Salicylanilides and Their Anticancer Properties
Abstract
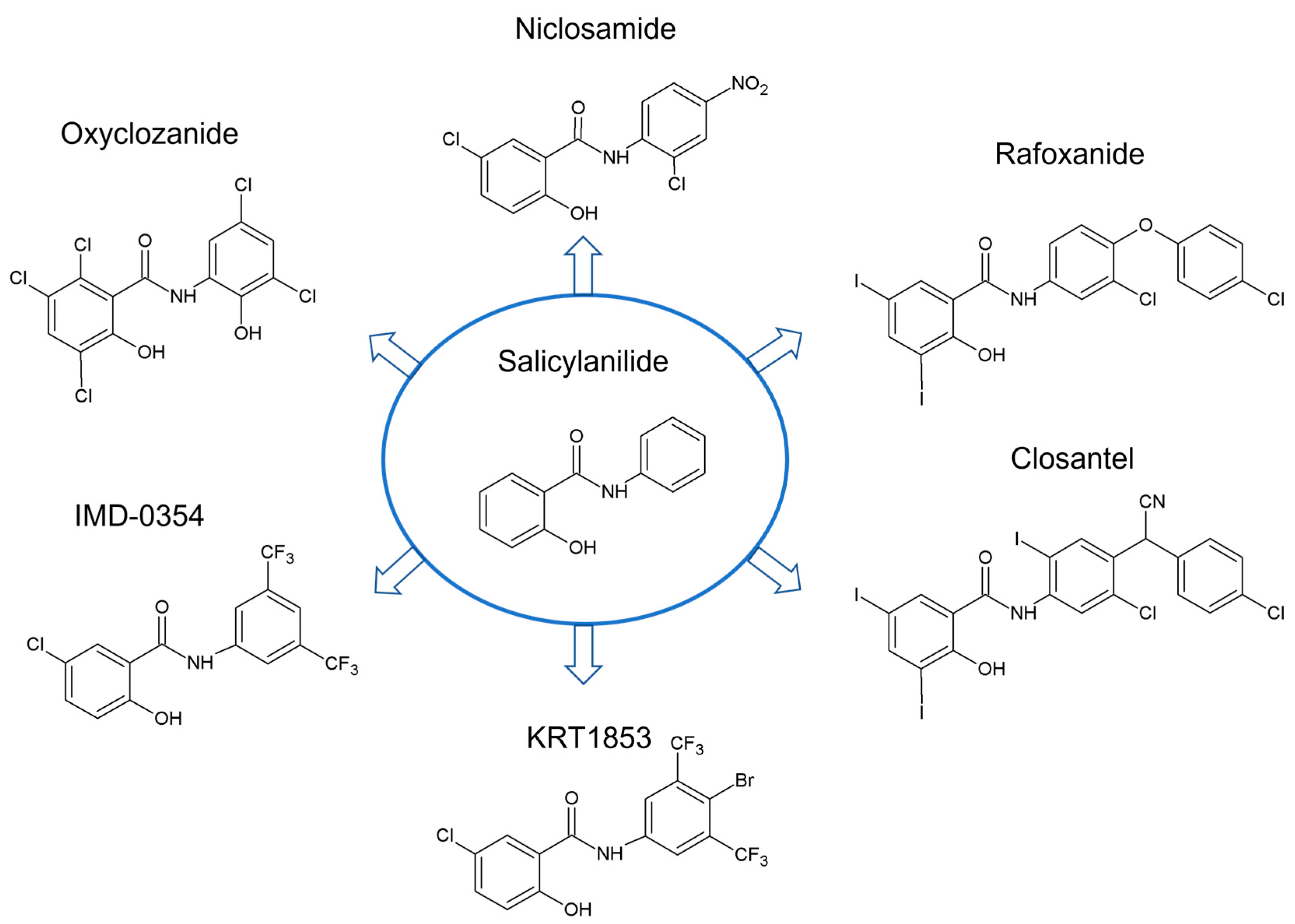
1. Introduction
2. Structural Features of Salicylanilides Related to Their Biological Activity
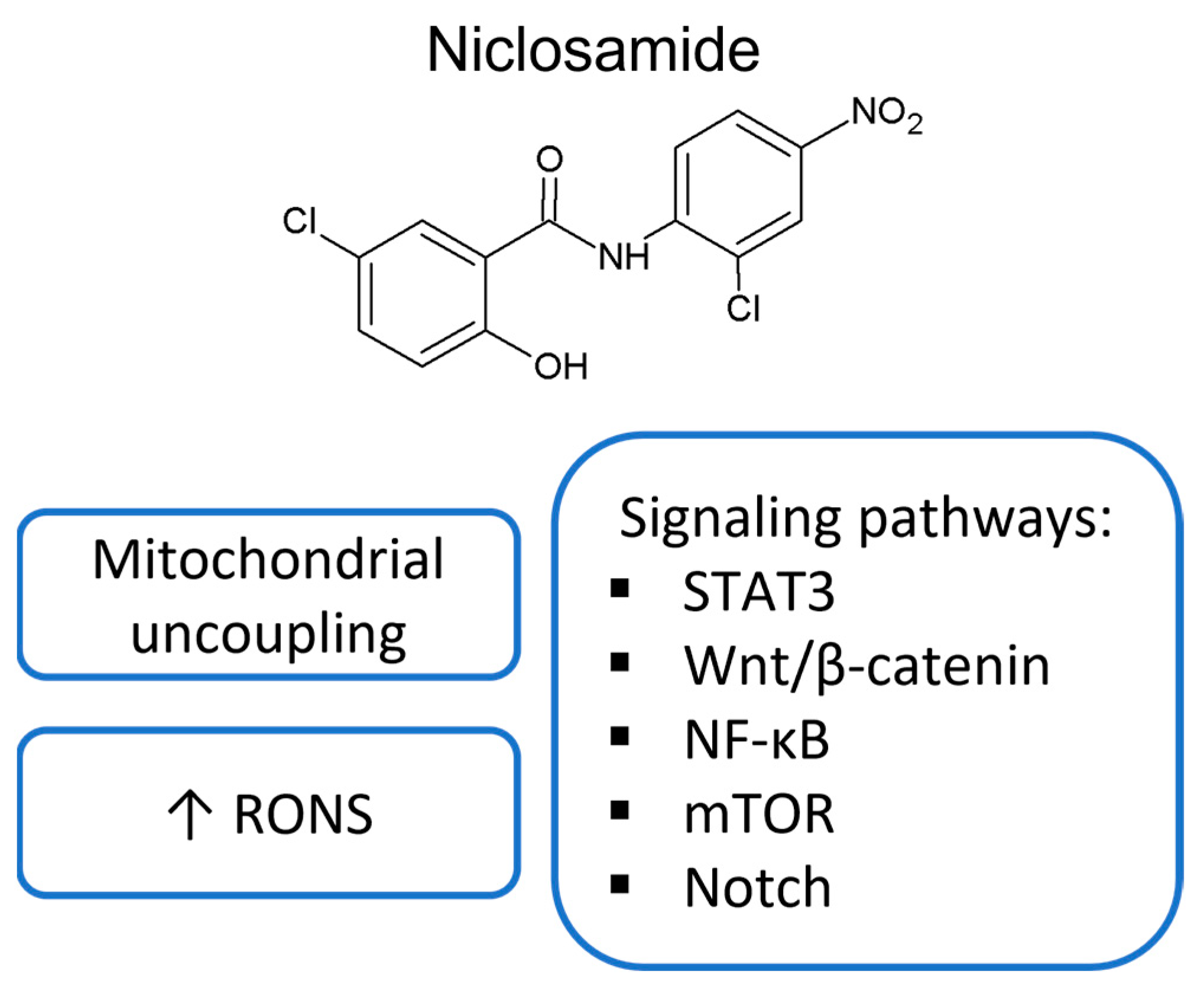
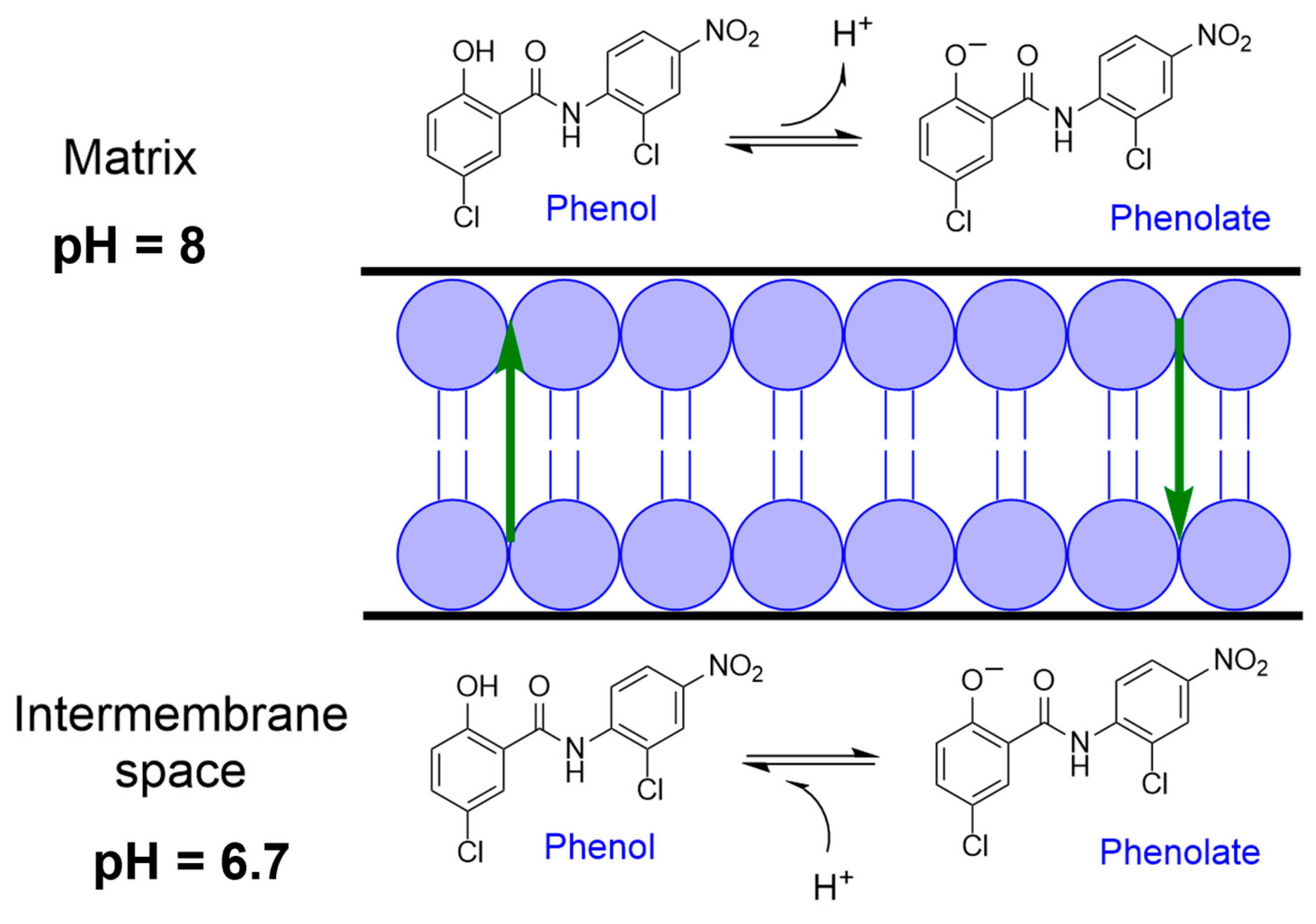
3. Mitochondrial Uncoupling
4. TK EGFR Inhibition
5. Signaling Pathways Modulated by Salicylanilides
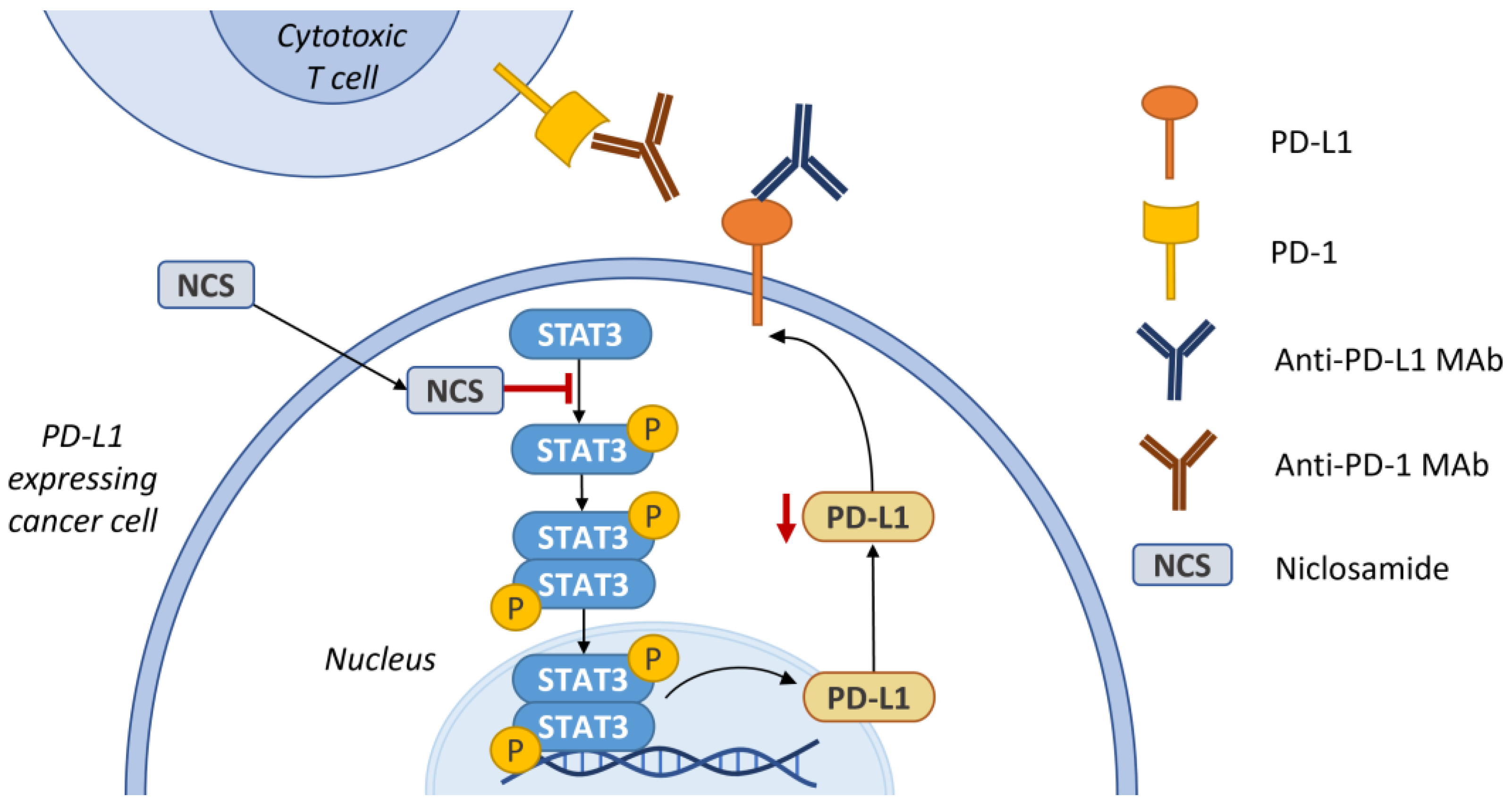
5.1. STAT3
5.2. Wnt Signaling Pathway
5.3. NF-κB Signaling Pathway
5.4. mTOR
5.5. Other Signaling Pathways or Tumor Suppressors as Targets of Salicylanilides
6. Synergism with Immune Checkpoint Inhibitors
7. Cancer Clinical Trials and Pharmacokinetic Properties
8. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Honkala, A.; Malhotra, S.V.; Kummar, S.; Junttila, M.R. Harnessing the predictive power of preclinical models for oncology drug development. Nat. Rev. Drug Discov. 2022, 21, 99–114. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P.; Vandeborne, L.; Bouche, G. A Database of Drug Repurposing Clinical Trials in Oncology. Front. Pharmacol. 2021, 12, 790952. [Google Scholar] [CrossRef] [PubMed]
- Alasadi, A.; Chen, M.; Swapna, G.V.T.; Tao, H.L.; Guo, J.J.; Collantes, J.; Fadhil, N.; Montelione, G.T.; Jin, S.K. Effect of mitochondrial uncouplers niclosamide ethanolamine (NEN) and oxyclozanide on hepatic metastasis of colon cancer. Cell Death Dis. 2018, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Duan, L.; He, Q.; Zhang, Z.; Zhou, Y.; Wu, D.; Pan, J.; Pei, D.; Ding, K. Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway. ACS Med. Chem. Lett. 2010, 1, 454–459. [Google Scholar] [CrossRef]
- Senkowski, W.; Zhang, X.; Olofsson, M.H.; Isacson, R.; Höglund, U.; Gustafsson, M.; Nygren, P.; Linder, S.; Larsson, R.; Fryknäs, M. Three-Dimensional Cell Culture-Based Screening Identifies the Anthelmintic Drug Nitazoxanide as a Candidate for Treatment of Colorectal Cancer. Mol. Cancer Ther. 2015, 14, 1504–1516. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, Z.; Chang, S.; Li, B.; Yu, D.; Wu, H.; Xie, Y.; Wang, Y.; Xie, B.; Sun, X.; et al. Rafoxanide, an organohalogen drug, triggers apoptosis and cell cycle arrest in multiple myeloma by enhancing DNA damage responses and suppressing the p38 MAPK pathway. Cancer Lett. 2019, 444, 45–59. [Google Scholar] [CrossRef]
- Tanaka, A.; Muto, S.; Konno, M.; Itai, A.; Matsuda, H. A new IkappaB kinase beta inhibitor prevents human breast cancer progression through negative regulation of cell cycle transition. Cancer Res. 2006, 66, 419–426. [Google Scholar] [CrossRef]
- Kim, S.; Ko, D.; Lee, Y.; Jang, S.; Lee, I.Y. Anti-cancer activity of the novel 2-hydroxydiarylamide derivatives IMD-0354 and KRT1853 through suppression of cancer cell invasion, proliferation, and survival mediated by TMPRSS4. Sci. Rep. 2019, 9, 10003. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, C.; Liu, Z.; Peng, F.; Chen, X.; Yang, G.; Zhang, D.; Yin, Z.; Ma, J.; Zheng, Z.; et al. Nitazoxanide, an antiprotozoal drug, inhibits late-stage autophagy and promotes ING1-induced cell cycle arrest in glioblastoma. Cell Death Dis. 2018, 9, 1032. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Huang, H.S.; Chen, K.C.; Saka, T.; Chiang, C.Y.; Chung, L.W.K.; Sung, S.Y. A Novel Salicylanilide Derivative Induces Autophagy Cell Death in Castration-Resistant Prostate Cancer via ER Stress-Activated PERK Signaling Pathway. Mol. Cancer Ther. 2020, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Z.; Ai, F.; Tang, W.K.; Zhu, G. A monofunctional platinum(II)-based anticancer agent from a salicylanilide derivative: Synthesis, antiproliferative activity, and transcription inhibition. J. Inorg. Biochem. 2015, 142, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Haugk, K.; McKiernan, J.S.; Gulati, R.; Cheng, H.H.; Maes, J.L.; Dumpit, R.F.; Nelson, P.S.; Montgomery, B.; McCune, J.S.; et al. A phase I study of niclosamide in combination with enzalutamide in men with castration-resistant prostate cancer. PloS ONE 2018, 13, e0198389. [Google Scholar] [CrossRef] [PubMed]
- Burock, S.; Daum, S.; Keilholz, U.; Neumann, K.; Walther, W.; Stein, U. Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: The NIKOLO trial. BMC Cancer 2018, 18, 297. [Google Scholar] [CrossRef]
- Felsher, I.M. Salicylanilide Therapy in Tinea Capitis. Arch. Dermatol. Syphilol. 1948, 58, 56–63. [Google Scholar] [CrossRef]
- Brain, R.T.; Crow, K.; Haber, H.; McKenny, C. Treatment of Ringworm of the Scalp. Br. Med. J. 1948, 1, 723–726. [Google Scholar] [CrossRef]
- Swan, G.E. The pharmacology of halogenated salicylanilides and their anthelmintic use in animals. J. S. Afr. Vet. Assoc. 1999, 70, 61–70. [Google Scholar] [CrossRef]
- Macielag, M.J.; Demers, J.P.; Fraga-Spano, S.A.; Hlasta, D.J.; Johnson, S.G.; Kanojia, R.M.; Russell, R.K.; Sui, Z.; Weidner-Wells, M.A.; Werblood, H.; et al. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J. Med. Chem. 1998, 41, 2939–2945. [Google Scholar] [CrossRef]
- Domalaon, R.; Okunnu, O.; Zhanel, G.G.; Schweizer, F. Synergistic combinations of anthelmintic salicylanilides oxyclozanide, rafoxanide, and closantel with colistin eradicates multidrug-resistant colistin-resistant Gram-negative bacilli. J. Antibiot. 2019, 72, 605–616. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J.; Novotna, E.; Mandikova, J.; Trejtnar, F.; Stolarikova, J. Antibacterial Activity of Salicylanilide 4-(Trifluoromethyl)benzoates. Molecules 2013, 18, 3674–3688. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J.; Novotna, E.; Mandikova, J.; Wsol, V.; Trejtnar, F.; Ulmann, V.; Stolarikova, J.; Fernandes, S.; Bhat, S.; et al. Salicylanilide derivatives block Mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. Tuberculosis 2012, 92, 434–439. [Google Scholar] [CrossRef]
- Waisser, K.; Bures, O.; Holý, P.; Kunes, J.; Oswald, R.; Jirásková, L.; Pour, M.; Klimesová, V.; Kubicová, L.; Kaustová, J. Relationship between the structure and antimycobacterial activity of substituted salicylanilides. Arch. Pharm. 2003, 336, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Imramovský, A.; Vinsová, J.; Férriz, J.M.; Dolezal, R.; Jampílek, J.; Kaustová, J.; Kunc, F. New antituberculotics originated from salicylanilides with promising in vitro activity against atypical mycobacterial strains. Bioorg. Med. Chem. 2009, 17, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Vinsova, J.; Ferriz, J.M.; Buchta, V.; Jampilek, J. Salicylanilide esters of N-protected amino acids as novel antimicrobial agents. Bioorg. Med. Chem. Lett. 2009, 19, 348–351. [Google Scholar] [CrossRef]
