Playing with Biophysics: How a Symphony of Different Electromagnetic Fields Acts to Reduce the Inflammation in Diabetic Derived Cells
Abstract
:1. Introduction
2. Results
2.1. Safety Following Insternational Standard Indication
2.2. ROS Production
2.3. Macrophage Polarization
2.4. Anti-Inflammatory Markers
2.5. Extracellular Matrix
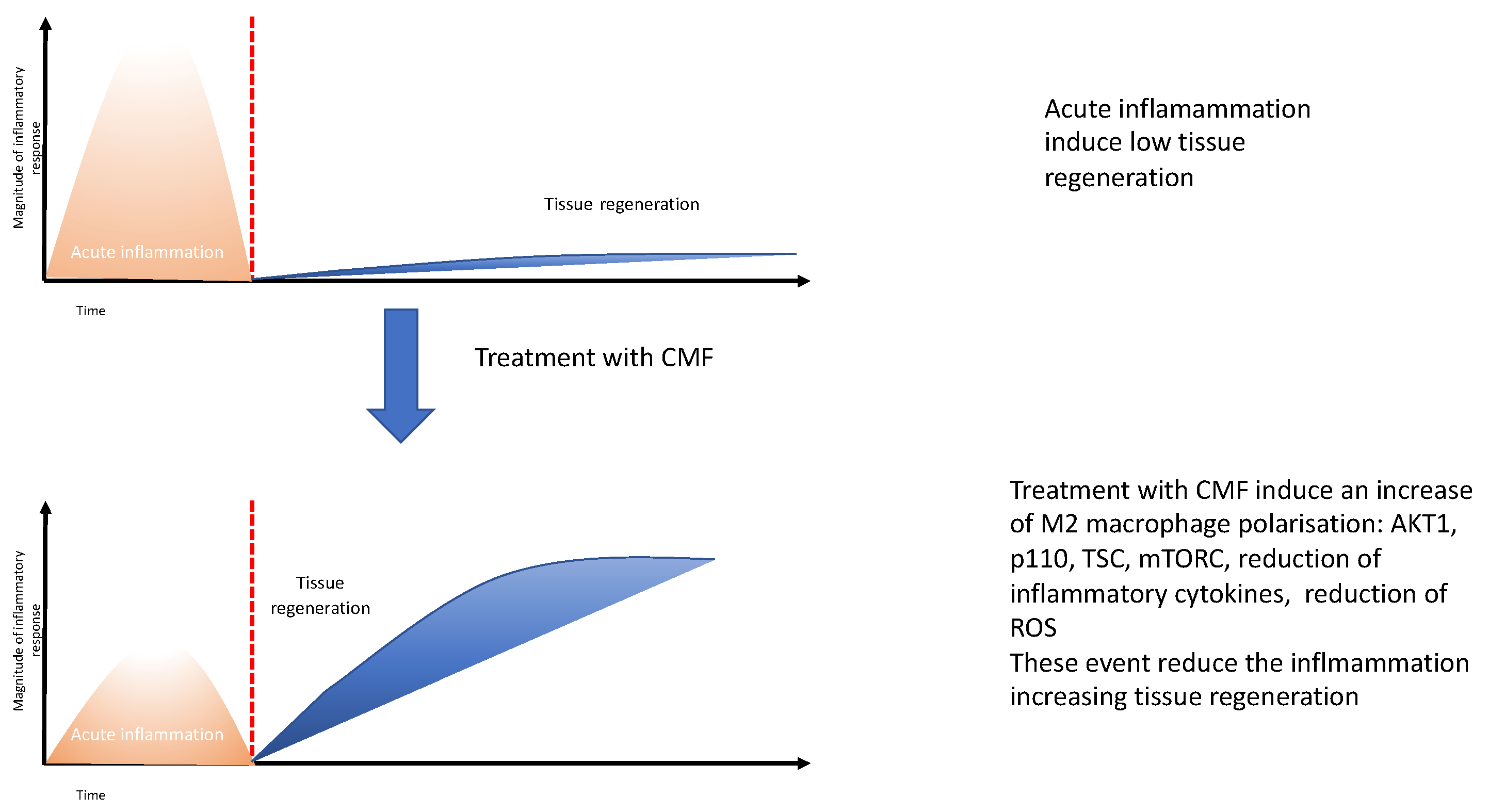
3. Discussion
4. Materials and Methods
4.1. Patients Recruitment and Samples Collection
4.2. Cell Isolation and Characterization
4.3. Treatment
4.4. In Vitro Cytotoxicity Test
4.5. Hemolysis Assay
4.6. Ames Test
4.7. Karyotype Analysis
4.8. Scanning Electron Microscopy (SEM)
4.9. RNA Extraction and Real-Time PCR Array
4.10. Reactive Oxygen Species (ROS) Measurements
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixon, D.; Edmonds, M. Managing Diabetic Foot Ulcers: Pharmacotherapy for Wound Healing. Drugs 2021, 81, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.A.; Guillén-Nieto, G.E.; Rodríguez-Rodríguez, N.; Mendoza-Mari, Y.; Bringas-Vega, M.L.; Berlanga-Saez, J.O.; García Del Barco Herrera, D.; Martinez-Jimenez, I.; Hernandez-Gutierrez, S.; Valdés-Sosa, P.A. Cellular Senescence as the Pathogenic Hub of Diabetes-Related Wound Chronicity. Front. Endocrinol. 2020, 11, 573032. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Bressan, E.; Ferroni, L.; Nalesso, E.; Vindigni, V.; Stellini, E.; Pinton, P.; Sivolella, S.; Zavan, B. In vitro concurrent endothelial and osteogenic commitment of adipose-derived stem cells and their genomical analyses through comparative genomic hybridization array: Novel strategies to increase the successful engraftment of tissue-engineered bone grafts. Stem Cells Dev. 2012, 21, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Gil, C.L.; Hooker, E.; Larrivée, B. Diabetic Kidney Disease, Endothelial Damage, and Podocyte-Endothelial Crosstalk. Kidney Med. 2020, 3, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Packer, C.F.D.U.; Ali, S.A.; Manna, B. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Enoch, S.; Grey, J.E.; Harding, K.G. ABC of wound healing: Recent advances and emerging treatments. Br. Med. J. 2006, 332, 962–965. [Google Scholar] [CrossRef] [Green Version]
- Tiengo, E.; Fermi, E.; Zanolla, I.; Zanotti, F.; Trentini, M.; Pasquino, E.; Palmieri, M.C.; Soliani, G.; Leo, S.; Tremoli, E.; et al. In Vitro Model for the Evaluation of Innovative Transcatheter Debridement Device (TDD): Pericardium-Based Scaffold and Stem Cells to Reproduce Calcificated Valves. Biomedicines 2022, 10, 2352. [Google Scholar] [CrossRef]
- Elpomme, D.; Irigaray, P. Why electrohypersensitivity and related symptoms are caused by non-ionizingman-made electromagnetic fields: An overview and medical assessment. Environ. Res. 2022, 212 Pt A, 113374. [Google Scholar] [CrossRef]
