The Bph45 Gene Confers Resistance against Brown Planthopper in Rice by Reducing the Production of Limonene
Abstract
:1. Introduction
2. Results
2.1. Recurrent Parent Genome Recovery Analysis of TNG71-Bph45
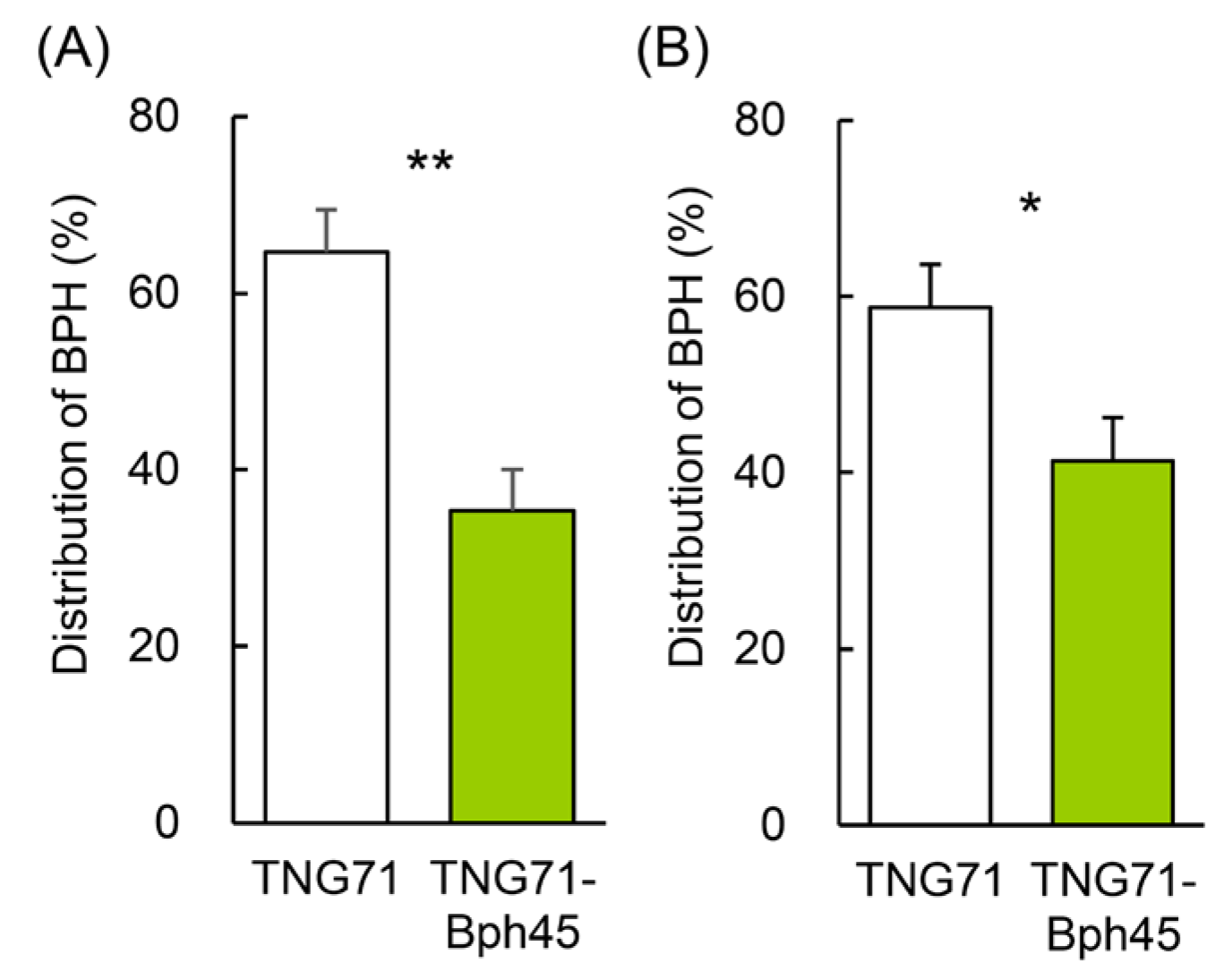
2.2. Host-Choice Settling Preference of BPH
2.3. Y-Maze Olfactometer Examination of Settling Preference of BPH
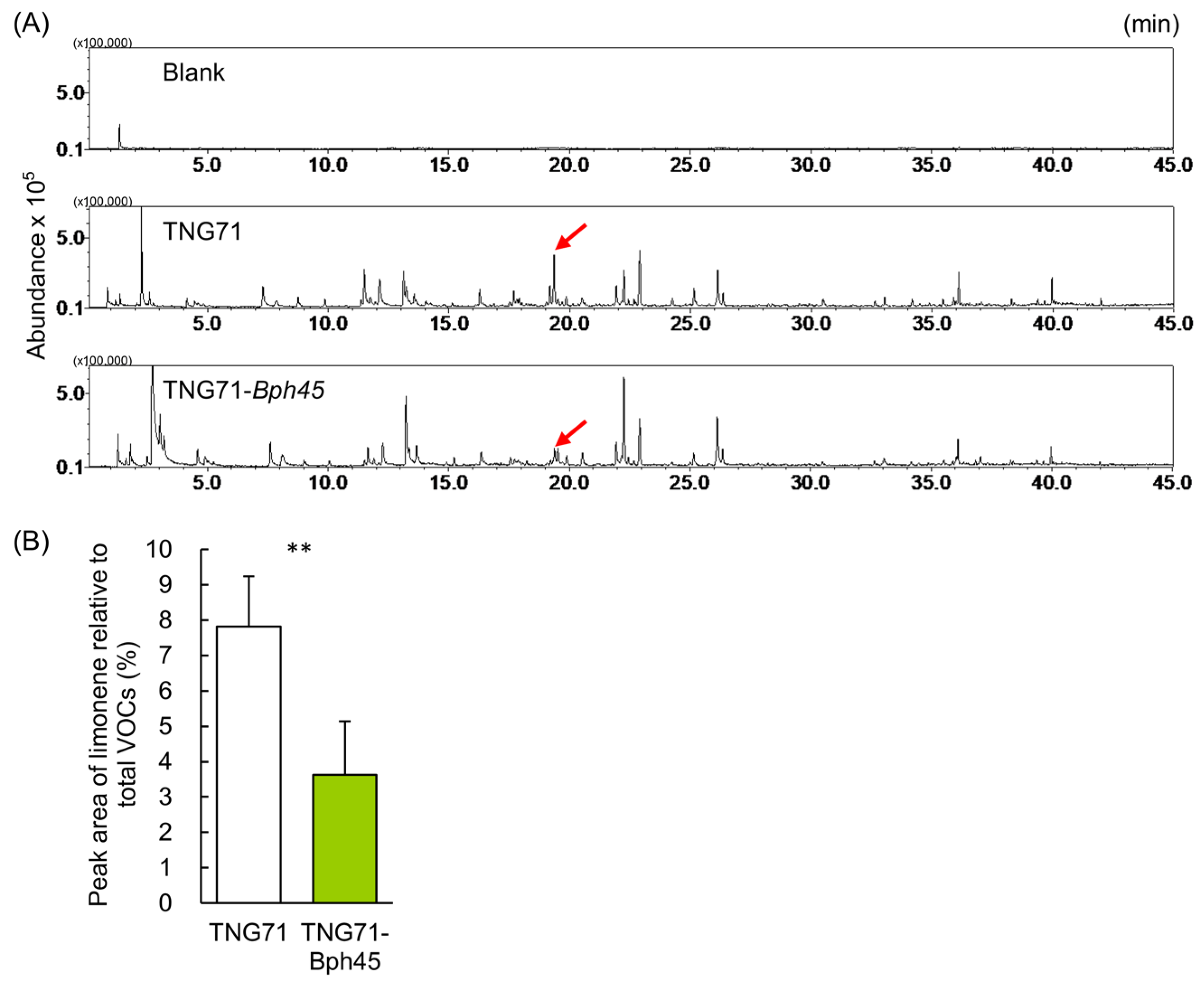
2.4. GC-MS Characterization of the Volatile Organic Compounds
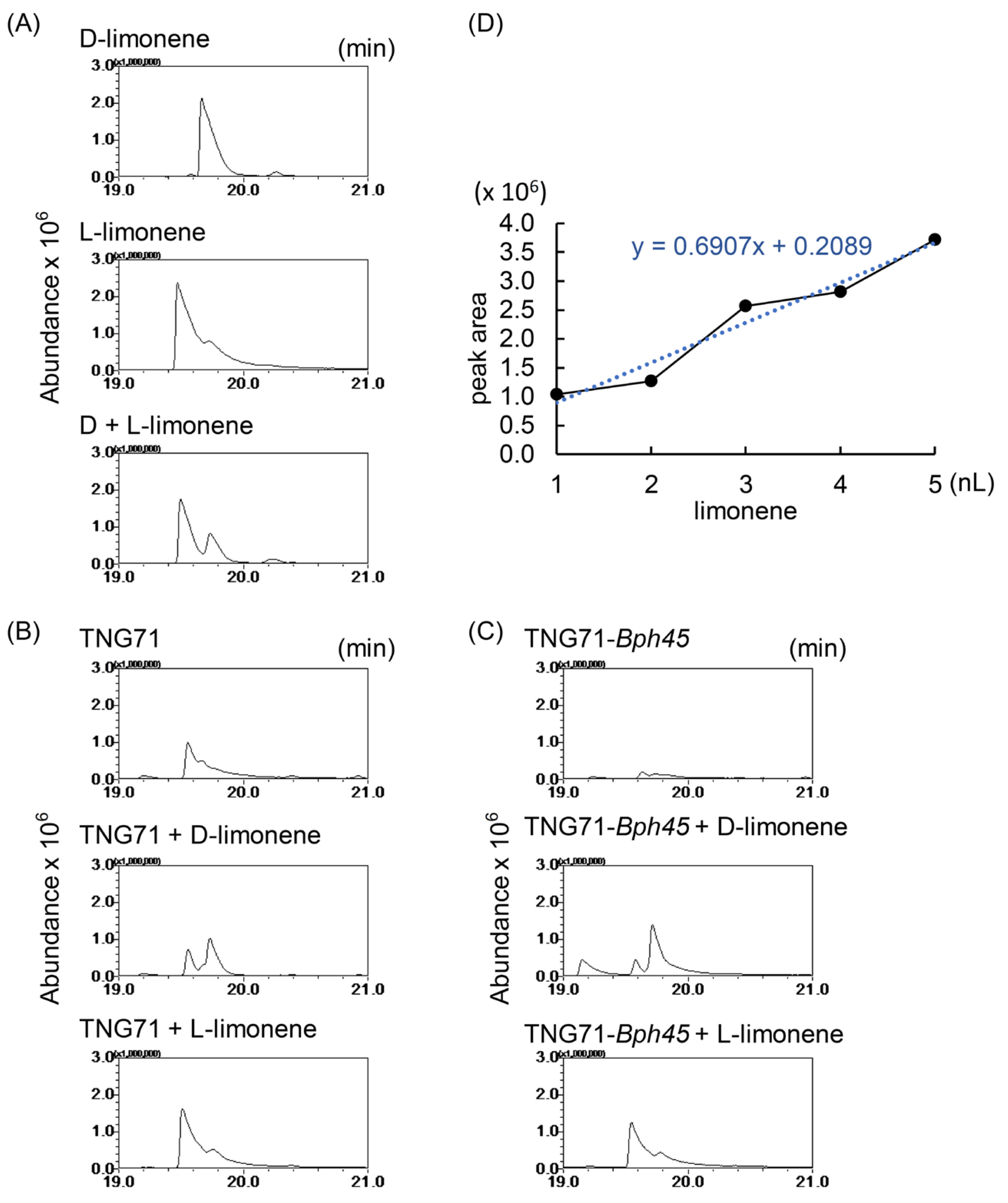
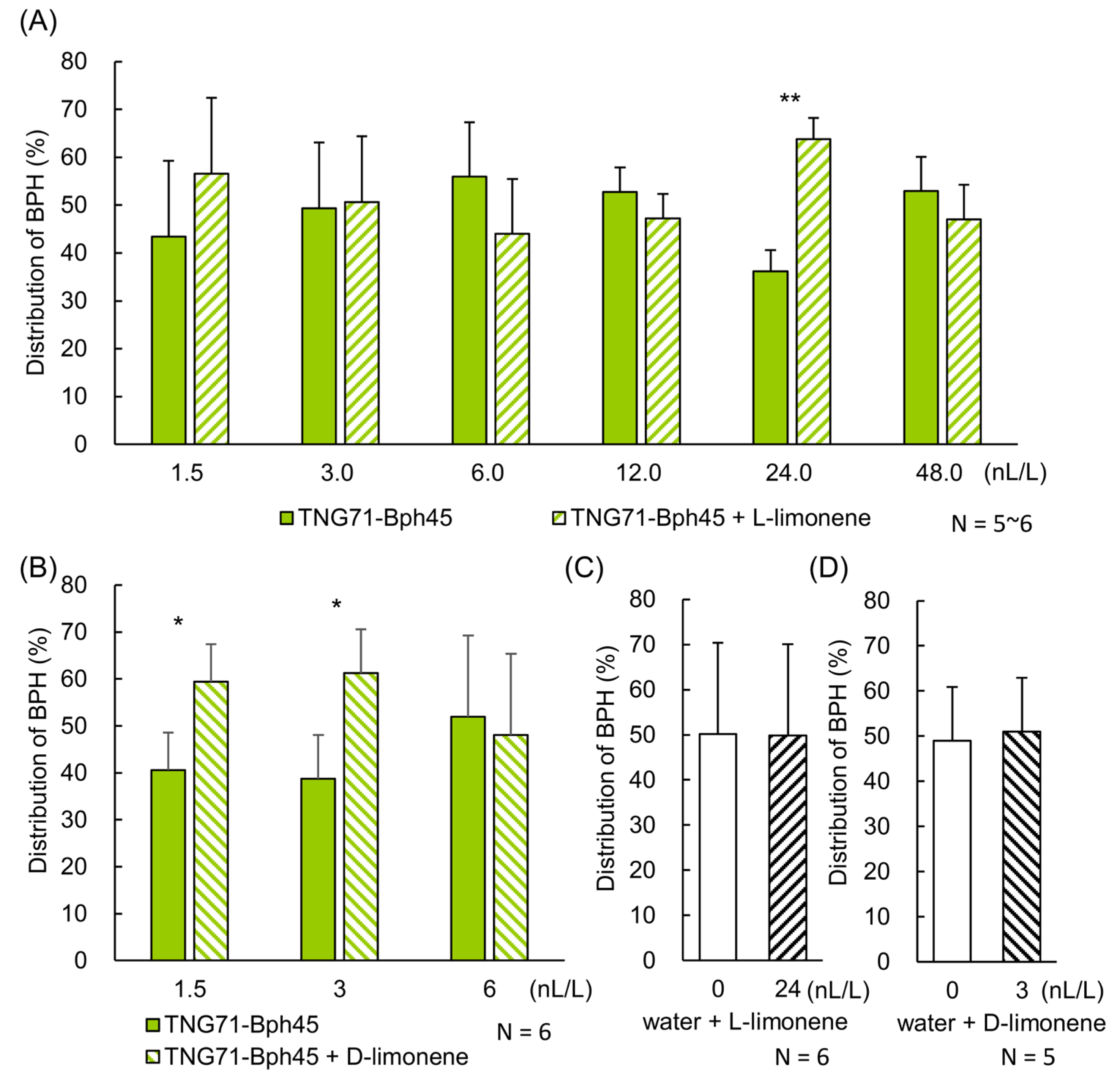
2.5. Confirmation of the Attractiveness of Limonene to BPH
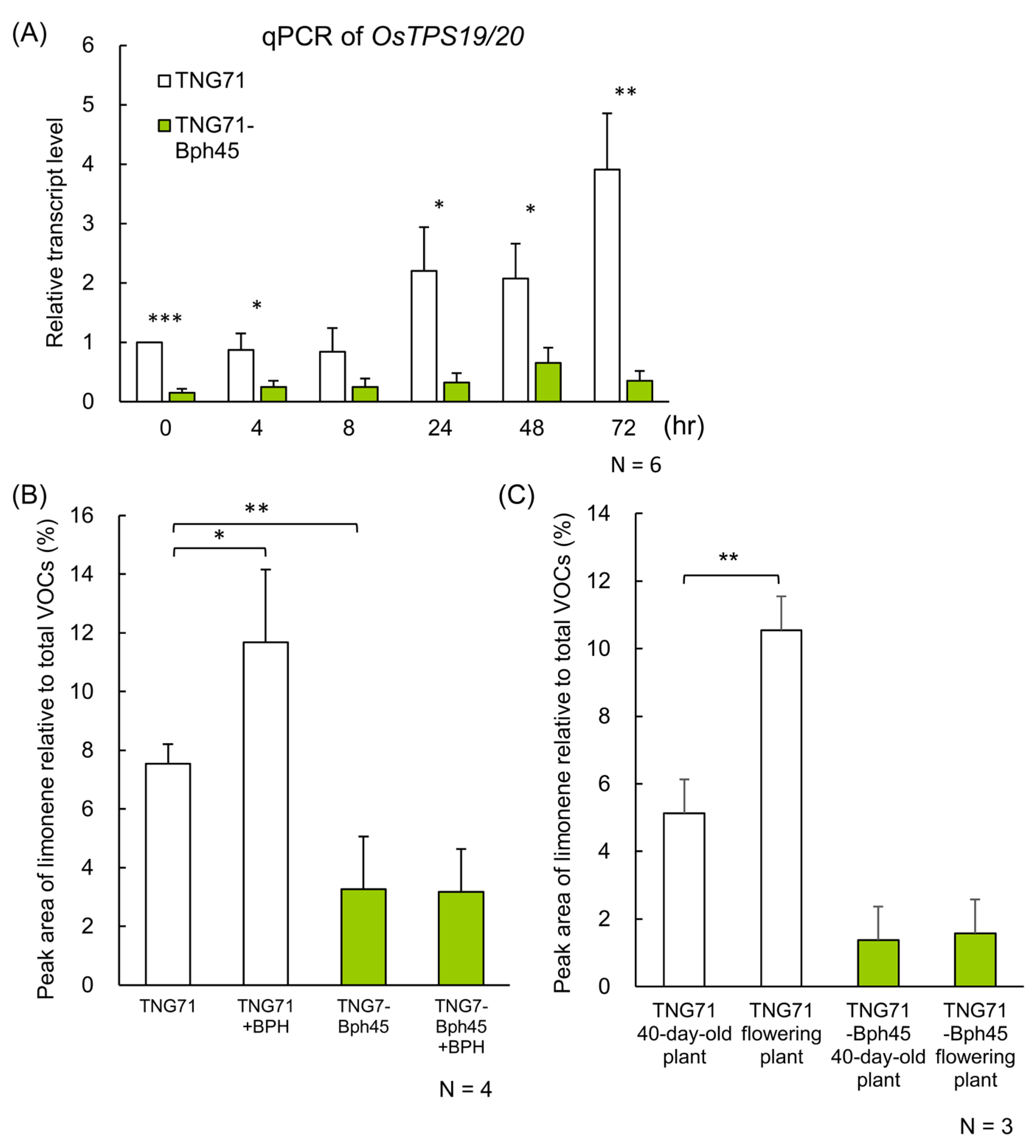
