The Sequence Characteristics and Binding Properties of the Odorant-Binding Protein 2 of Euplatypus parallelus to Semiochemicals
Abstract
1. Introduction
2. Results
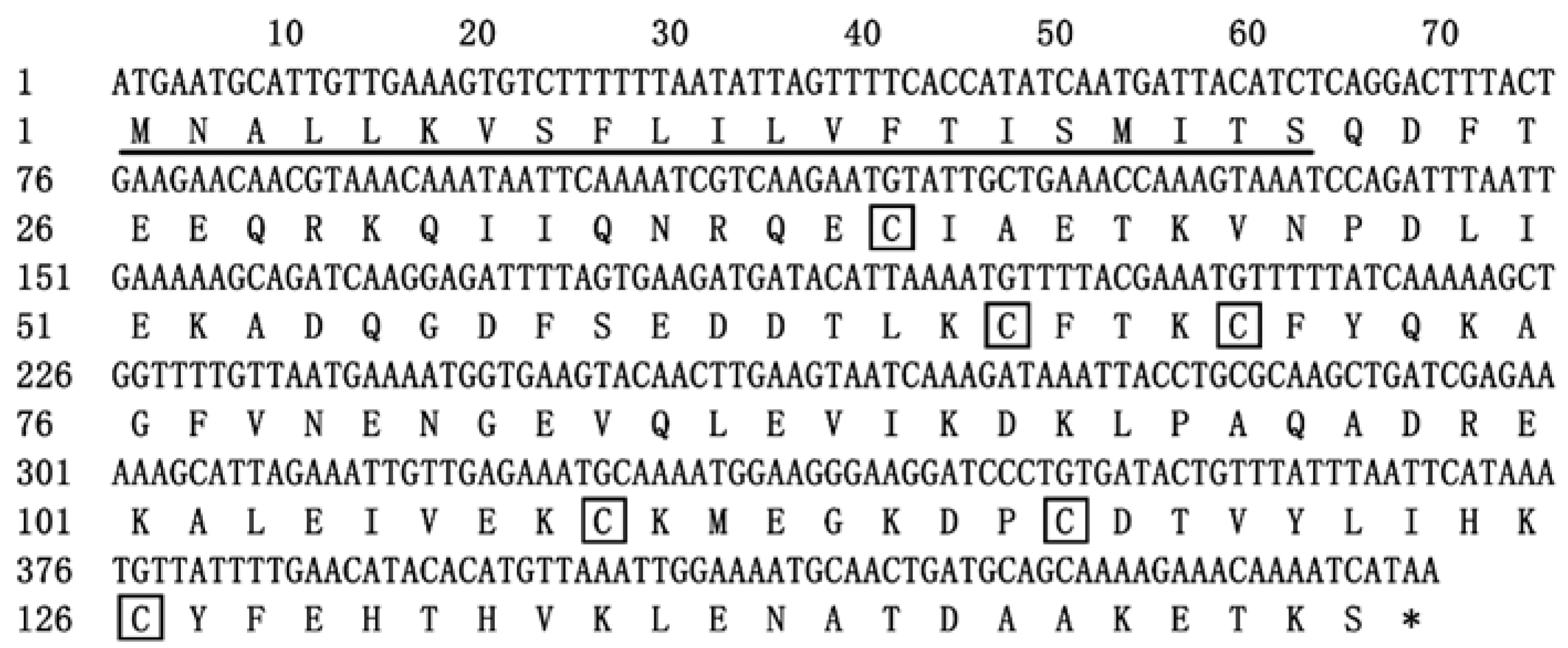
2.1. Sequence Analysis of EparOBP2
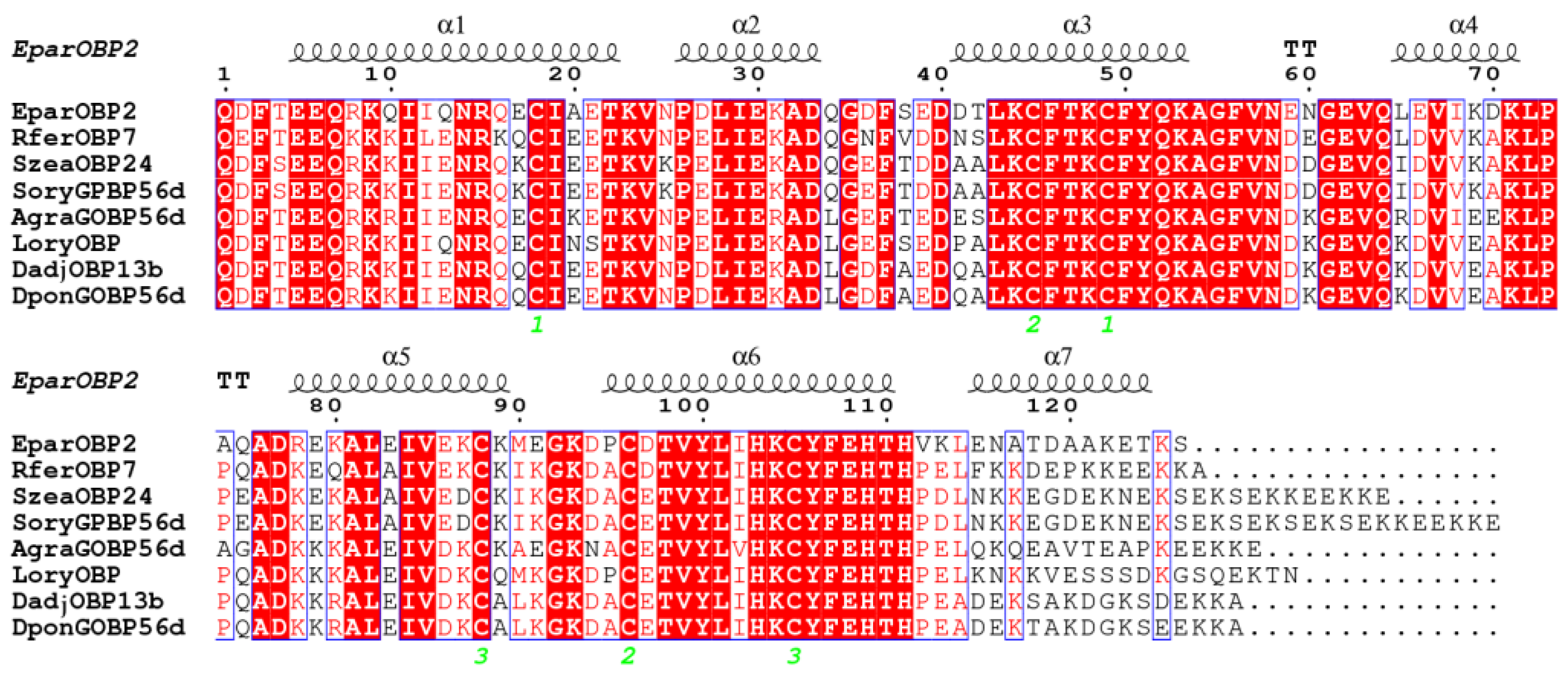
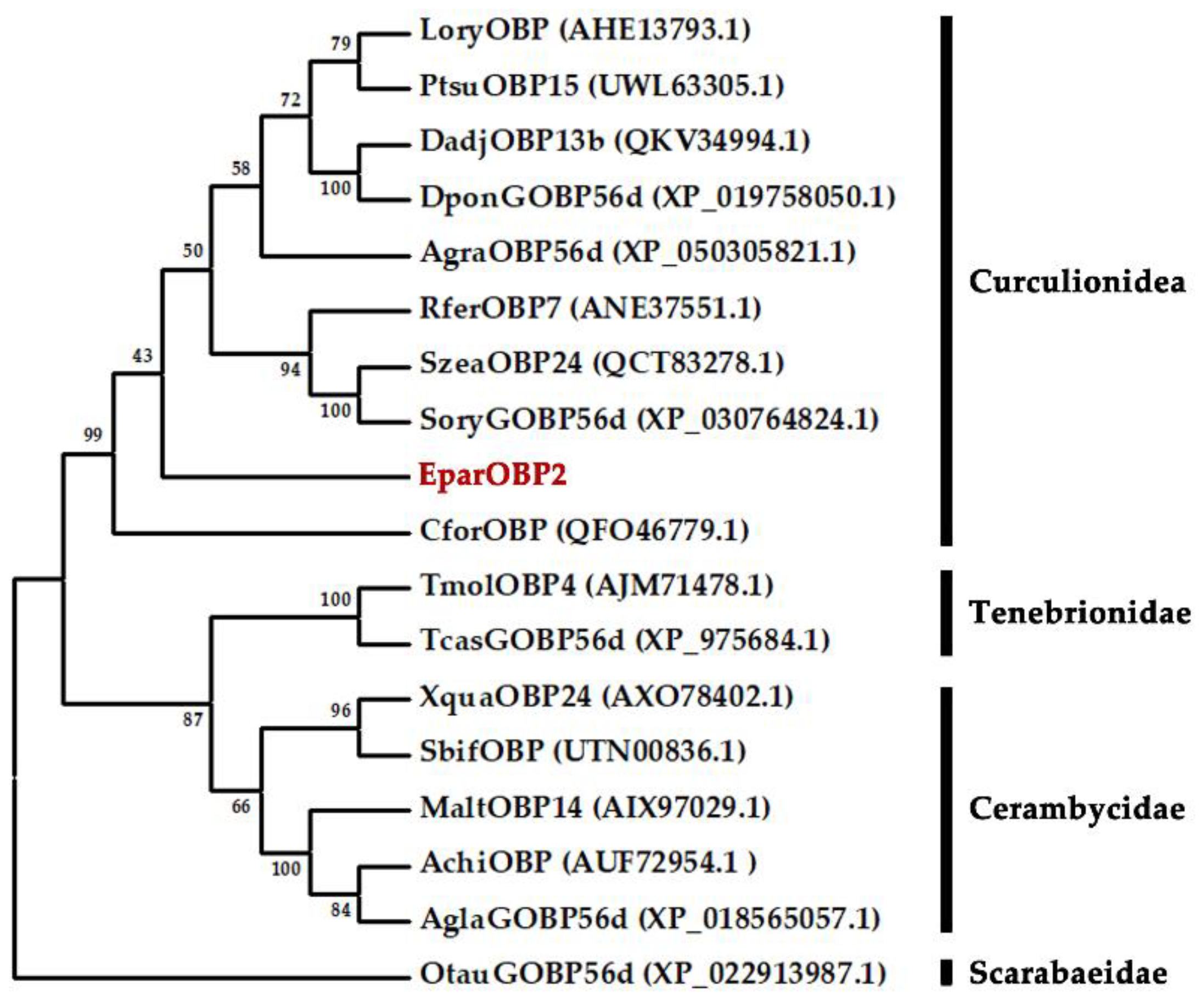
2.2. Sequence Alignment and Phylogenetic Analysis
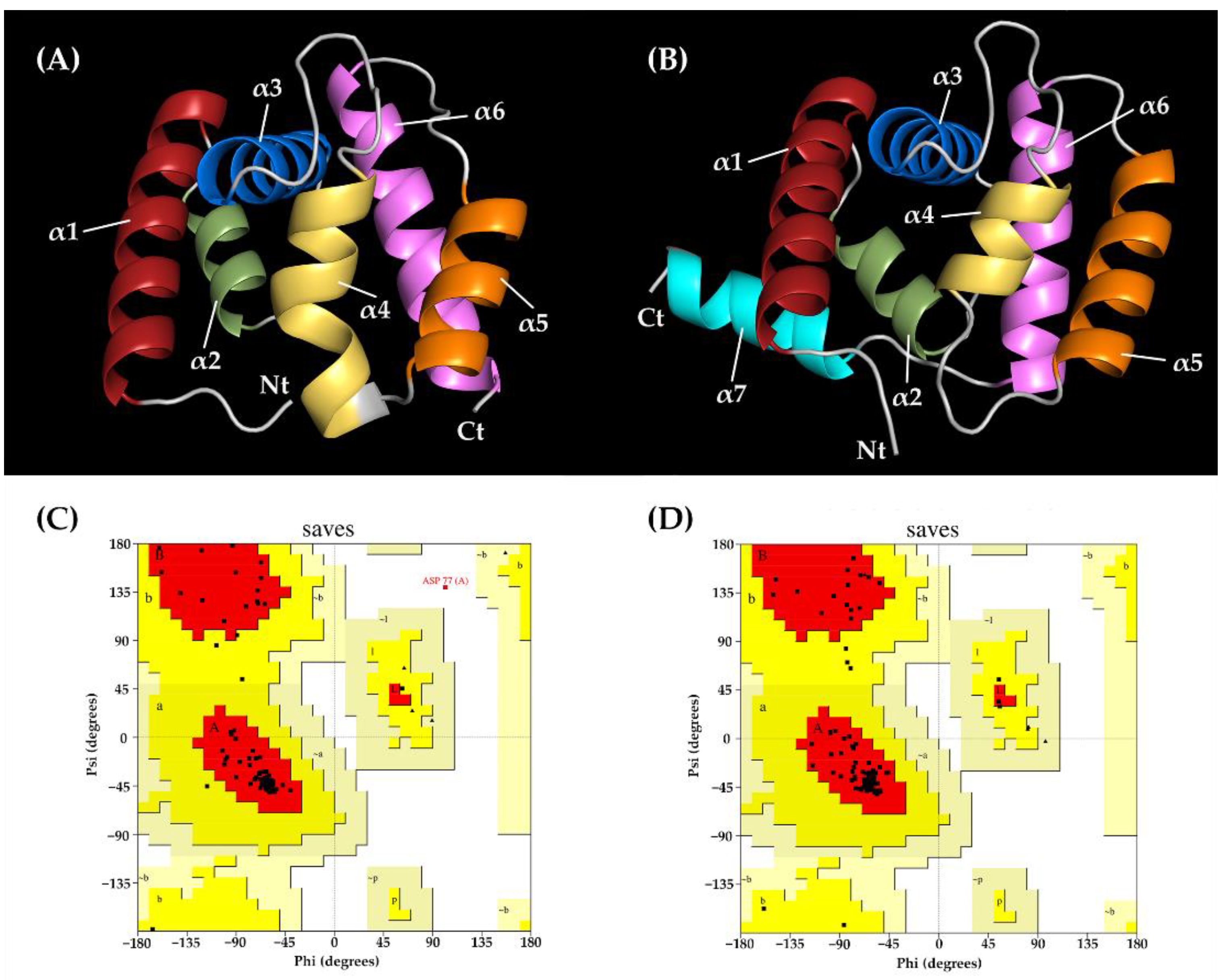
2.3. Three-Dimensional (3D) Modeling
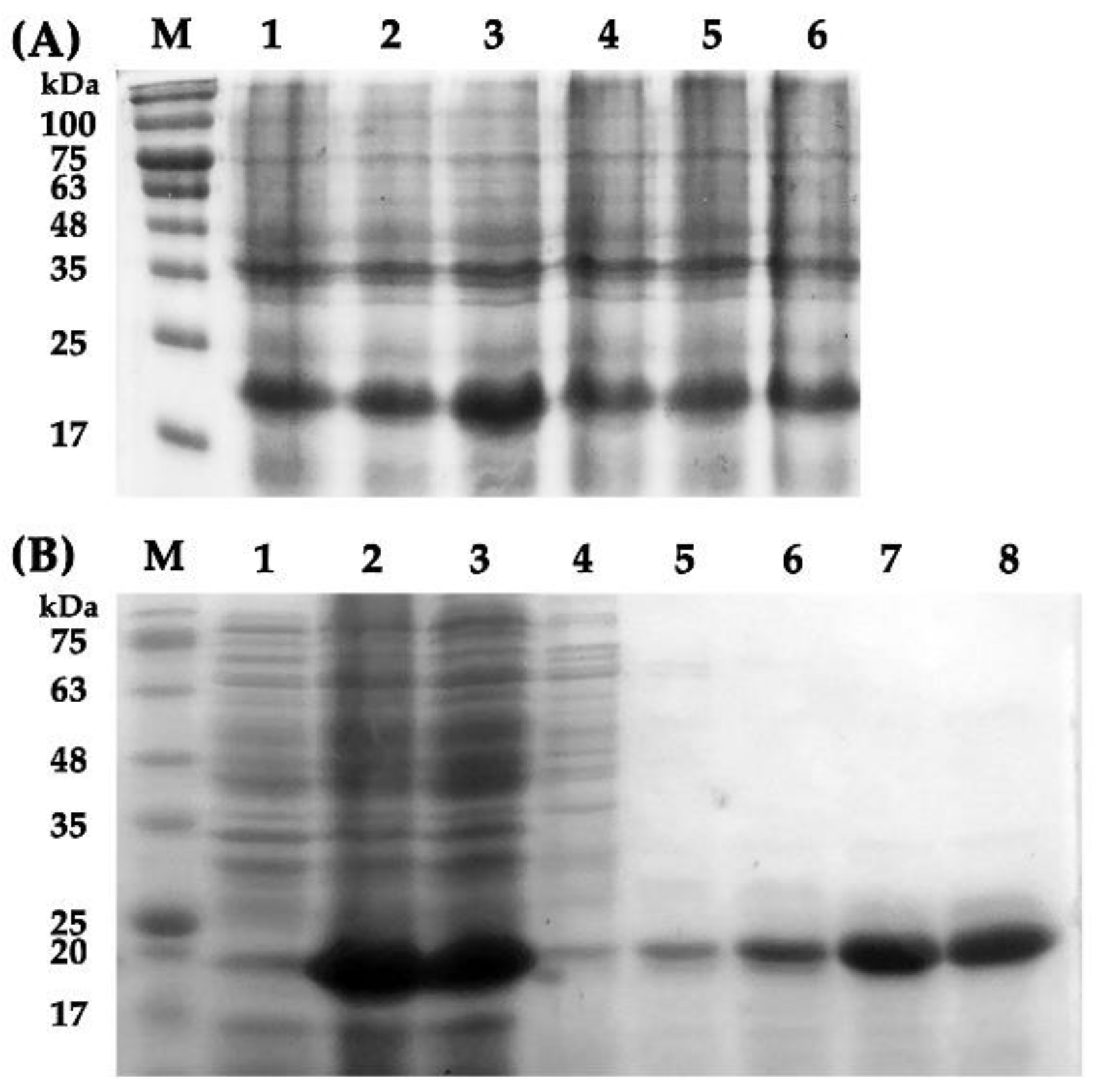
2.4. Expression and Purification of Recombinant EparOBP2
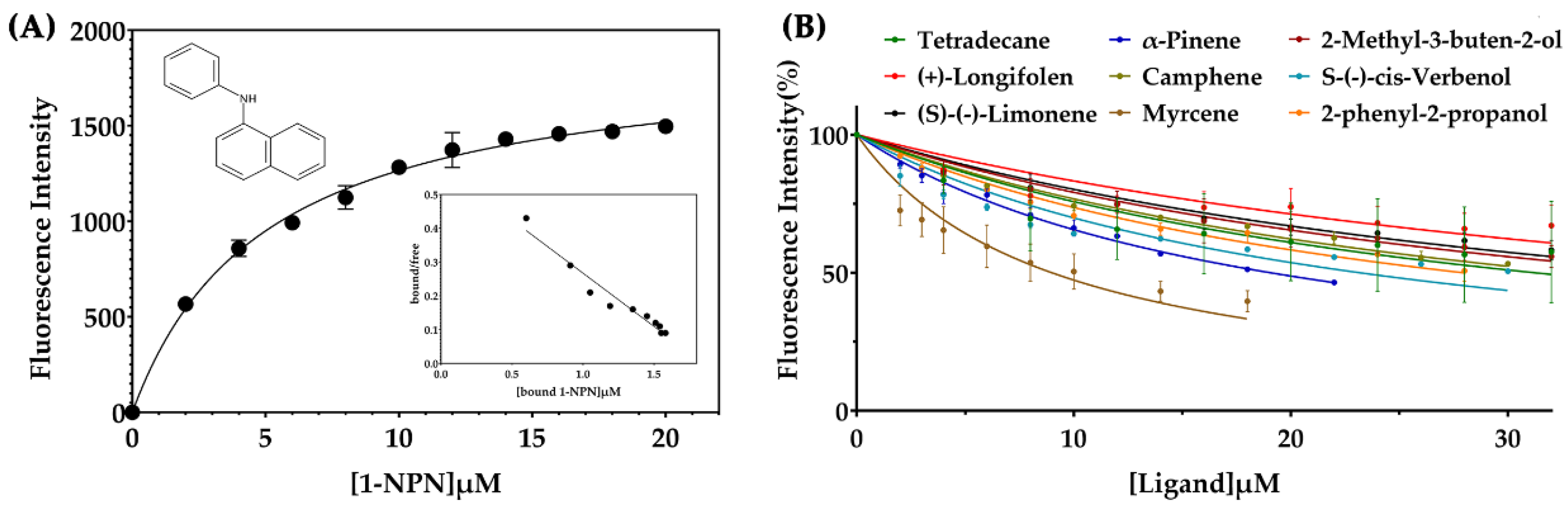
2.5. Fluorescence Competitive Binding Assays
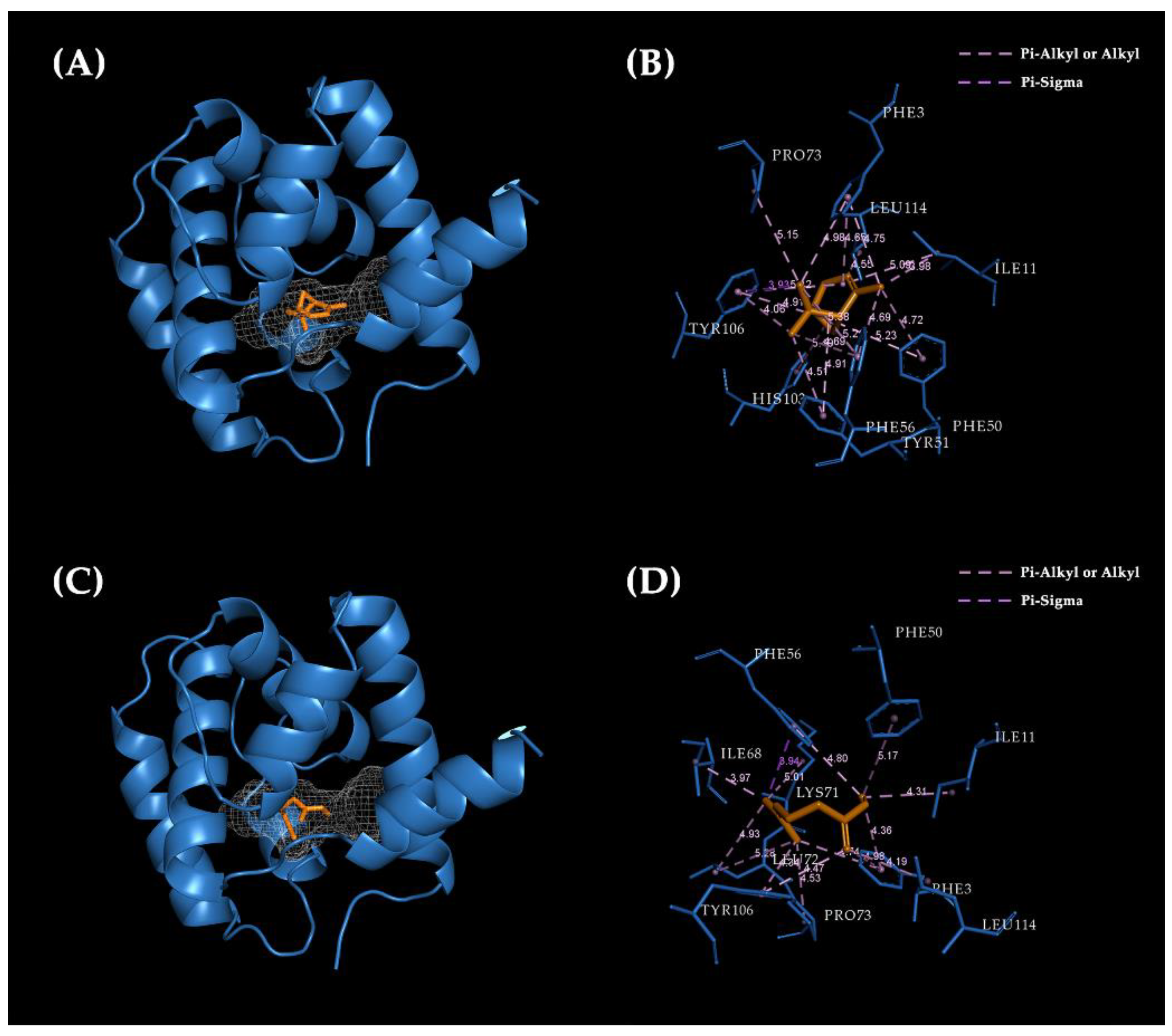
2.6. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Insect Collection
4.2. Sequencing Analysis
4.3. Expression and Purification of Recombinant EparOBP2
4.4. Fluorescence Competitive Binding Assays
4.5. Structure Modeling and Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wood, S.L.; Bright, D.E., Jr. A Catalog of Scolytidae and Platypodidae (Coleoptera), Part 1: Bibliography. In Great Basin Naturalist Memoirs; Brigham Young University: Provo, UT, USA, 1987; p. 685. [Google Scholar]
- Beaver, R.A. The invasive Neotropical ambrosia beetle Euplatypus parallelus (Fabricius 1801) in the Oriental region and its pest status (Coleoptera: Curculionidae: Platypodinae). Entomol. Mon. Mag. 2013, 149, 143–154. [Google Scholar]