- Markov, M.S. Expanding use of pulsed electromagnetic field therapies. Electromagn. Biol. Med. 2007, 26, 257–274. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Karabarbounis, A.; Margaritis, L.H. Mechanism for action of electromagnetic fields on cells. Biochem. Biophys. Res. Commun. 2002, 298, 95–102. [Google Scholar] [CrossRef]
- Funk, R.H.W.; Monsees, T.; Özkucur, N. Electromagnetic effects—From cell biology to medicine. Prog. Histochem. Cytochem. 2009, 43, 177–264. [Google Scholar] [CrossRef]
- Chalidis, B.; Sachinis, N.; Assiotis, A.; Maccauro, G. Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: Biologic responses and clinical implications. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. 2), 17–20. [Google Scholar] [CrossRef] [PubMed]
- Game, F.L.; Apelqvist, J.; Attinger, C.; Hartemann, A.; Hinchliffe, R.J.; Löndahl, M.; Price, P.E.; Jeffcoate, W.J.; International Working Group on the Diabetic Foot. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2016, 32, 154–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, A.W.; Graham, K.; Prato, F.S.; McKay, J.; Forster, P.M.; Moulin, D.E.; Chari, S. A randomized, double-blind, placebo-controlled clinical trial using a low-frequency magnetic field in the treatment of musculoskeletal chronic pain. Pain Res. Manag. 2007, 12, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Maya, I.C.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.C.; Ambrosio, L.; et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials 2021, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Brunello, G.; Zanotti, F.; Trentini, M.; Zanolla, I.; Pishavar, E.; Favero, V.; Favero, R.; Favero, L.; Bressan, E.; Bonora, M.; et al. Exosomes Derived from Dental Pulp Stem Cells Show Different Angiogenic and Osteogenic Properties in Relation to the Age of the Donor. Pharmaceutics 2022, 14, 908. [Google Scholar] [CrossRef]
- Merighi, S.; Gessi, S.; Bencivenni, S.; Battistello, E.; Vincenzi, F.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R.; Varani, K. Signaling pathways involved in anti-inflammatory effects of Pulsed Electromagnetic Field in microglial cells. Cytokine 2020, 125, 154777. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, J.; Zhen, C.; Wang, Y.; Wei, Y.; Ren, W.; Shang, P. Magnetic fields as a potential therapy for diabetic wounds based on animal experiments and clinical trials. Cell Prolif. 2021, 54, e12982. [Google Scholar] [CrossRef]
- Peng, L.; Fu, C.; Wang, L.; Zhang, Q.; Liang, Z.; He, C.; Wei, Q. The Effect of Pulsed Electromagnetic Fields on Angiogenesis. Bioelectromagnetics 2021, 42, 250–258. [Google Scholar] [CrossRef]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising application of Pulsed Electromagnetic Fields (PEMFs) in musculoskeletal disorders. Biomed. Pharm. 2020, 131, 110767. [Google Scholar] [CrossRef]
