Chitosan Composites Containing Boron-Dipyrromethene Derivatives for Biomedical Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. General Remarks and Visual Observation of Chitosan-BODIPY Composites
2.2. Infrared Spectroscopy
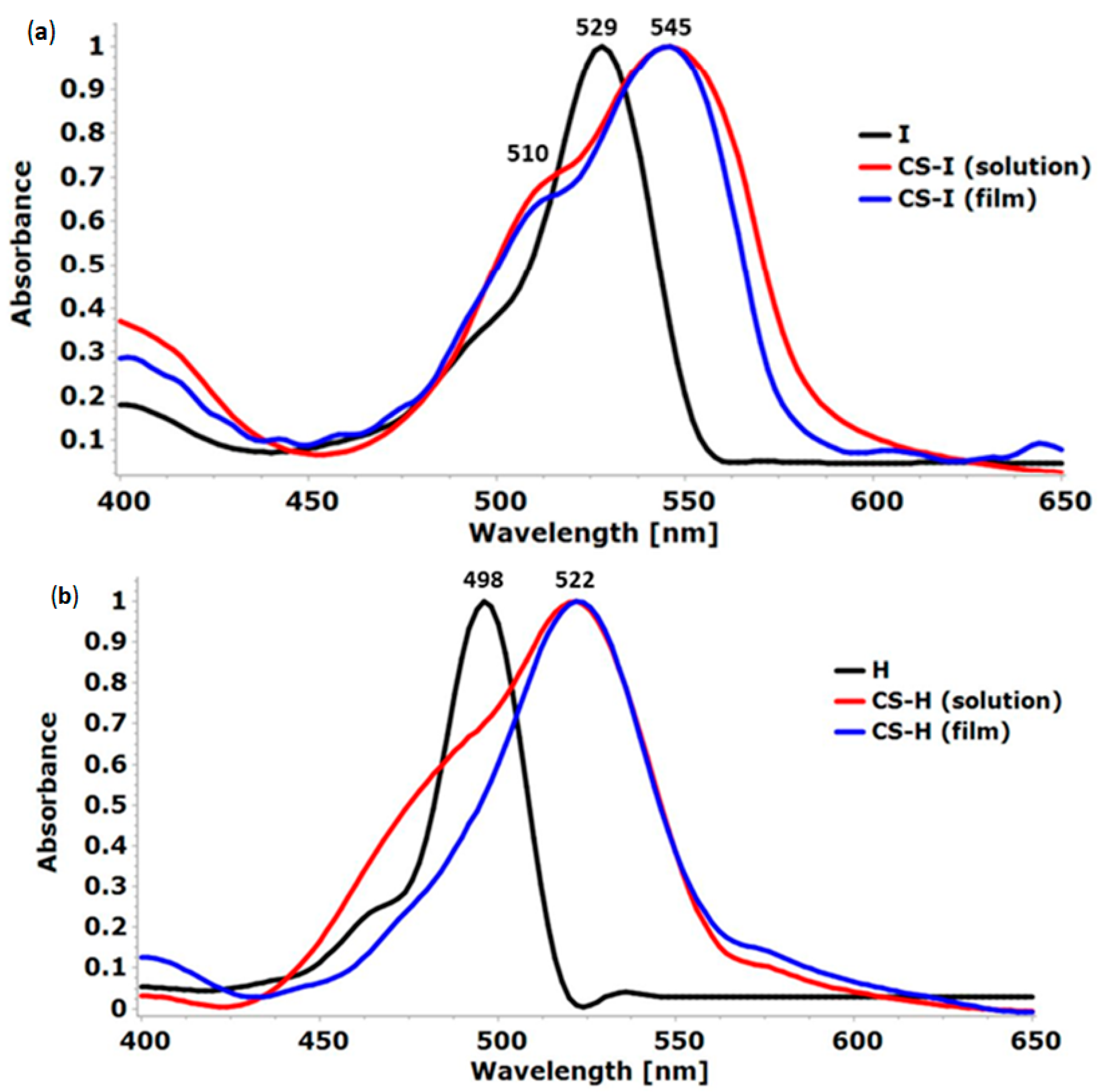
2.3. UV-Vis Absorption Spectroscopy
2.4. Fluorescence
2.5. Contact Angle Measurements
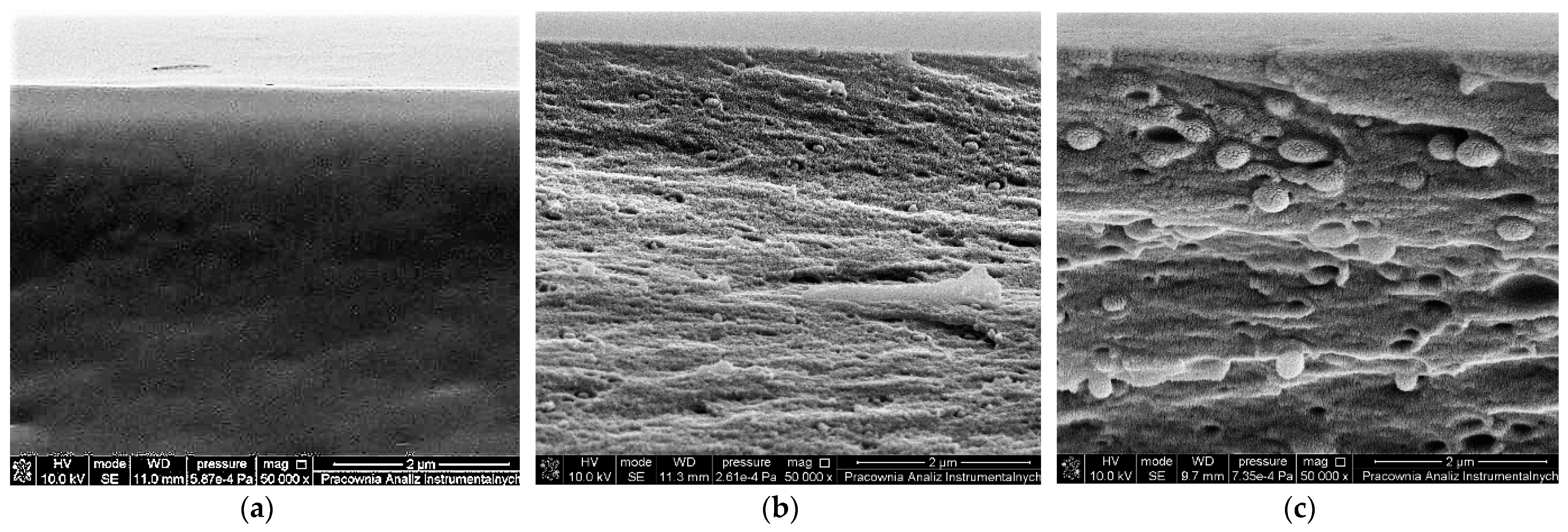
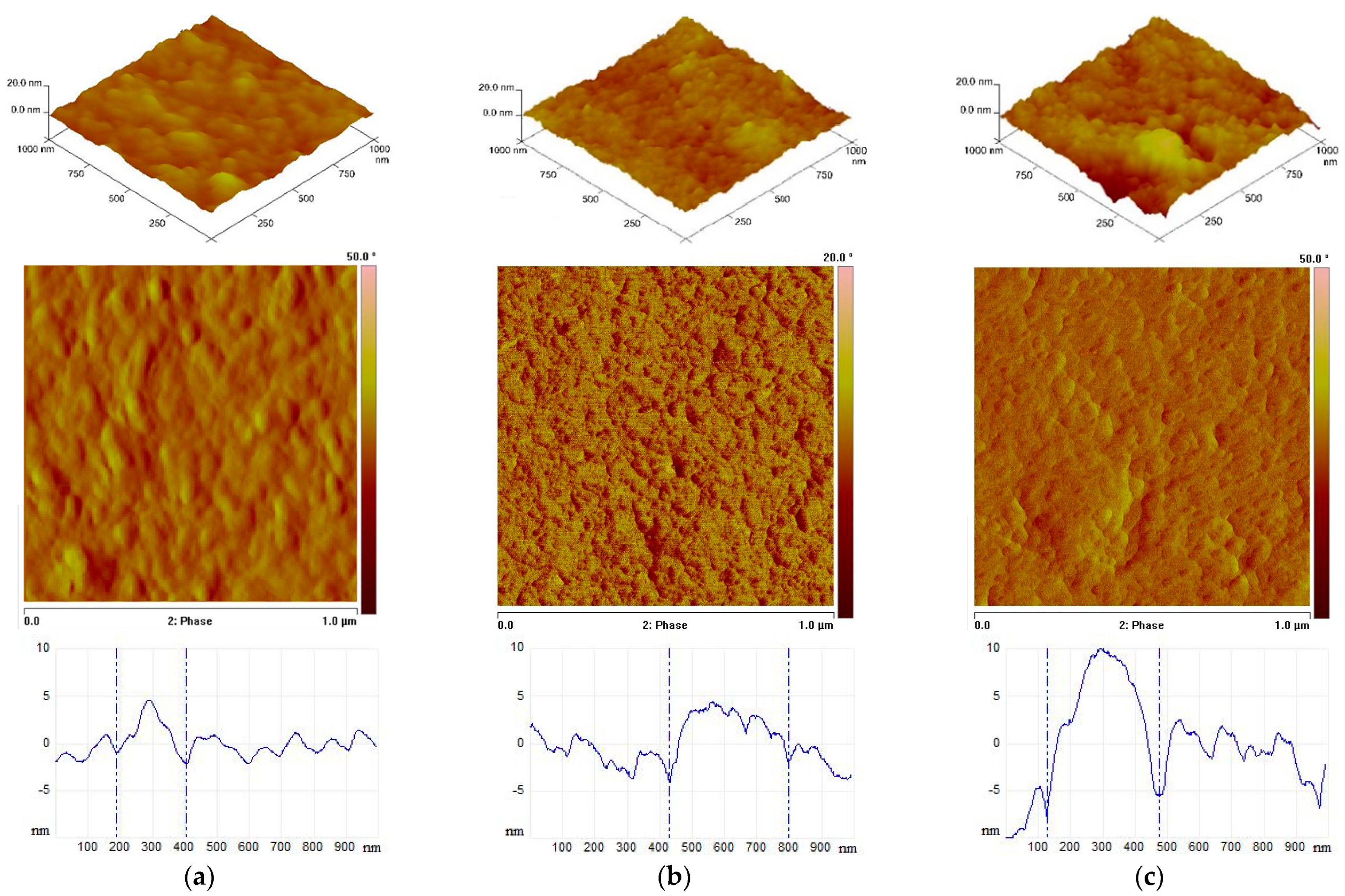
2.6. Morphology (SEM and AFM)
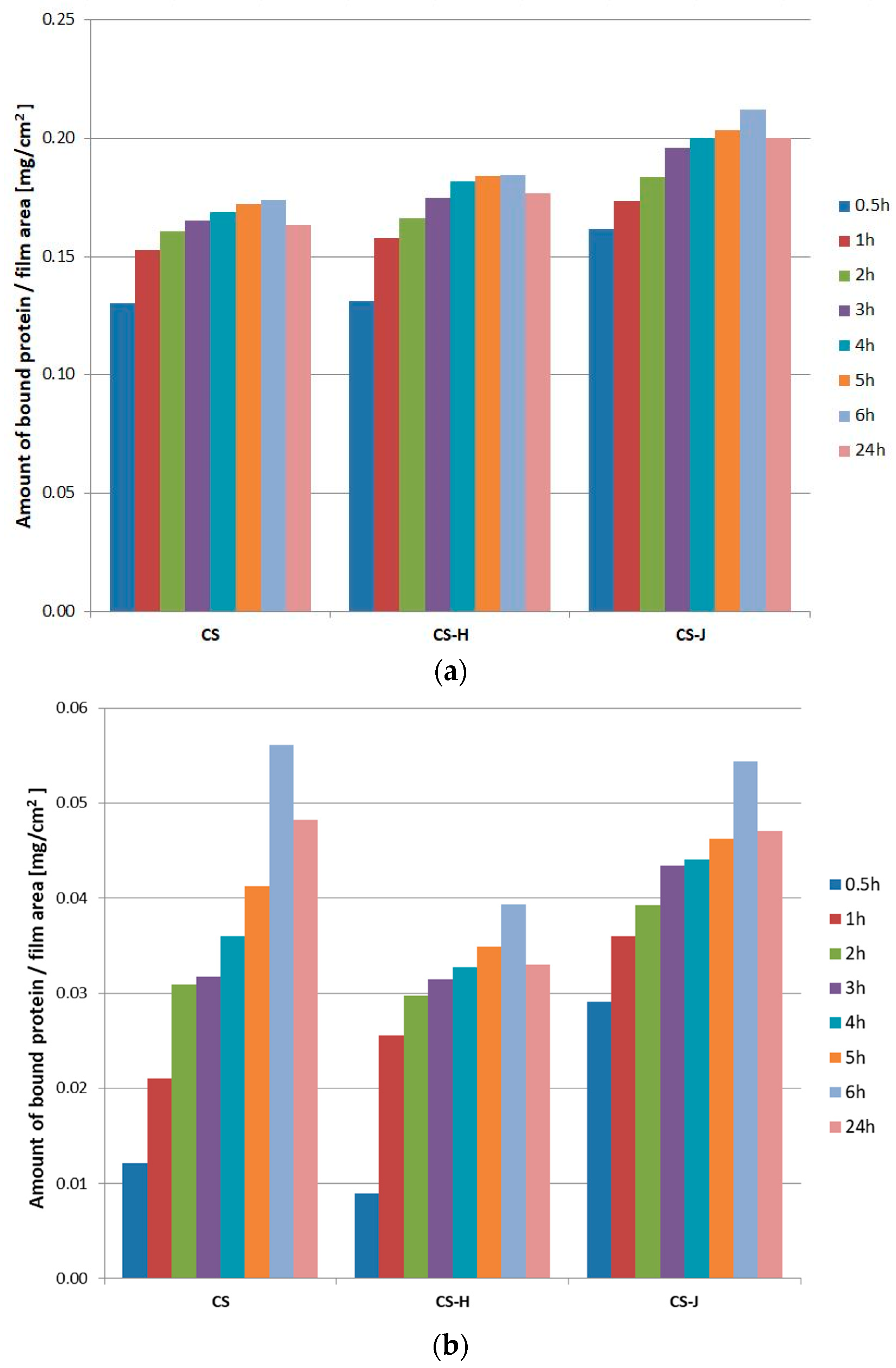
2.7. Protein Adsorption
2.8. Kinetics of the Dyes’ Release
2.9. Mechanical Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Chitosan Composites with BODIPY Dyes
3.3. Research Methodology
3.3.1. FTIR and UV-Vis Absorption Spectroscopy
3.3.2. Fluorescence and Quantum Yield Determination
3.3.3. Contact Angle Measurement
3.3.4. Morphology Analysis by SEM and AFM
3.3.5. Protein Adsorption
3.3.6. Kinetics of the Dyes’ Release
3.3.7. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Orzetti, S.; Tommasi, F.; Bertola, A.; Bortolin, G.; Caccin, E.; Cecco, S.; Ferrarin, E.; Giacomin, E.; Baldo, P. Genetic Therapy and Molecular Targeted Therapy in Oncology: Safety, Pharmacovigilance, and Perspectives for Research and Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3012. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Rybczyński, P.; Maćczak, P.; Smolarkiewicz-Wyczachowski, A.; Ziegler-Borowska, M. Chitosan as a Protective Matrix for the Squaraine Dye. Materials 2021, 14, 1171. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Tafelska-Kaczmarek, A.; Roszek, K.; Czarnecka, J.; Jędrzejewska, B.; Zblewska, K. Fluorescent Chitosan Modified with Heterocyclic Aromatic Dyes. Materials 2021, 14, 6429. [Google Scholar] [CrossRef]
