Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process
Abstract
1. Introduction
2. Results
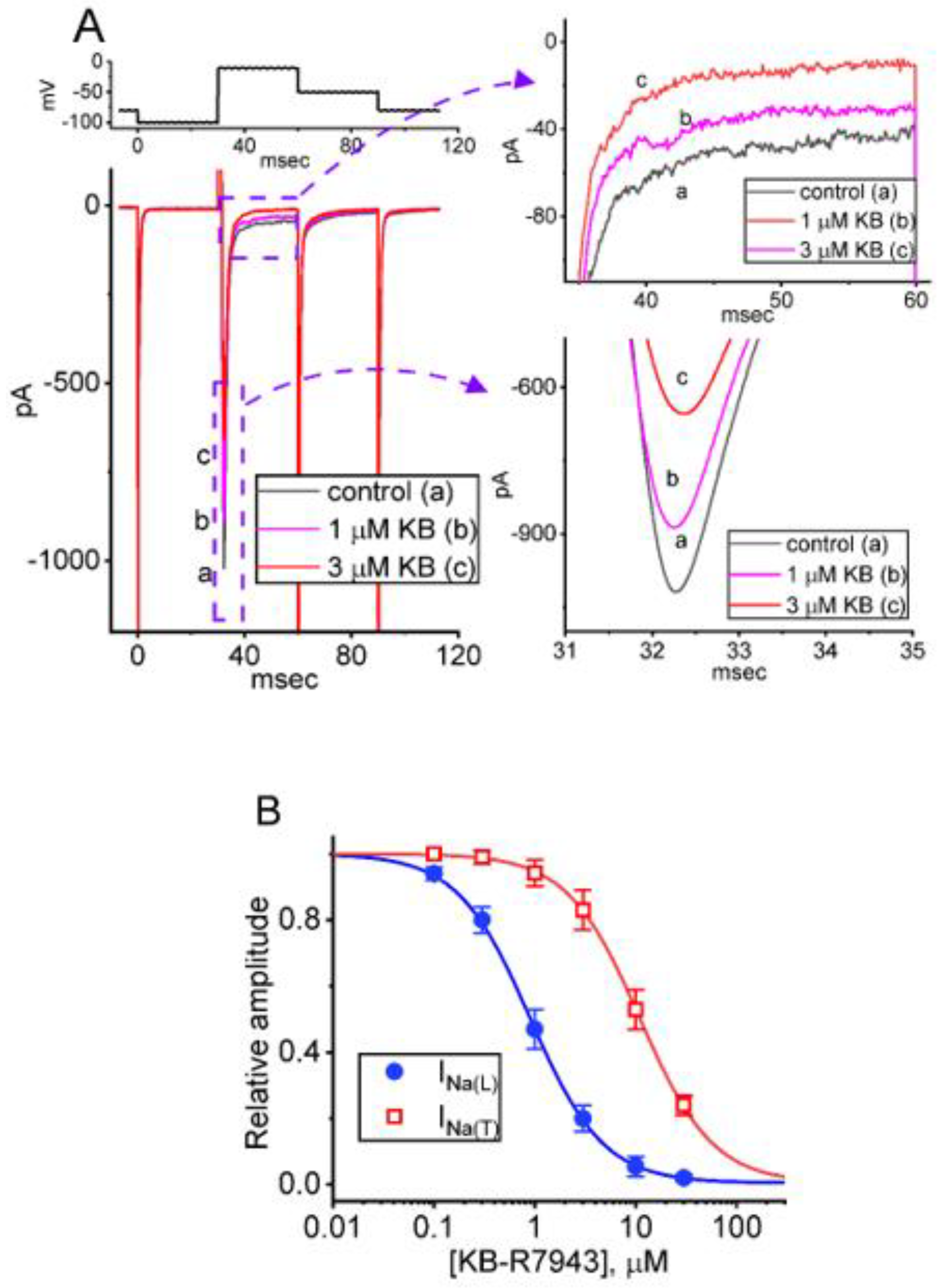
2.1. Suppressive Effect of KB-R7943 on Voltage-Gated Na+ Current (INa) Identified from Pituitary GH3 Cells
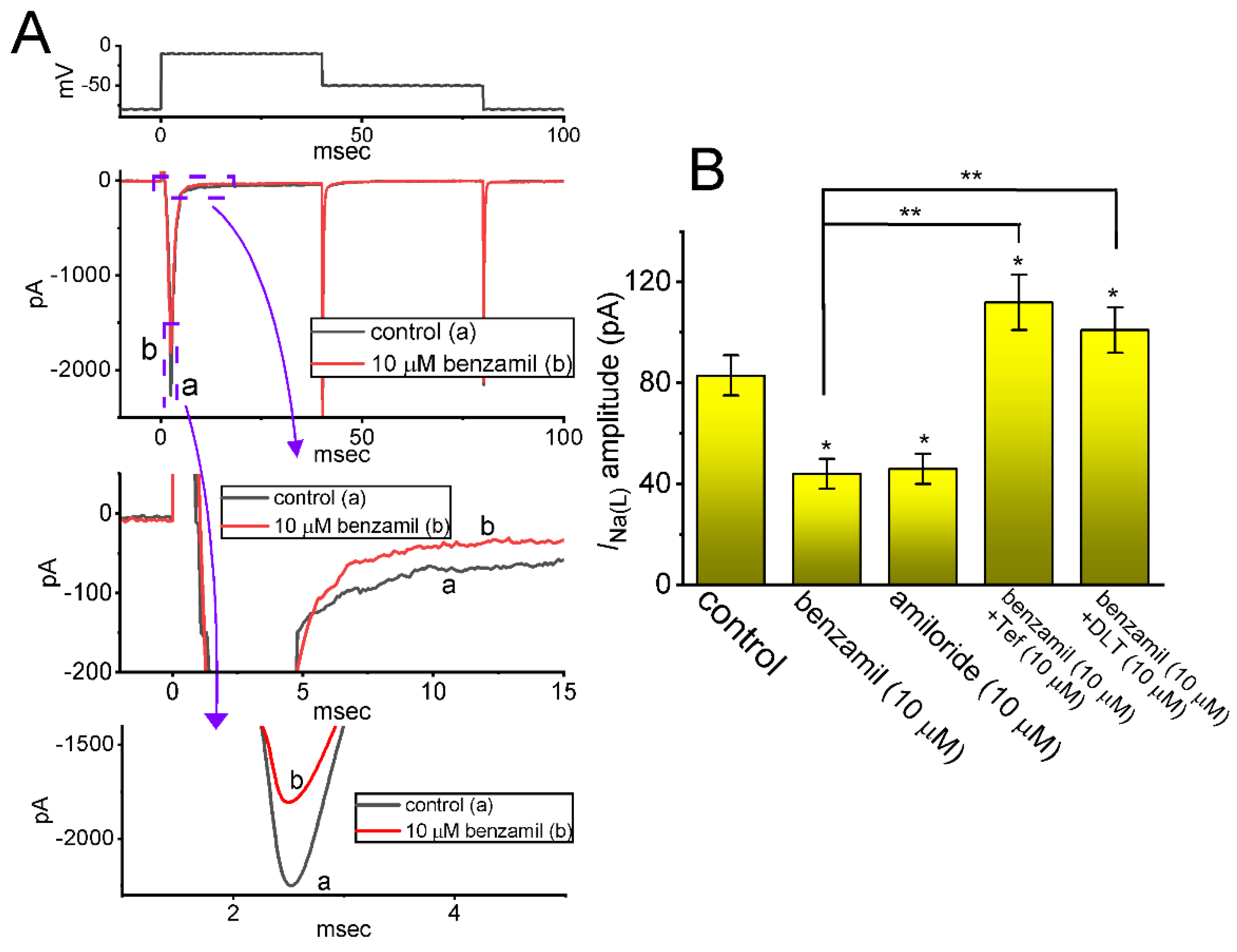
2.2. Comparison among Effects of Benzamil, Amiloride, Benzamil plus Tefluthrin (Tef), and Benzamil plus Deltamethrin (DLT) on INa(L) Amplitude Measured from GH3 Cells
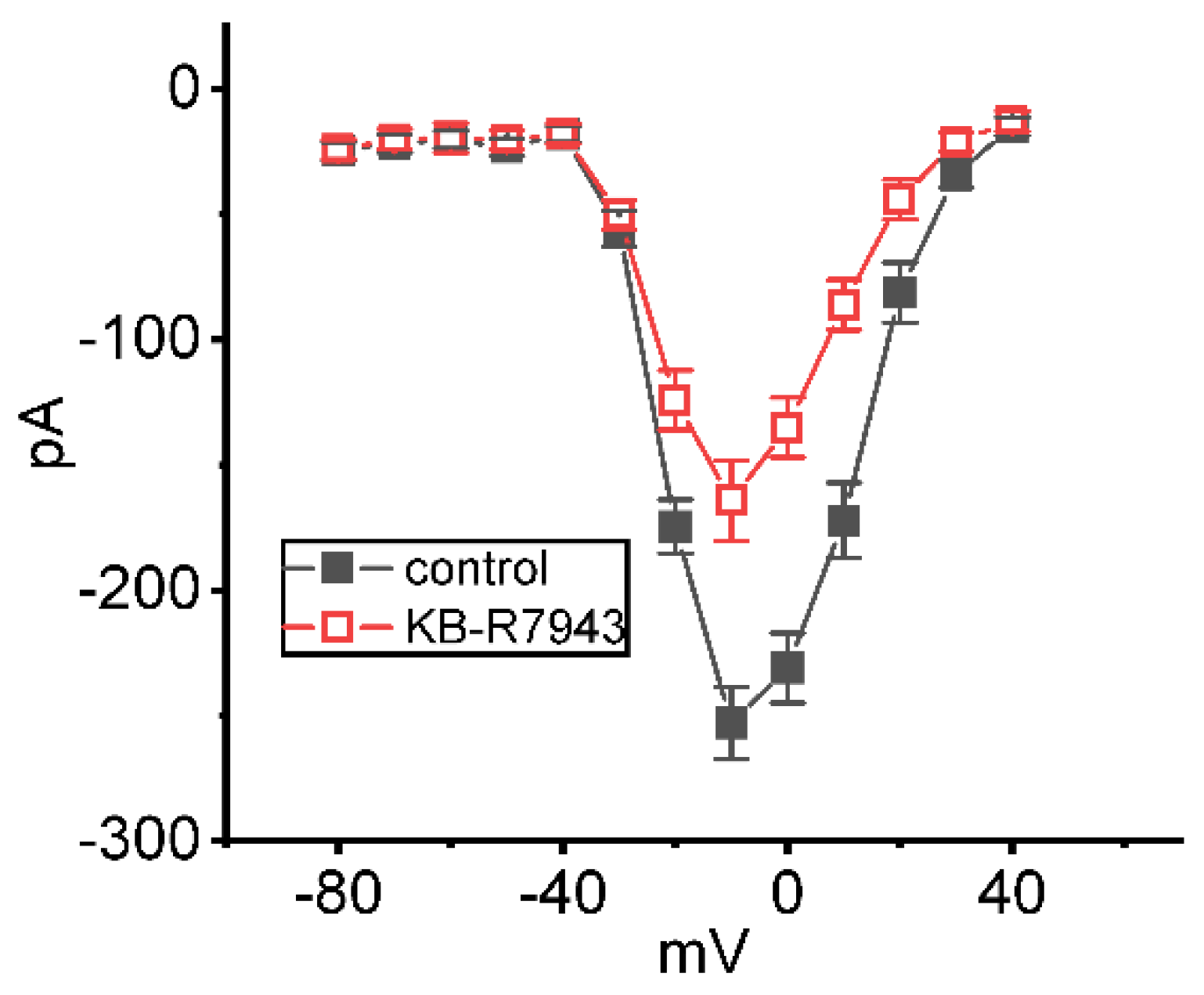
2.3. Inhibitory Effect of KB-R7943 on Average Steady-State Current Versus Voltage (I-V) Relationship of INa(T)
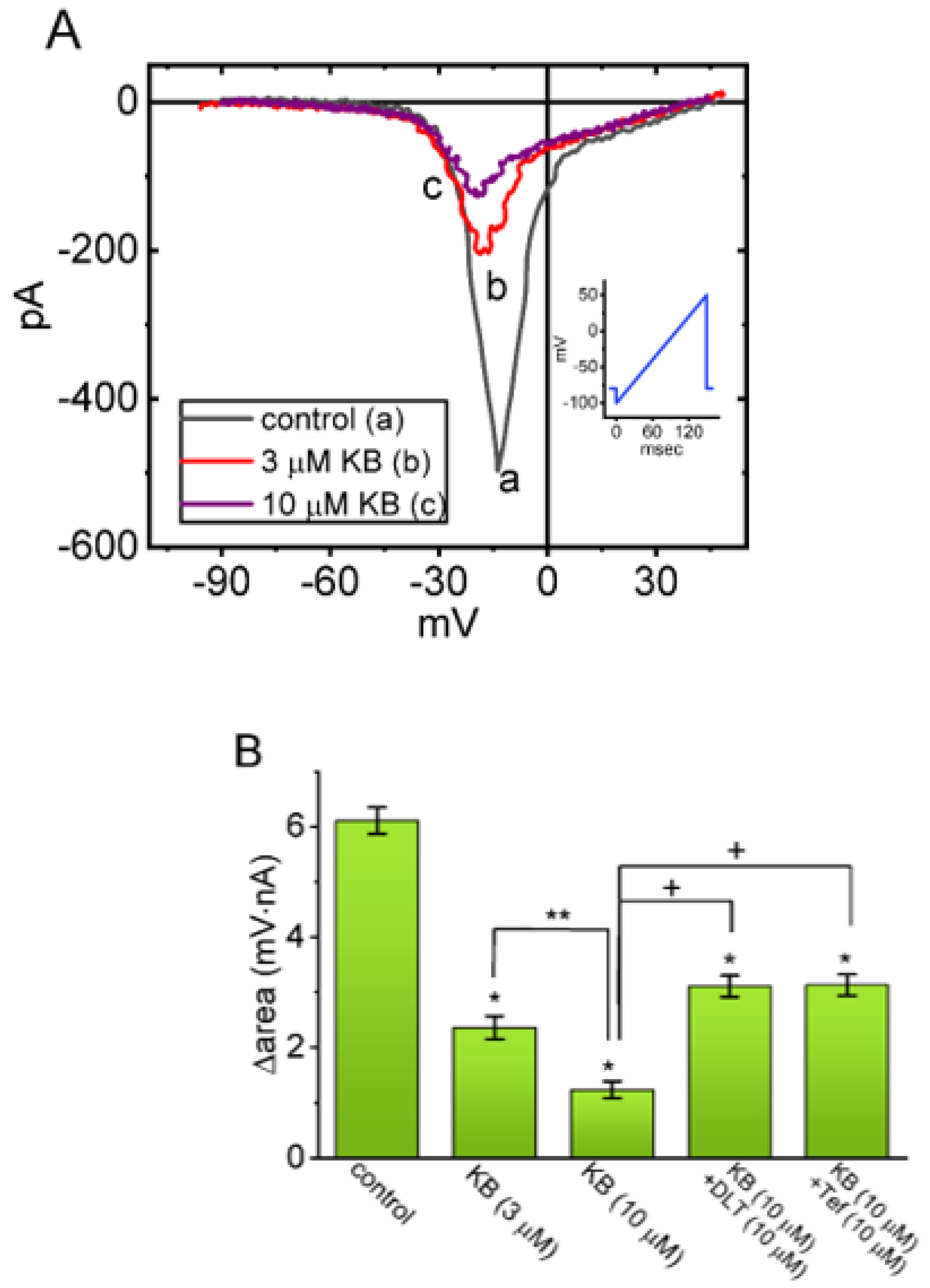
2.4. Suppressive Effect of KB-R7943 on the Window Component of INa (INa(W)) Measured from GH3 Cells
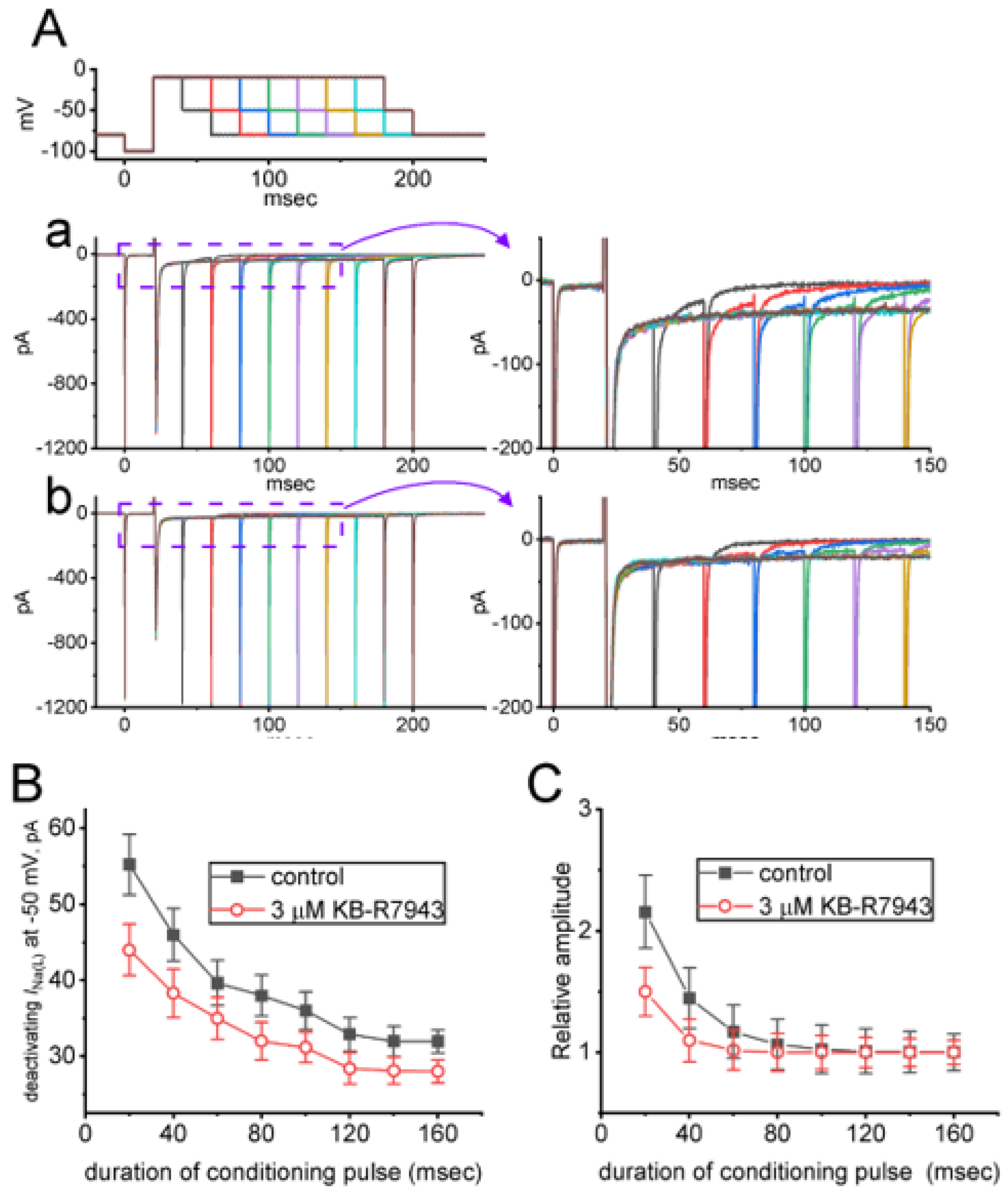
2.5. KB-R7943-Mediated Slowing in Recovery from INa(L) either during Prolonged Duration of Depolarizing Pulse or by the Envelope-of-Tail Test
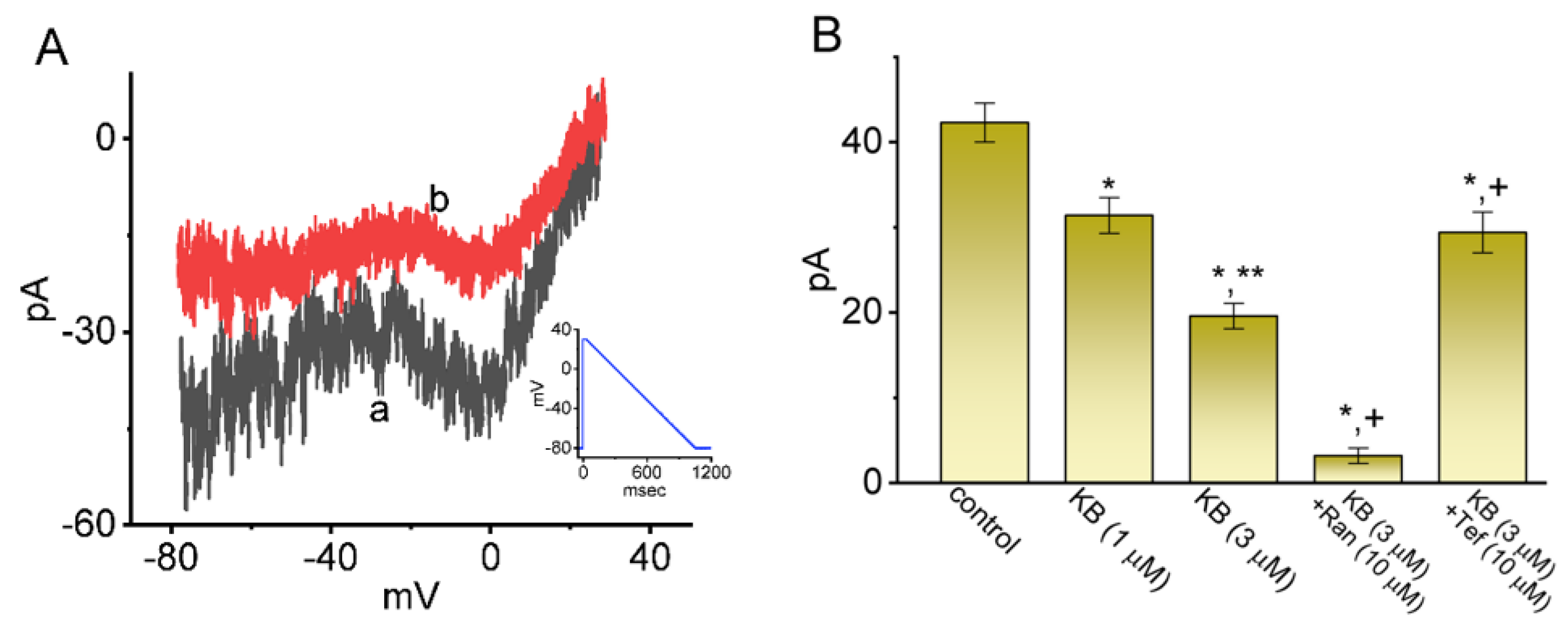
2.6. Modification of Nonlinear Resurgent Na+ Current (INa(R)) in Response to the Descending Vramp
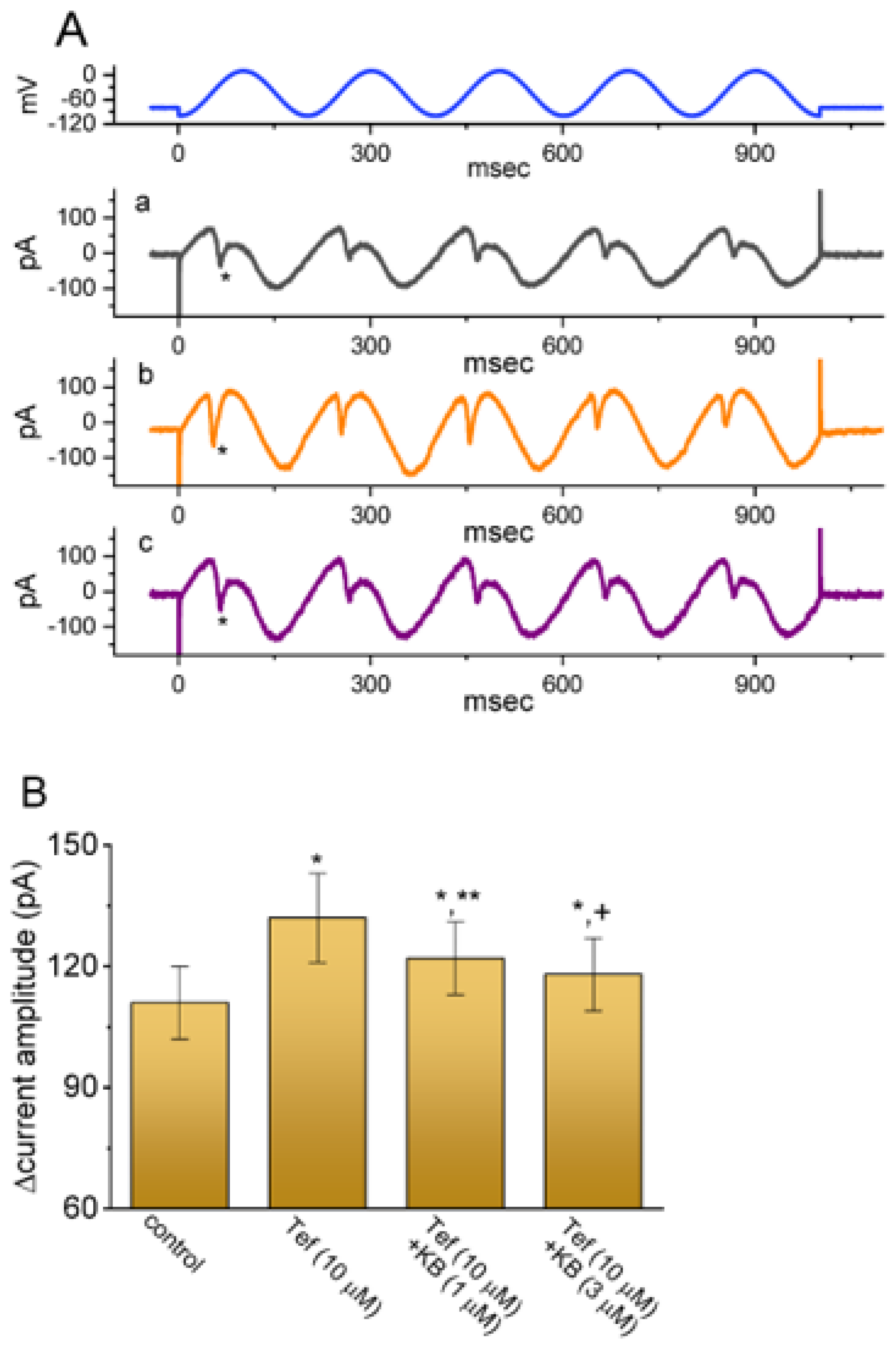
2.7. KB-R7943-Mediated Effect on Persistent Na+ Current (INa(P)) Evoked by Sinusoidal Voltage Waveform
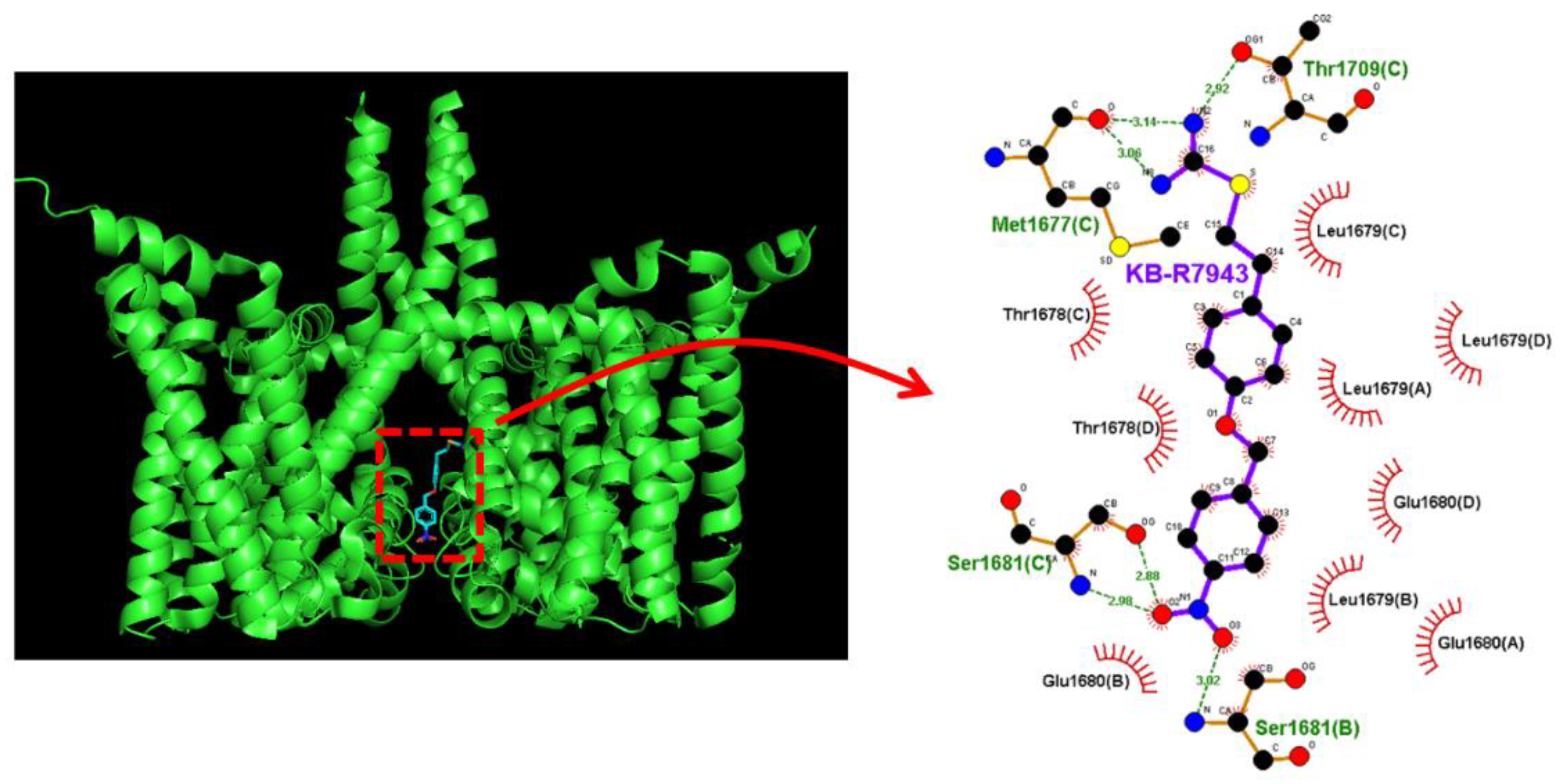
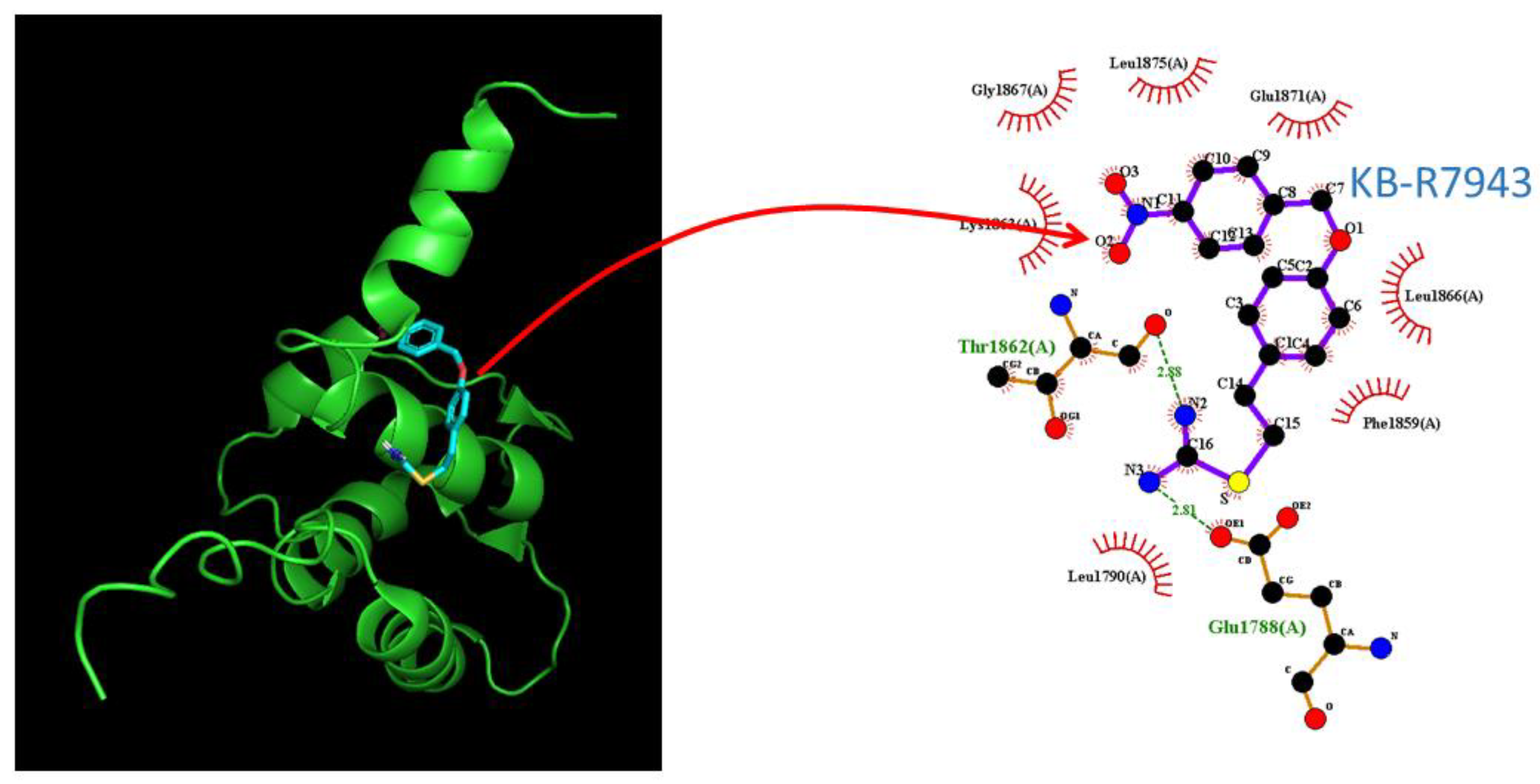
2.8. Docking Prediction of hNaV1.7 and KB-R7943
2.9. Docking Prediction of hNaV1.2 and KB-R7943
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs, and Reagents used in this Work
4.2. Cell Preparations
4.3. Electrophysiological Measurements
4.4. Whole-Cell Data Recordings with Patch-Clamp Technique
4.5. Data Analyses
4.6. Curve-Fitting Approximations and Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLT | deltamethrin |
| I-V | current versus voltage |
| IC50 | the concentration required for 50% inhibition |
| INa | voltage-gated Na+ current |
