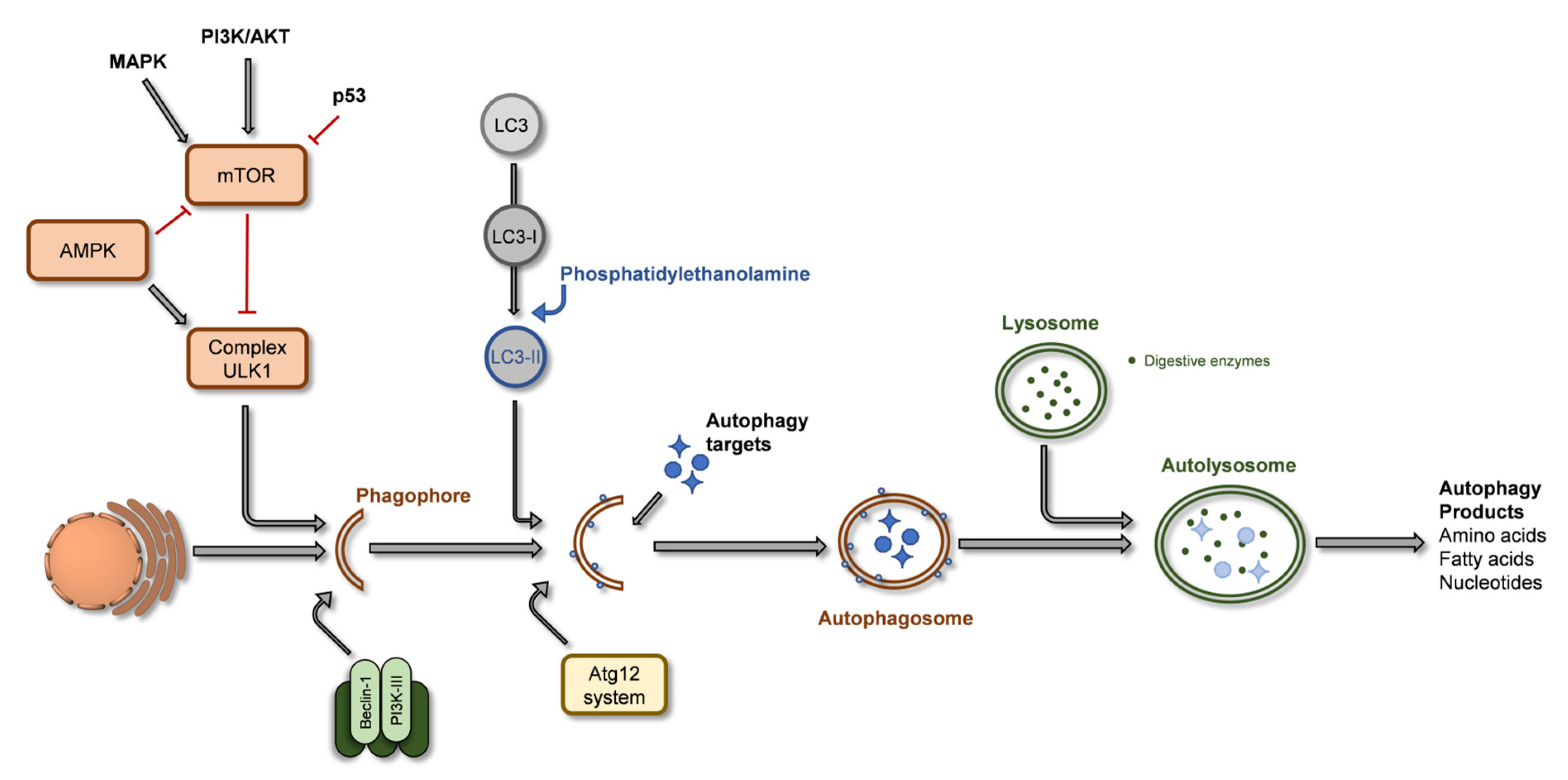
2.3. Autophagy
3. Modulation of Cell Proliferation and Cell Death by Thymus spp. Extracts
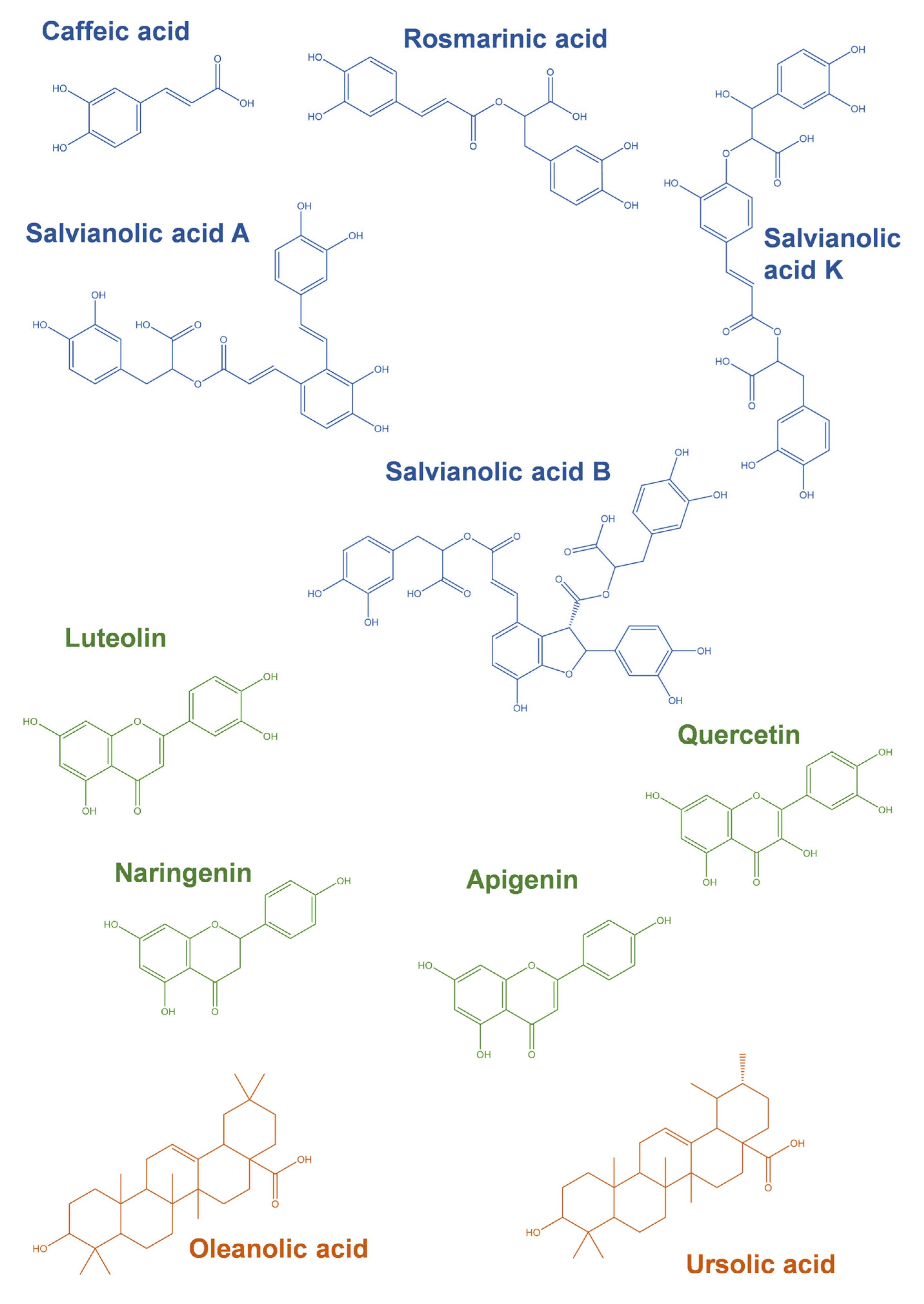
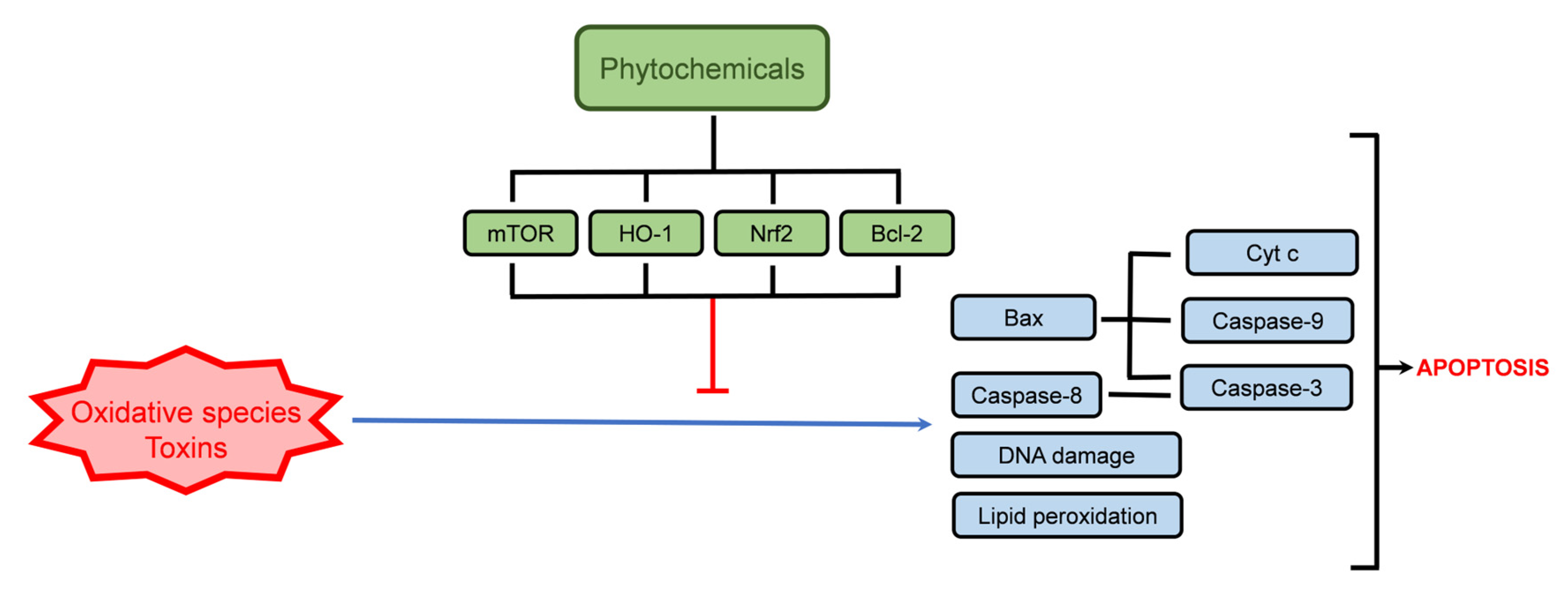
4. Modulation of Cell Survival and Cell Death Pathways by Phytochemicals Present in Thymus spp. Extracts
4.1. Cellular Protection
| Phenolic Acid | Concentration | Experimental Model | Observations | Ref. |
|---|---|---|---|---|
| Caffeic acid | 60 and 120 µM | Human peripheral blood mononuclear cells (PBMCs) | Reduced phosphatidylserine externalization in H2O2-induced apoptosis Reduced DNA fragmentation Reduced lipid peroxidation Bcl-2-independent mechanism | [108] |
| 50 µM | Human monocytic lymphoma cells (U937) | Inhibition of ceramide-induced apoptosis and NF-κB DNA-binding Tyrosine kinase inhibition | [109] | |
| 400 µM | Human neuroblastoma cells (SH-SY5Y) | Reduced cyclophosphamide-induced apoptosis and reduced DNA damage Increased Bcl-2 and reduced cyt C, Bax, and caspase-3 expression in cells exposed to cyclophosphamide | [107] | |
| Rosmarinic acid | 56 µM | Human neuroblastoma cells (SH-SY5Y) | Reduced H2O2-induced apoptosis Downregulation of caspase-3 and Bax Upregulation of Bcl-2 and HO-1 PKA and PI3K-dependent induction of HO-1 | [110] |
| 40 µM | Mouse proximal tubular epithelial cells | Reduced cadmium-induced apoptosis and DNA damage Reduced cyt c release, FAS activation, and the cleavage of caspase-3, -8 and -9 Reduced the expression of APAF-1, NF-kB, PKC and TNFR2 | [111] | |
| 6.25–50 μM | Rat liver fibroblasts (BRL-3A) | Reduced acrylamide-induced apoptosis Reduced ROS content Reduced Bax/Bcl-2 and cleaved caspase-3/caspase-3 ratio | [112] | |
| Salvianolic acid A | 50 μM | Human retinal pigmented epithelium cells (ARPE-19) | Reduced H2O2-induced apoptosis Reduced caspase-3 cleavage Upregulation of the mTORC1 (mammalian target of rapamycin complex 1) pathway Downregulation of MAPK pathway | [113] |
| 100 µg/kg | ICR mice | Protects the blood-brain barrier from apoptosis in induced ischemic brain Reduction of NF-κB pathway and cleaved caspase-3 expression Increased Bcl-2 expression | [123] | |
| 50 µM | Rat myocardium cells (H9c2) | Reduced H2O2-induced apoptosis Restored p-JNK/JNK ratio and increased thioredoxin expression | [124] | |
| Salvianolic acid B | 25–50 mg/kg | Sprague Dawley rats | Prevented myocardial infarction-induced ferroptosis Increased the expression of cystine/glutamate transporter (xCT), glutathione peroxidase 4, ferroportin 1(FPN1), and ferritin heavy chain (FTH1) | [115] |
| 10 µM | Rat myocardium cells (H9c2) | Reduced hypoxia-induced apoptosis | [125] | |
| 20 µM | Rat cerebral microvascular endothelial cells | Reduced H2O2-induced apoptosis Reduced caspase-3 and 9 activities PI3K/Akt/Raf/MEK/ERK pathway-dependent response | [114] | |
| 10 µM | Human neuroblastoma cells (SH-SY5Y) | Reduced 6-hydroxydopamine-induced apoptosis Prevented alterations in nucleus morphology Normalized intracellular calcium concentration and PKC phosphorylation | [126] | |
| Salvianolic acid C | 20 mg/kg | Liver tissue of ICR mice | Protection against acetaminophen-induced toxicity Decreased expression of Bax, cleaved caspase-3, and cyt C release | [116] |
| 5 mM | Human periodontal ligament stem cells | Decreased LPS-Induced apoptosis Cell cycle modulation Increased Bcl-2 expression Decreased Bax and caspase-3 expression | [117] | |
| Luteolin-7-O-glucoside | 10 and 20 µM | Rat myocardium cells (H9c2) | Reduced hypoxia/reoxygenation-induced apoptosis Downregulation of caspase-3, PARP, Fas, Fasl, p-ERK1/2 and p-JNK | [121] |
| 1 µM | Human neuroblastoma cells (SH-SY5Y) | Reduced 6-hydroxydopamine-induced apoptosis Decreased DNA damage and caspase-3 activity | [122] | |
| Quercetin-3-O-glucoside | 2.15–21.5 µM | Human skin fibroblasts (TIG-108) | Prevented advanced glycation end-products-induced apoptosis | [127] |
| Eriodictyol-7-O-glucoside | 30 mg/kg | Wistar rat model of cerebral ischemic injury | Reduced ischemia/reperfusion-induced apoptosis Upregulation of Nrf2, HO-1 | [128] |
4.2. Cell Death Induction
4.2.1. Phenolic Acids
| Phenolic Acid | Concentration | Experimental Model | Observations | Ref. |
|---|---|---|---|---|
| Caffeic acid | 200 µM | Human melanoma cells (SK-Mel-28) | Induced apoptosis Cell cycle arrest (G0/G1 phase) Increased caspase-1, -3, and -8 genes expression | [134] |
| 100 µM | Human breast adenocarcinoma cells (MDA-MB-231) | Induced apoptosis Cell cycle arrest (S phase) | [135] | |
| 10 mM | Human cervical cancer cells (HeLa) | Induced apoptosis Increased cleaved caspase-3 expression Induced cyt c release Downregulation of Bcl-2 Upregulation of p53 | [137] | |
| ≥200 µM | Human monocytic lymphoma cells (U937) | Apoptosis induction DNA fragmentation | [109] | |
| 300 µM | Human cervical adenocarcinoma cells (HeLa) | Anti-proliferative activity mediated by caspase-3, -7, -9 pathways | [141] | |
| 300 µM | Human cervical carcinoma cells (CaSki) | |||
| 100 µM | Human squamous carcinoma cells (Detroit 562) | Induced apoptosis Cell cycle arrest (G0/G1 phase) | [136] | |
| 400–800 μM | Human gastric carcinoma cells (SC-M1) | Apoptosis induction Ca2+-independent pathway | [138] | |
| 360 µM | Human tongue squamous cell carcinoma cells (CAL-27) | Proline dehydrogenase-dependent apoptosis Decreased DNA biosynthesis p53 and cleaved caspase-9 upregulation | [139] | |
| Rosmarinic acid | 60 μM | Human monocytic lymphoma cells (U937) | Increased TNF-α-induced apoptosis and DNA fragmentation Increased caspase-3 and -8 activity, PARP cleavage, and cyt c release Inhibited NF-kB pathway with TNF-α exposure | [140] |