- Calero, J.L.; Berríos, Z.; Suarez, O.M. Biodegradable Chitosan Matrix Composite Reinforced with Titanium Dioxide for Biocidal Applications. In Renewable and Sustainable Composites; Pereira, A., Fernandes, F., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2020, 282, 119132. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.R.; Isakoff, S.T.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol Photoimmunol Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Crous, A.; Abrahamse, H. Photodynamic therapy of lung cancer, where are we? Front. Pharmacol. 2022, 13, 932098. [Google Scholar] [CrossRef] [PubMed]
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.T.; Heidi, A. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photodiagn. Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, X.; Mo, J.; Zhao, W. Drug delivery using nanoparticles for cancer stem-like cell targeting. Front. Pharmacol. 2016, 7, 84. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, R.; He, X.; Wang, J.; Wang, M.; Qian, Y.; Wang, S. Enhanced photocytotoxicity of curcumin delivered by solid lipid nanoparticles. Int. J. Nanomed. 2017, 12, 167–178. [Google Scholar] [CrossRef]
- Chen, H.; Sun, X.; Wang, G.D.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J.; Shen, B. LiGa 5 O 8: Cr-Based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater. Horiz. 2017, 6, 1092–1101. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent advances of BODIPY based derivatives for optoelectronic applications. Coord. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. [Google Scholar] [CrossRef]