| KB | KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea) |
| INa(L) | late Na+ current |
| INa(R) | resurgent Na+ current |
| INa(T) | transient Na+ current |
| INa(W) | window Na+ current |
| KD | dissociation constant |
| NCX exchanger | Na+-Ca2+ exchanger |
| Ran | ranolazine |
| SEM | standard error of mean |
| τinact(S) | time constant in the slow component of current inactivation |
| TEA | tetraethylammonium chloride |
| Tef | tefluthrin |
| TTX | tetrodotoxin |
| Vramp | ramp voltage |
References
- Campbell, D.L.; Giles, W.R.; Robinson, K.; Shibata, E.F. Studies of the sodium-calcium exchanger in bull-frog atrial myocytes. J. Physiol. 1988, 403, 317–340. [Google Scholar] [CrossRef] [PubMed]
- Giles, W.; Shimoni, Y. Comparison of sodium-calcium exchanger and transient inward currents in single cells from rabbit ventricle. J. Physiol. 1989, 417, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.B.; Bouchard, R.A.; Giles, W.R. Action potential duration modulates calcium influx, Na(+)-Ca2+ exchange, and intracellular calcium release in rat ventricular myocytes. Ann. N. Y. Acad. Sci. 1996, 779, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Lachuer, J.; Bilbaut, A.; Georges, B.; Andrieu, J.L.; Diez, J.; Ojeda, C. Characterization of a Na+-Ca2+ exchanger NCX1 isoform in bovine fasciculata cells of adrenal gland. Mol. Cell. Biochem. 2001, 218, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sung, R.J.; Wu, Y.H.; Lai, N.H.; Teng, C.H.; Luo, C.H.; Tien, H.C.; Lo, C.P.; Wu, S.N. Beta-adrenergic modulation of arrhythmogenesis and identification of targeted sites of antiarrhythmic therapy in Timothy (LQT8) syndrome: A theoretical study. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H33–H44. [Google Scholar] [CrossRef] [PubMed]