- Lullini, G.; Cammisa, E.; Setti, S.; Sassoli, I.; Zaffagnini, S.; Muccioli, G.M.M. Role of pulsed electromagnetic fields after joint replacements. World J. Orthop. 2020, 11, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xie, Y.; Ni, Z.; Chen, L. Effects and Mechanisms of Exogenous Electromagnetic Field on Bone Cells: A Review. Bioelectromagnetics 2020, 41, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Carraro, A.; Ferroni, L.; Gardin, C.; Sbricoli, L.; Guazzo, R.; Stellini, E.; Roman, M.; Pinton, P.; Sivolella, S.; et al. Nanotechnology to drive stem cell commitment. Nanomedicine 2013, 8, 469–486. [Google Scholar] [CrossRef]
- Ehnert, S.; Schröter, S.; Aspera-Werz, R.H.; Eisler, W.; Falldorf, K.; Ronniger, M.; Nussler, A.K. Translational Insights into Extremely Low Frequency Pulsed ElectromagneticFields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery. J. Clin. Med. 2019, 8, 2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferroni, L.; Bellin, G.; Emer, V.; Rizzuto, R.; Isola, M.; Gardin, C.; Zavan, B. Treatment by Therapeutic Magnetic Resonance (TMR™) increases fibroblastic activity and keratinocyte differentiation in an in vitro model of 3D artificial skin. J. Tissue Eng. Regen. Med. 2017, 11, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Barak, S.; Matalon, S.; Dolkart, O.; Zavan, B.; Mortellaro, C.; Piattelli, A. Miniaturized Electromagnetic Device Abutment Improves Stability of the Dental Implants. J. Craniofac. Surg. 2019, 30, 1055–1057. [Google Scholar] [CrossRef] [Green Version]
- Ferroni, L.; Gardin, C.; De Pieri, A.; Sambataro, M.; Seganfreddo, E.; Goretti, C.; Iacopi, E.; Zavan, B.; Piaggesi, A. Treatment of diabetic foot ulcers with Therapeutic Magnetic Resonance (TMR®) improves the quality of granulation tissue. Eur. J. Histochem. 2017, 61, 2800. [Google Scholar] [CrossRef] [Green Version]
- Ferroni, L.; Gardin, C.; Dolkart, O.; Salai, M.; Barak, S.; Piattelli, A.; Amir-Barak, H.; Zavan, B. Pulsed electromagnetic fields increase osteogenetic commitment of MSCs via the mTOR pathway in TNF-α mediated inflammatory conditions: An in-vitro study. Sci. Rep. 2018, 8, 5108. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, J.; Qu, M.; Huang, X.; Yin, L.; Liao, Y.; Huang, F.; Ning, P.; Zhong, P.; Zeng, Y. Effect of the Pulsed Electromagnetic Field Treatment in a Rat Model of Senile Osteoporosis In Vivo. Bioelectromagnetics 2022, 43, 438–447. [Google Scholar] [CrossRef]