- Gümüş, E.; Ergün, A. Report of a pest risk analysis for Platypus parallelus (Fabricus, 1801) for Turkey. Eppo Bull. 2015, 45, 112–118. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Lai, S.; Yin, T.; Ji, Y.; Wang, S.; Wang, J.; Hulcr, J. First record of Euplatypus parallelus (Coleoptera: Curculionidae) in China. Fla. Entomol. 2018, 101, 141–143. [Google Scholar] [CrossRef]
- Tang, J.; Li, J.; Lu, H.; Lu, F.; Lu, B. Potential distribution of an invasive pest, Euplatypus parallelus, in China as predicted by Maxent. Pest Manag. Sci 2019, 75, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.; Putz, P.; de Carvalho Silveira, E.; Flechtmann, C.A.H. Biological aspects of Euplatypus parallelus (F.) (Coleoptera, Curculionidae, Platypodinae) attacking Hevea brasiliensis (Willd. ex A. Juss.) in São Paulo northwest, Brazil. In Proceedings of the 3rd Congresso Brasileiro de Heveicultura, Guarapari, Brazil, 24–26 July 2013; pp. 24–26. [Google Scholar]
- Zhou, X.; Guo, J.; Yin, T.; Gan, W.; Bai, C.; Xu, F.; Yang, S. Research progress on rubber bark beetles and trapping control technology in China. Chin. J. Trop. Crops 2021, 42, 3049–3054. [Google Scholar]
- Hiremath, S.; Prathapan, K. First report of the invasive South American pinhole borer, Euplatypus parallelus (Fabricius) (Coleoptera: Curculionidae: Platypodinae), on rubber in India. Coleopt. Bull. 2019, 73, 714–717. [Google Scholar] [CrossRef]
- Lai, S.; Wang, J.; Fu, Y.; Duan, B.; Hongchang, A.; Zhang, L.; Tarno, H. Infestation of Platypodine beetles (Coleoptera: Curculionidae) on rubber trees in China. Coleopt. Bull. 2020, 74, 626–631. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef]