2.6. Transcript Abundance of Limonene Synthase in Rice Plants
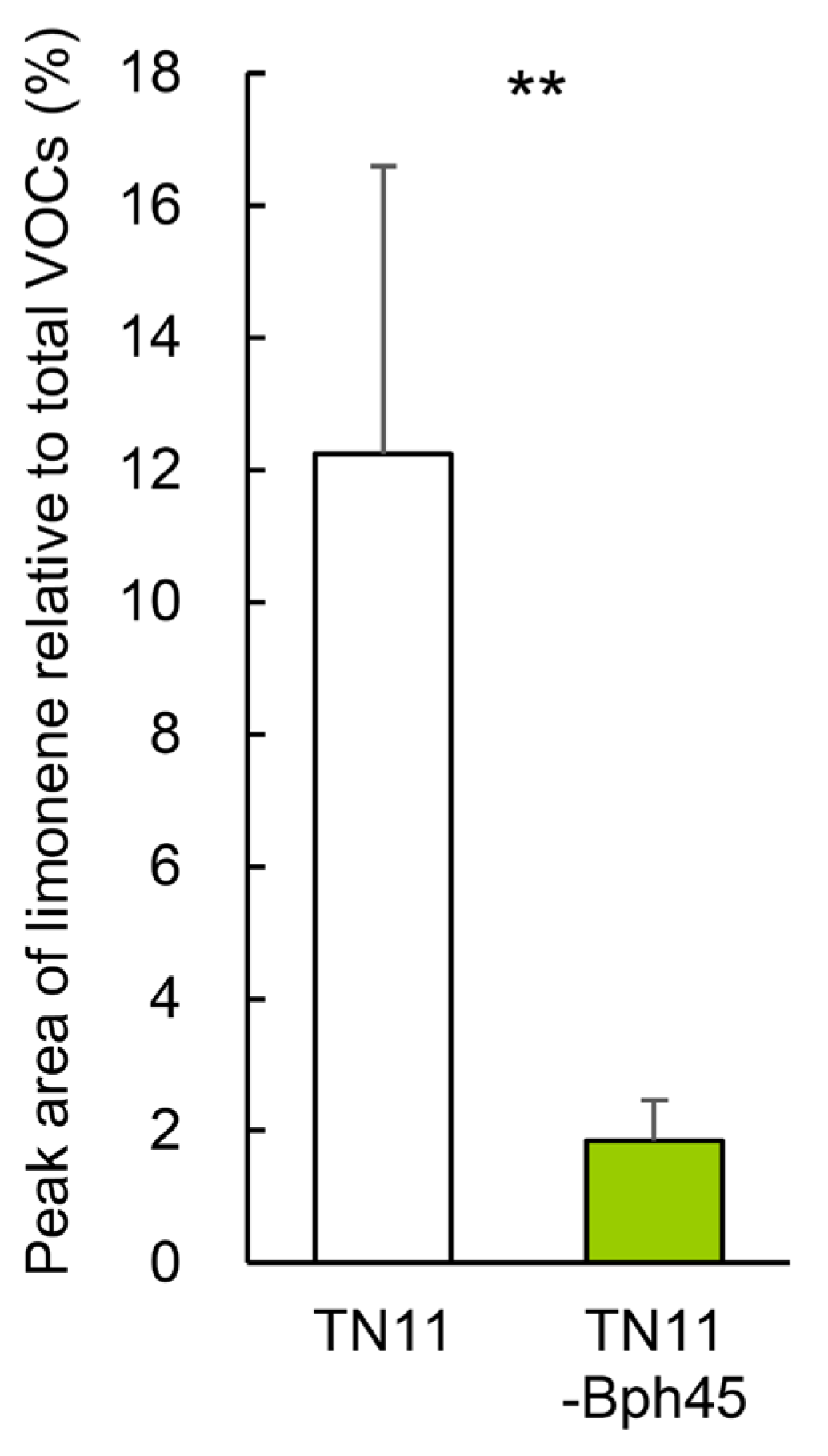
2.7. Introgression of the Bph45 Allele Affects the Limonene Content in Rice
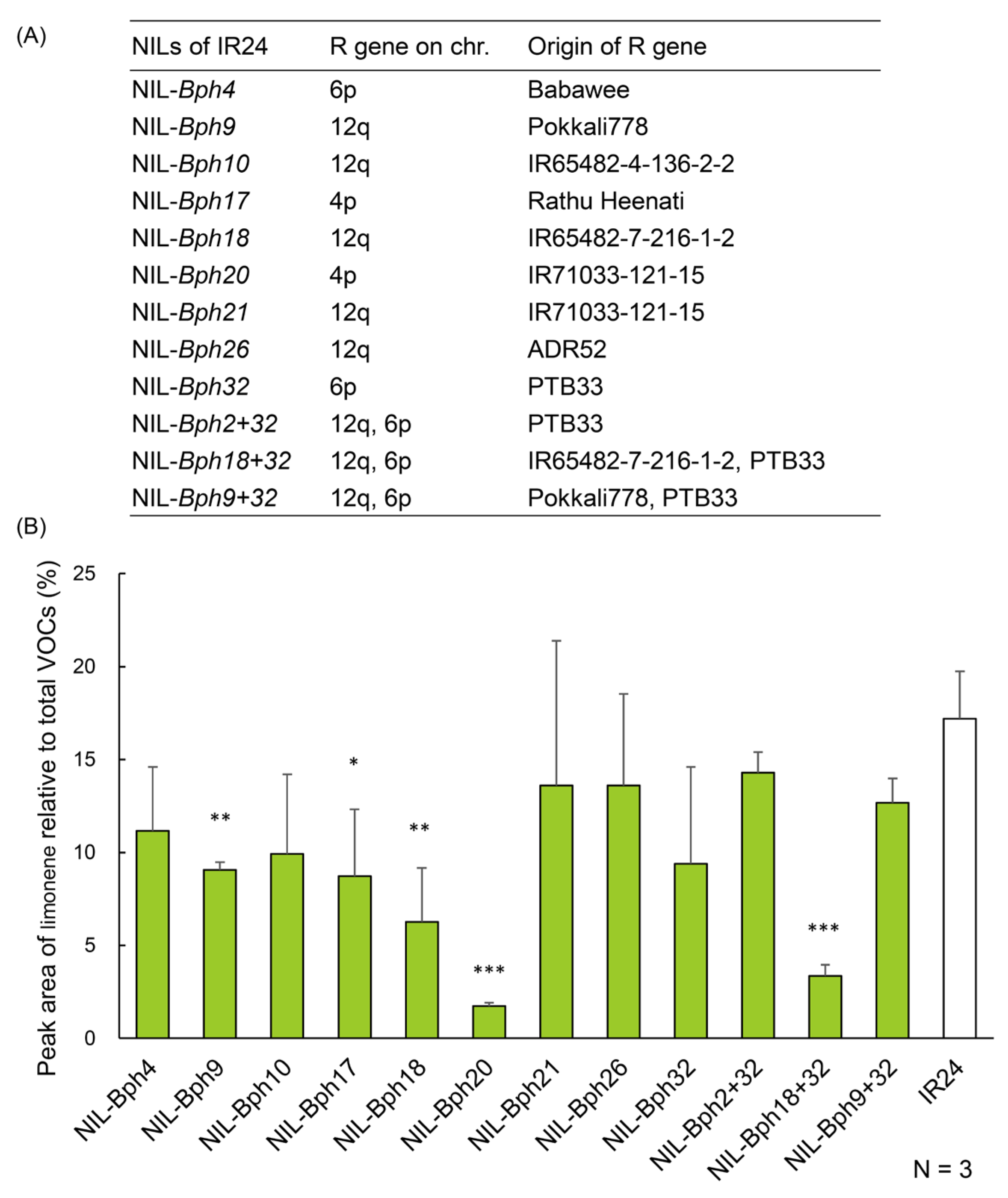
2.8. The Amount of Limonene Can Be Conferred by Various BPH-Resistance Genes
3. Discussion
3.1. Investigation of the Mechanism Underlying Antixenosis
3.2. Reduction of Limonene Emission and Attraction of BPH Simultaneously by the Bph45 Gene
3.3. Attraction of BPH by Limonene at Ppb Levels Emitted by Rice
3.4. Attraction of BPH by Both L- and D-Limonene