- Hegner, P.; Drzymalski, M.; Biedermann, A.; Memmel, B.; Durczok, M.; Wester, M.; Floerchinger, B.; Provaznik, Z.; Schmid, C.; Zausig, Y.; et al. SAR296968, a Novel Selective Na(+)/Ca(2+) Exchanger Inhibitor, Improves Ca(2+) Handling and Contractile Function in Human Atrial Cardiomyocytes. Biomedicines 2022, 10, 1932. [Google Scholar] [CrossRef]
- Iwamoto, T.; Watano, T.; Shigekawa, M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J. Biol. Chem. 1996, 271, 22391–22397. [Google Scholar] [CrossRef]
- Brustovetsky, T.; Brittain, M.K.; Sheets, P.L.; Cummins, T.R.; Pinelis, V.; Brustovetsky, N. KB-R7943, an inhibitor of the reverse Na+/Ca2+ exchanger, blocks N-methyl-D-aspartate receptor and inhibits mitochondrial complex I. Br. J. Pharmacol. 2011, 162, 255–270. [Google Scholar] [CrossRef]
- Wu, F.; Wei, G.Z.; Li, W.J.; Liu, B.; Zhou, J.J.; Wang, H.C.; Gao, F. Low extracellular K+ increases intracellular Ca2+ oscillation and injury by activating the reverse mode Na+-Ca2+ exchanger and inhibiting the Na+, K+ ATPase in rat cardiomyocytes. Int. J. Cardiol. 2010, 140, 161–168. [Google Scholar] [CrossRef]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef]
- Philippaert, K.; Kalyaanamoorthy, S.; Fatehi, M.; Long, W.; Soni, S.; Byrne, N.J.; Barr, A.; Singh, J.; Wong, J.; Palechuk, T.; et al. Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143, 2188–2204. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Huang, C.W. Editorial to the Special Issue “Electrophysiology”. Int. J. Mol. Sci. 2021, 22, 2956. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Onkal, R.; Theys, M.; Bosmans, F.; Djamgoz, M.B.A. Neonatal Na(V) 1.5 channels: Pharmacological distinctiveness of a cancer-related voltage-gated sodium channel splice variant. Br. J. Pharmacol. 2022, 179, 473–486. [Google Scholar] [CrossRef]
- Gamal El-Din, T.M.; Lenaeus, M.J. Fenestropathy of Voltage-Gated Sodium Channels. Front. Pharmacol. 2022, 13, 842645. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, J.; Xia, Z. Structural Advances in Voltage-Gated Sodium Channels. Front. Pharmacol. 2022, 13, 908867. [Google Scholar] [CrossRef] [PubMed]