- Barkawi, L.S.; Francke, W.; Blomquist, G.J.; Seybold, S.J. Frontalin: De novo biosynthesis of an aggregation pheromone component by Dendroctonus spp. bark beetles (Coleoptera: Scolytidae). Insect Biochem. Mol. Biol. 2003, 33, 773–788. [Google Scholar] [CrossRef]
- Chiu, C.C.; Keeling, C.I.; Bohlmann, J. Monoterpenyl esters in juvenile mountain pine beetle and sex-specific release of the aggregation pheromone trans-verbenol. Proc. Natl. Acad. Sci. USA 2018, 115, 3652–3657. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Xiangbo, K.; Zhang, Z.; Wang, H.; Jiao, X.; Duan, R.; Chen, J.; Li, J. Olfactory reception of conspecific aggregation pheromone and host volatile terpenes by asian larch bark beetle, Ips subelongatus. Sci. Silvae Sin. 2013, 49, 89–97. [Google Scholar]
- Xu, F.; Zhou, X.; Song, C. Effects of Volatile Components in Rubber Tree on Behavior of Xyleborus affinis. Genom. Appl. Biol. 2020, 39, 4145–4153. [Google Scholar]
- Xu, F. Research of Volatile Compounds of Rubber Tree and Its Behavioral Activities on Two Species of Bark Beetles; Hainan University: Haikou, China, 2019; pp. 20–24. [Google Scholar]
- Shore, T.; McLean, J. Attraction of Platypus wilsoni Swaine (Coleoptera: Platipodidae) to traps baited with sulcatol, ethanol and alpha-pinene. Can. For. Serv. Res. Notes 1983, 3, 24–25. [Google Scholar]
- Chen, Q.; Liang, X.; Wu, C.; Chen, Q.; Zhang, Z. Trapping effects of 12 aggregation pheromones on rubber bark beetles. Chin. J. Trop. Crops 2019, 40, 2432–2439. [Google Scholar]
- Byers, J.A. Chemical ecology of bark beetles. Experientia 1989, 45, 271–283. [Google Scholar] [CrossRef]