3.5. Controversial Functions of Limonene, Depending on the Forms and Concentrations Applied
3.6. Implicated Applications in Pyramid Breeding against BPH
3.7. Implicated Applications in Integrated Pest Management against BPH
3.8. Implicated BPH Resistance Mechanism by Bph45
4. Materials and Methods
4.1. Plant Materials
4.2. DNA Preparation and Marker Analysis
4.3. BPH Maintenance
4.4. Chemicals
4.5. Host-Choice Test of BPH
4.6. Y-Maze Olfactometer Examination of BPH Settling Preference
4.7. SPME–GC–MS Analysis of Rice Volatile Organic Compounds
4.8. Chiral SPME–GC–MS Analysis of Rice Volatile Organic Compounds
4.9. RNA Isolation and RT-qPCR
4.10. Data analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sani Haliru, B.; Rafii, M.Y.; Mazlan, N.; Ramlee, S.I.; Muhammad, I.; Silas Akos, I.; Halidu, J.; Swaray, S.; Rini Bashir, Y. Recent Strategies for Detection and Improvement of Brown Planthopper Resistance Genes in Rice: A Review. Plants 2020, 9, 1202. [Google Scholar] [CrossRef] [PubMed]
- Muduli, L.; Pradhan, S.K.; Mishra, A.; Bastia, D.N.; Samal, K.C.; Agrawal, P.K.; Dash, M. Understanding Brown Planthopper Resistance in Rice: Genetics, Biochemical and Molecular Breeding Approaches. Rice Sci. 2021, 28, 532–546. [Google Scholar] [CrossRef]