- Mai, D.K.; Kim, C.; Lee, J.; Vales, T.P.; Badon, I.W.; De, K.; Cho, S.; Yang, J.; Kim, H.J. BODIPY nanoparticles functionalized with lactose for cancer-targeted and fluorescence imaging-guided photodynamic therapy. Sci. Rep. 2022, 12, 2541. [Google Scholar] [CrossRef]
- Lv, F.; Li, H.; Wu, Q.; Guo, X.; Zhang, H.; Yu, C.; Jiao, L.; Hao, E. Silver-mediated, direct phosphorylation of BODIPY dyes at the 3- or 3,5-positions with H-phosphonates. Chem. Commun. 2022, 58, 3937–3940. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, H.; Xiong, Z.; Ran, X.; Tang, H.; Cao, D. Design, synthesis and applications of NIR-emissive scaffolds of diketopyrrolopyrrole-aza-BODIPY hybrids. Chem. Commun. 2022, 58, 5996. [Google Scholar] [CrossRef] [PubMed]
- Doria, S.; Taddei, M.; Cupellini, L.; Biagiotti, G.; Bartolini, P.; Bussotti, L.; Cicchi, S.; Foggi, P.; Mennucci, B.; Donato, M. Unravelling the ultrafast dynamics of a N-BODIPY compound. Dyes Pigm. 2022, 200, 110181. [Google Scholar] [CrossRef]
- Maleckaite, K.; Narkevičius, D.; Žilenait, R.; Dodonova-Vaitkunien, J.; Toliautas, S.; Tumkevicius, S.; Vyšniauskas, A. Give or Take: Effects of Electron-Accepting/-Withdrawing Groups in Red-Fluorescent BODIPY Molecular Rotors. Molecules 2022, 27, 23. [Google Scholar] [CrossRef] [PubMed]