- Rainho, H.L.; Silva, W.D.; Bento, J.M.S. Semiochemical-based attractant for the ambrosia pinhole borer Euplatypus parallelus. Agronomy 2021, 11, 266. [Google Scholar] [CrossRef]
- Raffa, K.; Andersson, M.N.; Schlyter, F. Host selection by bark beetles: Playing the odds in a high-stakes game. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–74. [Google Scholar]
- Andersson, M.N. Mechanisms of odor coding in coniferous bark beetles: From neuron to behavior and application. Psyche 2012, 2012, 149572. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Sachse, S.; Krieger, J. Olfaction in insects. e-Neuroforum 2011, 17, 49–60. [Google Scholar] [CrossRef]
- Mohapatra, P.; Menuz, K. Molecular profiling of the Drosophila antenna reveals conserved genes underlying olfaction in insects. G3 Genes Genomes Genet. 2019, 9, 3753–3771. [Google Scholar]
- Pelosi, P.; Iovinella, I.; Felicioli, A.; Dani, F.R. Soluble proteins of chemical communication: An overview across arthropods. Front. Physiol. 2014, 5, 320. [Google Scholar] [CrossRef] [PubMed]
- Bai, C. Identification and Binding Properties of Odorant-Binding Proteins OBP1 and OBP2 of Platypus parallelus; Hainan University: Haikou, China, 2021; pp. 16–17. [Google Scholar]
- Bickerstaff, J.R.; Smith, S.S.; Kent, D.S.; Beaver, R.A.; Seago, A.E.; Riegler, M. A review of the distribution and host plant associations of the platypodine ambrosia beetles (Coleoptera: Curculionidae: Platypodinae) of Australia, with an electronic species identification key. Zootaxa 2020, 4894, 69–80. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, H.A.F.; Faleiro, J.R. Semiochemicals and their potential use in pest management. In Biological Control of Pest and Vector Insects; Shields, V., Ed.; IntechOpen: London, UK, 2017; pp. 1–22. [Google Scholar]