- Ferroni, L.; Tocco, I.; De Pieri, A.; Menarin, M.; Fermi, E.; Piattelli, A.; Gardin, C.; Zavan, B. Pulsed magnetic therapy increases osteogenic differentiation of mesenchymal stem cells only if they are pre-committed. Life Sci. 2016, 152, 44–51. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Paola, L.D.; Campo, G.; Cimaglia, P.; Bellin, G.; Pinton, P.; Zavan, B. Characterization of Dermal Stem Cells of Diabetic Patients. Cells 2019, 8, 729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morganti, C.; Missiroli, S.; Lebiedzinska-Arciszewska, M.; Ferroni, L.; Morganti, L.; Perrone, M.; Ramaccini, D.; Occhionorelli, S.; Zavan, B.; Wieckowski, M.R.; et al. Regulation of PKCβ levels and autophagy by PML is essential for high-glucose-dependent mesenchymal stem cell adipogenesis. Int. J. Obes. 2019, 43, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Agnoletto, C.; Baldini, C.; Bononi, A.; Bonora, M.; Marchi, S.; Missiroli, S.; Patergnani, S.; Poletti, F.; Rimessi, A.; et al. Redox control of protein kinase C: Cell- and disease-specific aspects. Antioxid. Redox Signal. 2010, 13, 1051–1085. [Google Scholar] [CrossRef] [PubMed]
- Aguiari, P.; Leo, S.; Zavan, B.; Vindigni, V.; Rimessi, A.; Bianchi, K.; Franzin, C.; Cortivo, R.; Rossato, M.; Vettor, R.; et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1226–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mijiritsky, E.; Ferroni, L.; Gardin, C.; Peleg, O.; Gultekin, A.; Saglanmak, A.; Delogu, L.G.; Mitrecic, D.; Piattelli, A.; Tatullo, M.; et al. Presence of ROS in Inflammatory Environment of Peri-Implantitis Tissue: In Vitro and In Vivo Human Evidence. J. Clin. Med. 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials 2019, 12, 2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Wieckowski MRMitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef] [Green Version]
- Ferroni, L.; Zago, M.; Patergnani, S.; Campbell, S.E.; Hébert, L.; Nielsen, M.; Scarpa, C.; Bassetto, F.; Pinton, P.; Zavan, B. Fluorescent Light Energy (FLE) Acts on Mitochondrial Physiology Improving Wound Healing. Clin. Med. 2020, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Mattsson, M.O.; Simkó, M.; Scarfi, M.R.; Zeni, O. Editorial: Effects of combined EMF exposures and co-exposures, volume II. Front. Public Health 2022, 10, 1052639. [Google Scholar] [CrossRef]
- Scarfì, M.R.; Mattsson, M.O.; Simkó, M.; Zeni, O. Special Issue: Electric, Magnetic, and Electromagnetic Fields in Biology and Medicine: From Mechanisms to Biomedical Applications. Int. J. Environ. Res. Public Health 2019, 16, 4548. [Google Scholar] [CrossRef]
