Scd1 Deficiency in Early Embryos Affects Blastocyst ICM Formation through RPs-Mdm2-p53 Pathway
Abstract
1. Introduction
2. Results
2.1. Expression of Scd1 Gradually Increased as Embryonic Development
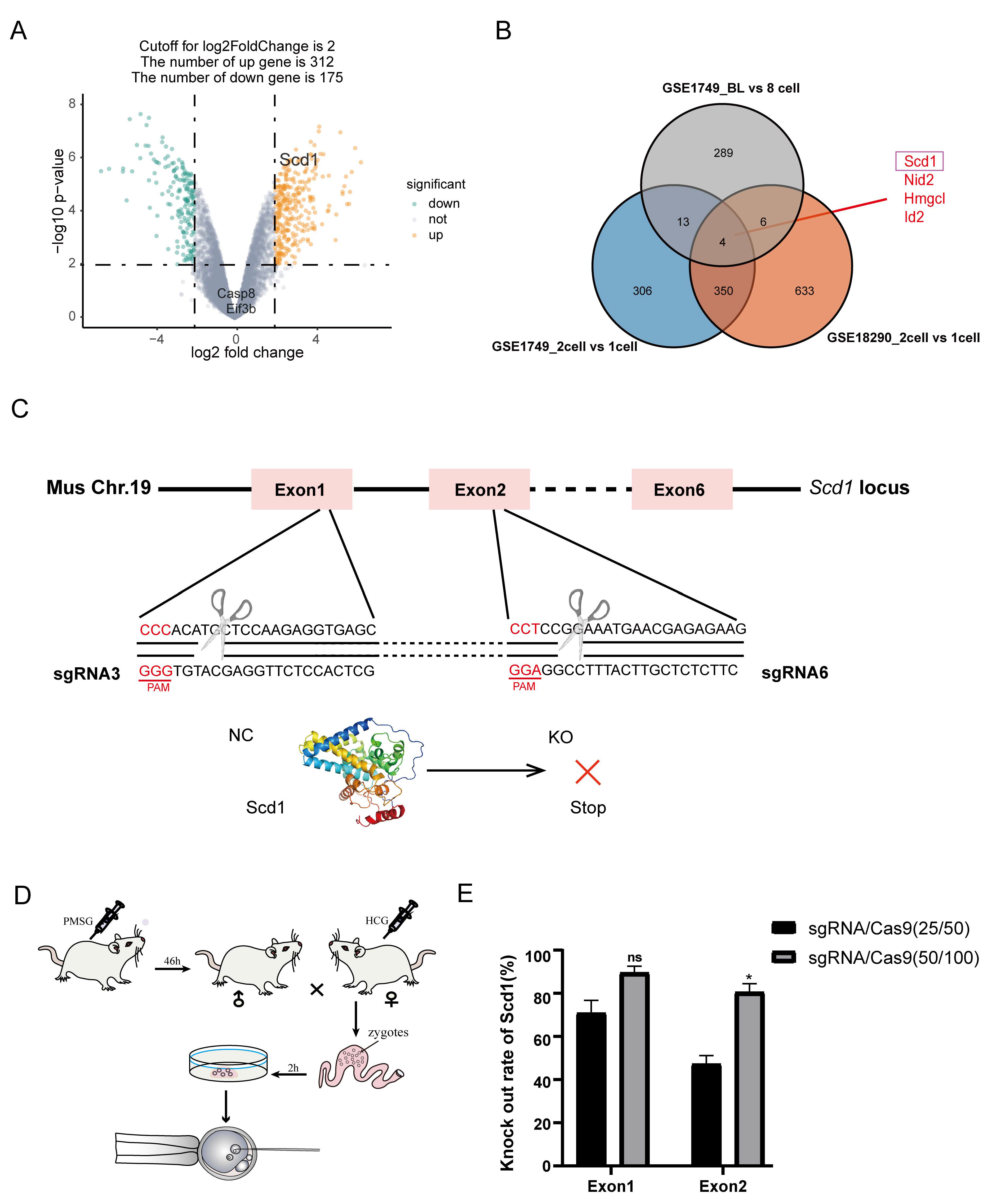
2.2. Scd1 Deficiency Caused Embryonic Development Arrest
2.3. Scd1 Regulated the Blastocyst Generation and Lipid Droplet Synthesis in Mouse Embryos
2.4. Single-Embryo RNA-Seq Atlas Exhibited the Increasing Requirement of Scd1 during Embryonic Development
2.5. Ribosome Biogenesis Was Suppressed in 2-Cell Stage Scd1−/− Embryos
2.6. Ribosome Biogenesis and RNA Translation Was Reversely Upregulated in 4-Cell Stage Scd1−/− Embryos
2.7. Ribosome Stress Stimulated the RPs-Mdm2-P53 Pathway in Scd1−/− Blastocysts
2.8. P53 Inhibition Rescued the Blastocyst Development in Scd1−/− Embryos
2.9. Scd1−/− Blastocyst Give Rise to Inner Cell Mess (ICM) Impairment and Embryo Development Arrest
3. Discussion
4. Materials and Methods
4.1. Cas9 mRNA Synthesis
4.2. sgRNA Synthesis
4.3. Animals
4.4. Microinjection and Culture of Pre-Implantation Embryos
4.5. Genotyping
4.6. DNA Library Preparation for Single Embryo RNA-Seq
4.7. RNA-Seq Data Analysis
4.8. Western Blot Analysis
4.9. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
4.10. Immunofluorescence Staining
4.11. Lipid Droplet Staining
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Liu, X.; Tang, B.; Li, C.; Kou, Z.; Li, L.; Liu, W.; Wu, Y.; Kou, X.; Li, J.; et al. Protein Expression Landscape of Mouse Embryos during Pre-implantation Development. Cell Rep. 2017, 21, 3957–3969. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Huang, K.; Cai, C.; Cai, L.; Jiang, C.Y.; Feng, Y.; Liu, Z.; Zeng, Q.; Cheng, L.; Sun, Y.E.; et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature 2013, 500, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Kremsky, I.; Corces, V.G. Protection from DNA re-methylation by transcription factors in primordial germ cells and pre-implantation embryos can explain trans-generational epigenetic inheritance. Genome Biol. 2020, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, G.; Wang, J.; Gao, Y.; Sun, R.; Cao, Z.; Chen, Z.; Zheng, X.; Yuan, J.; Luo, Y.; et al. 6mA-DNA-binding factor Jumu controls maternal-to-zygotic transition upstream of Zelda. Nat. Commun. 2019, 10, 2219. [Google Scholar] [CrossRef]
- Middelkamp, S.; van Tol, H.T.A.; Spierings, D.C.J.; Boymans, S.; Guryev, V.; Roelen, B.A.J.; Lansdorp, P.M.; Cuppen, E.; Kuijk, E.W. Sperm DNA damage causes genomic instability in early embryonic development. Sci. Adv. 2020, 6, eaaz7602. [Google Scholar] [CrossRef]
- Gardner, D.K. Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 1998, 49, 83–102. [Google Scholar] [CrossRef]
- Khurana, N.K.; Niemann, H. Energy metabolism in preimplantation bovine embryos derived in vitro or in vivo. Biol. Reprod. 2000, 62, 847–856. [Google Scholar] [CrossRef]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef]