- Krátký, M.; Vinšová, J.; Buchta, V. In vitro antibacterial and antifungal activity of salicylanilide pyrazine-2-carboxylates. Med. Chem. 2012, 8, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Fitzner, J.N.; Stevens, T.; Chin, W.; Wright, C.D.; Boyce, J.P. Salicylanilides: Selective inhibitors of interleukin-12p40 production. Bioorganic Med. Chem. 2016, 16, 8760–8764. [Google Scholar] [CrossRef]
- Sugita, A.; Ogawa, H.; Azuma, M.; Muto, S.; Honjo, A.; Yanagawa, H.; Nishioka, Y.; Tani, K.; Itai, A.; Sone, S. Antiallergic and anti-inflammatory effects of a novel I kappaB kinase beta inhibitor, IMD-0354, in a mouse model of allergic inflammation. Int. Arch. Allergy Immunol. 2009, 148, 186–198. [Google Scholar] [CrossRef]
- Williamson, R.L.; Metcalf, R.L. Salicylanilides: A new group of active uncouplers of oxidative phosphorylation. Science 1967, 158, 1694–1695. [Google Scholar] [CrossRef]
- Liechti, C.; Sequin, U.; Bold, G.; Furet, P.; Meyer, T.; Traxler, P. Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor. Eur. J. Med. Chem. 2004, 39, 11–26. [Google Scholar] [CrossRef]
- Kamath, S.; Buolamwini, J.K. Targeting EGFR and HER-2 receptor tyrosine kinases for cancer drug discovery and development. Med. Res. Rev. 2006, 26, 569–594. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Xia, B.; Liu, H.C.; Xu, Y.Q.; Huang, C.J.; Gao, J.M.; Dong, Q.X.; Li, C.Q. Closantel Suppresses Angiogenesis and Cancer Growth in Zebrafish Models. Assay. Drug Dev. Technol. 2016, 14, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, B.; Xu, Z.; Li, B.; Cai, T.; Zhang, X.; Yu, Y.; Wang, H.; Shi, J.; Zhu, W. Repositioning organohalogen drugs: A case study for identification of potent B-Raf V600E inhibitors via docking and bioassay. Sci. Rep. 2016, 6, 31074. [Google Scholar] [CrossRef] [PubMed]
- Luciano, V.; Proschak, E.; Langer, J.D.; Knapp, S.; Heering, J.; Marschalek, R. Closantel is an allosteric inhibitor of human Taspase1. iScience 2021, 24, 103524. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, H.; Shi, A.; Yao, H.; Ke, K.; Dong, C.; Zhu, Y.; Qin, Y.; Ding, Y.; He, Y.H.; et al. Discovery of rafoxanide as a dual CDK4/6 inhibitor for the treatment of skin cancer. Oncol. Rep. 2018, 40, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Hu, Y.L.; Feng, Y.; Guo, Y.B.; Liu, Y.F.; Yang, J.L.; Mao, Q.S.; Xue, W.J. Rafoxanide promotes apoptosis and autophagy of gastric cancer cells by suppressing PI3K /Akt/mTOR pathway. Exp. Cell Res. 2019, 385, 111691. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Grazia, A.; De Simone, V.; Cherubini, F.; Colantoni, A.; Ortenzi, A.; Franzè, E.; Dinallo, V.; Di Fusco, D.; Monteleone, I.; et al. Induction of endoplasmic reticulum stress and inhibition of colon carcinogenesis by the anti-helmintic drug rafoxanide. Cancer Lett. 2019, 462, 1–11. [Google Scholar] [CrossRef]
- Di Grazia, A.; Laudisi, F.; Di Fusco, D.; Franzè, E.; Ortenzi, A.; Monteleone, I.; Monteleone, G.; Stolfi, C. Rafoxanide Induces Immunogenic Death of Colorectal Cancer Cells. Cancers 2020, 12, 1314. [Google Scholar] [CrossRef]
- Laudisi, F.; Pacifico, T.; Maresca, C.; Luiz-Ferreira, A.; Antonelli, S.; Ortenzi, A.; Colantoni, A.; Di Grazia, A.; Franzè, E.; Colella, M.; et al. Rafoxanide sensitizes colorectal cancer cells to TRAIL-mediated apoptosis. Biomed. Pharm. 2022, 155, 113794. [Google Scholar] [CrossRef]
- He, W.; Xu, Z.; Song, D.; Zhang, H.; Li, B.; Gao, L.; Zhang, Y.; Feng, Q.; Yu, D.; Hu, L.; et al. Antitumor effects of rafoxanide in diffuse large B cell lymphoma via the PTEN/PI3K/Akt and JNK/c-Jun pathways. Life Sci. 2020, 243, 117249. [Google Scholar] [CrossRef]
- Tanaka, A.; Konno, M.; Muto, S.; Kambe, N.; Morii, E.; Nakahata, T.; Itai, A.; Matsuda, H. A novel NF-kappaB inhibitor, IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood 2005, 105, 2324–2331. [Google Scholar] [CrossRef]
- Kanduri, M.; Tobin, G.; Aleskog, A.; Nilsson, K.; Rosenquist, R. The novel NF-kappaB inhibitor IMD-0354 induces apoptosis in chronic lymphocytic leukemia. Blood Cancer J. 2011, 1, e12. [Google Scholar] [CrossRef] [PubMed]
- Uota, S.; Zahidunnabi Dewan, M.; Saitoh, Y.; Muto, S.; Itai, A.; Utsunomiya, A.; Watanabe, T.; Yamamoto, N.; Yamaoka, S. An IkappaB kinase 2 inhibitor IMD-0354 suppresses the survival of adult T-cell leukemia cells. Cancer Sci. 2012, 103, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, S.; Tanaka, A.; Matsuda, A.; Oida, K.; Jang, H.; Jung, K.; Amagai, Y.; Ahn, G.; Okamoto, N.; Ishizaka, S.; et al. A molecular targeting against nuclear factor-kappaB, as a chemotherapeutic approach for human malignant mesothelioma. Cancer Med. 2014, 3, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Saito, Y.; Saitoh, T.; Dewan, M.Z.; Shioya, A.; Kobayashi, M.; Kawachi, H.; Muto, S.; Itai, A.; Uota, S.; et al. Inhibition of IkappaB kinase beta restrains oncogenic proliferation of pancreatic cancer cells. J. Med. Dent. Sci. 2008, 55, 49–59. [Google Scholar]
- Feng, Y.; Pathria, G.; Heynen-Genel, S.; Jackson, M.; James, B.; Yin, J.; Scott, D.A.; Ronai, Z.A. Identification and Characterization of IMD-0354 as a Glutamine Carrier Protein Inhibitor in Melanoma. Mol. Cancer Ther. 2021, 20, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Olsen, J.R.; Yuan, X.; Cheng, P.F.; Levesque, M.P.; Brokstad, K.A.; Hoffman, P.S.; Oyan, A.M.; Zhang, W.; Kalland, K.H.; et al. Small molecule promotes β-catenin citrullination and inhibits Wnt signaling in cancer. Nat. Chem. Biol. 2018, 14, 94–101. [Google Scholar] [CrossRef]
- Fan-Minogue, H.; Bodapati, S.; Solow-Cordero, D.; Fan, A.; Paulmurugan, R.; Massoud, T.F.; Felsher, D.W.; Gambhir, S.S. A c-Myc activation sensor-based high-throughput drug screening identifies an antineoplastic effect of nitazoxanide. Mol. Cancer Ther. 2013, 12, 1896–1905. [Google Scholar] [CrossRef]
- Abd El-Fadeal, N.M.; Nafie, M.S.; K El-Kherbetawy, M.; El-Mistekawy, A.; Mohammad, H.M.F.; Elbahaie, A.M.; Hashish, A.A.; Alomar, S.Y.; Aloyouni, S.Y.; El-Dosoky, M.; et al. Antitumor Activity of Nitazoxanide against Colon Cancers: Molecular Docking and Experimental Studies Based on Wnt/β-Catenin Signaling Inhibition. Int. J. Mol. Sci. 2021, 22, 5213. [Google Scholar] [CrossRef]