- Wisedchaisri, G.; Gamal El-Din, T.M. Druggability of Voltage-Gated Sodium Channels-Exploring Old and New Drug Receptor Sites. Front. Pharmacol. 2022, 13, 858348. [Google Scholar] [CrossRef] [PubMed]
- Morinville, A.; Fundin, B.; Meury, L.; Juréus, A.; Sandberg, K.; Krupp, J.; Ahmad, S.; O’Donnell, D. Distribution of the voltage-gated sodium channel Na(v)1.7 in the rat: Expression in the autonomic and endocrine systems. J. Comp. Neurol. 2007, 504, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Forty Years of Sodium Channels: Structure, Function, Pharmacology, and Epilepsy. Neurochem. Res. 2017, 42, 2495–2504. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Bjelobaba, I.; Zemkova, H. Ion Channels of Pituitary Gonadotrophs and Their Roles in Signaling and Secretion. Front. Endocrinol. 2017, 8, 126. [Google Scholar] [CrossRef]
- Taddese, A.; Bean, B.P. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 2002, 33, 587–600. [Google Scholar] [CrossRef]
- Rybak, I.A.; Ptak, K.; Shevtsova, N.A.; McCrimmon, D.R. Sodium currents in neurons from the rostroventrolateral medulla of the rat. J. Neurophysiol. 2003, 90, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Wu, Y.H.; Chen, B.S.; Lo, Y.C.; Liu, Y.C. Underlying mechanism of actions of tefluthrin, a pyrethroid insecticide, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology 2009, 258, 70–77. [Google Scholar] [CrossRef]
- Guérineau, N.C.; Monteil, A.; Lory, P. Sodium background currents in endocrine/neuroendocrine cells: Towards unraveling channel identity and contribution in hormone secretion. Front. Neuroendocrinol. 2021, 63, 100947. [Google Scholar] [CrossRef] [PubMed]