- Najt, C.P.; Khan, S.A.; Heden, T.D.; Witthuhn, B.A.; Perez, M.; Heier, J.L.; Mead, L.E.; Franklin, M.P.; Karanja, K.K.; Graham, M.J.; et al. Lipid Droplet-Derived Monounsaturated Fatty Acids Traffic via PLIN5 to Allosterically Activate SIRT1. Mol. Cell 2020, 77, 810–824.e8. [Google Scholar] [CrossRef]
- Bradley, J.; Pope, I.; Masia, F.; Sanusi, R.; Langbein, W.; Swann, K.; Borri, P. Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy. Development 2016, 143, 2238–2247. [Google Scholar] [CrossRef]
- Xiang, J.; Xing, Y.; Long, C.; Hou, D.; Liu, F.; Zhang, Y.; Lu, Z.; Wang, J.; Zuo, Y.; Li, X. Fatty acid metabolism as an indicator for the maternal-to-zygotic transition in porcine IVF embryos revealed by RNA sequencing. Theriogenology 2020, 151, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Ntambi, J.M. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kindt, A.; Liebisch, G.; Clavel, T.; Haller, D.; Hörmannsperger, G.; Yoon, H.; Kolmeder, D.; Sigruener, A.; Krautbauer, S.; Seeliger, C.; et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat. Commun. 2018, 9, 3760. [Google Scholar] [CrossRef] [PubMed]
- Peláez, R.; Ochoa, R.; Pariente, A.; Villanueva-Martínez, Á.; Pérez-Sala, Á.; Larráyoz, I. Sterculic Acid Alters Adhesion Molecules Expression and Extracellular Matrix Compounds to Regulate Migration of Lung Cancer Cells. Cancers 2021, 13, 4370. [Google Scholar] [CrossRef]
- Yu, S.; Lu, Y.; Su, A.; Chen, J.; Li, J.; Zhou, B.; Liu, X.; Xia, Q.; Li, Y.; Li, J.; et al. A CD10-OGP Membrane Peptolytic Signaling Axis in Fibroblasts Regulates Lipid Metabolism of Cancer Stem Cells via SCD1. Adv. Sci. (Weinh. Baden-Wurtt. Ger.) 2021, 8, e2101848. [Google Scholar] [CrossRef]
- Luo, H.; Chen, C.; Li, X.; Zhang, X.; Su, C.; Liu, Y.; Cao, T.; Hao, L.; Wang, M.; Kang, J. Increased Lipogenesis Is Critical for Self-Renewal and Growth of Breast Cancer Stem Cells: Impact of Omega-3 Fatty Acids. Stem Cells (Dayt. Ohio) 2021, 39, 1660–1670. [Google Scholar] [CrossRef]
- Vivas-García, Y.; Falletta, P.; Liebing, J.; Louphrasitthiphol, P.; Feng, Y.; Chauhan, J.; Scott, D.A.; Glodde, N.; Chocarro-Calvo, A.; Bonham, S.; et al. Lineage-Restricted Regulation of SCD and Fatty Acid Saturation by MITF Controls Melanoma Phenotypic Plasticity. Mol. Cell 2020, 77, 120–137.e9. [Google Scholar] [CrossRef]
- Hur, S.; Mittapally, R.; Yadlapalli, S.; Reddy, P.; Meyhofer, E. Sub-nanowatt resolution direct calorimetry for probing real-time metabolic activity of individual C. elegans worms. Nat. Commun. 2020, 11, 2983. [Google Scholar] [CrossRef]
- Hao, J.W.; Wang, J.; Guo, H.; Zhao, Y.Y.; Sun, H.H.; Li, Y.F.; Lai, X.Y.; Zhao, N.; Wang, X.; Xie, C.; et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 2020, 11, 4765. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, X.; Zhou, Z.; Yang, J.; Xin, Q.; Ying, C.; Zhang, Y.; Tao, J. MiR-1908/EXO1 and MiR-203a/FOS, regulated by scd1, are associated with fracture risk and bone health in postmenopausal diabetic women. Aging 2020, 12, 9549–9584. [Google Scholar] [CrossRef]