- Yu, J.; Yang, K.; Zheng, J.; Zhao, W.; Sun, X. Synergistic tumor inhibition of colon cancer cells by nitazoxanide and obeticholic acid, a farnesoid X receptor ligand. Cancer Gene Ther. 2021, 28, 590–601. [Google Scholar] [CrossRef]
- Hiroko, S.; Minoru, H.; Tomoaki, Y.; Yoshiki, H.; Isao, T.; Michiharu, S. Studies on the Conformations of Antimicrobial Salicylanilide Derivatives by Spectroscopy. Bull. Chem. Soc. Jpn. 2000, 73, 2335–2339. [Google Scholar] [CrossRef]
- Deng, W.; Guo, Z.; Guo, Y.; Feng, Z.; Jiang, Y.; Chu, F. Acryloylamino-salicylanilides as EGFR PTK inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Q.L.; Jiang, Q.Q.; Jiang, Q.J.; Jiang, Y.B. Anion-triggered substituent-dependent conformational switching of salicylanilides. New hints for understanding the inhibitory mechanism of salicylanilides. J. Org. Chem. 2007, 72, 9947–9953. [Google Scholar] [CrossRef] [PubMed]
- Imramovský, A.; Pauk, K.; Padělková, Z.; Hanusek, J. Crystal Structure of the 5-Chloro Salicylamides: Three Different Types of the H-bonding Influenced Linear Chain Formation in the Solid State. Crystals 2012, 2, 349–361. [Google Scholar] [CrossRef]
- Spaczynska, E.; Mrozek-Wilczkiewicz, A.; Malarz, K.; Kos, J.; Gonec, T.; Oravec, M.; Gawecki, R.; Bak, A.; Dohanosova, J.; Kapustikova, I.; et al. Design and synthesis of anticancer 1-hydroxynaphthalene-2-carboxanilides with a p53 independent mechanism of action. Sci. Rep. 2019, 9, 6387. [Google Scholar] [CrossRef] [PubMed]
- Kauerova, T.; Kos, J.; Gonec, T.; Jampilek, J.; Kollar, P. Antiproliferative and Pro-Apoptotic Effect of Novel Nitro-Substituted Hydroxynaphthanilides on Human Cancer Cell Lines. Int. J. Mol. Sci. 2016, 17, 1219. [Google Scholar] [CrossRef]
- Kauerová, T.; Goněc, T.; Jampílek, J.; Hafner, S.; Gaiser, A.K.; Syrovets, T.; Fedr, R.; Souček, K.; Kollar, P. Ring-Substituted 1-Hydroxynaphthalene-2-Carboxanilides Inhibit Proliferation and Trigger Mitochondria-Mediated Apoptosis. Int. J. Mol. Sci. 2020, 21, 3416. [Google Scholar] [CrossRef]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef]
- Tang, Z.; Acuna, U.M.; Fernandes, N.F.; Chettiar, S.; Li, P.K.; EC, D.E.B. Structure-Activity Relationship of Niclosamide Derivatives. AntiCancer Res. 2017, 37, 2839–2843. [Google Scholar] [CrossRef]
- Kumar, R.; Coronel, L.; Somalanka, B.; Raju, A.; Aning, O.A.; An, O.; Ho, Y.S.; Chen, S.; Mak, S.Y.; Hor, P.Y.; et al. Mitochondrial uncoupling reveals a novel therapeutic opportunity for p53-defective cancers. Nat. Commun. 2018, 9, 3931. [Google Scholar] [CrossRef]
- Childress, E.S.; Alexopoulos, S.J.; Hoehn, K.L.; Santos, W.L. Small Molecule Mitochondrial Uncouplers and Their Therapeutic Potential. J. Med. Chem. 2018, 61, 4641–4655. [Google Scholar] [CrossRef]
- Menegazzi, M.; Masiello, P.; Novelli, M. Anti-Tumor Activity of. Antioxidants 2020, 10, 18. [Google Scholar] [CrossRef]
- Terada, H. The interaction of highly active uncouplers with mitochondria. Biochim. Biophys. Acta. 1981, 639, 225–242. [Google Scholar] [CrossRef]
- Terada, H.; Goto, S.; Yamamoto, K.; Takeuchi, I.; Hamada, Y.; Miyake, K. Structural requirements of salicylanilides for uncoupling activity in mitochondria: Quantitative analysis of structure-uncoupling relationships. Biochim. Biophys. Acta. 1988, 936, 504–512. [Google Scholar] [CrossRef]
- Tao, H.; Zhang, Y.; Zeng, X.; Shulman, G.I.; Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 2014, 20, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Weinbach, E.C.; Garbus, J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature 1969, 221, 1016–1018. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim Biophys Acta 2014, 1837, 418–426. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends. Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Wotring, J.W.; McCarty, S.M.; Shafiq, K.; Zhang, C.J.; Nguyen, T.; Meyer, S.R.; Fursmidt, R.; Mirabelli, C.; Clasby, M.C.; Wobus, C.E.; et al. In Vitro Evaluation and Mitigation of Niclosamide’s Liabilities as a COVID-19 Treatment. Vaccines 2022, 10, 1284. [Google Scholar] [CrossRef]
- Enoch, S.J.; Schultz, T.W.; Popova, I.G.; Vasilev, K.G.; Mekenyan, O.G. Development of a Decision Tree for Mitochondrial Dysfunction: Uncoupling of Oxidative Phosphorylation. Chem. Res. Toxicol. 2018, 31, 814–820. [Google Scholar] [CrossRef]
- Tollenaere, J.P. Structure-activity relationships of three groups of uncouplers of oxidative phosphorylation: Salicylanilides, 2-trifluoromethylbenzimidazoles, and phenols. J. Med. Chem. 1973, 16, 791–796. [Google Scholar] [CrossRef]
- Da Silva-Diz, V.; Cao, B.; Lancho, O.; Chiles, E.; Alasadi, A.; Aleksandrova, M.; Luo, S.; Singh, A.; Tao, H.; Augeri, D.; et al. A novel and highly effective mitochondrial uncoupling drug in T-cell leukemia. Blood 2021, 138, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Khanim, F.L.; Merrick, B.A.; Giles, H.V.; Jankute, M.; Jackson, J.B.; Giles, L.J.; Birtwistle, J.; Bunce, C.M.; Drayson, M.T. Redeployment-based drug screening identifies the anti-helminthic niclosamide as anti-myeloma therapy that also reduces free light chain production. Blood Cancer J. 2011, 1, e39. [Google Scholar] [CrossRef]
- Jiang, H.; Greathouse, R.L.; Tiche, S.J.; Zhao, M.; He, B.; Li, Y.; Li, A.M.; Forgo, B.; Yip, M.; Li, A.; et al. Mitochondrial uncoupling induces epigenome remodeling and promotes differentiation in neuroblastoma. Cancer Res. 2022, 83, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Corbett, J.R.; Goose, A.J. A possible biochemical mode of action of the fasciolicides nitroxynil, hexachlorophene and oxyclozanide. Pestic. Sci. 1971, 2, 119–121. [Google Scholar] [CrossRef]
- Bacon, J.A.; Ulrich, R.G.; Davis, J.P.; Thomas, E.M.; Johnson, S.S.; Conder, G.A.; Sangster, N.C.; Rothwell, J.T.; McCracken, R.O.; Lee, B.H.; et al. Comparative in vitro effects of closantel and selected beta-ketoamide anthelmintics on a gastrointestinal nematode and vertebrate liver cells. J. Vet. Pharmacol. Ther. 1998, 21, 190–198. [Google Scholar] [CrossRef]
- De Carvalho, L.P.; Darby, C.M.; Rhee, K.Y.; Nathan, C. Nitazoxanide Disrupts Membrane Potential and Intrabacterial pH Homeostasis of Mycobacterium tuberculosis. ACS Med. Chem. Lett. 2011, 2, 849–854. [Google Scholar] [CrossRef]