- Fettig, C.J.; McKelvey, S.R.; Dabney, C.P.; Huber, D.P. Responses of Dendroctonus brevicomis (Coleoptera: Curculionidae) in behavioral assays: Implications to development of a semiochemical-based tool for tree protection. J. Econ. Entomol. 2012, 105, 149–160. [Google Scholar] [CrossRef]
- Gillette, N.E.; Fettig, C.J. Semiochemicals for bark beetle (Coleoptera: Curculionidae) management in western North America: Where do we go from here? Can. Entomol. 2021, 153, 121–135. [Google Scholar] [CrossRef]
- Xu, Y.L.; He, P.; Zhang, L.; Fang, S.Q.; Dong, S.L.; Zhang, Y.J.; Li, F. Large-scale identification of odorant-binding proteins and chemosensory proteins from expressed sequence tags in insects. BMC Genom. 2009, 10, 632. [Google Scholar] [CrossRef]
- Pelosi, P.; Zhou, J.J.; Ban, L.; Calvello, M. Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. CMLS 2006, 63, 1658–1676. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Du, Z.; Su, H.; Wang, W.; Ye, L.; Wei, H.; Peng, Z.; Anishchenko, I.; Baker, D.; Yang, J. The trRosetta server for fast and accurate protein structure prediction. Nat. Protoc. 2021, 16, 5634–5651. [Google Scholar] [CrossRef]
- Kuhlman, B.; Bradley, P. Advances in protein structure prediction and design. Nat. Rev. Mol. Cell Biol. 2019, 20, 681–697. [Google Scholar] [CrossRef]