- Yu, Y.; Kim, H.; Choi, S.G.; Yu, J.S.; Lee, J.Y.; Lee, H.; Yoon, S.; Kim, W.Y. Targeting a Lipid Desaturation Enzyme, SCD1, Selectively Eliminates Colon Cancer Stem Cells through the Suppression of Wnt and NOTCH Signaling. Cells 2021, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.; Xi, H.; Cui, J.; Zhang, K.; Zhang, J.; Zhang, Y.; Xu, W.; Liang, W.; Zhuang, Z.; et al. Stearoyl-CoA-desaturase-1 regulates gastric cancer stem-like properties and promotes tumour metastasis via Hippo/YAP pathway. Br. J. Cancer 2020, 122, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yoo, Y.; Kim, H.; Lee, H.; Chung, H.; Nam, M.; Moon, J.; Lee, H.; Yoon, S.; Kim, W. Clinical and biochemical relevance of monounsaturated fatty acid metabolism targeting strategy for cancer stem cell elimination in colon cancer. Biochem. Biophys. Res. Commun. 2019, 519, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef]
- Fathi Maroufi, N.; Hasegawa, K.; Vahedian, V.; Nazari Soltan Ahmad, S.; Zarebkohan, A.; Miresmaeili Mazrakhondi, S.; Hosseini, V.; Rahbarghazi, R. A glimpse into molecular mechanisms of embryonic stem cells pluripotency: Current status and future perspective. J. Cell. Physiol. 2020, 235, 6377–6392. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Gan, Q.F.; Golan-Lev, T.; Arora, P.; Yanuka, O.; Oren, Y.S.; Leikin-Frenkel, A.; Graf, M.; Garippa, R.; Boehringer, M.; et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 2013, 12, 167–179. [Google Scholar] [CrossRef]
- Asghari, S.; Nouri, M.; Darabi, M.; Valizadeh, A. Steroid-depleted endometriosis serum improves oocyte maturation in IVM systems. J. Cell. Physiol. 2021, 236, 205–214. [Google Scholar] [CrossRef]
- Hosseini, V.; Kalantary-Charvadeh, A.; Hajikarami, M.; Fayyazpour, P.; Rahbarghazi, R.; Totonchi, M.; Darabi, M. A small molecule modulating monounsaturated fatty acids and Wnt signaling confers maintenance to induced pluripotent stem cells against endodermal differentiation. Stem Cell Res. Ther. 2021, 12, 550. [Google Scholar] [CrossRef]
- Tesfay, L.; Paul, B.; Konstorum, A.; Deng, Z.; Cox, A.; Lee, J.; Furdui, C.; Hegde, P.; Torti, F.; Torti, S. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019, 79, 5355–5366. [Google Scholar] [CrossRef]
- Zeng, F.; Baldwin, D.A.; Schultz, R.M. Transcript profiling during preimplantation mouse development. Dev. Biol. 2004, 272, 483–496. [Google Scholar] [CrossRef]
