Central Regulation of Brown Fat Thermogenesis in Response to Saturated or Unsaturated Long-Chain Fatty Acids
Abstract
1. Introduction
2. Results
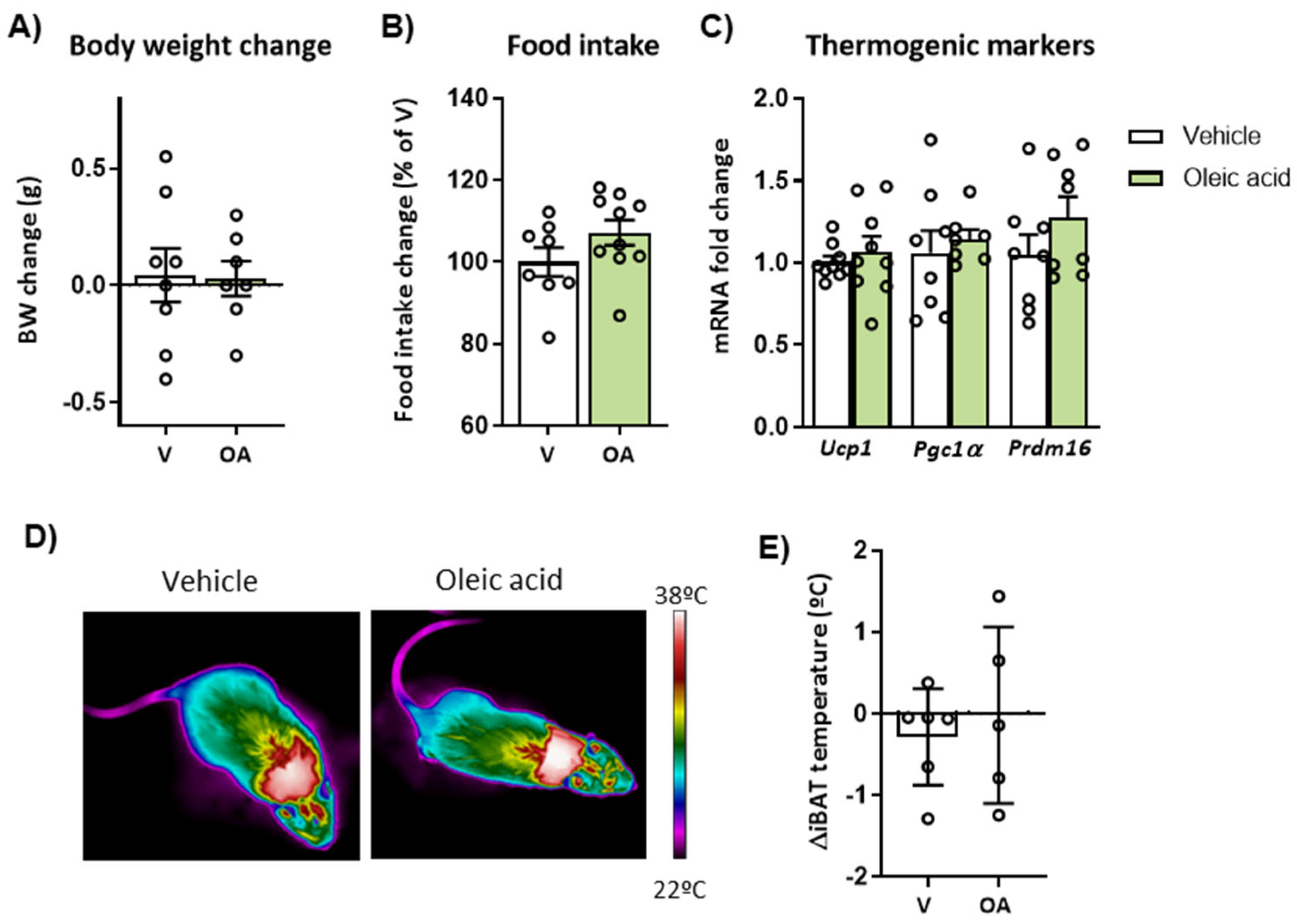
2.1. Short-Term Administration of Monounsaturated (MUFA) or Saturated FA (SFA)-Enriched HFD Differently Affects Body Weight, Feeding, BAT Thermogenesis and Neuronal Activation in the Hypothalamus
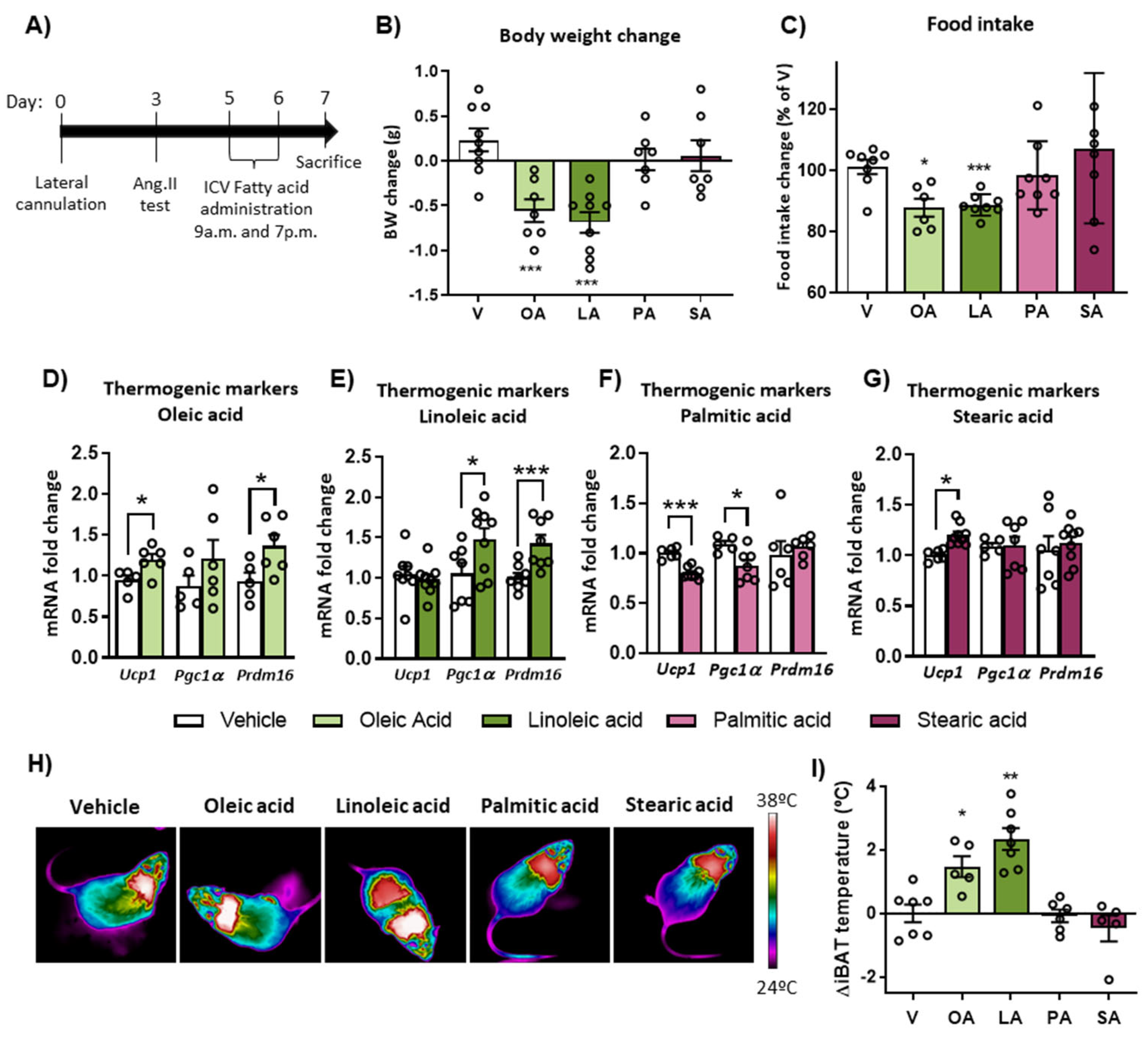
2.2. Intracerebroventricular (ICV) Injection of Unsaturated FA, but Not Saturated FA, Reduces Food Intake, Body Weight and Activates BAT Thermogenesis
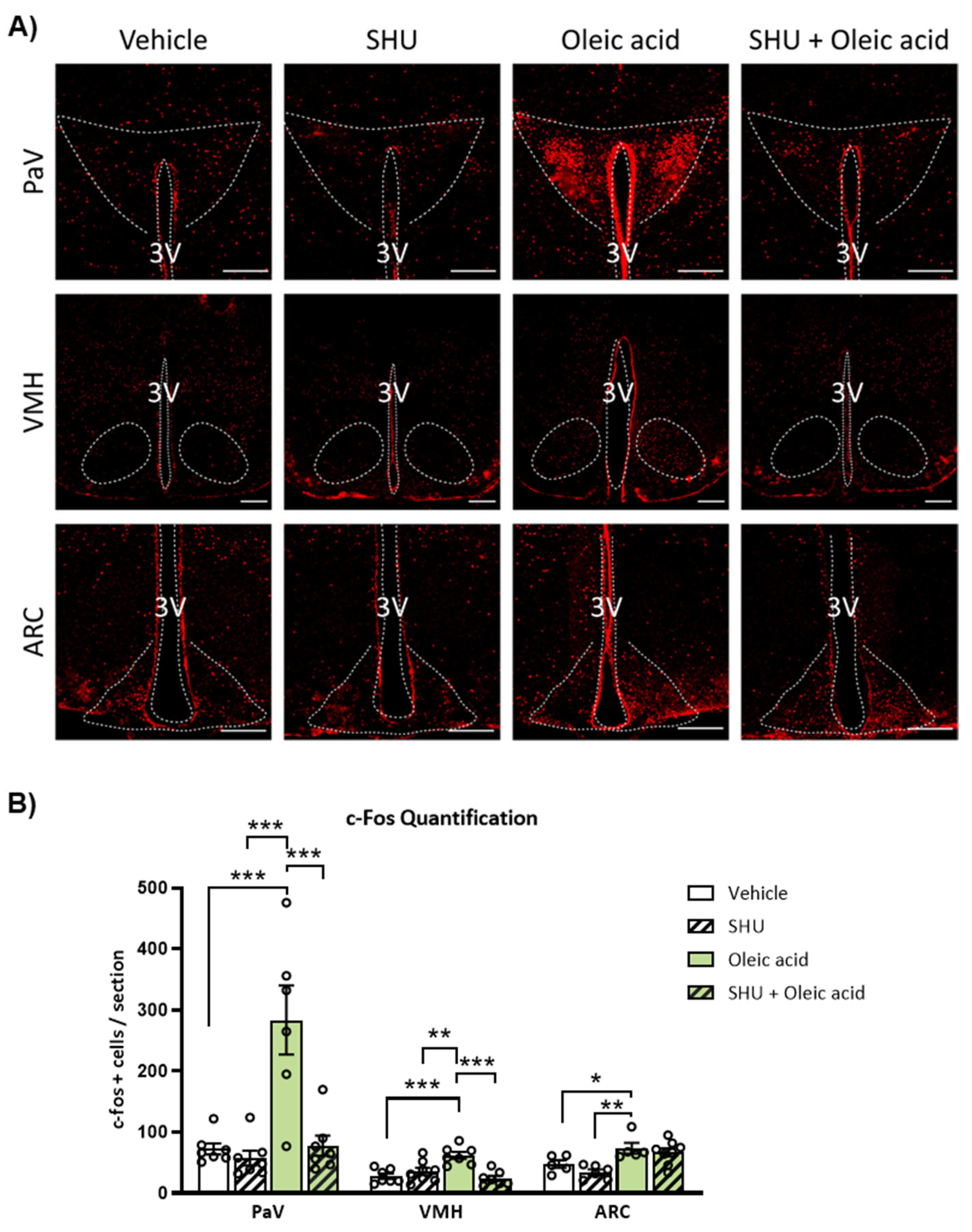