- Bordoli, L.; Kiefer, F.; Arnold, K.; Benkert, P.; Battey, J.; Schwede, T. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 2009, 4, 1–13. [Google Scholar] [CrossRef]
- Bedard, W.D.; Tilden, P.E.; Wood, D.L.; Silverstein, R.M.; Brownlee, R.G.; Rodin, J.O. Western pine beetle: Field response to its sex pheromone and a synergistic host terpene, myrcene. Science 1969, 164, 1284–1285. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.J.; FUu, N.N.; Xu, Q.; Liu, Y.S.; Ren, L.L.; Luo, Y.Q. Analysis of volatiles from seven conifer species and effects of main terpenois on the behavior responses of Dendroctonus valens. J. Environ. Entomol. 2021, 43, 48–59. [Google Scholar]
- Fang, J.X.; Zhang, S.F.; Liu, F.; Cheng, B.; Zhang, Z.; Zhang, Q.H.; Kong, X.B. Functional investigation of monoterpenes for improved understanding of the relationship between hosts and bark beetles. J. Appl. Entomol. 2021, 145, 303–311. [Google Scholar] [CrossRef]
- Hansen, E.M.; Vandygriff, J.C.; Cain, R.J.; Wakarchuk, D. Comparison of naturally and synthetically baited spruce beetle trapping systems in the central Rocky Mountains. J. Econ. Entomol. 2006, 99, 373–382. [Google Scholar] [CrossRef]
- Borden, J.H.; Pureswaran, D.S.; Pierre Lafontaine, J. Synergistic blends of monoterpenes for aggregation pheromones of the mountain pine beetle (Coleoptera: Curculionidae). J. Econ. Entomol. 2008, 101, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.R. Myrcene: A precursor of pheromones in Ips beetles. J. Insect Physiol. 1974, 20, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Vanderwel, D. Factors affecting pheromone production in beetles. Arch. Insect Biochem. Physiol. 1994, 25, 347–362. [Google Scholar] [CrossRef]