- Mattsson, M.O.; Simkó, M. Emerging medical applications based on non-ionizing electromagnetic fields from 0 Hz to 10 THz. Med. Devices 2019, 12, 347–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simkó, M.; Mattsson, M.O. 5G Wireless Communication and Health Effects—A Pragmatic Review Based on Available Studies Regarding 6 to 100 GHz. Int. J. Environ. Res. Public Health 2019, 16, 3406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simkó, M.; Mattsson, M.O. Activation of the intracellular temperature and ROS sensor membrane protein STIM1 as a mechanism underpinning biological effects of low-level low frequency magnetic fields. Med. Hypotheses 2019, 122, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, M.O.; Zeni, O.; Simkó, M.; Scarfì, M.R. Editorial: Effects of Combined EMF Exposures and Co-exposures. Front. Public Health 2018, 6, 230. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.M.; Simkó, M.; Mattsson, M.O.; Pioli, C. Immune-Modulating Perspectives for Low Frequency Electromagnetic Fields in Innate Immunity. Front. Public Health 2018, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeni, O.; Simkó, M.; Scarfi, M.R.; Mattsson, M.O. Cellular Response to ELF-MF and Heat: Evidence for a Common Involvement of Heat Shock Proteins? Front. Public Health 2017, 5, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Muri, J.; Kopf, M. Redox regulation of immunometabolism. Nat. Rev. Immunol. 2020, 21, 363–381. [Google Scholar] [CrossRef]
- Magnani, N.D.; Marchini, T.; Calabró, V.; Alvarez, S.; Evelson, P. Role of Mitochondria in the Redox Signaling Network and Its Outcomes in High Impact Inflammatory Syndromes. Front. Endocrinol. 2020, 11, 568305. [Google Scholar] [CrossRef]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. J. Immunobiol. 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Romano, M.; De Francesco, F.; Pirozzi, G.; Gringeri, E.; Boetto, R.; Di Domenico, M.; Zavan, B.; Ferraro, G.A.; Cillo, U. Expression of cancer stem cell biomarkers as a tool for a correct therapeutic approach to hepatocellular carcinoma. Oncoscience 2015, 2, 443–456. [Google Scholar] [CrossRef] [Green Version]
- Scialò, F.; Fernández-Ayala, D.J.; Sanz, A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, R.; Zhang, B.; Tan, B.; Lin, N. Long non-coding RNAs as the regulators and targets of macrophage M2 polarization. Life Sci. 2021, 266, 118895. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; Sivolella, S.; Brunello, G.; Berengo, M.; Piattelli, A.; Bressan, E.; Zavan, B. A hyaluronan-based scaffold for the in vitro construction of dental pulp-like tissue. Int. J. Mol. Sci. 2015, 16, 4666–4681. [Google Scholar] [CrossRef] [Green Version]
- Pasca, S.; Jurj, A.; Petrushev, B.; Tomuleasa, C.; Matei, D. microRNA-155 Implication in M1 Polarization and the Impact in Inflammatory Diseases. Front. Immunol. 2020, 11, 625. [Google Scholar] [CrossRef]
- Curtale, G.; Rubino, M.; Locati, M. MicroRNAs as Molecular Switches in Macrophage Activation. Front. Immunol. 2019, 10, 799. [Google Scholar] [CrossRef] [Green Version]