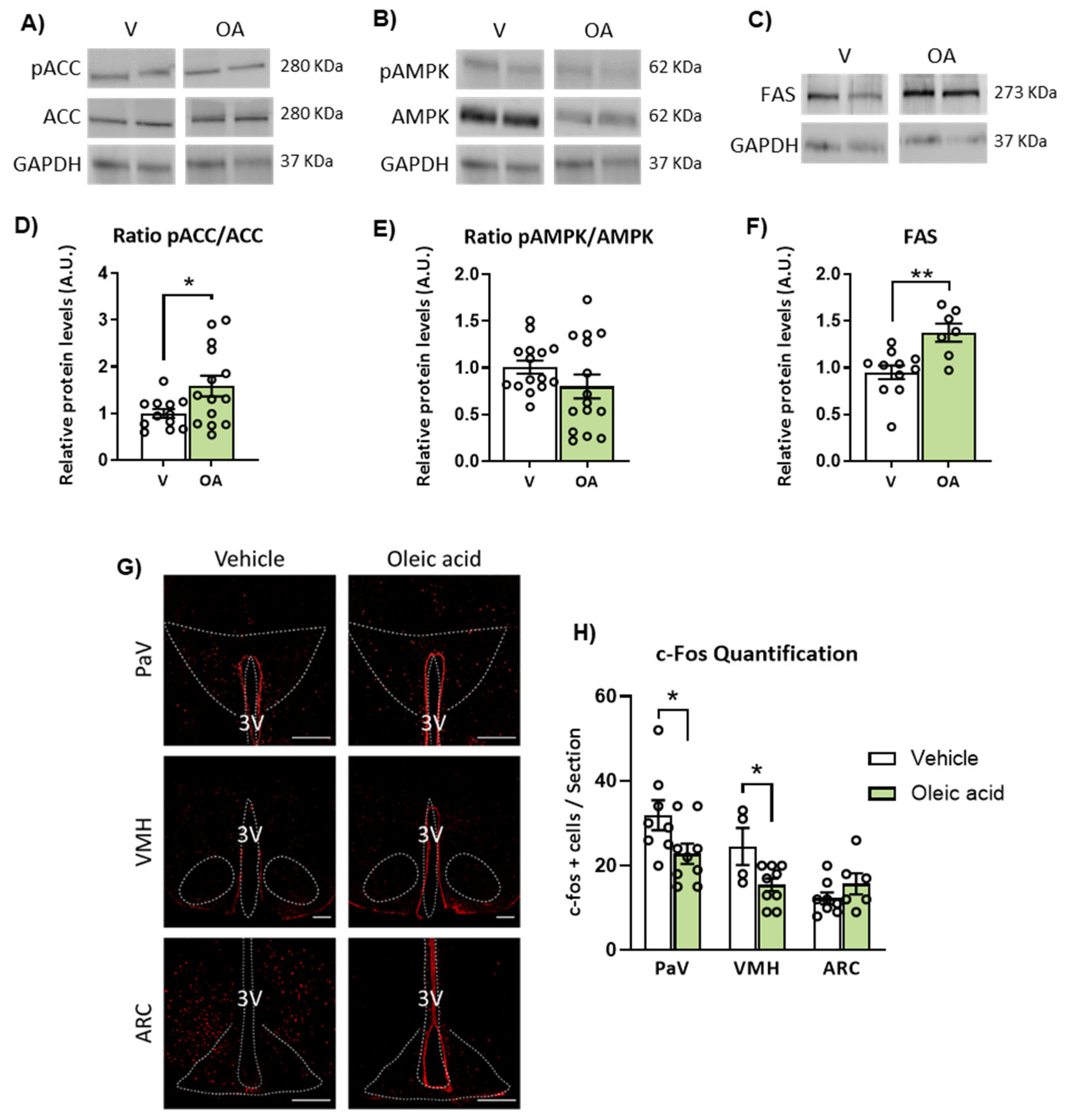
2.3. ICV Injection of Oleic Acid, but Not Palmitic Acid, Increases Neuronal Activation in Hypothalamic PaV, VMH and ARC Nuclei
2.4. Neuronal Activation Induced by Oleic Acid in the PaV and VMH Was Attenuated in the Presence of the Melanocortin 4 Receptor (MC4R) Antagonist
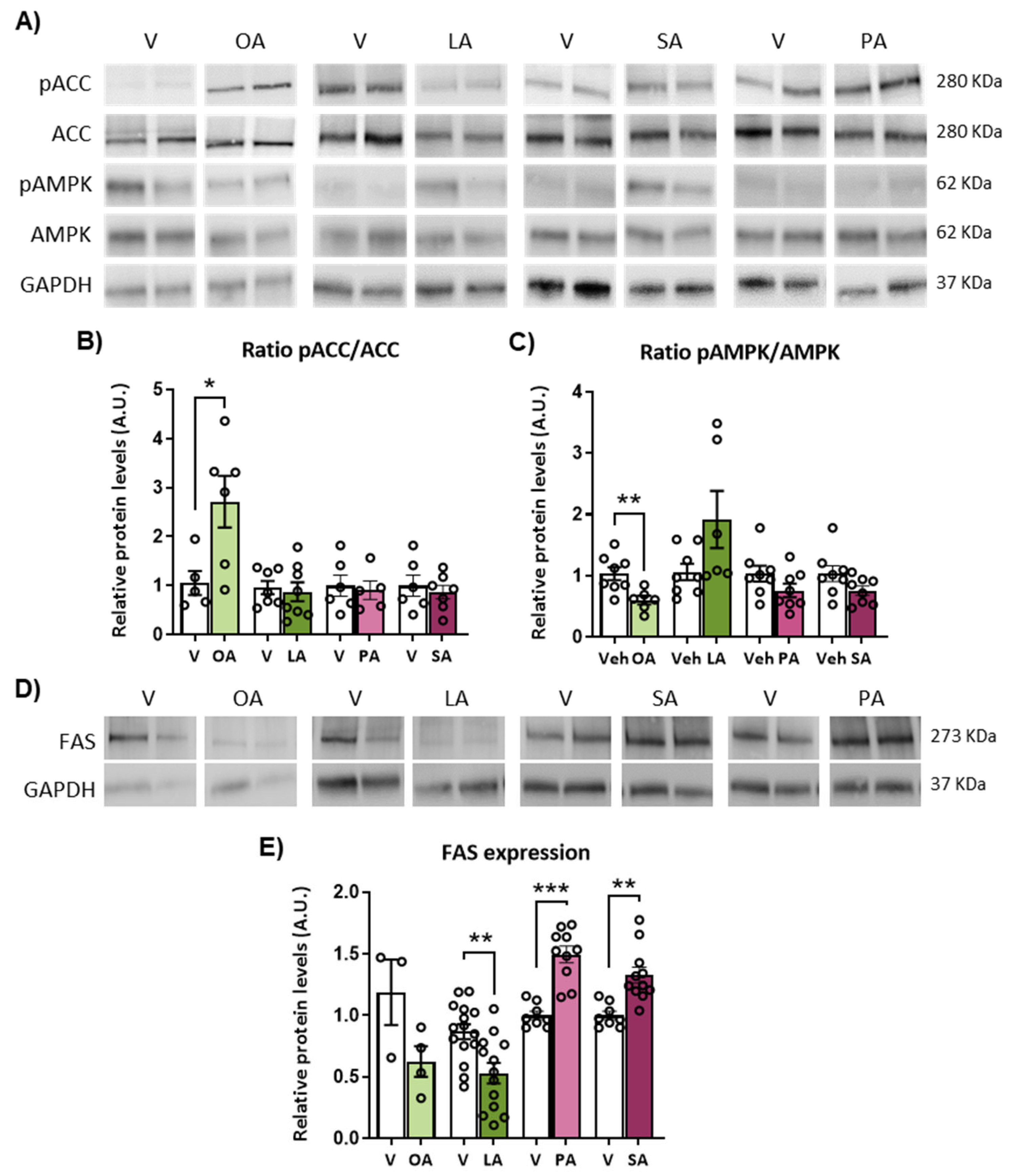
2.5. ICV Injection of Unsaturated versus Saturated FA Differently Affects AMPK/ACC Phosphorylation and Fatty Acid Synthase (FAS) Expression
2.6. Mice Deficient in CPT1C Showed a Reduced Effect of ICV Oleic Acid on Body Weight, Thermogenesis and Hypothalamic Neuronal Activation