- Hendry, L.; Piatek, B.; Browne, L.; Wood, D.; Byers, J.; Fish, R.; Hicks, R. In vivo conversion of a labelled host plant chemical to pheromones of the bark beetle Ips paraconfusus. Nature 1980, 284, 485. [Google Scholar] [CrossRef]
- Miller, D.R.; Lafontaine, J.P. cis-Verbenol: An aggregation pheromone for the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). J. Entomol. Soc. Br. Columbia 1991, 88, 34–38. [Google Scholar]
- Okoli, B.J.; Ladan, Z.; Mtunzi, F.; Hosea, Y.C. Vitex negundo L. Essential oil: Odorant binding protein efficiency using molecular docking approach and studies of the mosquito repellent. Insects 2021, 12, 1061. [Google Scholar] [CrossRef]
- Wang, S.N.; Shan, S.; Yu, G.Y.; Wang, H.; Dhiloo, K.H.; Khashaveh, A.; Zhang, F.; Zhang, Y.J. Identification of odorant-binding proteins and functional analysis of antenna-specific AplaOBP1 in the emerald ash borer, Agrilus planipennis. J. Pest Sci. 2020, 93, 853–865. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
| Ligands | Formula | 2D Structure | CAS Number | IC50 (μM) | Ki (μM) |
|---|---|---|---|---|---|
| Myrcene | C10H16 | 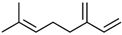 | 123-35-3 | 8.991 | 7.49 |
| S-(-)-cis-Verbenol | C10H16O | 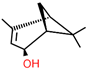 | 18881-04-4 | 23.18 | 19.30 |
| 2-Methyl-3-buten-2-ol | C5H10O |  | 115-18-4 | 37.9 | 31.56 |
| α-Pinene | C10H16 | 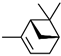 | 80-56-8 | 19.06 | 15.87 |