- Rossignol, J.F. Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antiviral. Res. 2014, 110, 94–103. [Google Scholar] [CrossRef]
- Ek, F.; Blom, K.; Selvin, T.; Rudfeldt, J.; Andersson, C.; Senkowski, W.; Brechot, C.; Nygren, P.; Larsson, R.; Jarvius, M.; et al. Sorafenib and nitazoxanide disrupt mitochondrial function and inhibit regrowth capacity in three-dimensional models of hepatocellular and colorectal carcinoma. Sci. Rep. 2022, 12, 8943. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z.X. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Kjær, I.M.; Olsen, D.A.; Brandslund, I.; Bechmann, T.; Jakobsen, E.H.; Bogh, S.B.; Madsen, J.S. Dysregulated EGFR pathway in serum in early-stage breast cancer patients: A case control study. Sci. Rep. 2020, 10, 6714. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Byeon, H.K.; Ku, M.; Yang, J. Beyond EGFR inhibition: Multilateral combat strategies to stop the progression of head and neck cancer. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Alqahtani, A.M.; Youssif, B.G.M.; Gouda, A.M. Globally Approved EGFR Inhibitors: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism. Molecules 2021, 26, 6677. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Moasser, M.M. The epidermal growth factor receptor family: Biology driving targeted therapeutics. Cell. Mol. Life Sci. 2008, 65, 1566–1584. [Google Scholar] [CrossRef]
- Kovacs, E.; Zorn, J.A.; Huang, Y.J.; Barros, T.; Kuriyan, J. A Structural Perspective on the Regulation of the Epidermal Growth Factor Receptor. Annu. Rev. Biochem. 2015, 84, 739–764. [Google Scholar] [CrossRef]
- Hedge, C.N.; Pierce, J. A diazine heterocycle replaces a six-membered hydrogen-bonded array in the active site of scytalone dehydratase. Bioorg. Med. Chem. Lett. 1993, 3, 1605–1608. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, L.; Sun, W.; Yu, Z.; Wang, J.; Gao, H.; Yang, G. Synthesis of p-O-Alkyl Salicylanilide Derivatives as Novel EGFR Inhibitors. Drug Dev. Res. 2016, 77, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ye, W.; Li, J.; Zhong, G.; He, G.; Xu, Q.; Zhang, Y. Synthesis and evaluation of salicylanilide derivatives as potential epidermal growth factor receptor inhibitors. Chem. Biol. Drug Des. 2015, 85, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.W.; Shi, L.; Ruan, X.M.; Yang, Y.; Li, H.Q.; Xu, S.P.; Zhu, H.L. Synthesis and antiproliferative activities against Hep-G2 of salicylanide derivatives: Potent inhibitors of the epidermal growth factor receptor (EGFR) tyrosine kinase. J. Enzyme. Inhib. Med. Chem. 2011, 26, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Zheng, Y.W.; Lu, S.M.; Li, Y.; Zhang, S.Q. Synthesis and biological evaluation of N-aryl salicylamides with a hydroxamic acid moiety at 5-position as novel HDAC-EGFR dual inhibitors. Bioorg. Med. Chem. 2012, 20, 4405–4412. [Google Scholar] [CrossRef]
- Furtek, S.L.; Backos, D.S.; Matheson, C.J.; Reigan, P. Strategies and Approaches of Targeting STAT3 for Cancer Treatment. Acs Chem. Biol. 2016, 11, 308–318. [Google Scholar] [CrossRef]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Wake, M.S.; Watson, C.J. STAT3 the oncogene—still eluding therapy? Febs. J. 2015, 282, 2600–2611. [Google Scholar] [CrossRef]
- Jarnicki, A.; Putoczki, T.; Ernst, M. Stat3: Linking inflammation to epithelial cancer—more than a “gut” feeling? Cell Div. 2010, 5, 14. [Google Scholar] [CrossRef]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Li, R.; You, S.; Hu, Z.; Chen, Z.G.; Sica, G.L.; Khuri, F.R.; Curran, W.J.; Shin, D.M.; Deng, X. Inhibition of STAT3 by niclosamide synergizes with erlotinib against head and neck cancer. PLoS ONE 2013, 8, e74670. [Google Scholar] [CrossRef]
- Lu, L.; Dong, J.L.; Wang, L.L.; Xia, Q.; Zhang, D.; Kim, H.J.; Yin, T.; Fan, S.J.; Shen, Q. Activation of STAT3 and Bcl-2 and reduction of reactive oxygen species (ROS) promote radioresistance in breast cancer and overcome of radioresistance with niclosamide. Oncogene 2018, 37, 5292–5304. [Google Scholar] [CrossRef]
- You, S.; Li, R.; Park, D.; Xie, M.; Sica, G.L.; Cao, Y.; Xiao, Z.Q.; Deng, X. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol. Cancer Ther. 2014, 13, 606–616. [Google Scholar] [CrossRef]
- Atsaves, V.; Tsesmetzis, N.; Chioureas, D.; Kis, L.; Leventaki, V.; Drakos, E.; Panaretakis, T.; Grander, D.; Medeiros, L.J.; Young, K.H.; et al. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia 2017, 31, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Luo, M.; Rong, Q.X.; Zhang, H.; Chen, Z.; Wang, F.; Zhao, H.Y.; Fu, L.W. Niclosamide, an antihelmintic drug, enhances efficacy of PD-1/PD-L1 immune checkpoint blockade in non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 245. [Google Scholar] [CrossRef]
- Wu, M.M.; Zhang, Z.; Tong, C.W.S.; Yan, V.W.; Cho, W.C.S.; To, K.K.W. Repurposing of niclosamide as a STAT3 inhibitor to enhance the anticancer effect of chemotherapeutic drugs in treating colorectal cancer. Life Sci. 2020, 262, 118522. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, X.; Xu, H.; Shi, X.; Zhao, J.; Yang, M.; Zhang, L.; Jin, X.; Hu, Y.; Li, X.; et al. Niclosamide Inhibits Cell Growth and Enhances Drug Sensitivity of Hepatocellular Carcinoma Cells via STAT3 Signaling Pathway. J. Cancer 2018, 9, 4150–4155. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Han, Z.; Wen, Y.; Ma, Z.; Wang, Y. Niclosamide acts as a new inhibitor of vasculogenic mimicry in oral cancer through upregulation of miR-124 and downregulation of STAT3. Oncol. Rep. 2018, 39, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, J.; Lee, Y.H.; Min, B.S.; Choi, J. Niclosamide reverses adipocyte induced epithelial-mesenchymal transition in breast cancer cells via suppression of the interleukin-6/STAT3 signalling axis. Sci. Rep. 2019, 9, 11336. [Google Scholar] [CrossRef]
- Cheng, B.; Morales, L.D.; Zhang, Y.; Mito, S.; Tsin, A. Niclosamide induces protein ubiquitination and inhibits multiple pro-survival signaling pathways in the human glioblastoma U-87 MG cell line. PloS ONE 2017, 12, e0184324. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ye, W.; Li, J.; Zhou, P.; Chu, Z.; Huang, W. The salicylanilide derivatives inhibit signal transducer and activator of transcription 3 pathways in A549 lung cancer cells. Anticancer Drugs 2016, 27, 41–47. [Google Scholar] [CrossRef]