- Milman, A.; Ventéo, S.; Bossu, J.L.; Fontanaud, P.; Monteil, A.; Lory, P.; Guérineau, N.C. A sodium background conductance controls the spiking pattern of mouse adrenal chromaffin cells in situ. J. Physiol. 2021, 599, 1855–1883. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Lo, Y.C.; Peng, H.; Hsu, T.I.; Wu, S.N. Effects of ranolazine, a novel anti-anginal drug, on ion currents and membrane potential in pituitary tumor GH(3) cells and NG108-15 neuronal cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; So, E.C.; Liao, Y.K.; Huang, Y.M. Reversal by ranolazine of doxorubicin-induced prolongation in the inactivation of late sodium current in rat dorsal root ganglion neurons. Pain Med. 2015, 16, 1032–1034. [Google Scholar] [CrossRef]
- Wu, C.L.; Chuang, C.W.; Cho, H.Y.; Chuang, T.H.; Wu, S.N. The Evidence for Effective Inhibition of I(Na) Produced by Mirogabalin ((1R,5S,6S)-6-(aminomethyl)-3-ethyl-bicyclo [3.2.0] hept-3-ene-6-acetic acid), a Known Blocker of Ca(V) Channels. Int. J. Mol. Sci. 2022, 23, 3845. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.M.; Cho, H.Y.; Chiang, C.W.; Chuang, T.H.; Wu, S.N.; Tu, Y.F. Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na(+) and Erg-Mediated K(+) Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line. Int. J. Mol. Sci. 2022, 23, 7892. [Google Scholar] [CrossRef]
- Wu, S.N.; Wu, C.L.; Cho, H.Y.; Chiang, C.W. Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents. Int. J. Mol. Sci. 2022, 23, 9453. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Characterization of Direct Perturbations on Voltage-Gated Sodium Current by Esaxerenone, a Nonsteroidal Mineralocorticoid Receptor Blocker. Biomedicines 2021, 9, 549. [Google Scholar] [CrossRef]