- Sarao, P.S.; Sahi, G.K.; Neelam, K.; Mangat, G.S.; Patra, B.C.; Singh, K. Donors for Resistance to Brown Planthopper Nilaparvata lugens (Stål) from Wild Rice Species. Rice Sci. 2016, 23, 219–224. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Sarao, P.S.; Bhatia, D.; Neelam, K.; Kaur, A.; Mangat, G.S.; Brar, D.S.; Singh, K. High-resolution genetic mapping of a novel brown planthopper resistance locus, Bph34 in Oryza sativa L. X Oryza nivara (Sharma & Shastry) derived interspecific F2 population. Theor. Appl. Genet. 2018, 131, 1163–1171. [Google Scholar] [CrossRef]
- Stein, J.C.; Yu, Y.; Copetti, D.; Zwickl, D.J.; Zhang, L.; Zhang, C.; Chougule, K.; Gao, D.; Iwata, A.; Goicoechea, J.L.; et al. Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover and innovation across the genus Oryza. Nat. Genet. 2018, 50, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Li, C.; Chen, J.; Huang, S.; Ku, H. Mapping of Brown Planthopper Resistance Gene Introgressed from Oryza nivara into Cultivated Rice, O. sativa. In Proceedings of the International Symposium on Rice Research in the Era of Global Warming, Taichung, Taiwan, 6–7 October 2009; pp. 56–65. Available online: https://scholars.tari.gov.tw/handle/123456789/16458 (accessed on 13 December 2022).