| (S)-(-)-Limonene | C10H16 | 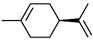 | 5989-54-8 | 40.44 | 33.68 |
| (+)-Longifolene | C15H24 |  | 475-20-7 | 49.61 | 41.31 |
| Tetradecane | C14H30 |  | 629-59-4 | 31.23 | 26.00 |
| 2-Phenyl-2-propanol | C9H12O | 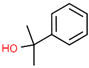 | 617-94-7 | 27.93 | 23.26 |
| Camphene | C10H16 | 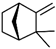 | 79-92-5 | 32.94 | 27.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, G.; Zhou, X.; Wang, Q.; Zhang, K.; Qin, L.; Guo, J. The Sequence Characteristics and Binding Properties of the Odorant-Binding Protein 2 of Euplatypus parallelus to Semiochemicals. Int. J. Mol. Sci. 2023, 24, 1714. https://doi.org/10.3390/ijms24021714
Cui G, Zhou X, Wang Q, Zhang K, Qin L, Guo J. The Sequence Characteristics and Binding Properties of the Odorant-Binding Protein 2 of Euplatypus parallelus to Semiochemicals. International Journal of Molecular Sciences. 2023; 24(2):1714. https://doi.org/10.3390/ijms24021714
Chicago/Turabian StyleCui, Guangchao, Xiang Zhou, Qian Wang, Kai Zhang, Lei Qin, and Jixing Guo. 2023. "The Sequence Characteristics and Binding Properties of the Odorant-Binding Protein 2 of Euplatypus parallelus to Semiochemicals" International Journal of Molecular Sciences 24, no. 2: 1714. https://doi.org/10.3390/ijms24021714
APA StyleCui, G., Zhou, X., Wang, Q., Zhang, K., Qin, L., & Guo, J. (2023). The Sequence Characteristics and Binding Properties of the Odorant-Binding Protein 2 of Euplatypus parallelus to Semiochemicals. International Journal of Molecular Sciences, 24(2), 1714. https://doi.org/10.3390/ijms24021714