- Chuang, T.H.; Cho, H.Y.; Wu, S.N. The Evidence for Sparsentan-Mediated Inhibition of I(Na) and I(K(erg)): Possibly Unlinked to Its Antagonism of Angiotensin II or Endothelin Type a Receptor. Biomedicines 2021, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- McCavera, S.J.; Soderlund, D.M. Differential state-dependent modification of inactivation-deficient Nav1.6 sodium channels by the pyrethroid insecticides S-bioallethrin, tefluthrin and deltamethrin. Neurotoxicology 2012, 33, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, S.N. Activation of voltage-gated sodium current and inhibition of erg-mediated potassium current caused by telmisartan, an antagonist of angiotensin II type-1 receptor, in HL-1 atrial cardiomyocytes. Clin. Exp. Pharmacol. Physiol. 2018, 45, 797–807. [Google Scholar] [CrossRef] [PubMed]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of I(Na) activation and inactivation as well as on resurgent and persistent I(Na) in a pituitary cell line (GH(3)). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Wu, S.N.; Huang, C.W. Telmisartan, an Antagonist of Angiotensin II Receptors, Accentuates Voltage-Gated Na(+) Currents and Hippocampal Neuronal Excitability. Front. Neurosci. 2020, 14, 902. [Google Scholar] [CrossRef] [PubMed]
- Bothe, S.N.; Lampert, A. The insecticide deltamethrin enhances sodium channel slow inactivation of human Nav1.9, Nav1.8 and Nav1.7. Toxicol. Appl. Pharmacol. 2021, 428, 115676. [Google Scholar] [CrossRef]
- Chuang, T.H.; Cho, H.Y.; Wu, S.N. Effective Accentuation of Voltage-Gated Sodium Current Caused by Apocynin (4′-Hydroxy-3′-methoxyacetophenone), a Known NADPH-Oxidase Inhibitor. Biomedicines 2021, 9, 1146. [Google Scholar] [CrossRef]
- Kaczorowski, G.J.; Dethmers, J.K.; Cragoe, E.J., Jr. Development of reversible and irreversible inhibitors of Na+/Ca2+ exchange in pituitary plasma membrane vesicles. Prog. Clin. Biol. Res. 1984, 168, 83–87. [Google Scholar]
- Korn, S.J.; Horn, R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J. Gen. Physiol. 1989, 94, 789–812. [Google Scholar] [CrossRef]