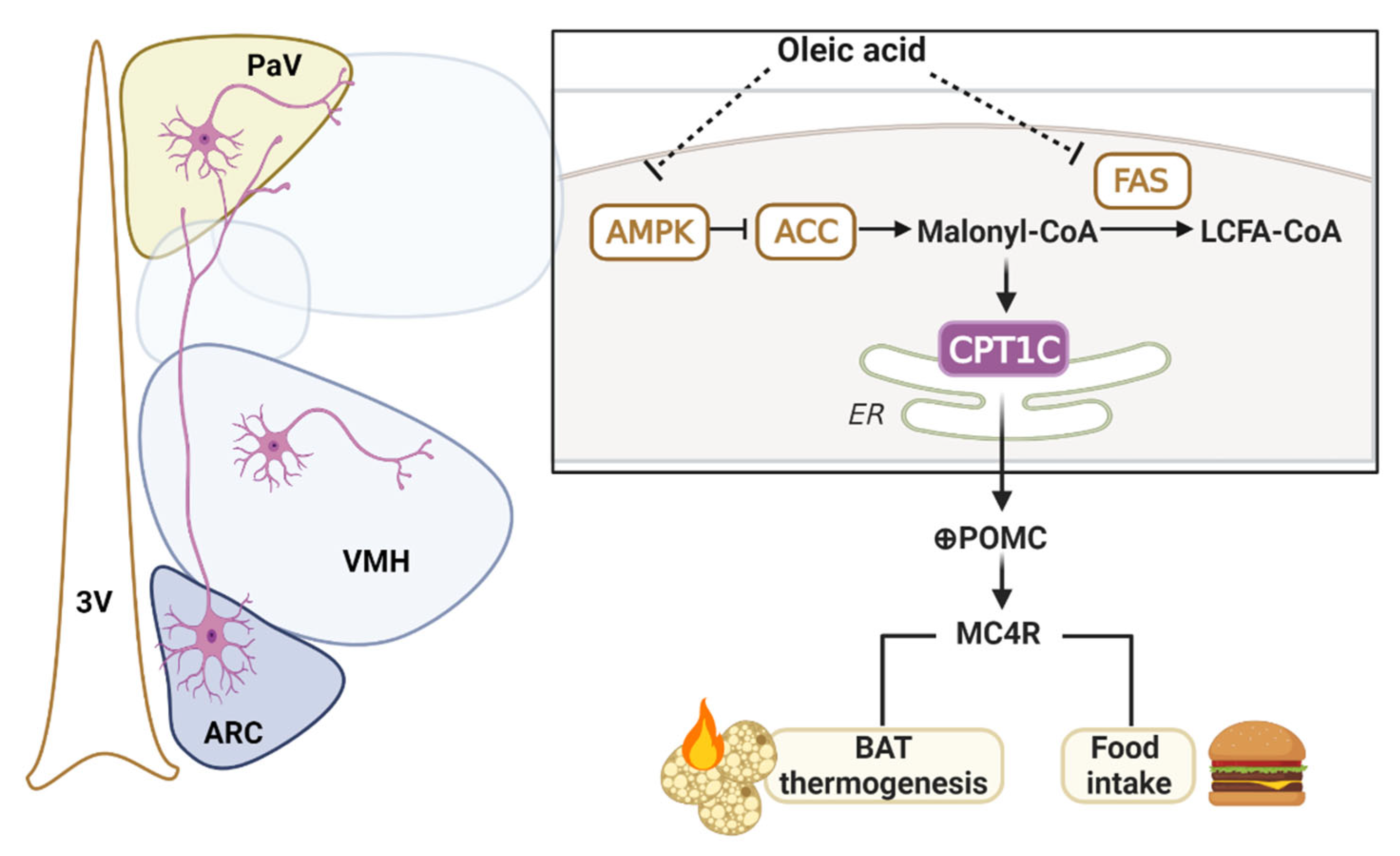
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. ICV Cannulation Surgery
4.3. Experimental Protocols
4.3.1. Administration of HFD
- HFD feeding for 7 days. During this period, body weight and the amount of each diet consumed was measured, and the caloric intake value was calculated. BAT temperature was also monitored. At the end of the experiment, mice were sacrificed by cervical dislocation, and hypothalamus and BAT were collected and stored at −80 °C until further processing.
- HFD feeding for 2 h. This protocol was designed to analyze neuronal activation in the hypothalamic nuclei. Mice were fasted for 3 h followed by a refeeding period of 2 h with MUFA, SFA or SD diet. After 2 h, mice were perfused for brain cryopreservation, as indicated below.
4.3.2. ICV Administration of FA
- ICV injection of FA for 2 days (2 injections/day). Injections were performed 1 h after the light phase and 1 h before the dark phase. During this period, body weight, food intake and BAT temperature were monitored. At the end of the experiment, 2 h after the dark phase, were sacrificed by cervical dislocation and hypothalamus, and BAT were collected and stored at −80 °C until further processing.
- ICV injection of FA for 2 h. This protocol was designed to analyze neuronal activation in the hypothalamic nuclei. FA were injected and during this period; animals did not have access to food. After 2 h, mice were perfused for brain cryopreservation, as indicated below. In some experiments, mice were previously treated with the MC4R antagonist SHU9119 (M4603, Sigma-Aldrich, St. Louis, MO, USA) (ICV administration; 0.5 nmol/dose) with the FA injection. SHU9119 was prepared in aCSF as previously described [10].