- Gargantilla, M.; Persoons, L.; Kauerová, T.; Del Río, N.; Daelemans, D.; Priego, E.M.; Kollar, P.; Pérez-Pérez, M.J. Hybridization Approach to Identify Salicylanilides as Inhibitors of Tubulin Polymerization and Signal Transducers and Activators of Transcription 3 (STAT3). Pharmaceuticals 2022, 15, 835. [Google Scholar] [CrossRef] [PubMed]
- Lü, Z.; Li, X.; Li, K.; Wang, C.; Du, T.; Huang, W.; Ji, M.; Li, C.; Xu, F.; Xu, P.; et al. Structure-Activity Study of Nitazoxanide Derivatives as Novel STAT3 Pathway Inhibitors. ACS Med. Chem. Lett. 2021, 12, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, J.; Lu, J.; Bond, M.C.; Ren, X.R.; Lyerly, H.K.; Barak, L.S.; Chen, W. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry 2009, 48, 10267–10274. [Google Scholar] [CrossRef]
- Sack, U.; Walther, W.; Scudiero, D.; Selby, M.; Kobelt, D.; Lemm, M.; Fichtner, I.; Schlag, P.M.; Shoemaker, R.H.; Stein, U. Novel effect of antihelminthic Niclosamide on S100A4-mediated metastatic progression in colon cancer. J. Natl. Cancer Inst. 2011, 103, 1018–1036. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.T. Wnt/beta-catenin pathway. Sci STKE 2005, 2005, cm1. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef]
- Monin, M.B.; Krause, P.; Stelling, R.; Bocuk, D.; Niebert, S.; Klemm, F.; Pukrop, T.; Koenig, S. The anthelmintic niclosamide inhibits colorectal cancer cell lines via modulation of the canonical and noncanonical Wnt signaling pathway. J. Surg. Res. 2016, 203, 193–205. [Google Scholar] [CrossRef]
- Wang, J.; Ren, X.R.; Piao, H.; Zhao, S.; Osada, T.; Premont, R.T.; Mook, R.A.; Morse, M.A.; Lyerly, H.K.; Chen, W. Niclosamide-induced Wnt signaling inhibition in colorectal cancer is mediated by autophagy. Biochem. J. 2019, 476, 535–546. [Google Scholar] [CrossRef]
- Osada, T.; Chen, M.; Yang, X.Y.; Spasojevic, I.; Vandeusen, J.B.; Hsu, D.; Clary, B.M.; Clay, T.M.; Chen, W.; Morse, M.A.; et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011, 71, 4172–4182. [Google Scholar] [CrossRef]
- Lu, W.; Lin, C.; Roberts, M.J.; Waud, W.R.; Piazza, G.A.; Li, Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/beta-catenin pathway. PLoS ONE 2011, 6, e29290. [Google Scholar] [CrossRef]
- Zhao, J.; He, Q.; Gong, Z.; Chen, S.; Cui, L. Niclosamide suppresses renal cell carcinoma by inhibiting Wnt/β-catenin and inducing mitochondrial dysfunctions. Springerplus 2016, 5, 1436. [Google Scholar] [CrossRef]
- Arend, R.C.; Londono-Joshi, A.I.; Samant, R.S.; Li, Y.; Conner, M.; Hidalgo, B.; Alvarez, R.D.; Landen, C.N.; Straughn, J.M.; Buchsbaum, D.J. Inhibition of Wnt/beta-catenin pathway by niclosamide: A therapeutic target for ovarian cancer. Gynecol. Oncol. 2014, 134, 112–120. [Google Scholar] [CrossRef]
- Wang, L.H.; Xu, M.; Fu, L.Q.; Chen, X.Y.; Yang, F. The Antihelminthic Niclosamide Inhibits Cancer Stemness, Extracellular Matrix Remodeling, and Metastasis through Dysregulation of the Nuclear β-catenin/c-Myc axis in OSCC. Sci. Rep. 2018, 8, 12776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jin, B.; Jin, Y.; Liu, Y.; Pan, J. The antihelminthic drug niclosamide effectively inhibits the malignant phenotypes of uveal melanoma. Theranostics 2017, 7, 1447–1462. [Google Scholar] [CrossRef]
- Ono, M.; Yin, P.; Navarro, A.; Moravek, M.B.; Coon V., J.S.; Druschitz, S.A.; Gottardi, C.J.; Bulun, S.E. Inhibition of canonical WNT signaling attenuates human leiomyoma cell growth. Fertil. Steril. 2014, 101, 1441–1449. [Google Scholar] [CrossRef]
- Londoño-Joshi, A.I.; Arend, R.C.; Aristizabal, L.; Lu, W.; Samant, R.S.; Metge, B.J.; Hidalgo, B.; Grizzle, W.E.; Conner, M.; Forero-Torres, A.; et al. Effect of niclosamide on basal-like breast cancers. Mol. Cancer Ther. 2014, 13, 800–811. [Google Scholar] [CrossRef] [PubMed]
- King, M.L.; Lindberg, M.E.; Stodden, G.R.; Okuda, H.; Ebers, S.D.; Johnson, A.; Montag, A.; Lengyel, E.; MacLean Ii, J.A.; Hayashi, K. WNT7A/β-catenin signaling induces FGF1 and influences sensitivity to niclosamide in ovarian cancer. Oncogene 2015, 34, 3452–3462. [Google Scholar] [CrossRef]
- Hemmati-Dinarvand, M.; Ahmadvand, H.; Seghatoleslam, A. Nitazoxanide and Cancer Drug Resistance: Targeting Wnt/β-catenin Signaling Pathway. Arch. Med. Res. 2022, 53, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Wei, M.; Huang, H.; Wang, Y.; Zhang, L.; Yang, C.; Huang, Y.; Luo, J. Nitazoxanide inhibits osteosarcoma cells growth and metastasis by suppressing AKT/mTOR and Wnt/β-catenin signaling pathways. Biol. Chem. 2022, 403, 929–943. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Signaling to NF-kappaB. Genes. Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Azuma, M.; Muto, S.; Nishioka, Y.; Honjo, A.; Tezuka, T.; Uehara, H.; Izumi, K.; Itai, A.; Sone, S. IkappaB kinase beta inhibitor IMD-0354 suppresses airway remodelling in a Dermatophagoides pteronyssinus-sensitized mouse model of chronic asthma. Clin. Exp. Allergy 2011, 41, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Noha, S.M.; Atanasov, A.G.; Schuster, D.; Markt, P.; Fakhrudin, N.; Heiss, E.H.; Schrammel, O.; Rollinger, J.M.; Stuppner, H.; Dirsch, V.M.; et al. Discovery of a novel IKK-β inhibitor by ligand-based virtual screening techniques. Bioorg. Med. Chem. Lett 2011, 21, 577–583. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, Z.; Ding, K.; Li, J.; Du, X.; Chen, C.; Sun, X.; Wu, Y.; Zhou, J.; Pan, J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: Inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010, 70, 2516–2527. [Google Scholar] [CrossRef]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Edwards, J.; Pepper, C.; Mackay, S. Inhibitory-κB Kinase (IKK) α and Nuclear Factor-κB (NFκB)-Inducing Kinase (NIK) as Anti-Cancer Drug Targets. Cells 2018, 7, 176. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Balgi, A.D.; Fonseca, B.D.; Donohue, E.; Tsang, T.C.; Lajoie, P.; Proud, C.G.; Nabi, I.R.; Roberge, M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS ONE 2009, 4, e7124. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.D.; Diering, G.H.; Bidinosti, M.A.; Dalal, K.; Alain, T.; Balgi, A.D.; Forestieri, R.; Nodwell, M.; Rajadurai, C.V.; Gunaratnam, C.; et al. Structure-activity analysis of niclosamide reveals potential role for cytoplasmic pH in control of mammalian target of rapamycin complex 1 (mTORC1) signaling. J. Biol. Chem. 2012, 287, 17530–17545. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, L.; Shen, H.; Lin, H.; Li, D. Anthelminthic drug niclosamide sensitizes the responsiveness of cervical cancer cells to paclitaxel via oxidative stress-mediated mTOR inhibition. Biochem. Biophys. Res. Commun. 2017, 484, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal. Transduct. Target Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Suliman, M.A.; Zhang, Z.; Na, H.; Ribeiro, A.L.; Zhang, Y.; Niang, B.; Hamid, A.S.; Zhang, H.; Xu, L.; Zuo, Y. Niclosamide inhibits colon cancer progression through downregulation of the Notch pathway and upregulation of the tumor suppressor miR-200 family. Int. J. Mol. Med. 2016, 38, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Zeyada, M.S.; Abdel-Rahman, N.; El-Karef, A.; Yahia, S.; El-Sherbiny, I.M.; Eissa, L.A. Niclosamide-loaded polymeric micelles ameliorate hepatocellular carcinoma in vivo through targeting Wnt and Notch pathways. Life Sci. 2020, 261, 118458. [Google Scholar] [CrossRef] [PubMed]
- Loo, E.; Khalili, P.; Beuhler, K.; Siddiqi, I.; Vasef, M.A. BRAF V600E Mutation Across Multiple Tumor Types: Correlation Between DNA-based Sequencing and Mutation-specific Immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 709–713. [Google Scholar] [CrossRef]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Hardcastle, I.R.; Golding, B.T.; Griffin, R.J. Designing inhibitors of cyclin-dependent kinases. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 325–348. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Fassl, A.; Geng, Y.; Sicinski, P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science 2022, 375, eabc1495. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, L.; Delgado, M.D.; Leon, J. MYC Oncogene Contributions to Release of Cell Cycle Brakes. Genes 2019, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Seviour, E.G.; Sehgal, V.; Lu, Y.; Luo, Z.; Moss, T.; Zhang, F.; Hill, S.M.; Liu, W.; Maiti, S.N.; Cooper, L.; et al. Functional proteomics identifies miRNAs to target a p27/Myc/phospho-Rb signature in breast and ovarian cancer. Oncogene 2016, 35, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Bretones, G.; Delgado, M.D.; León, J. Myc and cell cycle control. Biochim. Biophys. Acta. 2015, 1849, 506–516. [Google Scholar] [CrossRef]
- Wang, H.; Mannava, S.; Grachtchouk, V.; Zhuang, D.; Soengas, M.; Gudkov, A.; Prochownik, E.; Nikiforov, M. c-Myc depletion inhibits proliferation of human tumor cells at various stages of the cell cycle. Oncogene 2008, 27, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Mora, L.B.; Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002, 8, 945–954. [Google Scholar]
- Kikuchi, A. Regulation of beta-catenin signaling in the Wnt pathway. Biochem. Biophys. Res. Commun. 2000, 268, 243–248. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Harada, D.; Takigawa, N.; Kiura, K. The Role of STAT3 in Non-Small Cell Lung Cancer. Cancers 2014, 6, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhu, H.; Xiao, Y.; Guo, H.; Lin, M.; Yuan, Z.; Yang, X.; Huang, Y.; Zhang, Q.; Bai, Y. The anthelmintic drug niclosamide induces GSK-β-mediated β-catenin degradation to potentiate gemcitabine activity, reduce immune evasion ability and suppress pancreatic cancer progression. Cell Death Dis. 2022, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Zhang, T.; Zhang, J.; Wu, B. Significantly enhanced bioavailability of niclosamide through submicron lipid emulsions with or without PEG-lipid: A comparative study. J. Microencapsul. 2015, 32, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Hatamipour, M.; Jaafari, M.R.; Momtazi-Borojeni, A.A.; Ramezani, M.; Sahebkar, A. Nanoliposomal Encapsulation Enhances. Anticancer Agents Med. Chem. 2019, 19, 1618–1626. [Google Scholar] [CrossRef]
- Parikh, M.; Liu, C.; Wu, C.Y.; Evans, C.P.; Dall’Era, M.; Robles, D.; Lara, P.N.; Agarwal, N.; Gao, A.C.; Pan, C.X. Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer. Sci. Rep. 2021, 11, 6377. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Famta, P.; Fernandes, V.; Bagasariya, D.; Charankumar, K.; Kumar Khatri, D.; Bala Singh, S.; Srivastava, S. Quality by design steered development of Niclosamide loaded liposomal thermogel for Melanoma: In vitro and Ex vivo evaluation. Eur. J. Pharm. Biopharm. 2022, 180, 119–136. [Google Scholar] [CrossRef]
- Tsume, Y.; Mudie, D.M.; Langguth, P.; Amidon, G.E.; Amidon, G.L. The Biopharmaceutics Classification System: Subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur. J. Pharm. Sci. 2014, 57, 152–163. [Google Scholar] [CrossRef]
- Pardhi, V.; Chavan, R.B.; Thipparaboina, R.; Thatikonda, S.; Naidu, V.; Shastri, N.R. Preparation, characterization, and cytotoxicity studies of niclosamide loaded mesoporous drug delivery systems. Int. J. Pharm. 2017, 528, 202–214. [Google Scholar] [CrossRef]
- Lodagekar, A.; Borkar, R.M.; Thatikonda, S.; Chavan, R.B.; Naidu, V.G.M.; Shastri, N.R.; Srinivas, R.; Chella, N. Formulation and evaluation of cyclodextrin complexes for improved anticancer activity of repurposed drug: Niclosamide. Carbohydr. Polym. 2019, 212, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Jing, Z.; Ferreira, R.; Ren, P.; Zhang, F. Investigating the Association Mechanism between Rafoxanide and Povidone. Langmuir 2018, 34, 13971–13978. [Google Scholar] [CrossRef]
- Vega, A.F.; Medina-Torres, L.; Calderas, F.; Gracia-Mora, J.; Bernad-Bernad, M. Closantel nano-encapsulated polyvinyl alcohol (PVA) solutions. Pharm. Dev. Technol. 2016, 21, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Glisoni, R.J.; Sosnik, A. Encapsulation of the antimicrobial and immunomodulator agent nitazoxanide within polymeric micelles. J. Nanosci. Nanotechnol. 2014, 14, 4670–4682. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.J.; Löbenberg, R.; de Araujo, G.L.B.; Bou-Chacra, N.A. Niclosamide repositioning for treating cancer: Challenges and nano-based drug delivery opportunities. Eur. J. Pharm. Biopharm. 2019, 141, 58–69. [Google Scholar] [CrossRef]