- Kaur, P.; Neelam, K.; Sarao, P.S.; Babbar, A.; Kumar, K.; Vikal, Y.; Khanna, R.; Kaur, R.; Mangat, G.S.; Singh, K. Molecular mapping and transfer of a novel brown planthopper resistance gene bph42 from Oryza rufipogon (Griff.) To cultivated rice (Oryza sativa L.). Mol. Biol. Rep. 2022, 49, 8597–8606. [Google Scholar] [CrossRef]
- Guo, J.; Xu, C.; Wu, D.; Zhao, Y.; Qiu, Y.; Wang, X.; Ouyang, Y.; Cai, B.; Liu, X.; Jing, S.; et al. Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat. Genet. 2018, 50, 297–306. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Liu, Y.; Jiang, L.; Wu, H.; Kang, H.; Liu, S.; Chen, L.; Liu, X.; Cheng, X.; et al. High-resolution mapping of brown planthopper (BPH) resistance gene Bph27(t) in rice (Oryza sativa L.). Mol. Breed. 2012, 31, 549–557. [Google Scholar] [CrossRef]
- Huang, D.; Qiu, Y.; Zhang, Y.; Huang, F.; Meng, J.; Wei, S.; Li, R.; Chen, B. Fine mapping and characterization of BPH27, a brown planthopper resistance gene from wild rice (Oryza rufipogon Griff.). Theor. Appl. Genet. 2013, 126, 219–229. [Google Scholar] [CrossRef]
- Yang, M.; Lin, J.; Cheng, L.; Zhou, H.; Chen, S.; Liu, F.; Li, R.; Qiu, Y. Identification of a novel planthopper resistance gene from wild rice (Oryza rufipogon Griff.). Crop J. 2020, 8, 1057–1070. [Google Scholar] [CrossRef]
- Sogawa, K.; Pathak, M.D. Mechanisms of Brown Planthopper Resistance in Mudgo Variety of Rice (Hemiptera: Delphacidae). Appl. Entomol. Zool. 1970, 5, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.N.; Cohen, M.B. Detection and analysis of QTLs for resistance to the brown planthopper, Nilaparvata lugens, in a doubled-haploid rice population. Theor. Appl. Genet. 1998, 97, 1370–1379. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, J.; Jing, S.; Zhu, L.; He, G. High-resolution mapping of the brown planthopper resistance gene Bph6 in rice and characterizing its resistance in the 9311 and Nipponbare near isogenic backgrounds. Theor. Appl. Genet. 2010, 121, 1601–1611. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; He, J.; Liu, Y.; Jiang, L.; Liu, L.; Wang, C.; Cheng, X.; Wan, J. Fine mapping of brown planthopper (Nilaparvata lugens Stål) resistance gene Bph28(t) in rice (Oryza sativa L.). Mol. Breed. 2014, 33, 909–918. [Google Scholar] [CrossRef]
- Koch, K.G.; Chapman, K.; Louis, J.; Heng-Moss, T.; Sarath, G. Plant Tolerance: A Unique Approach to Control Hemipteran Pests. Front. Plant Sci. 2016, 7, 1363. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Wang, H.; Nie, L.; Tan, D.; Zhou, C.; Zhang, Q.; Li, Y.; Du, B.; Guo, J.; Huang, J.; et al. Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol. Plant 2021, 14, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, Y.; Wu, D.; Rao, W.; Guo, J.; Ma, Y.; Wang, Z.; Shangguan, X.; Wang, H.; Xu, C.; et al. The Coiled-Coil and Nucleotide Binding Domains of Brown Planthopper Resistance 14 Function in Signaling and Resistance against Planthopper in Rice. Plant Cell 2017, 29, 3157–3185. [Google Scholar] [CrossRef] [Green Version]
- Kamolsukyunyong, W.; Sukhaket, W.; Ruanjaichon, V.; Toojinda, T.; Vanavichit, A. Single-feature polymorphism mapping of isogenic rice lines identifies the influence of terpene synthase on brown planthopper feeding preferences. Rice 2013, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.W.; Lee, S.; Chung, M.S.; Jeong, Y.S.; Chung, B.Y. Rice terpene synthase 20 (OsTPS20) plays an important role in producing terpene volatiles in response to abiotic stresses. Protoplasma 2015, 252, 997–1007. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Yuan, J.S.; Kollner, T.G.; Chen, Y.; Guo, Y.; Zhuang, X.; Chen, X.; Zhang, Y.J.; Fu, J.; et al. The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol. J. 2018, 16, 1778–1787. [Google Scholar] [CrossRef]
- Li, Z.; Xue, Y.; Zhou, H.; Li, Y.; Usman, B.; Jiao, X.; Wang, X.; Liu, F.; Qin, B.; Li, R.; et al. High-resolution mapping and breeding application of a novel brown planthopper resistance gene derived from wild rice (Oryza rufipogon Griff). Rice 2019, 12, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarao, P.S.; Bentur, J.S. Antixenosis and Tolerance of Rice Genotypes Against Brown Planthopper. Rice Sci. 2016, 23, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, H.; Chen, H.; Liu, Y.; He, J.; Kang, H.; Sun, Z.; Pan, G.; Wang, Q.; Hu, J.; et al. A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat. Biotechnol. 2015, 33, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Guo, J.; Jing, S.; Zhu, L.; He, G. Development and characterization of japonica rice lines carrying the brown planthopper-resistance genes BPH12 and BPH6. Theor. Appl. Genet. 2012, 124, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Q.; Erb, M.; Turlings, T.C.; Ge, L.; Hu, L.; Li, J.; Han, X.; Zhang, T.; Lu, J.; et al. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef]