- Piskorz, J.; Dlugaszewska, J.; Porolnik, W.; Teubert, A.; Mielcarek, J. Boron-dipyrromethene derivatives bearing N-alkyl phthalimide and amine substituents of potential application in the photoinactivation of bacteria. Dyes Pigm. 2020, 178, 108332. [Google Scholar] [CrossRef]
- Júnior, J.; Holanda, V.N.; Gambôa, D.S.R.; Monte, T.V.S.; Araújo, H.D.A.; Araújo, J.A.A.N.J.V.F.S.; Callôu, M.A.M.; Assis, S.P.O.; Lima, V.L.M. Therapeutic Potential of Phthalimide Derivatives: A Review. Am. J. Biomed. Sci. Res. 2019, 4, 378–384. [Google Scholar] [CrossRef]
- Koksoy, B.; Kaya, E.N.; Hacıvelioglu, F.; Yesilot, S.; Durmus, M. Effect of iodine substitution pattern on the singlet oxygen generation and solvent depended ketoenol tautomerization behavior of BODIPY photosensitizers. Dyes Pigm. 2017, 140, 384–391. [Google Scholar] [CrossRef]
- Zhang, H.; Shin, B.G.; Lee, D.E.; Yoon, K.B. Preparation of PP/2D-Nanosheet Composites Using MoS2/MgCl2− and BN/MgCl2− Bisupported Ziegler–Natta Catalysts. Catalysts 2020, 10, 596. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, G.; Singh, P.; Singh, K.; Kumar, B.; Vij, A.; Kumar, M.; Bala, R.; Meena, R.; Singh, A.; et al. Nanostructured Boron Nitride with High Water Dispersibility for Boron Neutron Capture Therapy. Sci. Rep. 2016, 6, 35535. [Google Scholar]
- Tang, C.; Bando, Y.; Huang, Y.; Zhi, C.; Golberg, D. Synthetic routes and formation mechanisms of spherical boron nitride nanoparticles. Adv. Funct. Mater. 2008, 18, 3653–3661. [Google Scholar] [CrossRef]
- Advanced Organic Chemistry: Infrared Spectrum of 1-Iodobutane. Available online: https://docbrown.info/page06/spectra2/1-iodobutane-ir.htm (accessed on 9 November 2022).