- Trentini, M.; Zanotti, F.; Tiengo, E.; Camponogara, F.; Degasperi, M.; Licastro, D.; Lovatti, L.; Zavan, B. An Apple a Day Keeps the Doctor Away: Potential Role of miRNA 146 on Macrophages Treated with Exosomes Derived from Apples. Biomedicines 2022, 10, 415. [Google Scholar] [CrossRef]
- Li, H.; Jiang, T.; Li, M.Q.; Zheng, X.L.; Zhao, G.J. Transcriptional Regulation of Macrophages Polarization by MicroRNAs. Front. Immunol. 2018, 9, 1175. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage polarization and meta-inflammation. Trans. Res. 2018, 191, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Chachques, J.C.; Gardin, C.; Lila, N.; Ferroni, L.; Migonney, V.; Falentin-Daudre, C.; Zanotti, F.; Trentini, M.; Brunello, G.; Rocca, T.; et al. Elastomeric Cardiowrap Scaffolds Functionalized with Mesenchymal Stem Cells-Derived Exosomes Induce a Positive Modulation in the Inflammatory and Wound Healing Response of Mesenchymal Stem Cell and Macrophage. Biomedicines 2021, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Bosco, G.; Paganini, M.; Giacon, T.A.; Oppio, A.; Vezzoli, A.; Dellanoce, C.; Moro, T.; Paoli, A.; Zanotti, F.; Zavan, B.; et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. Int. J. Environ. Res. Public Health 2021, 18, 9755. [Google Scholar] [CrossRef] [PubMed]
- Selvam, R.; Ganesan, K.; Raju, K.V.N.; Gangadharan, A.C.; Manohar, B.M.; Puvanakrishnan, R. Low frequency and low intensity pulsed electromagnetic field exerts its antiinflammatory effect through restoration of plasma membrane calcium ATPase activity. Life Sci. 2007, 80, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Harrison, B.S. Effect of time-varied magnetic field on inflammatory response in macrophage cell line RAW 264.7. Electromagn. Biol. Med. 2013, 32, 59–69. [Google Scholar] [CrossRef]
- Selmaoui, B.; Lambrozo, J.; Sackett-Lundeen, L.; Haus, E.; Touitou, Y. Acute exposure to 50-Hz magnetic fields increases interleukin-6 in young healthy men. J. Clin. Immunol. 2011, 31, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Gyòrgyi, A.S. Interaction of non ionizing radiation with living systems. In Proceedings of the Internationai Symposium on Wave Tera-peutics, Paris, France, 19–20 May 1979; pp. 7–13. [Google Scholar]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the celi surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef]
- Maniotis, A.; Chen, C.S.; Ingber, D.E. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structures. Proc. Natl. Acad. Sci. USA 1997, 94, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, H. Coherence in the cytoskeleton: Implications for biological information processing. In Biological Coherence and Response to External Stimuli; Fròhuich, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 242–266. [Google Scholar]