- Lu, J.; Li, J.; Ju, H.; Liu, X.; Erb, M.; Wang, X.; Lou, Y. Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol. Plant 2014, 7, 1670–1682. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.W.; Chung, M.S.; Kang, M.; Chung, B.Y.; Lee, S. Direct suppression of a rice bacterial blight (Xanthomonas oryzae pv. oryzae) by monoterpene (S)-limonene. Protoplasma 2016, 253, 683–690. [Google Scholar] [CrossRef]
- Maruyama, T.; Ito, M.; Kiuchi, F.; Honda, G. Molecular cloning, functional expression and characterization of d-limonene synthase from Schizonepeta tenuifolia. Biol. Pharm. Bull. 2001, 24, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Rajaonarivony, J.I.M.; Gershenzon, J.; Croteau, R. Characterization and mechanism of (4S)-limonene synthase, A monoterpene cyclase from the glandular trichomes of peppermint (Mentha x piperita). Arch. Biochem. Biophys. 1992, 296, 49–57. [Google Scholar] [CrossRef]
- Environmental Protection Agency, U.S. R.E.D. FACTS Limonene; EPA-738-F-94-030; Enviromental Protection Agency: Washington, DC, USA, 1994. [Google Scholar]
- Karr, L.; Coats, J. Insecticidal Properties of d-Limonene. J. Pestic. Sci. 1988, 13, 287–290. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Liu, S.; Qiu, L.; Li, Y. Three odorant-binding proteins are involved in the behavioral response of Sogatella furcifera to rice plant volatiles. PeerJ 2019, 7, e6576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, B.; Liang, Y.; Liu, S.; Zhang, L.; Tang, G.; Ma, T.; Yao, Y. Behavioral responses of Aphis citricola (Hemiptera: Aphididae) and its natural enemy Harmonia axyridis (Coleoptera: Coccinellidae) to non-host plant volatiles. Fla. Entomol. 2017, 100, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Chen, R.; Guo, J.; He, G. Current understanding of the genomic, genetic, and molecular control of insect resistance in rice. Mol. Breed. 2020, 40, 24. [Google Scholar] [CrossRef] [Green Version]
- Khetnon, P.; Busarakam, K.; Sukhaket, W.; Niwaspragrit, C.; Kamolsukyeunyong, W.; Kamata, N.; Sanguansub, S. Mechanisms of Trichomes and Terpene Compounds in Indigenous and Commercial Thai Rice Varieties against Brown Planthopper. Insects 2022, 13, 427. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, J.H.; Fan, T.; Wang, C.; Liu, L.; Jin, H.; Foba, C.N.; Wang, M.Q. The control of the brown planthopper by the rice Bph14 gene is affected by nitrogen. Pest Manag. Sci. 2020, 76, 3649–3656. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Portaluri, V.; Woodcock, C.; Pickett, J.A.; Wang, S.; Zhou, J.J. Chemical Identity and Functional Characterization of Semiochemicals That Promote the Interactions between Rice Plant and Rice Major Pest Nilaparvata lugens. J. Agric. Food Chem. 2021, 69, 4635–4644. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, J.; Hamada, C.; Cai, W.; Khan, M.; Zou, Y.; Hua, H. Identification of attractants from plant essential oils for Cyrtorhinus lividipennis, an important predator of rice planthoppers. J. Pest Sci. 2018, 92, 769–780. [Google Scholar] [CrossRef]
- Lai, M.; Li, C.; Tseng, S.; Huang, H.; Chern, C.; Kuo, Y. Development of Aromatic Rice Variety Tainung 71 (Yihchuan aromatic rice). J. Agric. Res. China 2001, 50, 1–12. [Google Scholar] [CrossRef]
- Lin, G.C. Development of a New Japonic Rice Variety “Tainan No. 11”. Res. Bull. Tainan Dist. Agric. Improv. Stn. 2005, 45, 1–25. Available online: https://book.tndais.gov.tw/RBulletin/paper45-1.htm (accessed on 13 December 2022).
- Jena, K.K.; Hechanova, S.L.; Verdeprado, H.; Prahalada, G.D.; Kim, S.R. Development of 25 near-isogenic lines (NILs) with ten BPH resistance genes in rice (Oryza sativa L.): Production, resistance spectrum, and molecular analysis. Theor. Appl. Genet. 2017, 130, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-H.; Wu, H.-P.; Wang, C.-S.; Tseng, H.-Y.; Hwu, K.-K. Genome-wide InDel marker system for application in rice breeding and mapping studies. Euphytica 2013, 192, 131–143. [Google Scholar] [CrossRef]
- Maruyama, T.; Saeki, D.; Ito, M.; Honda, G. Molecular cloning, functional expression and characterization of d-limonene synthase from Agastache rugosa. Biol. Pharm. Bull. 2002, 25, 661–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-P.; Wu, D.-H.; Huang, S.-H.; Meng, M.; Shih, H.-T.; Lai, M.-H.; Chen, L.-J.; Jena, K.K.; Hechanova, S.L.; Ke, T.-J.; et al. The Bph45 Gene Confers Resistance against Brown Planthopper in Rice by Reducing the Production of Limonene. Int. J. Mol. Sci. 2023, 24, 1798. https://doi.org/10.3390/ijms24021798
Li C-P, Wu D-H, Huang S-H, Meng M, Shih H-T, Lai M-H, Chen L-J, Jena KK, Hechanova SL, Ke T-J, et al. The Bph45 Gene Confers Resistance against Brown Planthopper in Rice by Reducing the Production of Limonene. International Journal of Molecular Sciences. 2023; 24(2):1798. https://doi.org/10.3390/ijms24021798
Chicago/Turabian StyleLi, Charng-Pei, Dong-Hong Wu, Shou-Horng Huang, Menghsiao Meng, Hsien-Tzung Shih, Ming-Hsin Lai, Liang-Jwu Chen, Kshirod K. Jena, Sherry Lou Hechanova, Ting-Jyun Ke, and et al. 2023. "The Bph45 Gene Confers Resistance against Brown Planthopper in Rice by Reducing the Production of Limonene" International Journal of Molecular Sciences 24, no. 2: 1798. https://doi.org/10.3390/ijms24021798