| 60 μM | Human breast adenocarcinoma cells (MCF-7) | Increased TNF-α-induced apoptosis | [140] | |
| 60 μM | Human hepatic carcinoma cells (HepG2) | Increased TNF-α-induced apoptosis | [140] | |
| 200 and 400 μM | Human glioma cells (U251 and U343) | Induced apoptosis Reduction of PI3K, p-Akt, NF-κB, Fyn (Proto-oncogene tyrosine-protein kinase Fyn), and Bcl-2 expression Increased Bax and cleaved caspase-3 expression Reduced cell migration | [142] | |
| 200 μM | Human prostate adenocarcinoma cells (PC-3) | Induced apoptosis and DNA fragmentation Upregulation of p53, p21, caspase-3, cleaved PARP-1 and Bax Downregulation of histone deacetylase 2 (HDAC2), Bcl-2, cyclin D1 and cyclin E1 | [143] | |
| 200 μM | Human prostate carcinoma cells (DU145) | Induced apoptosis and necrosis Induced DNA fragmentation Upregulation of p53, caspase-3 and Bax Downregulation of histone deacetylase 2 (HDAC2), Bcl-2, cyclin D1 and cyclin E1 | [143] | |
| 125–400 μM | Human triple-negative breast adenocarcinoma cells (MDA-MB-231) | Induced apoptosis Cell cycle arrest (G0/G1 phase) Upregulation of HRK (activator of apoptosis harakiri), TNFRSF25 (TNF receptor superfamily member 25), and BNIP3 (Bcl-2 interacting protein 3) genes Downregulation of TNFRSF11B (TNF receptor superfamily member 11b) gene | [144] | |
| 125–400 μM | Human triple-negative breast adenocarcinoma cells (MDA-MB-468) | Induced apoptosis Cell cycle arrest (S phase) Upregulation of TNF, GADD45A (Growth Arrest and DNA damage-inducible alpha), and BNIP3 genes Downregulation of TNFSF10 (TNF Superfamily Member 10) and BIRC5 (Survivin/Baculoviral inhibitor of apoptosis repeat containing 5) genes | [144] | |
| 100 µM | Human colorectal adenocarcinoma cells (HCT-15) | Induced apoptosis Downregulation of phospho-ERK pathway | [145] | |
| 100 µM | Human colorectal adenocarcinoma cells (CO115) | Induced apoptosis | [145] | |
| Salvianolic acid A | 50 µM | Human acute monocytic leukemia cells (THP-1) | Induced apoptosis Increased cleaved caspase-3 and cleaved PARP Decreased Bcl-xL and p-Akt expression | [146] |
| 50 µM | Human acute myelogenous leukemia cells (KG-1) | |||
| 50 µM | Human acute myeloblastic leukemia cells (Kasumi-1) | |||
| Salvianolic acid B | 100 and 200 µM | Human hepatic adenocarcinoma or endothelial cells (SK-Hep-1) * | Induced apoptosis and autophagy Loss of mitochondria membrane depolarization Increased expression of cleaved caspase-3, cleaved caspase-9, and cleaved PARP Increased cyt C release Decreased p-Akt expression | [147] |
| 100 and 200 µM | Human liver carcinoma or HeLa derivative (Bel-7404) * | [147] | ||
| 200 µM | Human colorectal carcinoma cells (HCT116) | Induced apoptosis and autophagy Formation of autophagosomes and expression of LC3 Increased expression of cleaved caspase-3 and -9, and cleaved PARP Decreased expression of p-Akt and p-mTOR | [148] | |
| 200 µM | Human colorectal adenocarcinoma cells (HT29) | |||
| 10–100 µM | Human glioma cells (U87) | Induced apoptosis Increased expression of cleaved caspase-3, p-p38 and p-p53 | [149] |
4.2.2. Glycoside Derivatives of Flavonoids
| Flavonoid | Concentration | Experimental Model | Observations | Ref. |
|---|---|---|---|---|
| Luteolin-7-O-glucoside | 80 µM | Human nasopharyngeal carcinoma cells (NPC-039 and NPC-BM) | Induced apoptosis Cell cycle arrest (S and G2/M phases) Increased DNA condensation Increased FAS, TNFR1, RIP (ribosome-inactivating protein), DR5 (death receptor 5), cleaved caspase-3, 8 and -9, Bax, t-BID, cleaved PARP and p21 expression Reduced Bcl-xL and Bcl-2 expression Modulation of Akt pathway | [158] |
| 200 µM | Human hepatic carcinoma cells (HepG2) | Induced apoptosis and DNA damage Condensed chromatin and apoptotic bodies Increased cleaved PARP expression Upregulation of the JNK pathway Caspase-independent mechanism Cell cycle arrest (G2/M phase) | [159] | |
| 120 µM | Human colorectal adenocarcinoma cells (COLO 320 DM) | Induced apoptosis Reduction of β-catenin expression | [160] | |
| Quercetin-3-O-glucoside | 100 µM | Human hepatic carcinoma cells (HepG2) | Induced apoptosis Cell cycle arrest (S phase) Inhibition of DNA topoisomerase II Increase caspase-3 activity | [161] |
| Quercetin-3-O-glucuronide | 40 and 60 µM | Human embryonic neural stem cells | Increased cell proliferation and migration Increased p-Akt/Akt ratio Increased cyclin D1 and Brain-derived neurotrophic factor (BDNF) expression Increased C-X-C chemokine receptor type 4 gene (CXCR4) expression | [162] |
| 50 µM | Human hepatic carcinoma cells (HepG2) | Reduced doxorubicin resistance Increased DOX-induced apoptosis | [163] | |
| 100 µM | Human breast adenocarcinoma cells (MCF-7) | Induced apoptosis Cell cycle arrest (S phase) | [164] | |
| Quercetin-3-O-glucuronide + Quercetin-7-O-glucuronide + Quercetin-4′-O-glucuronide | 2.5–10 µM | Human lung carcinoma cells (NCL-H209) | Induced apoptosis Cell cycle arrest (S and G2/M phases) Increased caspase-3 activity Increased p21, Bak, and Bax expression Increased cyt C release Reduced Bcl-2 expression | [165] |
| Apigenin-7-O-glucoside | 25–100 µM | Human gastric adenocarcinoma cells (AGS) | Induced pathway apoptosis Increased expression of cleaved caspase-3, -8 and PARP, FasL Fas Induced autophagy Increased expression of LC3, p-JNK, Beclin-1 and p62 Cell cycle arrest (G2/M phase) Reduced expression of cyclin B1, M-phase inducer phosphatase 3 (CDC25C), Cyclin-dependent kinase 1 (CDK1), p-PI3K, p-Akt and p-mTOR | [166] |