- Jara, M.O.; Warnken, Z.N.; Williams, R.O. Amorphous Solid Dispersions and the Contribution of Nanoparticles to In Vitro Dissolution and In Vivo Testing: Niclosamide as a Case Study. Pharmaceutics 2021, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.B.; Kerr, D.L.; Spasojevic, I.; Tovmasyan, A.; Hsu, D.S.; Brigman, B.E.; Somarelli, J.A.; Needham, D.; Eward, W.C. Preclinical Testing of a Novel Niclosamide Stearate Prodrug Therapeutic (NSPT) Shows Efficacy Against Osteosarcoma. Mol. Cancer Ther. 2020, 19, 1448–1461. [Google Scholar] [CrossRef]
- Sood, S.; Maddiboyina, B.; Rawat, P.; Garg, A.K.; Foudah, A.I.; Alam, A.; Aldawsari, H.M.; Riadi, Y.; Singh, S.; Kesharwani, P. Enhancing the solubility of nitazoxanide with solid dispersions technique: Formulation, evaluation, and cytotoxicity study. J. Biomater. Sci. Polym. Ed. 2021, 32, 477–487. [Google Scholar] [CrossRef]
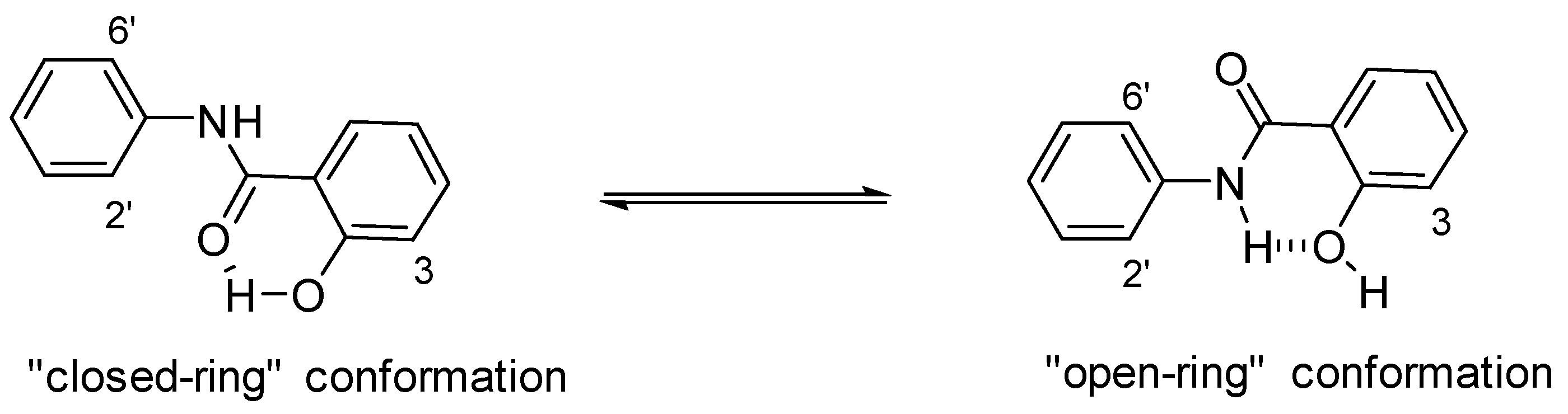
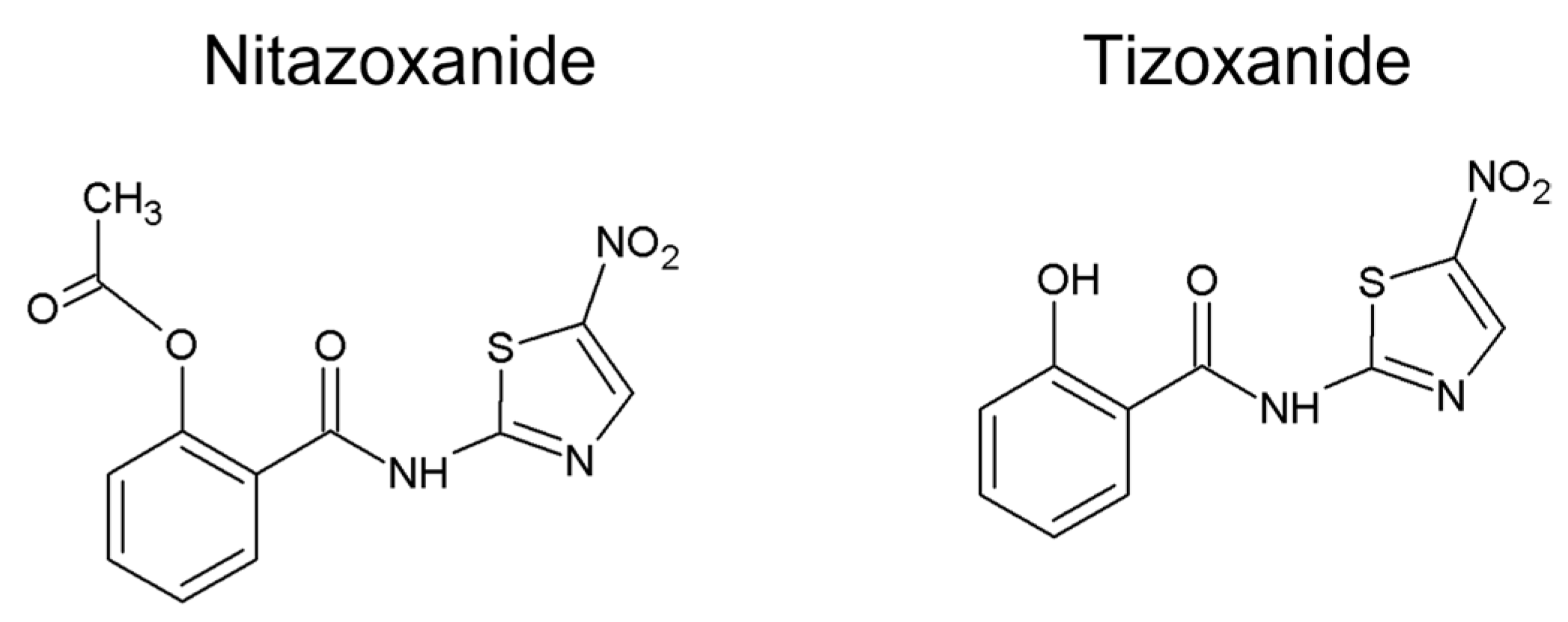

| Salicylanilide | Proposed Mechanism of Action | Model/Methods | Effects | Reference |
|---|---|---|---|---|
| Closantel | Antiangiogenic activity | Ramos, Hela, PANC-1, and HepG2 cell xenografts in zebrafish | Inhibited growth of xenotransplanted cells in zebrafish | [31] |
| Closantel | B-Raf V600E inhibition | ELISA-based assay molecular docking with halogen bonding scoring function | IC50 = 1.90 μM | [32] |
| Closantel | Mitochondrial dysfunction | HCT116 and HT-29 cells grown in monolayer and in vitro 3D model; tumor spheroids of HCT116 GFP and HT-29 GFP cell lines | Inhibited colony formation, inhibited mitochondrial respiration, decreased oxygen consumption, induced depolarization of mitochondrial membrane | [6] |
| Closantel | Taspase1 inhibition | Cell-free system (cfs-Taspase1), E. coli-produced Taspase1, HEK293T cells expressing GFP-CS2-BFP and Taspase1 | IC50 = 1.6 μM 1 (cfs-produced Taspase1) IC50 = 3.9 μM (E. coli-produced Taspase1) | [33] |
| Oxyclozanide | Mitochondrial uncoupling | MC38, HCT116 and C2C12 cell lines; MC38 xenografts in NSG mice | In vitro: decreased oxygen consumption, AMPK activation, inhibition of the mTOR activity In vivo: diminished hepatic metastases | [4] |
| Rafoxanide | CDK4/6 dual-inhibition | A375 and A431 cell lines; A375 xenografts in BALB/C nude mice | In vitro: decreased expression of CDK4/6, cyclin D, Rb, pho-CDK4/6, pho-Rb induced apoptosis, cell cycle arrest in G1 phase In vivo: reduced the growth of tumors | [34] |
| Rafoxanide | DNA damage responses, suppression of p38 MAPK pathway | Multiple myeloma cell lines; H929 xenografts in BALB/C nude mice | In vitro: antiproliferative effect, induced apoptosis, decreased mitochondrial membrane potential MMP, cell cycle arrest in G1 phase; synergistic activity with bortezomib or lenalidomide In vivo: tumor growth inhibition | [7] |
| Rafoxanide | PI3K /Akt/mTOR pathway suppression | SGC-7901 and BGC-823 cell lines; SGC-7901 xenografts in BALB/c nude mice | In vitro: antiproliferative effect, cell cycle arrest in G1 phase, promoted apoptosis and autophagy In vivo: tumor growth inhibition | [35] |
| Rafoxanide | B-Raf V600E inhibition | ELISA-based assay molecular docking with halogen bonding scoring function | IC50 = 0.07 μM | [32] |
| Rafoxanide | Endoplasmic reticulum stress induction | HCT-116 and HT-29 cell lines, human CRC Explants | In vitro: decreased p-ERK expression, antiproliferative effect, cell cycle arrest in G1 phase, induced cell death In vivo: reduced colonic tumorigenesis, induced apoptosis, eIF2α phosphorylation | [36] |
| Rafoxanide | bona fide immunogenic cell death (ICD) induction | HCT-116, DLD1 and CT26 cell lines, tumor vaccination model rafoxanide-treated CT26 cells | In vitro: induction of ecto-calreticulin exposure, adenosine triphosphate (ATP)/high mobility group box 1 (HMGB1) release In vivo: no visible sign of tumor growth or only tiny tumor masses in animals vaccinated with rafoxanide-treated CT26 cells | [37] |
| Rafoxanide | Restoration of sensitivity to TRAIL | DLD-1 and SW480 cell lines, BALB/c mice with CT26-derived grafts | In vitro: decreased c-FLIP and surviving expression In vivo: synergism with TRAIL in inhibiting the growth of CT26-derived tumors | [38] |
| Rafoxanide | PTEN/PI3K/AKT and JNK/c-Jun pathways suppression | Diffuse large B-cell lymphoma (DLBCL) cell lines (NU-DUL-1 and OCI-LY8 cells), nude mice with OCI-LY8-derived grafts | In vitro: induced apoptosis, decreased MMP, increased reactive oxygen species (ROS) generation, induced DNA damage In vivo: inhibition of tumor growth, induction of TUNEL positive cells production | [39] |
| IMD-0354 | NF-κB inhibition | HMC-1 cell line | decreased translocation of NF-κB to the nucleus, antiproliferative effect, cell cycle arrest in G1 phase, decreased cyclin D3 expression and pRb phosphorylation | [40] |
| IMD-0354 | NF-κB inhibition | MCF-7, MDA-MB-231, HMC1-8 cell lines, MDA-MB-231 xenografts in BALB/c nude mice | In vitro: decreased NF-κB activity without affecting Akt phosphorylation, antiproliferative effect, cell cycle arrest, down-regulation of D-type cyclins, decreased Rb phosphorylation and expression Bcl family proteins and MDM2 In vivo: suppressed tumor growth without any serious side effects and toxicity | [8] |
| IMD-0354 | NF-κB inhibition | Chronic lymphocytic leukemia (CLL) cell samples from untreated CLL patients | induced apoptosis, decreased survival index in vitro, inhibition of NF-κB activation, induction of apoptotic-related gene expression | [41] |
| IMD-0354 | NF-κB inhibition | Peripheral blood mononuclear cells (PBMCs) from adult T-cell leukemia (ATL) patients, ATL-derived cell lines, human T-cell leukemia virus type I (HTLV-I)-infected, and HTLV-I free T-cell lines, ATL-43Tb(-) xenografts NOG mice | In vitro: selective decrease of viability of CD4+CD25+ primary ATL cells in vitro (IC50 = 2.87 µM; caspase 3/7 activation), decreased growth and transcriptional activity of NF-κB in HTLV-I-infected cells In vivo: Suppressed tumor growth | [42] |
| IMD-0354 | IKKβ inhibition | MSTO-211H, NCI-H2052 and NCI-H28 cell lines, MSTO-211H xenografts in BALB/c-nu/nu mice or SCID mice | In vitro: decreased activation of NF-κB by IKKβ inhibition, cell cycle arrest in G1 phase, decreased cyclin D3 expression In vivo: Suppressed tumor growth | [43] |
| IMD-0354 | IKKβ inhibition | Panc-1, PK8 cell lines, Panc-1 xenografts in NOG mice | In vitro: decreased NF-κB activity, antiproliferative effect In vivo: suppressed tumor growth without toxicity | [44] |