- Yoshihashi, K.; Habara, Y. Contribution of Na+/Ca2+ exchanger to glucose-induced [Ca2+]i increase in rat pancreatic islets. Jpn. J. Physiol. 1999, 49, 71–80. [Google Scholar] [CrossRef]
- Fiekers, J.F. The contributions of plasma membrane Na+-Ca2+-exchange and the Ca2+-ATPase to the regulation of cytosolic calcium ([Ca2+]i) in a clonal pituitary cell line (AtT-20) of mouse corticotropes. Life Sci. 2001, 70, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Hamming, K.S.; Soliman, D.; Webster, N.J.; Searle, G.J.; Matemisz, L.C.; Liknes, D.A.; Dai, X.Q.; Pulinilkunnil, T.; Riedel, M.J.; Dyck, J.R.; et al. Inhibition of beta-cell sodium-calcium exchange enhances glucose-dependent elevations in cytoplasmic calcium and insulin secretion. Diabetes 2010, 59, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Herchuelz, A.; Nguidjoe, E.; Jiang, L.; Pachera, N. Na(+)/Ca (2+) exchange and the plasma membrane Ca(2+)-ATPase in β-cell function and diabetes. Adv. Exp. Med. Biol. 2013, 961, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Li, H.F.; Jan, C.R. Regulation of Ca2+-activated nonselective cationic currents in rat pituitary GH3 cells: Involvement in L-type Ca2+ current. Brain Res. 1998, 812, 133–141. [Google Scholar] [CrossRef]
- Martiszus, B.J.; Tsintsadze, T.; Chang, W.; Smith, S.M. Enhanced excitability of cortical neurons in low-divalent solutions is primarily mediated by altered voltage-dependence of voltage-gated sodium channels. Elife 2021, 10, e67914. [Google Scholar] [CrossRef]
- Wang, X.; Takeya, K.; Aaronson, P.I.; Loutzenhiser, K.; Loutzenhiser, R. Effects of amiloride, benzamil, and alterations in extracellular Na+ on the rat afferent arteriole and its myogenic response. Am. J. Physiol. Renal Physiol. 2008, 295, F272–F282. [Google Scholar] [CrossRef]
- Morris, C.E.; Boucher, P.A.; Joós, B. Left-shifted nav channels in injured bilayer: Primary targets for neuroprotective nav antagonists? Front. Pharmacol. 2012, 3, 19. [Google Scholar] [CrossRef]