4.4. BAT Temperature Measurements
4.5. RNA Preparation and Quantitative RT-PCR
4.6. Western Blotting
4.7. Brain Immunofluorescence
4.8. Data Processing and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obici, S.; Feng, Z.; Morgan, K.; Stein, D.; Karkanias, G.; Rossetti, L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002, 51, 271–275. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Model. Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Lelliott, C.J.; Vidal-Puig, A. Hypothalamic fatty acid metabolism: A housekeeping pathway that regulates food intake. BioEssays 2007, 29, 248–261. [Google Scholar] [CrossRef]
- Magnan, C.; Levin, B.E.; Luquet, S. Brain lipid sensing and the neural control of energy balance. Mol. Cell. Endocrinol. 2015, 418, 3–8. [Google Scholar] [CrossRef]
- Enriori, P.J.; Evans, A.E.; Sinnayah, P.; Jobst, E.E.; Tonelli-Lemos, L.; Billes, S.K.; Glavas, M.M.; Grayson, B.E.; Perello, M.; Nillni, E.A.; et al. Diet-Induced Obesity Causes Severe but Reversible Leptin Resistance in Arcuate Melanocortin Neurons. Cell Metab. 2007, 5, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Tracey, T.J.; Steyn, F.J.; Wolvetang, E.J.; Ngo, S.T. Neuronal lipid metabolism: Multiple pathways driving functional outcomes in health and disease. Front. Mol. Neurosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Bruce, K.D.; Zsombok, A.; Eckel, R.H. Lipid Processing in the Brain: A Key Regulator of Systemic Metabolism. Front. Endocrinol. 2017, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Fadó, R.; Rodríguez-Rodríguez, R.; Casals, N. The return of malonyl-CoA to the brain: Cognition and other stories. Prog. Lipid Res. 2021, 81, 101071. [Google Scholar] [CrossRef]
- Lane, M.D.; Wolfgang, M.; Cha, S.-H.; Dai, Y. Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA. Int. J. Obes. 2008, 32, S49–S54. [Google Scholar] [CrossRef]
- Schwinkendorf, D.R.; Tsatsos, N.G.; Gosnell, B.A.; Mashek, D.G. Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int. J. Obes. 2011, 35, 336–344. [Google Scholar] [CrossRef]
- Lam, T.K.T.; Pocai, A.; Gutierrez-Juarez, R.; Obici, S.; Bryan, J.; Aguilar-Bryan, L.; Schwartz, G.J.; Rossetti, L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat. Med. 2005, 11, 320–327. [Google Scholar] [CrossRef]
- Le Foll, C.; Irani, B.G.; Magnan, C.; Dunn-Meynell, A.A.; Levin, B.E. Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R655. [Google Scholar] [CrossRef] [PubMed]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.L.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef]
- Benoit, S.C.; Kemp, C.J.; Elias, C.F.; Abplanalp, W.; Herman, J.P.; Migrenne, S.; Lefevre, A.L.; Cruciani-Guglielmacci, C.; Magnan, C.; Yu, F.; et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-θ subcellular localization in rodents. J. Clin. Investig. 2009, 119, 2577–2589. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Williams, L.M. Potential relationship between dietary long-chain saturated fatty acids and hypothalamic dysfunction in obesity. Nutr. Rev. 2020, 78, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Araujo, E.P.; Moraes, J.C.; Cintra, D.E.; Velloso, L.A. MECHANISMS IN ENDOCRINOLOGY: Hypothalamic inflammation and nutrition. Eur. J. Endocrinol. 2016, 175, R97–R105. [Google Scholar] [CrossRef] [PubMed]
- Rust, B.; Raatz, S.; Casperson, S.; Duke, S.; Picklo, M. Dietary Fat Chain Length, Saturation, and PUFA Source Acutely Affect Diet-Induced Thermogenesis but Not Satiety in Adults in a Randomized, Crossover Trial. Nutrients 2021, 13, 2615. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Fuente-Martin, E.; Finan, B.; Kim, M.; Frank, A.; Garcia-Caceres, C.; Navas, C.R.; Gordillo, R.; Neinast, M.; Kalainayakan, S.P.; et al. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep. 2014, 9, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Miralpeix, C.; Fosch, A.; Casas, J.; Baena, M.; Herrero, L.; Serra, D.; Rodríguez-Rodríguez, R.; Casals, N. Hypothalamic endocannabinoids inversely correlate with the development of diet-induced obesity in male and female mice. J. Lipid Res. 2019, 60, 1260–1269. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Whittle, A.J.; López, M.; Vidal-Puig, A. Using brown adipose tissue to treat obesity—The central issue. Trends Mol. Med. 2011, 17, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; Nogueiras, R.; Diéguez, C.; Rahmouni, K.; López, M. Traveling from the hypothalamus to the adipose tissue: The thermogenic pathway. Redox Biol. 2017, 12, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Fosch, A.; Zagmutt, S.; Casals, N.; Rodríguez-Rodríguez, R. New Insights of SF1 Neurons in Hypothalamic Regulation of Obesity and Diabetes. Int. J. Mol. Sci. 2021, 22, 6186. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Zhao, L.; Donato, J.; Kohno, D.; Xu, Y.; Elias, C.F.; Lee, C.; Parker, K.L.; Elmquist, J.K. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc. Natl. Acad. Sci. USA 2011, 108, 10673–10678. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Rodríguez-Rodríguez, R.; Betz, M.J.; Rensen, P.C.N. Editorial: Current Challenges for Targeting Brown Fat Thermogenesis to Combat Obesity. Front. Endocrinol. 2020, 11, 845. [Google Scholar] [CrossRef]