- Xie, D.; Chen, C.C.; Ptaszek, L.M.; Xiao, S.; Cao, X.; Fang, F.; Ng, H.H.; Lewin, H.A.; Cowan, C.; Zhong, S. Rewirable gene regulatory networks in the preimplantation embryonic development of three mammalian species. Genome Res. 2010, 20, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Niu, H.; Luo, J.; Yao, W.; Gao, W.; Wen, Y.; Cheng, M.; Lei, A.; Hua, J. Effects of CRISPR/Cas9-mediated stearoyl-Coenzyme A desaturase 1 knockout on mouse embryo development and lipid synthesis. PeerJ 2022, 10, e13945. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, T.; Abe, S.; Inoue, K.; Yeung, W.K.A.; Miki, Y.; Ogura, A.; Sasaki, H. Reprogramming of the histone H3.3 landscape in the early mouse embryo. Nat. Struct. Mol. Biol. 2021, 28, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Hamatani, T.; Carter, M.G.; Sharov, A.A.; Ko, M.S. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell 2004, 6, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, Q.; Wang, Q.; Feng, S.; Xie, W. The landscape of RNA Pol II binding reveals a stepwise transition during ZGA. Nature 2020, 587, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, S.; Shu, W.; Guo, Y.; Guan, Y.; Zeng, J.; Wang, H.; Han, L.; Zhang, J.; Liu, X.; et al. Characterization of Metabolic Patterns in Mouse Oocytes during Meiotic Maturation. Mol. Cell 2020, 80, 525–540.e9. [Google Scholar] [CrossRef]
- Arena, R.; Bisogno, S.; Gąsior, Ł.; Rudnicka, J.; Bernhardt, L.; Haaf, T.; Zacchini, F.; Bochenek, M.; Fic, K.; Bik, E.; et al. Lipid droplets in mammalian eggs are utilized during embryonic diapause. Proc. Natl. Acad. Sci. USA 2021, 118, e2018362118. [Google Scholar] [CrossRef]
- Lee, D.K.; Choi, K.H.; Hwang, J.Y.; Oh, J.N.; Kim, S.H.; Lee, C.K. Stearoyl-coenzyme A desaturase 1 is required for lipid droplet formation in pig embryo. Reproduction 2019, 157, 235–243. [Google Scholar] [CrossRef]
- Taylor, A.M.; Macari, E.R.; Chan, I.T.; Blair, M.C.; Zon, L.I. Calmodulin inhibitors improve erythropoiesis in Diamond-Blackfan anemia. Sci. Transl. Med. 2020, 12, eabb5831. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, H.; Liu, T.; Zhou, S.; Du, Y.; Xiong, J.; Yi, S.; Qu, C.K.; Fu, H.; Zhou, M. Discovery of Dual Inhibitors of MDM2 and XIAP for Cancer Treatment. Cancer Cell 2016, 30, 623–636. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H. Signaling to p53: Ribosomal proteins find their way. Cancer Cell 2009, 16, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Panić, L.; Tamarut, S.; Sticker-Jantscheff, M.; Barkić, M.; Solter, D.; Uzelac, M.; Grabusić, K.; Volarević, S. Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol. Cell. Biol. 2006, 26, 8880–8891. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Xu, E.; Mohibi, S.; de Anda, D.M.; Jiang, Y.; Zhang, J.; Chen, X. Rbm24, a target of p53, is necessary for proper expression of p53 and heart development. Cell Death Differ. 2018, 25, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Sulic, S.; Panic, L.; Barkic, M.; Mercep, M.; Uzelac, M.; Volarevic, S. Inactivation of S6 ribosomal protein gene in T lymphocytes activates a p53-dependent checkpoint response. Genes Dev. 2005, 19, 3070–3082. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, V.H.; Cachay, E.; Ramers, C.; Vodkin, I.; Bassirian, S.; Singh, S.; Mangla, N.; Bettencourt, R.; Aldous, J.L.; Park, D.; et al. MRI Assessment of Treatment Response in HIV-associated NAFLD: A Randomized Trial of a Stearoyl-Coenzyme-A-Desaturase-1 Inhibitor (ARRIVE Trial). Hepatology (Baltim. Md.) 2019, 70, 1531–1545. [Google Scholar] [CrossRef]