| IMD-0354 | Modulation of glutamine metabolism, mTOR signaling suppression | A431 and A375 cell lines; prostate, pancreatic, colon, lung, and kidney tumor–derived cell lines; SW1 melanoma xenografts in C3H mice | In vitro: inhibition of SLC1A5 localization at the plasma membrane, decreased glutamine uptake, decreased glutamine-dependent amino acid pathways, decreased mTOR signaling, antiproliferative effect, induced apoptosis and autophagy; dose-dependent viability inhibition In vivo: xenograft growth inhibition, decreased mTOR activity, induced apoptosis | [45] |
| IMD-0354 KRT1853 | TMPRSS4 serine protease inhibition | Prostate, colon, and lung cancer cell lines | In vitro: decreased cancer cell invasion, migration, and proliferation in TMPRSS4-expressing cells, induced apoptosis, decreased TMPRSS4-mediated signaling activity | [9] |
| KRT1853 | TMPRSS4 serine protease inhibition | DU145 and HCT116 cell xenografts in nude mice | In vivo: suppressed tumor growth | [9] |
| Nitazoxanide | Mitochondrial dysfunction AMPK pathway activation, c-Myc, mTOR, and Wnt signaling downregulation | HCT116 and HT-29 cells grown in monolayer and in vitro 3D model –tumor spheroids of HCT116 GFP and HT-29 GFP cell line HCT116 xenografts in NMRI nu/nu mice | In vitro: inhibited colony formation, inhibited mitochondrial respiration, decreased oxygen consumption, induced depolarization of mitochondrial membrane, AMPK, downregulation In vivo: potentiation of irinotecan effect inducing reduction in tumor growth and volume | [6] |
| Nitazoxanide | Inhibition of Wnt/β-catenin signaling through PAD2, independent of GSK3β and APC | 293Ft and SW480 cell line Murine model for familial adenomatous polyposis (Apcmin/+ mice) with spontaneously generate tumors | In vitro: dose-dependent decrease of β-catenin, stabilization of PAD2 and increase in protein citrullination In vivo: Decreased numbers of micro- and macroadenomas, reduction of β-catenin | [46] |
| Nitazoxanide | c-Myc inhibition | Quantitative high throughput screening for c-Myc inhibition, SKBR3 cell line, SKBR3 cell xenografts in nude mice (nu/nu) | 80.5% c-Myc activity inhibition at 10 µM, inhibition of c-Myc in SKBR3 cells (IC50 ~100 nM) (luciferase activity assay), inhibition of phospho c-Myc level cell lines SKBR3 (IC50 = 122 nM), lymphoma (IC50 = 440 nM) and osteosarcoma-derived cell line In vivo: decreased c-Myc activity and suppressed tumor growth | [47] |
| Nitazoxanide | ING1 upregulation (increased expression and decreased cleavage) | LN229, U87, A172, and HUVEC cell lines, LN229 xenografts in BALB/C nude mice (s.c. injected), orthotopic brain tumor model of LN229 xenografts in BALB/C nude mice (injected into caudate nucleus) | In vitro: Antiproliferative effect, cycle arrest in G1 phase, blockage of late-stage autophagic flux In vivo: glioma growth inhibition, increased levels of ING1, LC3 and SQSTM1; inhibition of intracranial tumor growth, survival time prolongation | [10] |
| Nitazoxanide | Downregulation of Wnt/β-catenin/GSK-3β signaling | HCT-116 cell line, (FHC normal colon cell line), 1,2-dimethylhydrazine (DMH) induced colon cancer in Swiss albino mice | In vitro: Cytotoxic activity (HCT-116 IC50 = 11.07 μM; FHC IC50 = 48.4 μM), induced apoptosis, increased mRNA expression of p53, BAX, caspases 3, 8 and 9 In vivo: decreased Wnt, β-catenin and GSK-3β levels, decrease in number of PCNA positive nuclei, reduction of pathologic signs based on histopathologic scoring data | [48] |
| Nitazoxanide | β-catenin inhibition, synergistic effects with obeticholic acid (OCA) | SW403, SW480, DLD-1, and HT-29 cell lines, SW403 and SW480 xenografts in BALB/c-nude mice | In vitro: growth inhibition IC50 = 2.764 μM (SW403), IC50 = 2.294 μM (SW480), IC50 = 2.149 μM (DLD-1), IC50 = 1.930 μM (HT-29); decreased β-catenin expression, synergistic effects with OCA (repressed colony formation, cell cycle arrest in G1 phase, induced apoptosis) In vivo: Synergistic effect with OCA inducing tumor growth reduction | [49] |
| Name of Drug | Type of Cancer | Phase | Clinical Trials.gov Identifier | Current Clinical Trial Status | Location | Reference |
|---|---|---|---|---|---|---|
| Niclosamide | Colon Cancer | Phase 1 | NCT02687009 | Terminated (low accrual) | Duke University, NC, US | - |
| Niclosamide | Colorectal Cancer | Phase 2 | NCT02519582 | Unknown † | Charite University, Berlin, Germany | [14] |
| Niclosamide (w/enzalutamide) | Castration-Resistant Prostate Cancer | Phase 1 | NCT03123978 | Active, not recruiting | University of California Davis, Comprehensive Cancer Center, CA, US | - |
| Niclosamide (w/abiraterone acetate and prednisone) | Hormone-Resistant Prostate Cancer | Phase 2 | NCT02807805 | Active, not recruiting | University of California Davis, Comprehensive Cancer Center, CA, US | - |
| Niclosamide (w/ enzalutamide) | Castration-Resistant Prostate Carcinoma Metastatic Prostate Carcinoma Recurrent Prostate Carcinoma | Phase 1 | NCT02532114 | Completed | Fred Hutch/University of Washington Cancer Consortium, WA, US | [13] |
| Niclosamide | Familial Adenomatous Polyposis | Phase 2 | NCT04296851 | Recruiting | Department of Internal Medicine, Yonsei University College of Medicine Seoul, Republic of Korea | - |
| Niclosamide | Acute Myeloid Leukemia | Phase 1 | NCT05188170 | Recruiting | Stanford University, CA, US | - |
| Nitazoxanide (Antibacterial Agents Antifungal Agents Antiprotozoal Agents) | Neoplasms | Phase 2 | NCT02366884 | Recruiting | Dr. Frank Arguello Cancer Clinic, Instituto de Ciencia y Medicina Genomica, Mexico | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauerová, T.; Pérez-Pérez, M.-J.; Kollar, P. Salicylanilides and Their Anticancer Properties. Int. J. Mol. Sci. 2023, 24, 1728. https://doi.org/10.3390/ijms24021728
Kauerová T, Pérez-Pérez M-J, Kollar P. Salicylanilides and Their Anticancer Properties. International Journal of Molecular Sciences. 2023; 24(2):1728. https://doi.org/10.3390/ijms24021728
Chicago/Turabian StyleKauerová, Tereza, María-Jesús Pérez-Pérez, and Peter Kollar. 2023. "Salicylanilides and Their Anticancer Properties" International Journal of Molecular Sciences 24, no. 2: 1728. https://doi.org/10.3390/ijms24021728
APA StyleKauerová, T., Pérez-Pérez, M.-J., & Kollar, P. (2023). Salicylanilides and Their Anticancer Properties. International Journal of Molecular Sciences, 24(2), 1728. https://doi.org/10.3390/ijms24021728