- Labbé, S.M.; Caron, A.; Lanfray, D.; Monge-Rofarello, B.; Bartness, T.J.; Richard, D. Hypothalamic control of brown adipose tissue thermogenesis. Front. Syst. Neurosci. 2015, 9, 150. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Miralpeix, C.; Fosch, A.; Pozo, M.; Calderón-Domínguez, M.; Perpinyà, X.; Vellvehí, M.; López, M.; Herrero, L.; Serra, D.; et al. CPT1C in the ventromedial nucleus of the hypothalamus is necessary for brown fat thermogenesis activation in obesity. Mol. Metab. 2019, 19, 75–85. [Google Scholar] [CrossRef]
- Casals, N.; Zammit, V.; Herrero, L.; Fadó, R.; Rodríguez-Rodríguez, R.; Serra, D.; Fado, R.; Rodriguez-Rodriguez, R.; Serra, D.; Fadó, R.; et al. Carnitine palmitoyltransferase 1C: From cognition to cancer. Prog. Lipid Res. 2016, 61, 134–148. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Fosch, A.; Garcia-Chica, J.; Zagmutt, S.; Casals, N. Targeting carnitine palmitoyltransferase 1 isoforms in the hypothalamus: A promising strategy to regulate energy balance. J. Neuroendocrinol. 2023, e13234. [Google Scholar] [CrossRef]
- Ramírez, S.; Martins, L.; Jacas, J.; Carrasco, P.; Pozo, M.; Clotet, J.; Serra, D.; Hegardt, F.G.; Diéguez, C.; López, M.; et al. Hypothalamic ceramide levels regulated by CPT1C mediate the orexigenic effect of ghrelin. Diabetes 2013, 62, 2329–2337. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, G.; Gao, X.; Wu, D.; Carrasco, P.; Casals, N.; Hegardt, F.G.; Moran, T.H.; Lopaschuk, G.D. Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proc. Natl. Acad. Sci. USA 2011, 108, 9691–9696. [Google Scholar] [CrossRef]
- Pozo, M.; Rodrguez-Rodrguez, R.; Ramírez, S.; Seoane-Collazo, P.; López, M.; Serra, D.; Herrero, L.; Casals, N. Hypothalamic regulation of liver and muscle nutrient partitioning by brain-specific carnitine palmitoyltransferase 1c in male mice. Endocrinology 2017, 158, 2226–2238. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, M.J.; Kurama, T.; Dai, Y.; Suwa, A.; Asaumi, M.; Matsumoto, S.-I.; Cha, S.H.; Shimokawa, T.; Lane, M.D. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc. Natl. Acad. Sci. USA 2006, 103, 7282–7287. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.F.; Chen, W.; Kong, X.P.; Xu, A.M.; Wang, Z.G.; Sweeney, G.; Wu, D. Enhanced susceptibility of Cpt1c knockout mice to glucose intolerance induced by a high-fat diet involves elevated hepatic gluconeogenesis and decreased skeletal muscle glucose uptake. Diabetologia 2009, 52, 912–920. [Google Scholar] [CrossRef]
- Saito, M.; Matsushita, M.; Yoneshiro, T.; Okamatsu-Ogura, Y. Brown Adipose Tissue, Diet-Induced Thermogenesis, and Thermogenic Food Ingredients: From Mice to Men. Front. Endocrinol. 2020, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Silva-e-Silva, A.C.A.G.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur. J. Nutr. 2016, 55, 159–169. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X. Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem. Biophys. Res. Commun. 2014, 450, 1446–1451. [Google Scholar] [CrossRef]
- Shin, S.; Ajuwon, K.M. Effects of Diets Differing in Composition of 18-C Fatty Acids on Adipose Tissue Thermogenic Gene Expression in Mice Fed High-Fat Diets. Nutrients 2018, 10, 256. [Google Scholar] [CrossRef]
- Adermark, L.; Gutierrez, S.; Lagström, O.; Hammarlund, M.; Licheri, V.; Johansson, M.E. Weight gain and neuroadaptations elicited by high fat diet depend on fatty acid composition. Psychoneuroendocrinology 2021, 126, 105143. [Google Scholar] [CrossRef]
- Cintra, D.E.; Ropelle, E.R.; Moraes, J.C.; Pauli, J.R.; Morari, J.; de Souza, C.T.; Grimaldi, R.; Stahl, M.; Carvalheira, J.B.; Saad, M.J.; et al. Unsaturated Fatty Acids Revert Diet-Induced Hypothalamic Inflammation in Obesity. PLoS ONE 2012, 7, e30571. [Google Scholar] [CrossRef]