4.2.3. Pentacyclic Triterpenoids
| Terpenoid | Concentration | Experimental Model | Observations | Ref. |
|---|---|---|---|---|
| Oleanolic acid | 87.6–131.3 µM | Human pancreatic adenocarcinoma cells (Panc-28) | Induced apoptosis Cell cycle arrest (S and G2/M phases) Downregulation of p21, survivin, Bcl-2 Induced depolarization of mitochondrial membrane potential, cyt C release, PARP cleavage, caspase-3 and-9 activation | [175] |
| 4 and 8 µM | Human hepatic carcinoma cells (Huh7) | Induced apoptosis DNA fragmentation Induced depolarization of mitochondrial membrane potential Reduced Na+/K+-ATPase activity Increased caspase-3 and -8 activity | [176] | |
| Human hepatic carcinoma cells (Hep3B) | ||||
| Human hepatic carcinoma cells (HepG2) | ||||
| Human hepatic carcinoma cells (HA22T) | ||||
| 40 µM | Human hepatic carcinoma cells (HepG2) | Induced apoptosis Increased Bax expression, cyt c release, and cleavage of PARP Increased caspase-3 and -9 activity Reduced Bcl-2, p-Akt, and p-mTOR expression Cell cycle arrest in G2/M | [177] | |
| 219 µM | Human lung carcinoma cells (A549) | Induced apoptosis Increased p-38 MAPK, p-JNK, and p-ERK Increased cyt c release and cleavage of PARP and caspase-3 and -9 Promoted mitochondrial translocation of Bax and Bim | [178] | |
| 219 µM | Human pancreas adenocarcinoma cells (BXPC3) | Induced apoptosis Increased p-38 MAPK, p-JNK expression Increased cyt c release and cleavage of PARP and caspase-3 and -9 Promoted mitochondrial translocation of Bax and Bim | ||
| 20 µM | Human hepatic carcinoma cells (Huh7) | Induced apoptosis Induced depolarization of mitochondrial membrane potential Increased Bax expression and cyt c release Reduced Bcl-2 expression | [179] | |
| 80 µM | Human myeloid leukemia cells (HL60) | Induced apoptosis Cell cycle arrest (G1 phase) Increased caspase-3 and PARP cleavage | [180] | |
| 30 and 60 µM | Human hepatic carcinoma cells (SMMC-7721) | Induced apoptosis and autophagy Induced depolarization of mitochondrial membrane potential Increased Bax, Beclin, and LC3 expression Reduced p-mTOR, p-Akt, Bcl-2, and p62 expression | [181] | |
| 36 µM | Human papillomavirus-related endocervical adenocarcinoma cells (SGC-7901) * | Induced autophagy Increase in p-AMK, Beclin-1, and LC3-II expression Decreased p-PI3K, p-Akt, p-ERK1/2, p-p38 and p-mTOR expression | [182] | |
| 36 µM | Human gastric mucinous adenocarcinoma cells (MGC-803) | |||
| 36 µM | Human papillomavirus-related endocervical adenocarcinoma cells (BGC-823) * | |||
| Ursolic acid | 20 µM | Human hepatic carcinoma cells (Huh7) | Induced apoptosis Induced depolarization of mitochondrial membrane potential Increased Bax expression, cyt c release, cleavage of PARP, and caspase-3 and -9 activity Reduced Bcl-2 expression | [179] |
| 4 and 8 µM | Human hepatic carcinoma cells (Huh7) | Induced apoptosis DNA fragmentation Induced depolarization of mitochondrial membrane potential Reduced Na+/K+-ATPase activity Increased caspase-3 and -8 activity | [176] | |
| Human hepatic carcinoma cells (Hep3B) | ||||
| Human hepatic carcinoma cells (HepG2) | ||||
| Human hepatic carcinoma cells (HA22T) | ||||
| 10–30 µM | Mouse lymphoblast hybridoma (TC-1) | Induce autophagy Increased LC3-II and Autophagy related 5 (Atg5) expression | [183] | |
| 30 µM | Human cervix adenocarcinoma (HeLa) | Induce apoptosis DNA damage | ||
| 40 µM | Human glioblastoma cells (U87MG) | Cell cycle arrest (G1 phase) Decreased expression of cyclin D1, D3 and E, and cyclin-dependent kinase 4 (CDK4) Induced autophagy Increased LC3-II expression, p21 and p27 Modulation of CaMKK-AMPK-mTOR kinase pathway | [184] | |
| 20 µM | Human lung carcinoma cells (A549) | Induced apoptosis Cell cycle arrest (G1 phase) Increased p53, p21/WAF1, Fas/APO-1, Fas and Bax expression Decreased expression of Bcl-2, Bcl-Xl cyclin D1, D2 and E, CDK2, CDK4 and CDK6 Inhibition of NF-kB activity | [185] | |
| 53 µM | Human breast adenocarcinoma cells (MCF-7) | Induced apoptosis Induced PARP cleavage Downregulation of Bcl-2 | [186] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.; Amini-Nik, S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int. J. Mol. Sci. 2017, 18, 1068. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Li, Y.; Li, X.; Zhou, D.; Wang, Y.; Wen, X.; Wang, C.; Liu, X.; Feng, Y.; Li, B.; et al. Functional foods and intestinal homeostasis: The perspective of in vivo evidence. Trends Food Sci. Technol. 2021, 111, 475–482. [Google Scholar] [CrossRef]
- Jain, A.; Madu, C.O.; Lu, Y. Phytochemicals in Chemoprevention: A Cost-Effective Complementary Approach. J. Cancer 2021, 12, 3686–3700. [Google Scholar] [CrossRef]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential Lead Molecules for MDR Reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef]
- Beutler, J.A. Natural Products as a Foundation for Drug Discovery. Curr. Protoc. Pharmacol. 2019, 86, e67. [Google Scholar] [CrossRef]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parasuraman, S. Herbal Drug Discovery: Challenges and Perspectives. Curr. Pharm. Pers. Med. 2018, 16, 63–68. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef] [PubMed]
- International Union for Conservation of Nature ‘s Red List of Threatened Species, Number of Species Evaluated in Relation to the Overall Number of Described Species, and Numbers of Threatened Species by Major Groups of Organisms (Table 1a; 9 December 2022 update). Available online: https://nc.iucnredlist.org/redlist/content/attachment_files/2022-2_RL_Stats_Table_1a.pdf (accessed on 28 December 2022).