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.M.; Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A. Does the use of chitosan contribute to oxalate kidney stone formation? Mar Drugs. 2014, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 3, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, A.B.; Ashokkumar, P.; Shen, Z.; Rurack, K. On the Aggregation Behaviour and Spectroscopic Properties of Alkylated and Annelated Boron-Dipyrromethene (BODIPY) Dyes in Aqueous Solution. ChemPhotoChem. 2020, 4, 120–131. [Google Scholar] [CrossRef]
- Li, K.; Duan, X.; Jiang, Z.; Ding, D.; Chen, Y.; Zhang, G.-Q.; Liu, Z. J-aggregates of meso-[2.2]paracyclophanyl-BODIPY dye for NIR-II imaging. Nat. Commun. 2021, 12, 2376. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zhang, Y.; Liu, G.; Xing, G. J- and H-aggregates of heavy-atom-free Aza-BODIPY dyes with high 1O2 generation efficiency and photodynamic therapy potential. Dyes Pigm. 2022, 208, 110813. [Google Scholar] [CrossRef]
- Kaufman, N.E.; Meng, Q.; Griffin, K.E.; Singh, S.S.; Dahal, A.; Zhou, Z.; Fronczek, F.R.; Mathis, J.M.; Jois, S.D.; Vicente, M.G.H. Characterization, and Evaluation of Near-IR Boron Dipyrromethene Bioconjugates for Labeling of Adenocarcinomas by Selectively Targeting the Epidermal Growth Factor Receptor. J. Med. Chem. 2019, 62, 3323–3335. [Google Scholar] [CrossRef]
- Bobrov, A.V.; Kishalova, M.V.; Merkushev, D.A.; Marfin, Y.S. BODIPY in matrices: Brief review. Phys. Chem. Chem. Phys. 2021, 23, 12033–12044. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Marcus, Y. The Properties of Solvents; Marcel Dekker: New York, NY, USA, 2002; Volume 186. [Google Scholar]
- Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi, A.; Piergiovanni, L. Wetting of Biopolymer Coatings: Contact Angle Kinetics and Image Analysis Investigation. Langmuir 2011, 27, 7563–7574. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, X.; Ling, Y.; Wang, X.; Sun, R. Facile fabrication of chitosan active film with xylan via direct immersion. Cellulose 2014, 21, 1873–1883. [Google Scholar] [CrossRef]