- Dziewulska, A.; Dobosz, A.M.; Dobrzyn, A.; Smolinska, A.; Kolczynska, K.; Ntambi, J.M.; Dobrzyn, P. SCD1 regulates the AMPK/SIRT1 pathway and histone acetylation through changes in adenine nucleotide metabolism in skeletal muscle. J. Cell. Physiol. 2020, 235, 1129–1140. [Google Scholar] [CrossRef]
- Dan, X.; Babbar, M.; Moore, A.; Wechter, N.; Tian, J.; Mohanty, J.G.; Croteau, D.L.; Bohr, V.A. DNA damage invokes mitophagy through a pathway involving Spata18. Nucleic Acids Res. 2020, 48, 6611–6623. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Liu, X.; Cai, S.; Zhang, C.; Liu, Z.; Luo, J.; Xing, B.; Du, X. Deacetylation of NAT10 by Sirt1 promotes the transition from rRNA biogenesis to autophagy upon energy stress. Nucleic Acids Res. 2018, 46, 9601–9616. [Google Scholar] [CrossRef]
- Sirén, J.; Välimäki, N.; Mäkinen, V. Indexing Graphs for Path Queries with Applications in Genome Research. IEEE/ACM Trans. Comput. Biol. Bioinform. 2014, 11, 375–388. [Google Scholar] [CrossRef]






| sgRNA | Sequence (Bases in Bold Are PAM Sequence) |
|---|---|
| sgRNA3 | GCTCACCTCTTGGAGCATGTGGG |
| sgRNA6 | CTTCTCTCGTTCATTTCCGGAGG |
| sgRNA-NC | ACCGGAAGAGCGACCTCTTCT |
| Group | Total Number (tn) | Morula Number (mn) | Morula Rate (%) | Blastocyst Number (bn) | Blastocyst Rate (%) |
|---|---|---|---|---|---|
| Scd1−/− | 234 | 42 | 17.95 ± 1.81 | 109 | 46.58 ± 3.96 a |
| WT | 161 | 16 | 9.66 ± 1.79 | 97 | 60.84 ± 4.47 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, H.; Lei, A.; Tian, H.; Yao, W.; Liu, Y.; Li, C.; An, X.; Chen, X.; Zhang, Z.; Wu, J.; et al. Scd1 Deficiency in Early Embryos Affects Blastocyst ICM Formation through RPs-Mdm2-p53 Pathway. Int. J. Mol. Sci. 2023, 24, 1750. https://doi.org/10.3390/ijms24021750
Niu H, Lei A, Tian H, Yao W, Liu Y, Li C, An X, Chen X, Zhang Z, Wu J, et al. Scd1 Deficiency in Early Embryos Affects Blastocyst ICM Formation through RPs-Mdm2-p53 Pathway. International Journal of Molecular Sciences. 2023; 24(2):1750. https://doi.org/10.3390/ijms24021750
Chicago/Turabian StyleNiu, Huimin, Anmin Lei, Huibin Tian, Weiwei Yao, Ying Liu, Cong Li, Xuetong An, Xiaoying Chen, Zhifei Zhang, Jiao Wu, and et al. 2023. "Scd1 Deficiency in Early Embryos Affects Blastocyst ICM Formation through RPs-Mdm2-p53 Pathway" International Journal of Molecular Sciences 24, no. 2: 1750. https://doi.org/10.3390/ijms24021750
APA StyleNiu, H., Lei, A., Tian, H., Yao, W., Liu, Y., Li, C., An, X., Chen, X., Zhang, Z., Wu, J., Yang, M., Huang, J., Cheng, F., Zhao, J., Hua, J., Liu, S., & Luo, J. (2023). Scd1 Deficiency in Early Embryos Affects Blastocyst ICM Formation through RPs-Mdm2-p53 Pathway. International Journal of Molecular Sciences, 24(2), 1750. https://doi.org/10.3390/ijms24021750