- Jeiinek, P.; Pokorny, J. Microtubuies in bioiogical cells as circullar waveguides and resonators. Electromagnet 2001, 20, 75–80. [Google Scholar]
- Obermeier, A.; Matl, F.D.; Friess, W.; Stemberger, A. Growth inhibition of Staphylococcus aureus induced by low-frequency electric and electromagnetic fields. Bioelectromagnetics 2009, 30, 270–279. [Google Scholar] [CrossRef]
- Juncker, R.B.; Lazazzera, B.A.; Billi, F. Pulsed Electromagnetic Fields Disrupt Staphylococcus epidermidis Biofilms and Enhance the Antibiofilm Efficacy of Antibiotics. Microbiol. Spectr. 2022, 10, e0194922. [Google Scholar] [CrossRef]
- D’Ercole, S.; Di Lodovico, S.; Iezzi, G.; Pierfelice, T.; D’Amico, E.; Cipollina, A.; Piattelli, A.; Cellini, L.; Petrini, M. Article Complex Electromagnetic Fields Reduce Candida albicans Planktonic Growth and Its Adhesion to Titanium Surfaces. Biomedicines 2021, 9, 1261. [Google Scholar] [CrossRef]
- Petrini, M.; Di Lodovico, S.; Iezzi, G.; Cipollina, A.; Piattelli, A.; Cellini, L.; D’Ercole, S. Effects of Complex Electromagnetic Fields on Candida albicans Adhesion and Proliferation on Polyacrylic Resin. Appl. Sci. 2021, 11, 6786. [Google Scholar] [CrossRef]
- Nossol, B.; Buse, G.; Silny, J. Influence of weak static and 50 Hz magnetic fields on the redox activity of cytochrome-C oxidase. Bioelectromagnetics 1993, 14, 361. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, N.; Canseven, A.G. In vivo effects of ELF MFs on collagen synthesis, free radical processes, natural antioxidant system, respiratory burst system, immune system activities, and electrolytes in the skin, plasma, spleen, lung, kidney, and brain tissues. Electromagn. Biol. Med. 2006, 25, 291–305. [Google Scholar] [CrossRef]
- Carmody, S.; Wu, X.L.; Lin, H.; Blank, M.; Skopicki, H.; Goodman, R. Cytoprotection by electromagnetic field-induced hsp70: A model for clinical application. J. Cell Biochem. 2000, 79, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Callaghan, M.J.; Chang, E.I.; Galiano, R.D.; Bhatt, K.A.; Baharestani, S.; Gan, J.; Simon, B.; Hopper, R.A.; Levine, J.P.; et al. Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J. 2004, 18, 1231–1233. [Google Scholar] [CrossRef]
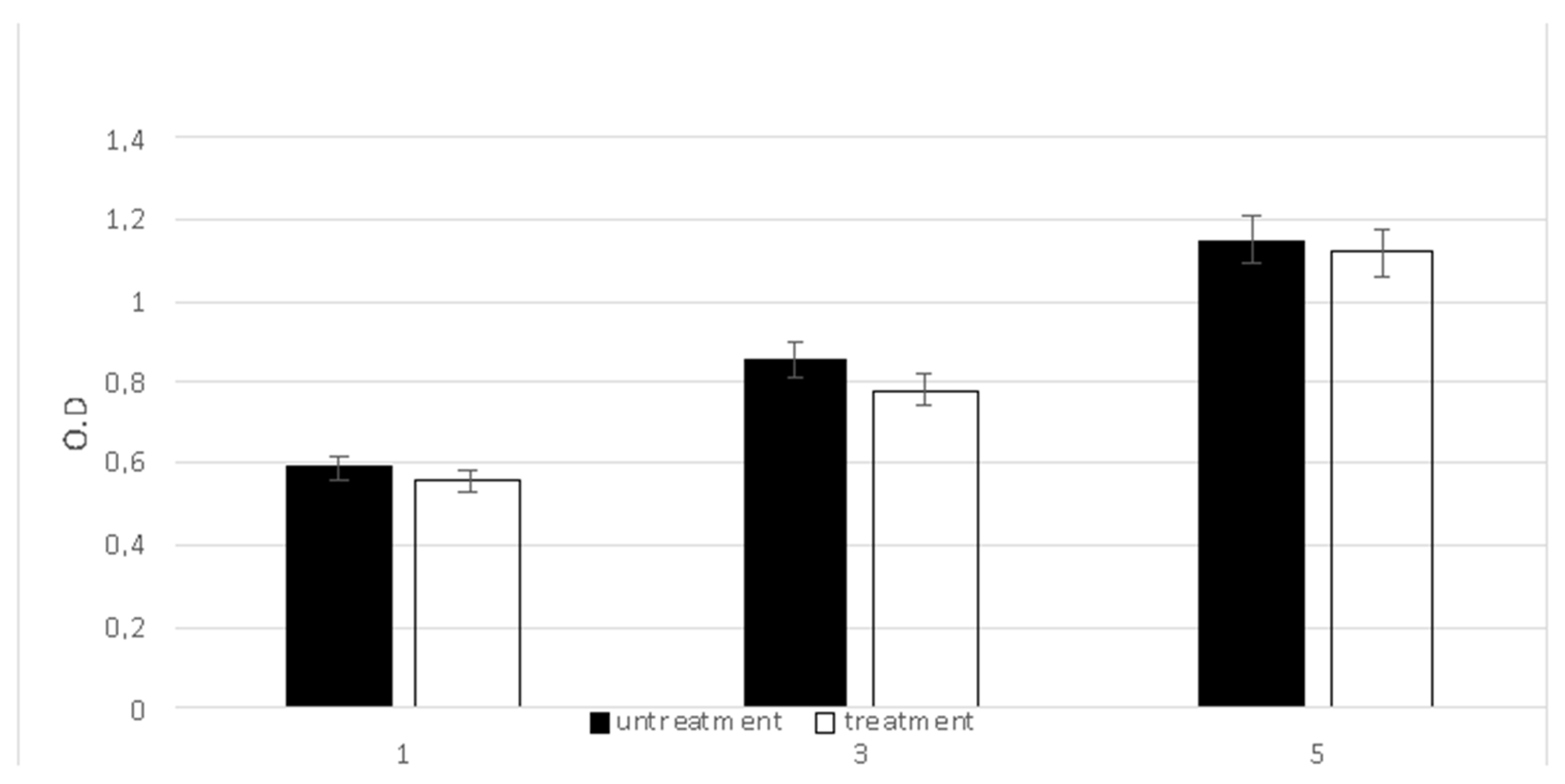

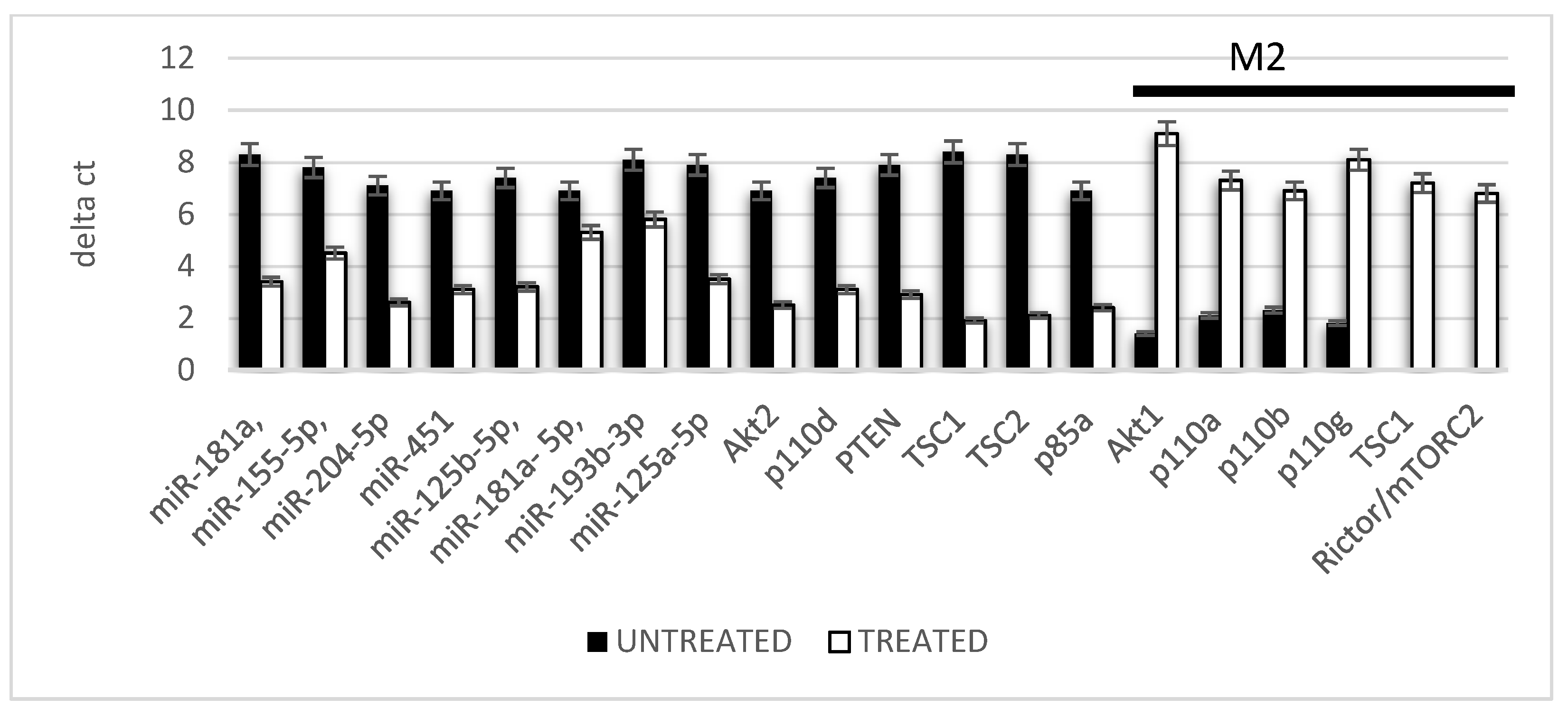

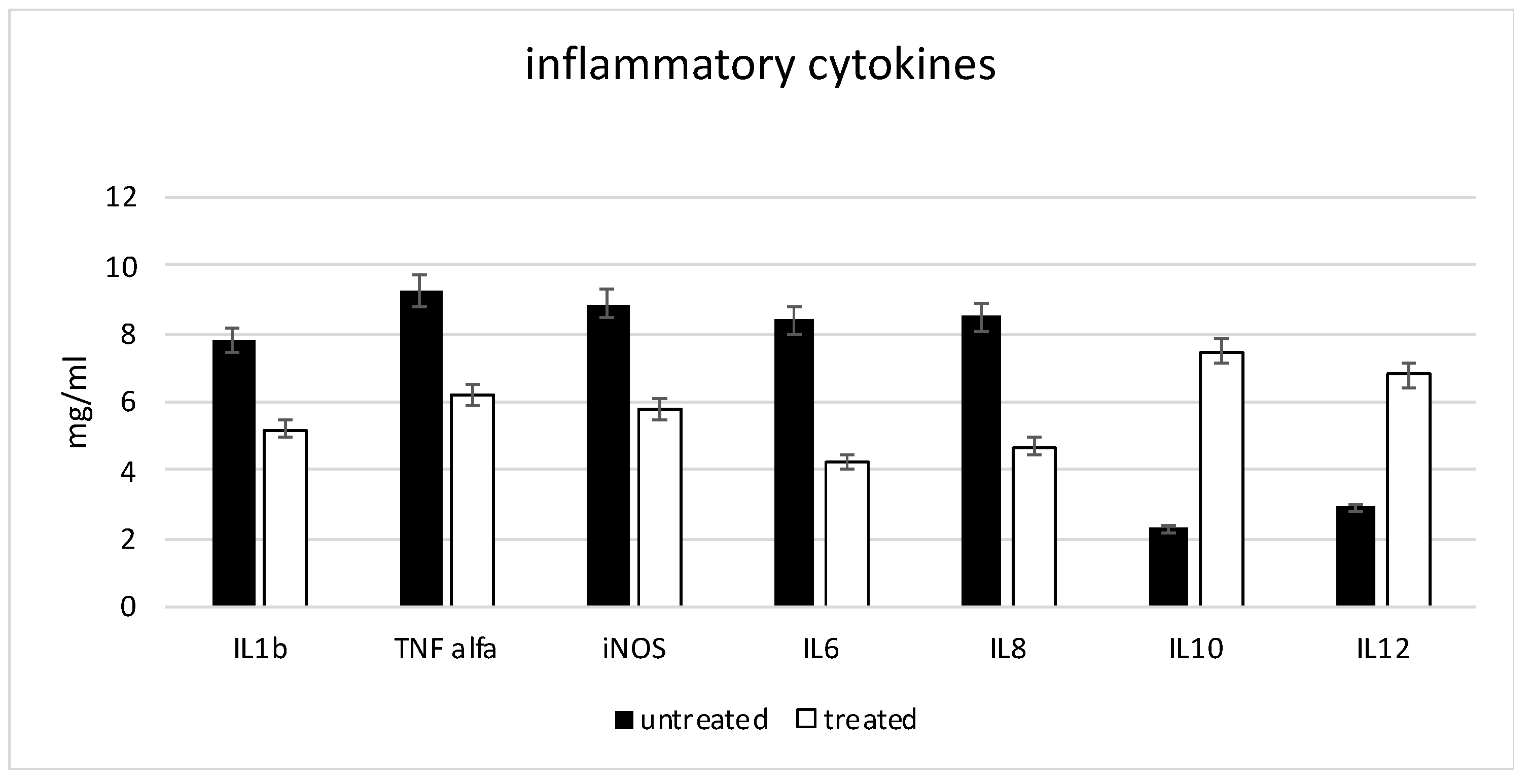
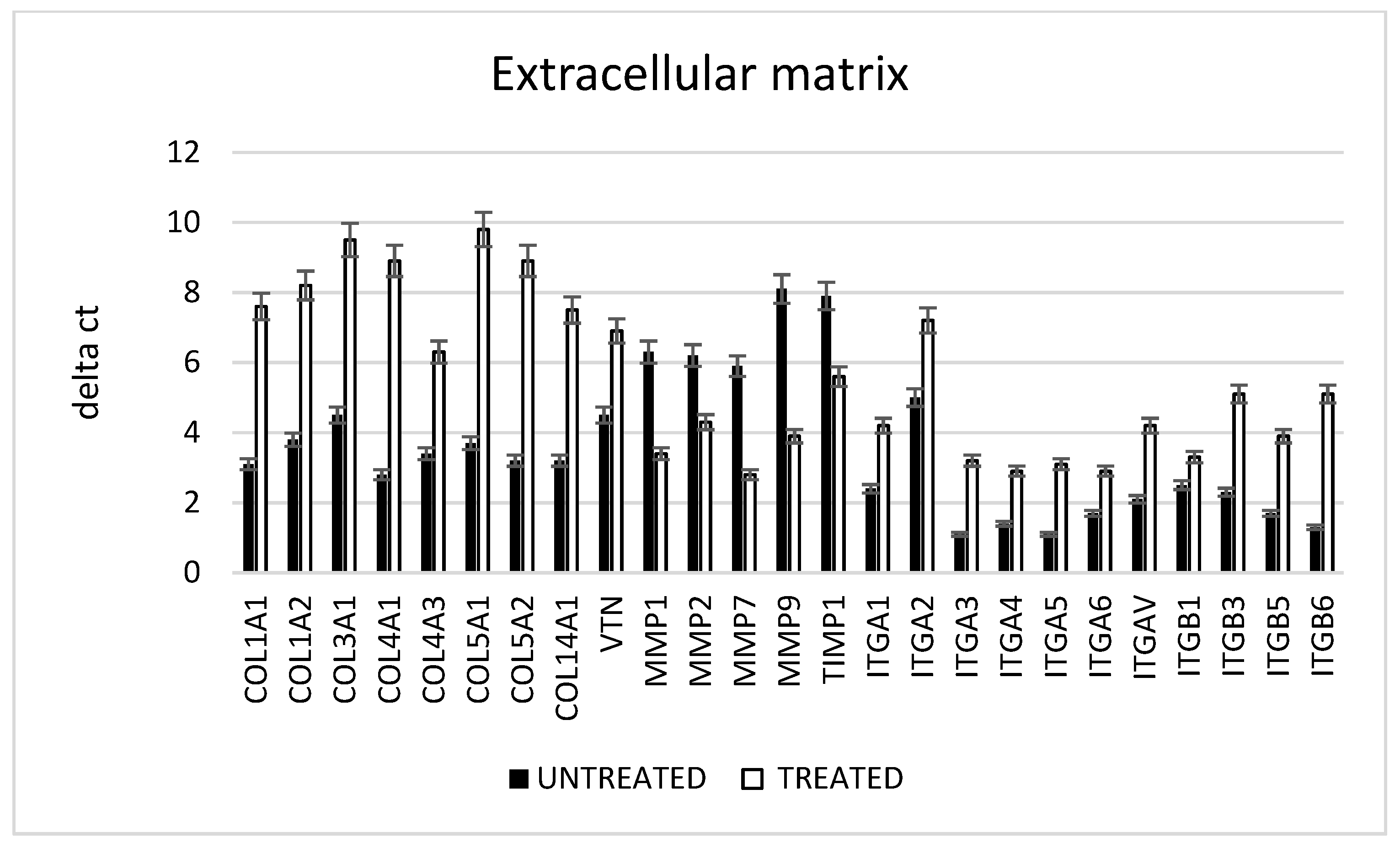

| Sample | OD | Hemolysis Index | Results |
|---|---|---|---|
| Positive control | 0.834 +/− 0.011 | 100% | Hemolytic |
| Negative control | 0.0103 +/− 0.023 | 0% | Non Hemolitic |
| CMF treatment | 0.0142 +/− 0.018 | 0.031% | Non Hemolitic |
| No treatment | 0.0131 +/− 0.022 | 0.045% | Non Hemolitic |