- Almeida, E.V.R.; Frollini, E.; Castellan, A.; Coma, V. Chitosan, sisal cellulose, and biocomposite chitosan/sisal, cellulose films prepared from thiourea/NaOH aqueoussolution. Carbohydr. Polym. 2010, 80, 655–664. [Google Scholar] [CrossRef]
- Cunha, A.G.; Fernandes, S.; Freire, C.; Silvestre, A.; Neto, C.; Gandini, A. What Is the Real Value of Chitosan’s Surface Energy? Biomacromolecules 2008, 9, 610–614. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Zastosowanie pomiarów kata zwilżania i swobodnej energii powierzchniowej do charakterystyki powierzchni polimerów wykorzystywanych w medycynie. Polim. Med. 2014, 1, 29–37. [Google Scholar]
- Nosal, W.H.; Thompson, D.W.; Yan, L.; Sarkar, S.; Subramanian, A.; Woollam, J.A. UV–vis–infrared optical and AFM study of spin-cast chitosan films. Colloids Surf. B Biointerfaces 2005, 43, 131–137. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; Remuñán López, C.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Y.; Hao-Ying, L. Chitosan-based spray-dried mucoadhesive microspheres for sustained oromucosal drug delivery. Powder Technol. 2017, 312, 124–132. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Cui, W.; Lu, X.; Cui, K.; Wu, J.; Wei, Y.; Lu, Q. Fluorescent nanoparticles of chitosan complex for real-time monitoring drug release. Langmuir 2011, 13, 8384–8390. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Rybczynski, P.; Smolarkiewicz-Wyczachowski, A.; Mlynarczyk, D.T.; Wegrzynowska-Drzymalska, K.; Ilnicka, A.; Goslinski, T.; Marszałł, M.P.; Ziegler-Borowska, M. Photosensitizing potential of tailored magnetite hybrid nanoparticles functionalized with levan and zinc (II) phthalocyanine. Appl. Surf. Sci. 2020, 524, 146602. [Google Scholar] [CrossRef] [PubMed]