- Martins-Gomes, C.; Souto, E.B.; Silva, A.M. Chapter 15—Nanophytosomes: A novel approach for the delivery of herbal drugs. In Systems of Nanovesicular Drug Delivery; Nayak, A.K., Hasnain, M.S., Aminabhavi, T.M., Torchilin, V.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 239–257. [Google Scholar] [CrossRef]
- Berghe, T.V.; Vanlangenakker, N.; Parthoens, E.; Deckers, W.; Devos, M.; Festjens, N.; Guerin, C.J.; Brunk, U.T.; Declercq, W.; Vandenabeele, P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010, 17, 922–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Tonnus, W.; Meyer, C.; Paliege, A.; Belavgeni, A.; von Mässenhausen, A.; Bornstein, S.R.; Hugo, C.; Becker, J.U.; Linkermann, A. The pathological features of regulated necrosis. J. Pathol. 2019, 247, 697–707. [Google Scholar] [CrossRef]
- Morgan, M.J.; Kim, Y.-S. Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp. Mol. Med. 2022, 54, 1695–1704. [Google Scholar] [CrossRef]
- Dai, W.; Cheng, J.; Leng, X.; Hu, X.; Ao, Y. The potential role of necroptosis in clinical diseases (Review). Int. J. Mol. Med. 2021, 47, 89. [Google Scholar] [CrossRef]
- Philipp, S.; Sosna, J.; Adam, D. Cancer and necroptosis: Friend or foe? Cellular and Molecular Life Sciences 2016, 73, 2183–2193. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, W.; Gong, F.; Chen, Y.; Chen, E. The Role and Mechanism of Pyroptosis and Potential Therapeutic Targets in Sepsis: A Review. Front. Immunol. 2021, 12, 711939. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, C.; Wang, J.; Hu, W.; Feng, Z. The Regulation of Ferroptosis by Tumor Suppressor p53 and its Pathway. Int. J. Mol. Sci. 2020, 21, 8387. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, S.J.; Stockwell, B.R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Cell Signaling Technology, I. Necrotic Cell Death. Available online: https://www.cellsignal.com/pathways/necrotic-cell-death-pathway (accessed on 21 October 2022).
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Q.; Liu, H.; Zhang, X. Interplay Between Non-Canonical NF-κB Signaling and Hepatitis B Virus Infection. Front. Immunol. 2021, 12, 4035. [Google Scholar] [CrossRef]
- Zargarian, S.; Shlomovitz, I.; Erlich, Z.; Hourizadeh, A.; Ofir-Birin, Y.; Croker, B.A.; Regev-Rudzki, N.; Edry-Botzer, L.; Gerlic, M. Phosphatidylserine externalization, “necroptotic bodies” release, and phagocytosis during necroptosis. PLoS Biol. 2017, 15, e2002711. [Google Scholar] [CrossRef] [PubMed]
- Vande Walle, L.; Lamkanfi, M. Pyroptosis. Curr. Biol. 2016, 26, R568–R572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compton, M.M. A biochemical hallmark of apoptosis: Internucleosomal degradation of the genome. Cancer Metastasis Rev. 1992, 11, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letai, A. Apoptosis and Cancer. Annu. Rev. Cancer Biol. 2017, 1, 275–294. [Google Scholar] [CrossRef]
- Chaabane, W.; User, S.D.; El-Gazzah, M.; Jaksik, R.; Sajjadi, E.; Rzeszowska-Wolny, J.; Łos, M.J. Autophagy, Apoptosis, Mitoptosis and Necrosis: Interdependence Between Those Pathways and Effects on Cancer. Arch. Immunol. Et Ther. Exp. 2013, 61, 43–58. [Google Scholar] [CrossRef] [Green Version]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Savitskaya, M.A.; Onishchenko, G.E. Mechanisms of apoptosis. Biochemistry 2015, 80, 1393–1405. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Colombo, G.; Gagliano, N.; Colombo, R.; Giustarini, D.; Rossi, R.; Milzani, A. S-Glutathiolation in life and death decisions of the cell. Free. Radic. Res. 2011, 45, 3–15. [Google Scholar] [CrossRef]
- Alenzi, F.Q.; Lotfy, M.; Wyse, R. Swords of cell death: Caspase activation and regulation. Asian Pac. J. Cancer Prev. 2010, 11, 271–280. [Google Scholar]
- Iranpour, M.; Moghadam, A.R.; Yazdi, M.; Ande, S.R.; Alizadeh, J.; Wiechec, E.; Lindsay, R.; Drebot, M.; Coombs, K.M.; Ghavami, S. Apoptosis, autophagy and unfolded protein response pathways in Arbovirus replication and pathogenesis. Expert Rev. Mol. Med. 2016, 18, e1. [Google Scholar] [CrossRef] [PubMed]
- Cell Signaling Technology, I. Cell Signaling Technology, I. Death Receptor Signaling Pathways Diagram. Available online: https://www.cellsignal.com/pathways/death-receptor-signaling (accessed on 21 October 2022).
- Cell Signaling Technology, I.; Inc. Mitochondrial Control of Apoptosis Pathways Diagram. Available online: https://www.cellsignal.com/pathways/mitochondrial-control-of-apoptosis (accessed on 21 October 2022).
- Cell Signaling Technology, I. Regulation of Apoptosis Pathways Diagram. Available online: https://www.cellsignal.com/pathways/regulation-of-apoptosis-pathway (accessed on 21 October 2022).
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speidel, D. Transcription-independent p53 apoptosis: An alternative route to death. Trends Cell Biol. 2010, 20, 14–24. [Google Scholar] [CrossRef]
- Ola, M.S.; Nawaz, M.; Ahsan, H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol. Cell. Biochem. 2011, 351, 41–58. [Google Scholar] [CrossRef]
- Lindsay, J.; Esposti, M.D.; Gilmore, A.P. Bcl-2 proteins and mitochondria—Specificity in membrane targeting for death. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011, 1813, 532–539. [Google Scholar] [CrossRef]
- Brenner, D.; Mak, T.W. Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 2009, 21, 871–877. [Google Scholar] [CrossRef]
- Jiang, P.; Du, W.; Heese, K.; Wu, M. The Bad Guy Cooperates with Good Cop p53: Bad Is Transcriptionally Up-Regulated by p53 and Forms a Bad/p53 Complex at the Mitochondria To Induce Apoptosis. Mol. Cell. Biol. 2006, 26, 9071–9082. [Google Scholar] [CrossRef] [Green Version]
- Widłak, P. The DFF40/CAD endonuclease and its role in apoptosis. Acta Biochim. Pol. 2000, 47, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Tanida, I. Autophagy basics. Microbiol. Immunol. 2011, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- White, E. The role for autophagy in cancer. J. Clin. Investig. 2015, 125, 42–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. In Autophagosome and Phagosome; Deretic, V., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 77–88. [Google Scholar] [CrossRef]
- Keller, C.W.; Münz, C.; Lünemann, J.D. Autophagy Pathways in CNS Myeloid Cell Immune Functions. Trends Neurosci. 2020, 43, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Kocaturk, N.M.; Gozuacik, D. Crosstalk Between Mammalian Autophagy and the Ubiquitin-Proteasome System. Front. Cell Dev. Biol. 2018, 6, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Guo, H.; Yang, Y.; Feng, M.; Liu, B.; Ren, X.; Zhou, H. Autophagy modulation in bladder cancer development and treatment (Review). Oncol. Rep. 2019, 42, 1647–1655. [Google Scholar] [CrossRef]
- Liang, C. Negative regulation of autophagy. Cell Death Differ. 2010, 17, 1807–1815. [Google Scholar] [CrossRef] [Green Version]
- Cell Signaling Technology, I. Autophagy Signaling. Available online: https://www.cellsignal.com/pathways/autophagy-signaling-pathway (accessed on 29 December 2022).