| STDisc™ TA1535 | STDisc™ TA1537 | STDisc™ TA98 | STDisc™ TA100 | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | Revertant Colonies | Mutagenic | Revertant Colonies | Mutagenic | Revertant Colonies | Mutagenic | Revertant Colonies | Mutagenic |
| Blank | 4 ± 3 | no | 5 ± 3 | no | 4 ± 2 | no | 5 ± 2 | no |
| Negative control | 3 ± 2 | no | 3 ± 2 | no | 3± 2 | no | 2 ± 2 | no |
| Positive control: ICR191 | 947 ± 85 | yes | 973 ± 66 | yes | 971 ± 79 | yes | 965 ± 69 | yes |
| Positive control: Sodium Azide | 853 ± 51 | yes | 876 ± 52 | yes | 893 ± 59 | yes | 879 ± 64 | yes |
| CMF treatment | 3 ± 2 | no | 2 ± 2 | no | 3 ± 2 | no | 3 ± 2 | no |
| No treatment | 3 ± 1 | no | 3 ± 2 | no | 2 ± 2 | no | 5 ± 2 | no |
| Mechanism of Action of Program: Wound Healing | ||
|---|---|---|
| Program Step | Target | Bibliography |
| 1 | Anti-inflammatory | [63,64,65] |
| 2 | Normalization Intracellular cell communication | [66,67,68] |
| 3 | Antibacterial and anti fungal | [69,70,71,72,73] |
| 4 | ROS modulation | [74,75,76] |
| 5 | Normalization Intracellular cell communication | [66,67,68] |
| 6 | Vascularization and tissue engineering regeneration | [77,78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanotti, F.; Trentini, M.; Zanolla, I.; Tiengo, E.; Mantarro, C.; Dalla Paola, L.; Tremoli, E.; Sambataro, M.; Sambado, L.; Picari, M.; et al. Playing with Biophysics: How a Symphony of Different Electromagnetic Fields Acts to Reduce the Inflammation in Diabetic Derived Cells. Int. J. Mol. Sci. 2023, 24, 1754. https://doi.org/10.3390/ijms24021754
Zanotti F, Trentini M, Zanolla I, Tiengo E, Mantarro C, Dalla Paola L, Tremoli E, Sambataro M, Sambado L, Picari M, et al. Playing with Biophysics: How a Symphony of Different Electromagnetic Fields Acts to Reduce the Inflammation in Diabetic Derived Cells. International Journal of Molecular Sciences. 2023; 24(2):1754. https://doi.org/10.3390/ijms24021754
Chicago/Turabian StyleZanotti, Federica, Martina Trentini, Ilaria Zanolla, Elena Tiengo, Chiara Mantarro, Luca Dalla Paola, Elena Tremoli, Maria Sambataro, Luisa Sambado, Massimo Picari, and et al. 2023. "Playing with Biophysics: How a Symphony of Different Electromagnetic Fields Acts to Reduce the Inflammation in Diabetic Derived Cells" International Journal of Molecular Sciences 24, no. 2: 1754. https://doi.org/10.3390/ijms24021754
APA StyleZanotti, F., Trentini, M., Zanolla, I., Tiengo, E., Mantarro, C., Dalla Paola, L., Tremoli, E., Sambataro, M., Sambado, L., Picari, M., Leo, S., Ferroni, L., & Zavan, B. (2023). Playing with Biophysics: How a Symphony of Different Electromagnetic Fields Acts to Reduce the Inflammation in Diabetic Derived Cells. International Journal of Molecular Sciences, 24(2), 1754. https://doi.org/10.3390/ijms24021754