- Gadgey, C.H.; Sharma, G.S. Investigation of mechanical properties of chitosan based films: A review. Int. J. Adv. Res. Eng. Technol. IJARET 2017, 8, 93–102. [Google Scholar]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Influence of plasticiser type and nanoclay on the properties of chitosan-based materials. Eur. Polym. J. 2021, 144, 110225. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Wendt, R. Estimating the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
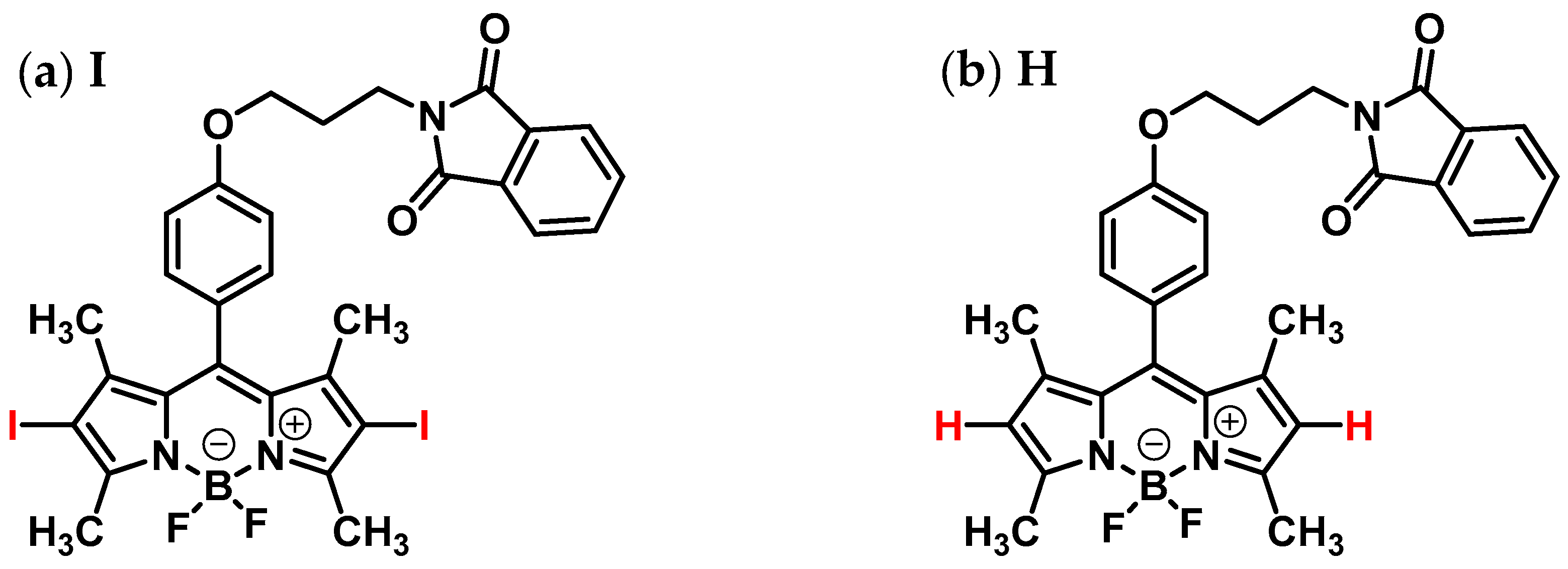


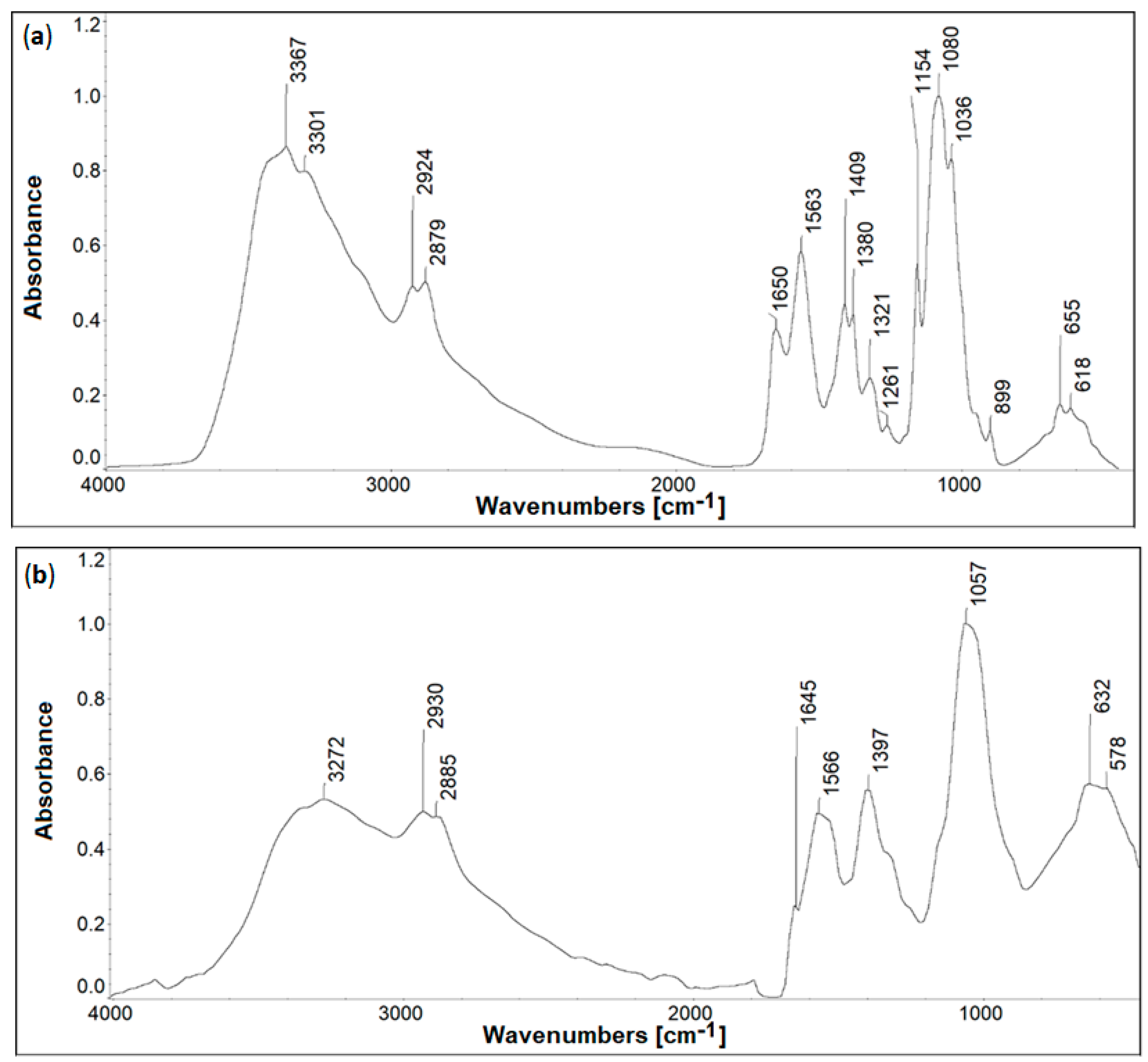
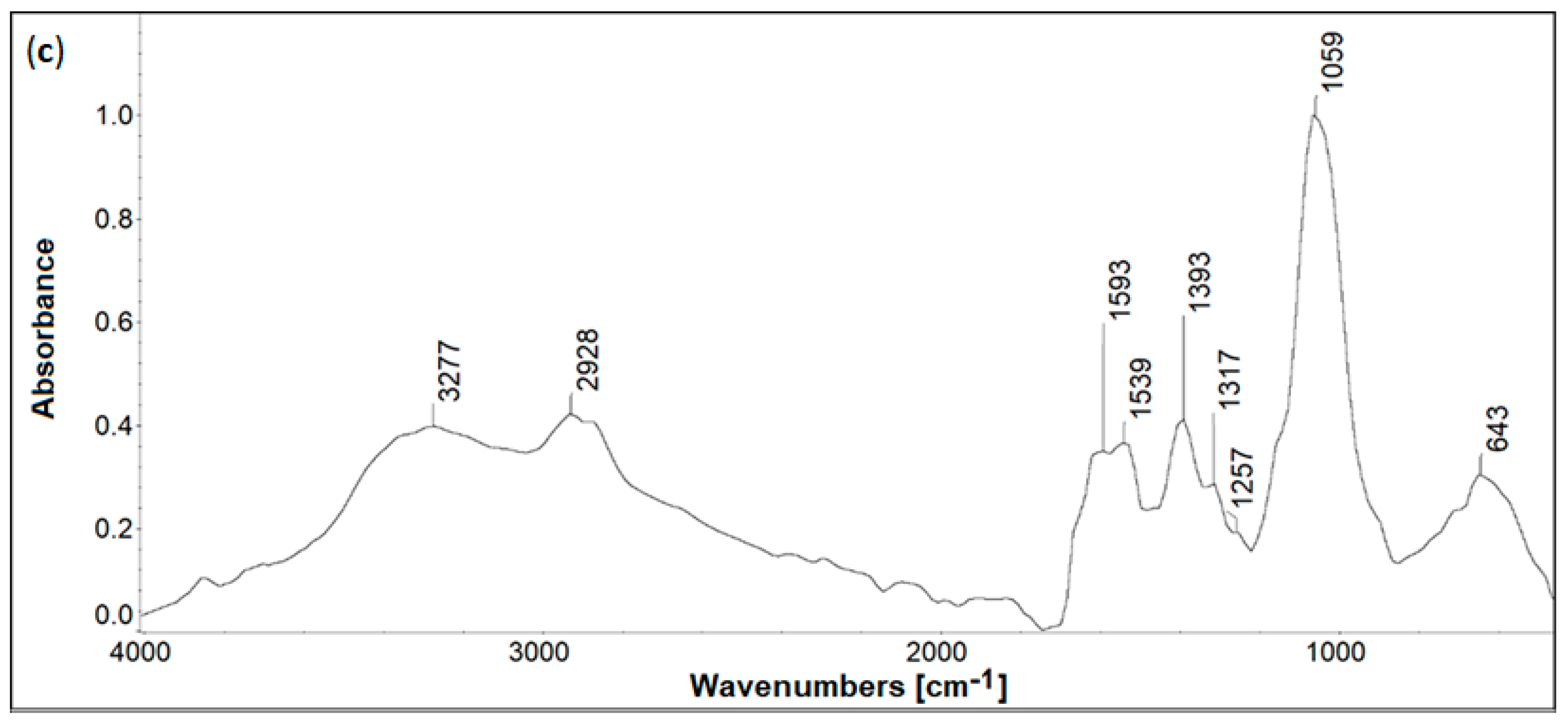
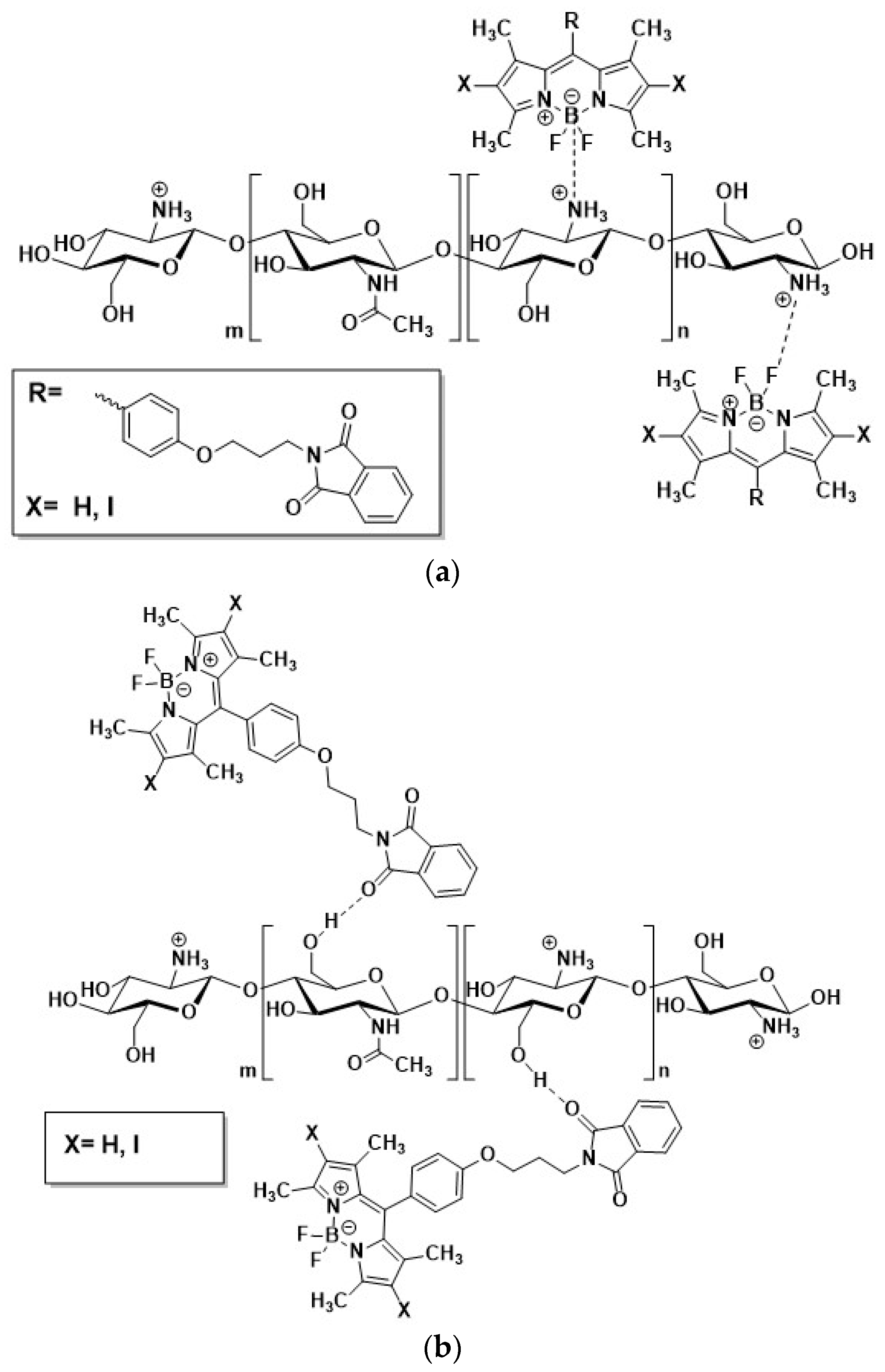


| IR Band Position, cm−1 | Assignment [29,30,31,32] | |
|---|---|---|
| I | H | |
| 3044 | 3083 | C-H aromatic and heterocyclic, stretching |
| 2928 | 2947 | C-H aliphatic, stretching |
| 1690 | 1710 | C=O (amide I) stretching |
| 1507 | 1489 | N-H (amide II), C=C aromatic,. stretching |
| 1380 | 1387 | C-H deformation, B-N stretching |
| 1163 | 1177 | C-O stretching |
| 1095 | 1054 | C-O stretching |
| 990 | 983 | C-C stretching |
| - | 822 | C-H deformation |
| 718 | 712 | C-H, B-N deformation |
| 523 | - | C-I stretching |
| IR Band Position, cm−1 | Assignment [33,34,35] | ||
|---|---|---|---|
| CS | CS-I | CS-H | |
| 3367 | 3272 | 3277 | O-H/N-H stretching |
| 2924, 2879 | 2930, 2885 | 2928, 2875 | C-H stretching (symmetric and asymmetric) |
| 1650 | 1645 (arm of amide II band) | 1663 (arm of amide II band) | C=O (amide I) stretching |
| 1563 | 1566 | 1539 | N-H (amide II) stretching |
| 1409 | 1397 | 1393 | C-H/C-N deformation |
| 1080 | 1057 | 1059 | C-O-C deformation |
| 899 | barely visible | barely visible | C-H bending saccharide ring, out of the plane |
| 655 | 632 | 643 | C-H deformation |
| - | 578 | - | C-I stretching |
| Sample | λabs, nm | λem, nm | Δλ, nm | ΦF, % |
|---|---|---|---|---|
| I solution | 529 | 553 | 24 | 2.60 |
| CS-I solution | 545 | 571 | 26 | 1.95 |
| CS-I film | 545 | 603 | 58 | 0.99 |
| H solution | 498 | 513 | 15 | 19.4 |
| CS-H solution | 522 | 546/570 | 24/48 | 2.97 |
| CS-H film | 522 | 570/602 | 48/80 | 2.45 |