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef]
- Gump, J.M.; Thorburn, A. Autophagy and apoptosis: What is the connection? Trends Cell Biol. 2011, 21, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Getia, M.; Korkotadze, T.; Moshiashvili, G.; Tabatadze, N.; Legault, J.; Mshvildadze, V. Composition and Cytotoxicity of Essential Oils from Aerial Parts of Thymus tiflisiensis and T. collinus Growing in Georgia. Chem. Nat. Compd. 2022, 58, 959–961. [Google Scholar] [CrossRef]
- Morales, R. Synopsis of the genus Thymus L. in the Mediterranean area. Lagascalia 1997, 19, 249–262. [Google Scholar]
- Taghouti, M.; Martins-Gomes, C.; Félix, L.M.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Polyphenol composition and biological activity of Thymus citriodorus and Thymus vulgaris: Comparison with endemic Iberian Thymus species. Food Chem. 2020, 331, 127362. [Google Scholar] [CrossRef] [PubMed]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Chemical Characterization and Bioactivity of Extracts from Thymus mastichina: A Thymus with a Distinct Salvianolic Acid Composition. Antioxidants 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Félix, L.M.; Teixeira, I.; Martins-Gomes, C.; Schäfer, J.; Souto, E.B.; Santos, D.J.; Bunzel, M.; Nunes, F.M. Orange thyme: Phytochemical profiling, in vitro bioactivities of extracts and potential health benefits. Food Chem. X 2021, 12, 100171. [Google Scholar] [CrossRef]
- Khouya, T.; Ramchoun, M.; Hmidani, A.; Amrani, S.; Harnafi, H.; Benlyas, M.; Filali Zegzouti, Y.; Alem, C. Anti-inflammatory, anticoagulant and antioxidant effects of aqueous extracts from Moroccan thyme varieties. Asian Pac. J. Trop. Biomed. 2015, 5, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, A.A.; Djordjević, V.B.; Petrović, P.M.; Pljevljakušić, D.S.; Zdunić, G.M.; Šavikin, K.P.; Bugarski, B.M. The influence of different extraction conditions on polyphenol content, antioxidant and antimicrobial activities of wild thyme. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100328. [Google Scholar] [CrossRef]
- Asghari, B.; Habibzadeh, F.; Ghorbani Nohooji, M. Persian Thyme (Thymus persicus): A Plant Containing Active Metabolites with Antioxidant, Anti-diabetic and Anti-Alzheimer Effects. J. Med. Plants 2019, 18, 97–109. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus pulegioides L. as a rich source of antioxidant, anti-proliferative and neuroprotective phenolic compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Taghouti, M.; Schäfer, J.; Bunzel, M.; Silva, A.M.; Nunes, F.M. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods 2018, 43, 154–164. [Google Scholar] [CrossRef]
- Hajializadeh, Z.; Nasri, S.; Kaeidi, A.; Sheibani, V.; Rasoulian, B.; Esmaeili-Mahani, S. Inhibitory effect of Thymus caramanicus Jalas on hyperglycemia-induced apoptosis in in vitro and in vivo models of diabetic neuropathic pain. J. Ethnopharmacol. 2014, 153, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, E.; Atmaca, H.; Kisim, A.; Uzunoglu, S.; Uslu, R.; Karaca, B. Effects of Thymus serpyllum Extract on Cell Proliferation, Apoptosis and Epigenetic Events in Human Breast Cancer Cells. Nutr. Cancer 2012, 64, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- N. Adham, A.; F. Hegazy, M.E.; Naqishbandi, A.M.; Efferth, T. Induction of Apoptosis, Autophagy and Ferroptosis by Thymus vulgaris and Arctium lappa Extract in Leukemia and Multiple Myeloma Cell Lines. Molecules 2020, 25, 5016. [Google Scholar] [CrossRef] [PubMed]
- Al-seragy, I.M.H.; Kharat, K.R.; Dhabe, A.S. Cell Cycle Arrest and Induction of Apoptosis in Human Breast Cancer Cells (T-47D) by Annona squamosa L. and Thymus vulgaris L. Ethanolic Extract. J. Biol. Act. Prod. Nat. 2019, 9, 47–56. [Google Scholar] [CrossRef]
- Heidari, Z.; Salehzadeh, A.; Sadat Shandiz, S.A.; Tajdoost, S. Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech 2018, 8, 177. [Google Scholar] [CrossRef]
- Al-Menhali, A.; Al-Rumaihi, A.; Al-Mohammed, H.; Al-Mazrooey, H.; Al-Shamlan, M.; AlJassim, M.; Al-Korbi, N.; Eid, A.H. Thymus vulgaris (Thyme) Inhibits Proliferation, Adhesion, Migration, and Invasion of Human Colorectal Cancer Cells. J. Med. Food 2014, 18, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Souto, E.B.; Cosme, F.; Nunes, F.M.; Silva, A.M. Thymus carnosus extracts induce anti-proliferative activity in Caco-2 cells through mechanisms that involve cell cycle arrest and apoptosis. J. Funct. Foods 2019, 54, 128–135. [Google Scholar] [CrossRef]
- Oliviero, M.; Romilde, I.; Beatrice, M.M.; Matteo, V.; Giovanna, N.; Consuelo, A.; Claudio, C.; Giorgio, S.; Filippo, M.; Massimo, N. Evaluations of thyme extract effects in human normal bronchial and tracheal epithelial cell lines and in human lung cancer cell line. Chem. Biol. Interact. 2016, 256, 125–133. [Google Scholar] [CrossRef]
- Soliman, M.M.; Aldhahrani, A.; Metwally, M.M.M. Hepatoprotective effect of Thymus vulgaris extract on sodium nitrite-induced changes in oxidative stress, antioxidant and inflammatory marker expression. Sci. Rep. 2021, 11, 5747. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; Refaat, B.; Qasem, A.H.; Khan, A.; Ghaith, M.; Almasmoum, H.; Mahbub, A.; Almaimani, R.A. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Res. 2019, 26, 22736–22746. [Google Scholar] [CrossRef]
- Ustuner, O.; Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Diren Sigirci, B.; Ak, S.; Akpulat, H.A.; Donmez, C.; Koca-Caliskan, U. In Vitro Evaluation of Antioxidant, Anti-Inflammatory, Antimicrobial and Wound Healing Potential of Thymus Sipyleus Boiss. Subsp. Rosulans (Borbas) Jalas. Molecules 2019, 24, 3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy). Ind. Crops Prod. 2015, 63, 256–263. [Google Scholar] [CrossRef]
- Kumar, S.; Yadav, A.; Yadav, M.; Yadav, J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res. Notes 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef] [PubMed]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a matter of time: The impact of daily and seasonal rhythms on phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Galasso, S.; Pacifico, S.; Kretschmer, N.; Pan, S.-P.; Marciano, S.; Piccolella, S.; Monaco, P.; Bauer, R. Influence of seasonal variation on Thymus longicaulis C. Presl chemical composition and its antioxidant and anti-inflammatory properties. Phytochemistry 2014, 107, 80–90. [Google Scholar] [CrossRef]
- Raudone, L.; Zymone, K.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V.; Janulis, V. Phenological changes in triterpenic and phenolic composition of Thymus L. species. Ind. Crops Prod. 2017, 109, 445–451. [Google Scholar] [CrossRef]
- Gordo, J.; Máximo, P.; Cabrita, E.; Lourenço, A.; Oliva, A.; Almeida, J.; Filipe, M.; Cruz, P.; Barcia, R.; Santos, M.; et al. Thymus mastichina: Chemical Constituents and their Anti-Cancer Activity. Nat. Prod. Commun. 2012, 7, 1934578X1200701120. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Righi, N.; Boumerfeg, S.; Fernandes, P.A.R.; Deghima, A.; Baali, F.; Coelho, E.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Thymus algeriensis Bioss & Reut: Relationship of phenolic compounds composition with in vitro/in vivo antioxidant and antibacterial activity. Food Res. Int. 2020, 136, 109500. [Google Scholar] [CrossRef]
- Sobeh, M.; Rezq, S.; Cheurfa, M.; Abdelfattah, M.A.O.; Rashied, R.M.H.; El-Shazly, A.M.; Yasri, A.; Wink, M.; Mahmoud, M.F. Thymus algeriensis and Thymus fontanesii: Chemical Composition, In Vivo Antiinflammatory, Pain Killing and Antipyretic Activities: A Comprehensive Comparison. Biomolecules 2020, 10, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdi, I.; Bakrim, W.B.; Bitchagno, G.T.M.; Annaz, H.; Mahmoud, M.F.; Sobeh, M. Unraveling the Phytochemistry, Traditional Uses, and Biological and Pharmacological Activities of Thymus algeriensis Boiss. & Reut. Oxidative Med. Cell. Longev. 2022, 2022, 6487430. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds characterization by LC-DAD- ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Nabet, N.; Gilbert-López, B.; Madani, K.; Herrero, M.; Ibáñez, E.; Mendiola, J.A. Optimization of microwave-assisted extraction recovery of bioactive compounds from Origanum glandulosum and Thymus fontanesii. Ind. Crops Prod. 2019, 129, 395–404. [Google Scholar] [CrossRef]