- Dalvi, P.S.; Chalmers, J.A.; Luo, V.; Han, D.Y.; Wellhauser, L.; Liu, Y.; Tran, D.Q.; Castel, J.; Luquet, S.; Wheeler, M.B.; et al. High fat induces acute and chronic inflammation in the hypothalamus: Effect of high-fat diet, palmitate and TNF-α on appetite-regulating NPY neurons. Int. J. Obes. 2017, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; He, W.; Chen, J.T.C.; Wellhauser, L.; Hopperton, K.E.; Bazinet, R.P.; Belsham, D.D. Palmitate-mediated induction of neuropeptide Y expression occurs through intracellular metabolites and not direct exposure to proinflammatory cytokines. J. Neurochem. 2021, 159, 574–589. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Su, Y.; Gutierrez-Juarez, R.; Chua, S. Oleic Acid Directly Regulates POMC Neuron Excitability in the Hypothalamus. J. Neurophysiol. 2009, 101, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Nogueiras, R.; Tena-Sempere, M.; Diéguez, C. Hypothalamic AMPK: A canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Collazo, P.; Roa, J.; Rial-Pensado, E.; Liñares-Pose, L.; Beiroa, D.; Ruíz-Pino, F.; López-González, T.; Morgan, D.A.; Pardavila, J.Á.; Sánchez-Tapia, M.J.; et al. SF1-Specific AMPKα1 Deletion Protects Against Diet-Induced Obesity. Diabetes 2018, 67, 2213–2226. [Google Scholar] [CrossRef]
- Milbank, E.; Dragano, N.R.V.; González-García, I.; Garcia, M.R.; Rivas-Limeres, V.; Perdomo, L.; Hilairet, G.; Ruiz-Pino, F.; Mallegol, P.; Morgan, D.A.; et al. Small extracellular vesicle-mediated targeting of hypothalamic AMPKα1 corrects obesity through BAT activation. Nat. Metab. 2021, 3, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Varela, L.; Vázquez, M.J.; Rodríguez-Cuenca, S.; González, C.R.; Velagapudi, V.R.; Morgan, D.A.; Schoenmakers, E.; Agassandian, K.; Lage, R.; et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010, 16, 1001–1008. [Google Scholar] [CrossRef]
- Wolfgang, M.J.; Cha, S.H.; Millington, D.S.; Cline, G.; Shulman, G.I.; Suwa, A.; Asaumi, M.; Kurama, T.; Shimokawa, T.; Lane, M.D. Brain-specific carnitine palmitoyl-transferase-1c: Role in CNS fatty acid metabolism, food intake, and body weight. J. Neurochem. 2008, 105, 1550–1559. [Google Scholar] [CrossRef]
- Okamoto, S.; Sato, T.; Tateyama, M.; Kageyama, H.; Maejima, Y.; Nakata, M.; Hirako, S.; Matsuo, T.; Kyaw, S.; Shiuchi, T.; et al. Activation of AMPK-Regulated CRH Neurons in the PVH is Sufficient and Necessary to Induce Dietary Preference for Carbohydrate over Fat. Cell Rep. 2018, 22, 706–721. [Google Scholar] [CrossRef]
- Miralpeix, C.; Reguera, A.C.; Fosch, A.; Casas, M.; Lillo, J.; Navarro, G.; Franco, R.; Casas, J.; Alexander, S.P.H.; Casals, N.; et al. Carnitine palmitoyltransferase 1C negatively regulates the endocannabinoid hydrolase ABHD6 in mice, depending on nutritional status. Br. J. Pharmacol. 2021, 178, 1507–1523. [Google Scholar] [CrossRef]
- Miralpeix, C.; Reguera, A.C.; Fosch, A.; Zagmutt, S.; Casals, N.; Cota, D.; Rodríguez-Rodríguez, R. Hypothalamic endocannabinoids in obesity: An old story with new challenges. Cell. Mol. Life Sci. 2021, 78, 7469–7490. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.; Fadó, R.; Domínguez, J.L.; Roig, A.; Kaku, M.; Chohnan, S.; Solé, M.; Unzeta, M.; Miñano-Molina, A.J.; Rodríguez-Álvarez, J.; et al. Sensing of nutrients by CPT1C controls SAC1 activity to regulate AMPA receptor trafficking. J. Cell Biol. 2020, 219, e201912045. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, P.; Sahún, I.; McDonald, J.; Ramírez, S.; Jacas, J.; Gratacós, E.; Sierra, A.Y.; Serra, D.; Herrero, L.; Acker-Palmer, A.; et al. Ceramide levels regulated by carnitine palmitoyltransferase 1C control dendritic spine maturation and cognition. J. Biol. Chem. 2012, 287, 21224–21232. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fosch, A.; Rodriguez-Garcia, M.; Miralpeix, C.; Zagmutt, S.; Larrañaga, M.; Reguera, A.C.; Garcia-Chica, J.; Herrero, L.; Serra, D.; Casals, N.; et al. Central Regulation of Brown Fat Thermogenesis in Response to Saturated or Unsaturated Long-Chain Fatty Acids. Int. J. Mol. Sci. 2023, 24, 1697. https://doi.org/10.3390/ijms24021697
Fosch A, Rodriguez-Garcia M, Miralpeix C, Zagmutt S, Larrañaga M, Reguera AC, Garcia-Chica J, Herrero L, Serra D, Casals N, et al. Central Regulation of Brown Fat Thermogenesis in Response to Saturated or Unsaturated Long-Chain Fatty Acids. International Journal of Molecular Sciences. 2023; 24(2):1697. https://doi.org/10.3390/ijms24021697
Chicago/Turabian StyleFosch, Anna, Maria Rodriguez-Garcia, Cristina Miralpeix, Sebastián Zagmutt, Maite Larrañaga, Ana Cristina Reguera, Jesus Garcia-Chica, Laura Herrero, Dolors Serra, Nuria Casals, and et al. 2023. "Central Regulation of Brown Fat Thermogenesis in Response to Saturated or Unsaturated Long-Chain Fatty Acids" International Journal of Molecular Sciences 24, no. 2: 1697. https://doi.org/10.3390/ijms24021697
APA StyleFosch, A., Rodriguez-Garcia, M., Miralpeix, C., Zagmutt, S., Larrañaga, M., Reguera, A. C., Garcia-Chica, J., Herrero, L., Serra, D., Casals, N., & Rodriguez-Rodriguez, R. (2023). Central Regulation of Brown Fat Thermogenesis in Response to Saturated or Unsaturated Long-Chain Fatty Acids. International Journal of Molecular Sciences, 24(2), 1697. https://doi.org/10.3390/ijms24021697