| Sample | Contact Angle (θ,°) | Surface Free Energy (mJ/m2) | |||
|---|---|---|---|---|---|
| Test Liquid | |||||
| Glycerin | Diiodomethane | SFE | SFE(D) | SFE(P) | |
| CS | 82.0 | 56.0 | 30.70 | 27.46 | 3.23 |
| CS-I | 72.9 | 61.1 | 30.71 | 22.07 | 8.64 |
| CS-H | 78.4 | 58.8 | 30.00 | 24.76 | 5.24 |
| Sample | Roughness Parameters [nm] for 1 × 1 µm2 and 10 × 10 µm2 | ||
|---|---|---|---|
| Rq | Ra | Rmax | |
| CS | 1.40/4.30 | 1.08/2.94 | 11.7/54.0 |
| CS-I | 1.66/1.98 | 1.31/1.51 | 15.1/41.8 |
| CS-H | 3.43/26.1 | 2.56/13.2 | 26.7/477 |
| Sample | Breaking Stress, MPa | Ultimate Elongation, % | Young’s Modulus, MPa |
|---|---|---|---|
| CS | 28.6 ± 0.8 | 2.9 ± 0.3 | 1169 ± 137 |
| CS-I | 22.5 ± 4.8 | 1.8 ± 0.2 | 423 ± 53 |
| CS-H | 8.6 ± 5.0 | 2.8 ± 0.8 | 767 ± 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolarkiewicz-Wyczachowski, A.; Kaczmarek, H.; Piskorz, J.; Nowak, P.; Ziegler-Borowska, M. Chitosan Composites Containing Boron-Dipyrromethene Derivatives for Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 1770. https://doi.org/10.3390/ijms24021770
Smolarkiewicz-Wyczachowski A, Kaczmarek H, Piskorz J, Nowak P, Ziegler-Borowska M. Chitosan Composites Containing Boron-Dipyrromethene Derivatives for Biomedical Applications. International Journal of Molecular Sciences. 2023; 24(2):1770. https://doi.org/10.3390/ijms24021770
Chicago/Turabian StyleSmolarkiewicz-Wyczachowski, Aleksander, Halina Kaczmarek, Jaroslaw Piskorz, Pawel Nowak, and Marta Ziegler-Borowska. 2023. "Chitosan Composites Containing Boron-Dipyrromethene Derivatives for Biomedical Applications" International Journal of Molecular Sciences 24, no. 2: 1770. https://doi.org/10.3390/ijms24021770
APA StyleSmolarkiewicz-Wyczachowski, A., Kaczmarek, H., Piskorz, J., Nowak, P., & Ziegler-Borowska, M. (2023). Chitosan Composites Containing Boron-Dipyrromethene Derivatives for Biomedical Applications. International Journal of Molecular Sciences, 24(2), 1770. https://doi.org/10.3390/ijms24021770