- Bendif, H.; Peron, G.; Miara, M.D.; Sut, S.; Dall’Acqua, S.; Flamini, G.; Maggi, F. Total phytochemical analysis of Thymus munbyanus subsp. coloratus from Algeria by HS-SPME-GC-MS, NMR and HPLC-MSn studies. J. Pharm. Biomed. Anal. 2020, 186, 113330. [Google Scholar] [CrossRef] [PubMed]
- Haile, T.; Cardoso, S.M.; de Oliveira Raphaelli, C.; Pereira, O.R.; Pereira, E.d.S.; Vizzotto, M.r.; Nora, L.; Asfaw, A.A.; Periasamy, G.; Karim, A. Chemical Composition, Antioxidant Potential, and Blood Glucose Lowering Effect of Aqueous Extract and Essential Oil of Thymus Serrulatus Hochst. Ex Benth. Front. Pharmacol. 2021, 12, 621536. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Nunes, F.M.; Sampaio, A.; Souto, E.B.; Silva, A.M. Rosmarinic acid: Sources, bioactivities and health benefits. In Phytochemicals: Plant Sources and Potential Health Benefits; Ryan, I., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2019; pp. 109–146. [Google Scholar]
- Stanoeva, J.P.; Stefova, M.; Andonovska, K.B.; Stafilov, T. LC/DAD/MSn and ICP-AES Assay and Correlations between Phenolic Compounds and Toxic Metals in Endemic Thymus alsarensis from the Thallium Enriched Allchar Locality. Nat. Prod. Commun. 2017, 12, 1934578X1701200206. [Google Scholar] [CrossRef] [Green Version]
- Ayna, A.; Özbolat, S.N.; Darendelioglu, E. Quercetin, chrysin, caffeic acid and ferulic acid ameliorate cyclophosphamide-induced toxicities in SH-SY5Y cells. Mol. Biol. Rep. 2020, 47, 8535–8543. [Google Scholar] [CrossRef]
- Khanduja, K.L.; Avti, P.K.; Kumar, S.; Mittal, N.; Sohi, K.K.; Pathak, C.M. Anti-apoptotic activity of caffeic acid, ellagic acid and ferulic acid in normal human peripheral blood mononuclear cells: A Bcl-2 independent mechanism. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 283–289. [Google Scholar] [CrossRef]
- Nardini, M.; Leonardi, F.; Scaccini, C.; Virgili, F. Modulation of ceramide-induced NF-κB binding activity and apoptotic response by caffeic acid in U937 cells: Comparison with other antioxidants. Free. Radic. Biol. Med. 2001, 30, 722–733. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, H.-S.; Park, E.; Kim, S.; Lee, S.-Y.; Kim, C.-S.; Kim, D.K.; Kim, S.-J.; Chun, H.S. Rosmarinic acid protects human dopaminergic neuronal cells against hydrogen peroxide-induced apoptosis. Toxicology 2008, 250, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Joardar, S.; Dewanjee, S.; Bhowmick, S.; Dua, T.K.; Das, S.; Saha, A.; De Feo, V. Rosmarinic Acid Attenuates Cadmium-Induced Nephrotoxicity via Inhibition of Oxidative Stress, Apoptosis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Z.; Minghua, W.; Bo, N.; Chaoyue, Y.; Haiyang, Y.; Haiqing, Y.; Chunyu, X.; Yan, Z.; Yuan, Y. Rosmarinic acid attenuates acrylamide induced apoptosis of BRL-3A cells by inhibiting oxidative stress and endoplasmic reticulum stress. Food Chem. Toxicol. 2021, 151, 112156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.-Y.; Jiang, Q.; Li, K.-R.; Zhao, Y.-X.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free. Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef]
- Liu, C.-L.; Xie, L.-X.; Li, M.; Durairajan, S.S.K.; Goto, S.; Huang, J.-D. Salvianolic Acid B Inhibits Hydrogen Peroxide-Induced Endothelial Cell Apoptosis through Regulating PI3K/Akt Signaling. PLoS ONE 2007, 2, e1321. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Shen, X.; Wang, S.; Zhang, Y.; Wang, Y.; Ding, Y.; Shen, J.; Zhao, J.; Qin, H.; Xu, Y.; et al. Protective effects of Salvianolic acid B on rat ferroptosis in myocardial infarction through upregulating the Nrf2 signaling pathway. Int. Immunopharmacol. 2022, 112, 109257. [Google Scholar] [CrossRef]
- Wu, C.-T.; Deng, J.-S.; Huang, W.-C.; Shieh, P.-C.; Chung, M.-I.; Huang, G.-J. Salvianolic Acid C against Acetaminophen-Induced Acute Liver Injury by Attenuating Inflammation, Oxidative Stress, and Apoptosis through Inhibition of the Keap1/Nrf2/HO-1 Signaling. Oxidative Med. Cell. Longev. 2019, 2019, 9056845. [Google Scholar] [CrossRef]
- Duan, Y.; An, W.; Wu, H.; Wu, Y. Salvianolic Acid C Attenuates LPS-Induced Inflammation and Apoptosis in Human Periodontal Ligament Stem Cells via Toll-Like Receptors 4 (TLR4)/Nuclear Factor kappa B (NF-κB) Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 9499–9508. [Google Scholar] [CrossRef]
- Bayrami, Z.; Khalighi-Sigaroodi, F.; Rahimi, R.; Farzaei, M.H.; Hodjat, M.; Baeeri, M.; Rahimifard, M.; Navaei-nigjeh, M.; Abdollahi, M.; Hajiaghaee, R. In vitro wound healing activity of luteolin. Res. J. Pharmacogn. 2017, 4, 7. [Google Scholar]
- Chen, L.-Y.; Cheng, H.-L.; Kuan, Y.-H.; Liang, T.-J.; Chao, Y.-Y.; Lin, H.-C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef]
- Chang, H.; Li, C.; Huo, K.; Wang, Q.; Lu, L.; Zhang, Q.; Wang, Y.; Wang, W. Luteolin Prevents H2O2-Induced Apoptosis in H9C2 Cells through Modulating Akt-P53/Mdm2 Signaling Pathway. BioMed Res. Int. 2016, 2016, 5125836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Yang, B.; Xu, Y.; Rong, Y.; Qiu, Y. Protection of Luteolin-7-O-glucoside against apoptosis induced by hypoxia/reoxygenation through the MAPK pathways in H9c2 cells. Mol. Med. Rep. 2018, 17, 7156–7162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehfeldt, S.C.H.; Silva, J.; Alves, C.; Pinteus, S.; Pedrosa, R.; Laufer, S.; Goettert, M.I. Neuroprotective Effect of Luteolin-7-O-Glucoside against 6-OHDA-Induced Damage in Undifferentiated and RA-Differentiated SH-SY5Y Cells. Int. J. Mol. Sci. 2022, 23, 2914. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-Y.; Chuang, C.-H.; Chern, C.-M.; Liou, K.-T.; Liu, D.-Z.; Hou, Y.-C.; Shen, Y.-C. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free. Radic. Biol. Med. 2016, 99, 508–519. [Google Scholar] [CrossRef]
- Zhou, R.; Gao, J.; Xiang, C.; Liu, Z.; Zhang, Y.; Zhang, J.; Yang, H. Salvianolic acid A attenuated myocardial infarction–induced apoptosis and inflammation by activating Trx. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 991–1002. [Google Scholar] [CrossRef]
- Xu, L.; Deng, Y.; Feng, L.; Li, D.; Chen, X.; Ma, C.; Liu, X.; Yin, J.; Yang, M.; Teng, F.; et al. Cardio-Protection of Salvianolic Acid B through Inhibition of Apoptosis Network. PLoS ONE 2011, 6, e24036. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.-L.; Wang, X.-J.; Sun, Y.-N.; Li, C.-R.; Xing, Y.-L.; Zhao, H.-B.; Duan, M.; Zhou, Z.; Wang, S.-Q. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents 6-hydroxydopamine induced apoptosis in SH-SY5Y cells. Int. J. Biochem. Cell Biol. 2008, 40, 409–422. [Google Scholar] [CrossRef]
- Shimoda, H.; Nakamura, S.; Morioka, M.; Tanaka, J.; Matsuda, H.; Yoshikawa, M. Effect of Cinnamoyl and Flavonol Glucosides Derived from Cherry Blossom Flowers on the Production of Advanced Glycation End Products (AGEs) and AGE-induced Fibroblast Apoptosis. Phytother. Res. 2011, 25, 1328–1335. [Google Scholar] [CrossRef]
- Jing, X.; Ren, D.; Wei, X.; Shi, H.; Zhang, X.; Perez, R.G.; Lou, H.; Lou, H. Eriodictyol-7-O-glucoside activates Nrf2 and protects against cerebral ischemic injury. Toxicol. Appl. Pharmacol. 2013, 273, 672–679. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Z.; Wang, Z.; Wang, X. Topical application of quercetin improves wound healing in pressure ulcer lesions. Exp. Dermatol. 2018, 27, 779–786. [Google Scholar] [CrossRef]
- Özyurt, H.; Çevik, Ö.; Özgen, Z.; Özden, A.S.; Çadırcı, S.; Elmas, M.A.; Ercan, F.; Gören, M.Z.; Şener, G. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free. Radic. Res. 2014, 48, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Ma, J.-Q.; Sun, Y.-Z. Quercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environ. Toxicol. Pharmacol. 2010, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, D.D.; Wang, L.; Lou, H.; Ren, D. Eriodictyol-7-O-glucoside, a novel Nrf2 activator, confers protection against cisplatin-induced toxicity. Food Chem. Toxicol. 2012, 50, 1927–1932. [Google Scholar] [CrossRef]
- Han, X.; Ren, D.; Fan, P.; Shen, T.; Lou, H. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9C2 cells. Eur. J. Pharmacol. 2008, 581, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.B.M.; Pillat, M.M.; Mânica, A.; Stefanello, N.; Weis, G.C.C.; de Oliveira Alves, A.; de Andrade, C.M.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzębska-Stojko, Ż.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules 2017, 22, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziedzic, A.; Kubina, R.; Kabała-Dzik, A.; Tanasiewicz, M. Induction of Cell Cycle Arrest and Apoptotic Response of Head and Neck Squamous Carcinoma Cells (Detroit 562) by Caffeic Acid and Caffeic Acid Phenethyl Ester Derivative. Evid. Based Complement. Altern. Med. 2017, 2017, 6793456. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.-C.; Hsieh, C.-H.; Hsiao, M.-W.; Lin, W.-C.; Hung, Y.-C.; Ye, J.-C. Caffeic Acid Induces Apoptosis in Human Cervical Cancer Cells Through the Mitochondrial Pathway. Taiwan. J. Obstet. Gynecol. 2010, 49, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.T.; Chen, I.L.; Chou, C.T.; Liang, W.Z.; Kuo, D.H.; Shieh, P.; Jan, C.R. Effect of caffeic acid on Ca(2+) homeostasis and apoptosis in SCM1 human gastric cancer cells. Arch. Toxicol. 2013, 87, 2141–2150. [Google Scholar] [CrossRef]
- Celińska-Janowicz, K.; Zaręba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Pałka, J.; Miltyk, W. Constituents of Propolis: Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27). Front. Pharmacol. 2018, 9, 336. [Google Scholar] [CrossRef] [Green Version]
- Moon, D.-O.; Kim, M.-O.; Lee, J.-D.; Choi, Y.H.; Kim, G.-Y. Rosmarinic acid sensitizes cell death through suppression of TNF-α-induced NF-κB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 2010, 288, 183–191. [Google Scholar] [CrossRef]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Tang, H.; Pan, Y.; Hu, B.; Huang, G. Rosmarinic acid inhibits cell proliferation, migration, and invasion and induces apoptosis in human glioma cells. Int. J. Mol. Med. 2021, 47, 67. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-G.; Hwang, K.-A.; Choi, K.-C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef] [Green Version]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F.A. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia Fruticosa, Salvia Officinalis, and Rosmarinic Acid Induce Apoptosis and Inhibit Proliferation of Human Colorectal Cell Lines: The Role in MAPK/ERK Pathway. Nutr. Cancer 2009, 61, 564–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, R.; Si, T.; Lu, Y.; Zhou, J.X.; Jiang, L. Salvianolic acid A, a novel PI3K/Akt inhibitor, induces cell apoptosis and suppresses tumor growth in acute myeloid leukemia. Leuk. Lymphoma 2018, 59, 1959–1967. [Google Scholar] [CrossRef]
- Gong, L.; Di, C.; Xia, X.; Wang, J.; Chen, G.; Shi, J.; Chen, P.; Xu, H.; Zhang, W. AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int. J. Oncol. 2016, 49, 2538–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, Z.; Fei, W.; Zhou, J.; Zhang, L.; Chen, L.; Zhang, X.; Liang, X.; Xie, J.; Fang, Y.; Sui, X.; et al. Salvianolic acid B, a novel autophagy inducer, exerts antitumor activity as a single agent in colorectal cancer cells. Oncotarget 2016, 7, 61509–61519. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-S.; Luo, P.; Dai, S.-H.; Liu, Z.-B.; Zheng, X.-R.; Chen, T. Salvianolic Acid B Induces Apoptosis in Human Glioma U87 Cells Through p38-Mediated ROS Generation. Cell. Mol. Neurobiol. 2013, 33, 921–928. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef] [PubMed]
- Rebouissou, S.; Zucman-Rossi, J.; Moreau, R.; Qiu, Z.; Hui, L. Note of caution: Contaminations of hepatocellular cell lines. J. Hepatol. 2017, 67, 896–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Jia, Y.; Pan, H.; Ding, H. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother. Pharmacol. 2017, 79, 1031–1041. [Google Scholar] [CrossRef]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017, 43, 1803–1812. [Google Scholar] [CrossRef]
- Wang, S.; Wuniqiemu, T.; Tang, W.; Teng, F.; Bian, Q.; Yi, L.; Qin, J.; Zhu, X.; Wei, Y.; Dong, J. Luteolin inhibits autophagy in allergic asthma by activating PI3K/Akt/mTOR signaling and inhibiting Beclin-1-PI3KC3 complex. Int. Immunopharmacol. 2021, 94, 107460. [Google Scholar] [CrossRef]
- Ho, H.-Y.; Chen, P.-J.; Lo, Y.-S.; Lin, C.-C.; Chuang, Y.-C.; Hsieh, M.-J.; Chen, M.-K. Luteolin-7-O-glucoside inhibits cell proliferation and modulates apoptosis through the AKT signaling pathway in human nasopharyngeal carcinoma. Environ. Toxicol. 2021, 36, 2013–2024. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Lee, E.J.; Kim, H.R.; Hwang, K.A. Molecular mechanisms of luteolin-7-O-glucoside-induced growth inhibition on human liver cancer cells: G2/M cell cycle arrest and caspase-independent apoptotic signaling pathways. BMB Rep. 2013, 46, 611–616. [Google Scholar] [CrossRef] [Green Version]
- Baskar, A.A.; Ignacimuthu, S.; Michael, G.P.; Al Numair, K.S. Cancer Chemopreventive Potential of Luteolin-7-O-Glucoside Isolated From Ophiorrhiza mungos Linn. Nutr. Cancer 2011, 63, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Sudan, S.; Rupasinghe, H.P.V. Quercetin-3-O-glucoside Induces Human DNA Topoisomerase II Inhibition, Cell Cycle Arrest and Apoptosis in Hepatocellular Carcinoma Cells. Anticancer Res. 2014, 34, 1691. [Google Scholar] [PubMed]
- Baral, S.; Pariyar, R.; Kim, J.; Lee, H.-S.; Seo, J. Quercetin-3-O-glucuronide promotes the proliferation and migration of neural stem cells. Neurobiol. Aging 2017, 52, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Yang, M.-Y.; Wang, C.-J. Quercetin-3-O-glucuronide inhibits doxorubicin resistance by reducing endoplasmic reticulum stress in hepatocellular carcinoma cells. J. Funct. Foods 2019, 54, 301–309. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Yang, J.-H.; Hsia, T.-C.; Kuo, H.-M.; Chao, P.-D.L.; Chou, C.-C.; Wei, Y.-H.; Chung, J.-G. Inhibition of lung cancer cell growth by qyercetin glucoronides via G2/M arrest and induction of apoptosis. Drug Metab. Dispos. 2006, 34, 296. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Vetrivel, P.; Ha, S.E.; Kim, H.H.; Kim, J.-A.; Kim, G.S. Apigetrin induces extrinsic apoptosis, autophagy and G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS human gastric cancer cell. J. Nutr. Biochem. 2020, 83, 108427. [Google Scholar] [CrossRef]
- Zheng, S.-Y.; Li, Y.; Jiang, D.; Zhao, J.; Ge, J.-F. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol. Med. Rep. 2012, 5, 822–826. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhang, K.; Zhang, Q.; Mei, J.; Chen, C.-j.; Feng, Z.-z.; Yu, D.-h. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol. In Vitro 2012, 26, 221–228. [Google Scholar] [CrossRef]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin Suppresses Twist to Induce Apoptosis in MCF-7 Breast Cancer Cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Long, C.; Junming, T.; Qihuan, L.; Youshun, Z.; Chan, Z. Quercetin-induced apoptosis of HL-60 cells by reducing PI3K/Akt. Mol. Biol. Rep. 2012, 39, 7785–7793. [Google Scholar] [CrossRef] [PubMed]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39, BSR20190720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Liu, R.; Li, J.; Mao, J.; Lei, Y.; Wu, J.; Zeng, J.; Zhang, T.; Wu, H.; Chen, L.; et al. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy 2011, 7, 966–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, L.; Orazizadeh, M.; Niazvand, F.; Abbaspour, M.R.; Mansouri, E.; Khodadadi, A. Quercetin induces apoptosis and necroptosis in MCF-7 breast cancer cells. Bratisl. Lek. Listy 2017, 118, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Liu, M.; Liu, H.; Wang, H.; Wang, F.; Zhang, Y.; Han, L.; Lin, X. Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J. Appl. Toxicol. 2013, 33, 756–765. [Google Scholar] [CrossRef]
- Yan, S.-L.; Huang, C.-Y.; Wu, S.-T.; Yin, M.-C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N.; Zhang, W.; Wang, Z.; Hai, C. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK–p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef]
- Liu, J.; Wu, N.; Ma, L.N.; Zhong, J.T.; Liu, G.; Zheng, L.H.; Lin, X.K. p38 MAPK signaling mediates mitochondrial apoptosis in cancer cells induced by oleanolic acid. Asian Pac. J. Cancer Prev. 2014, 15, 4519–4525. [Google Scholar] [CrossRef] [Green Version]
- Shyu, M.-H.; Kao, T.-C.; Yen, G.-C. Oleanolic Acid and Ursolic Acid Induce Apoptosis in HuH7 Human Hepatocellular Carcinoma Cells through a Mitochondrial-Dependent Pathway and Downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef]
- Zhang, P.; Li, H.; Chen, D.; Ni, J.; Kang, Y.; Wang, S. Oleanolic Acid Induces Apoptosis in Human Leukemia Cells through Caspase Activation and Poly(ADP-ribose) Polymerase Cleavage. Acta Biochim. Biophys. Sin. 2007, 39, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Zeng, X.; Wu, X. Effect of Oleanolic Acid on Apoptosis and Autophagy of SMMC-7721 Hepatoma Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921606. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wang, Y.; Qin, Y.; Gong, X.-G. Oleanolic acid induces autophagic death in human gastric cancer cells in vitro and in vivo. Cell Biol. Int. 2016, 40, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Hao, Y.; Du, D.; Xie, S.; Hong, L.; Gu, H.; Zhu, X.; Zhang, J.; Fan, D.; Kung, H.-f. Ursolic acid promotes cancer cell death by inducing Atg5-dependent autophagy. Int. J. Cancer 2013, 133, 2781–2790. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, Y.; Zhang, R.; Tu, X.; Gong, X. Ursolic acid induces autophagy in U87MG cells via ROS-dependent endoplasmic reticulum stress. Chem. Biol. Interact. 2014, 218, 28–41. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, C.-C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef]
- Kassi, E.; Sourlingas, T.G.; Spiliotaki, M.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Moutsatsou, P. Ursolic Acid Triggers Apoptosis and Bcl-2 Downregulation in MCF-7 Breast Cancer Cells. Cancer Investig. 2009, 27, 723–733. [Google Scholar] [CrossRef]
- Oparina, N.; Erlandsson, M.C.; Fäldt Beding, A.; Parris, T.; Helou, K.; Karlsson, P.; Einbeigi, Z.; Bokarewa, M.I. Prognostic Significance of BIRC5/Survivin in Breast Cancer: Results from Three Independent Cohorts. Cancers 2021, 13, 2209. [Google Scholar] [CrossRef]
- Reisman, S.A.; Aleksunes, L.M.; Klaassen, C.D. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem. Pharmacol. 2009, 77, 1273–1282. [Google Scholar] [CrossRef] [Green Version]
- Ehrhardt, H.; Fulda, S.; Führer, M.; Debatin, K.M.; Jeremias, I. Betulinic acid-induced apoptosis in leukemia cells. Leukemia 2004, 18, 1406–1412. [Google Scholar] [CrossRef]
- Schmidt, M.L.; Kuzmanoff, K.L.; Ling-Indeck, L.; Pezzuto, J.M. Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur. J. Cancer 1997, 33, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Kroemer, G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov. Today 2009, 14, 885–890. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Potze, L.; Mullauer, F.B.; Colak, S.; Kessler, J.H.; Medema, J.P. Betulinic acid-induced mitochondria-dependent cell death is counterbalanced by an autophagic salvage response. Cell Death Dis. 2014, 5, e1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Li, S.; Qu, Z.; Luo, Y.; Chen, R.; Wei, S.; Yang, X.; Wang, Q. Betulinic acid induces autophagy-mediated apoptosis through suppression of the PI3K/AKT/mTOR signaling pathway and inhibits hepatocellular carcinoma. Am. J. Transl. Res. 2019, 11, 6952–6964. [Google Scholar] [PubMed]
- Sung, B.; Kang, Y.J.; Kim, D.H.; Hwang, S.Y.; Lee, Y.; Kim, M.; Yoon, J.-H.; Kim, C.M.; Chung, H.Y.; Kim, N.D. Corosolic acid induces apoptotic cell death in HCT116 human colon cancer cells through a caspase-dependent pathway. Int. J. Mol. Med. 2014, 33, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Nho, K.J.; Chun, J.M.; Kim, H.K. Corosolic acid induces apoptotic cell death in human lung adenocarcinoma A549 cells in vitro. Food Chem. Toxicol. 2013, 56, 8–17. [Google Scholar] [CrossRef]
- Xu, Y.; Ge, R.; Du, J.; Xin, H.; Yi, T.; Sheng, J.; Wang, Y.; Ling, C. Corosolic acid induces apoptosis through mitochondrial pathway and caspases activation in human cervix adenocarcinoma HeLa cells. Cancer Lett. 2009, 284, 229–237. [Google Scholar] [CrossRef]
- Guo, X.; Cui, R.; Zhao, J.; Mo, R.; Peng, L.; Yan, M. Corosolic acid protects hepatocytes against ethanol-induced damage by modulating mitogen-activated protein kinases and activating autophagy. Eur. J. Pharmacol. 2016, 791, 578–588. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, H.; Wang, Y.; Zhao, A.; Zhu, Z.; Bao, X.; Sun, Y.; Li, L.; Zhang, Q. Corosolic acid, a natural triterpenoid, induces ER stress-dependent apoptosis in human castration resistant prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and TRIB3. J. Exp. Clin. Cancer Res. 2018, 37, 210. [Google Scholar] [CrossRef]
- Falé, P.L.; Ascensão, L.; Serralheiro, M.L. Effect of luteolin and apigenin on rosmarinic acid bioavailability in Caco-2 cell monolayers. Food Funct. 2013, 4, 426–431. [Google Scholar] [CrossRef] [PubMed]


| Thymus spp. | Main Phytochemicals | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Acids | Flavonoid Derivatives | Terpenoids | ||||||||||
| CA | RA | SA | E | A | L | Q | C | N | OA | UA | ||
| Thymus pulegioides | • | • | SAI | -di-O-H -O-H -O-Hrn | -O-Hrn | -O-H -O-Hrn | - | -O-H | -O-H | • | • | [77,95] |
| Thymus. fragrantissimus | - | • | SAK SAI | -O-Hrn | -O-Hrn | -O-H -O-Hrn | r. | r. | r. | - | - | [73] |
| Thymus carnosus | • | • | SAA iso SAA SAK SAI | -O-H | r. | -O-H -O-H-P | r. | r. | - | • | • | [78] |
| Thymus mastichina | • | • | SAA iso SAE/B SAI | r. | r. | -O-H | -O-H | -O-Hrn | r. | • | • | [71,96] |
| Thymus zygis | • | • | SAK SAI | -O-H | r. | -O-H -O-Hrn | -O-H -O-A-H | r. | r. | • | • | [72] |
| Thymus vulgaris | • | • | SAA iso SAK SAI | -O-H | r. | -O-H -O-Hrn | - | r. | r. | • | • | [59,86,87] |
| Thymus sibthorpii | • | • | - | - | -O-H | -O-H -O-Rut | - | - | - | • | • | [95] |
| Thymus serpyllum | • | • | SAK SAI | - | -O-H | -O-H -O-Rut | - | - | - | • | • | [95,97] |
| Thymus praecox | • | • | - | - | -O-H | -O-H -O-Rut | - | - | - | • | • | [95] |
| Thymus austriacus | • | • | - | - | r. | -O-Rut | - | - | - | • | • | [95] |
| Thymus × oblongifolius | • | • | - | - | - | -O-Rut | - | - | - | • | • | [95] |
| Thymus longicaulis | • | • | SAA iso SAK SAK iso | - | - | -O-H -O-P -O-Hrn -O-Rut | -O-H | - | - | • | • | [94,95] |
| Thymus algeriensis | • | • -O-H | SAA SAK SAK iso SAE iso SAB | -O-H | -O-H -O-Hrn | -O-H -O-Hrn | -O-H | - | r. | - | • | [98,99,100,101] |
| Thymus fontanesii | - | • | SAA SAA iso SAK | -O-H | O-Hrn | -O-Hrn | O-Hrn | - | - | - | - | [99,102] |
| Thymus munbyanus | - | • | SAA | -O-H | - | -O-H -O-Hrn | -O-Hrn | - | - | • | • | [103] |
| Thymus serrulatus | - | • | SAA SAB SAF SAK iso SAK iso | -O-H | r. | -O-H -O-Hrn | -O-H -O-Hrn | - | - | - | - | [104] |
| Thymus × citriodorus | • | • | SAK SAI | -O-H | r. | -O-H -O-Hrn | r. | -O-H | r. | • | • | [59,84,86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Modulation of Cell Death Pathways for Cellular Protection and Anti-Tumoral Activity: The Role of Thymus spp. Extracts and Their Bioactive Molecules. Int. J. Mol. Sci. 2023, 24, 1691. https://doi.org/10.3390/ijms24021691
Martins-Gomes C, Nunes FM, Silva AM. Modulation of Cell Death Pathways for Cellular Protection and Anti-Tumoral Activity: The Role of Thymus spp. Extracts and Their Bioactive Molecules. International Journal of Molecular Sciences. 2023; 24(2):1691. https://doi.org/10.3390/ijms24021691
Chicago/Turabian StyleMartins-Gomes, Carlos, Fernando M. Nunes, and Amélia M. Silva. 2023. "Modulation of Cell Death Pathways for Cellular Protection and Anti-Tumoral Activity: The Role of Thymus spp. Extracts and Their Bioactive Molecules" International Journal of Molecular Sciences 24, no. 2: 1691. https://doi.org/10.3390/ijms24021691
APA StyleMartins-Gomes, C., Nunes, F. M., & Silva, A. M. (2023). Modulation of Cell Death Pathways for Cellular Protection and Anti-Tumoral Activity: The Role of Thymus spp. Extracts and Their Bioactive Molecules. International Journal of Molecular Sciences, 24(2), 1691. https://doi.org/10.3390/ijms24021691