- Yu, N.; Morris, C.E.; Joós, B.; Longtin, A. Spontaneous excitation patterns computed for axons with injury-like impairments of sodium channels and Na/K pumps. PLoS Comput. Biol. 2012, 8, e1002664. [Google Scholar] [CrossRef]
- Moreau, A.; Gosselin-Badaroudine, P.; Delemotte, L.; Klein, M.L.; Chahine, M. Gating pore currents are defects in common with two Nav1.5 mutations in patients with mixed arrhythmias and dilated cardiomyopathy. J. Gen. Physiol. 2015, 145, 93–106. [Google Scholar] [CrossRef]
- Zylbertal, A.; Yarom, Y.; Wagner, S. The Slow Dynamics of Intracellular Sodium Concentration Increase the Time Window of Neuronal Integration: A Simulation Study. Front. Comput. Neurosci. 2017, 11, 85. [Google Scholar] [CrossRef]
- Raman, I.M.; Bean, B.P. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J. Neurosci. 1997, 17, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, Z.M.; Gouwens, N.W.; Raman, I.M. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: An experimental and modeling study. J. Neurosci. 2003, 23, 4899–4912. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Pablo, J.L.; Wang, C.; Pitt, G.S. FGF14 modulates resurgent sodium current in mouse cerebellar Purkinje neurons. Elife 2014, 3, e04193. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Lu, T.; Wang, X.; Wang, Y.; Sanchez, J.T. Resurgent sodium current promotes action potential firing in the avian auditory brainstem. J. Physiol. 2018, 596, 423–443. [Google Scholar] [CrossRef]
- Lewis, A.H.; Raman, I.M. Resurgent current of voltage-gated Na(+) channels. J. Physiol. 2014, 592, 4825–4838. [Google Scholar] [CrossRef]
- Cross, K.P.; Robertson, R.M. Ionic mechanisms maintaining action potential conduction velocity at high firing frequencies in an unmyelinated axon. Physiol. Rep. 2016, 4, e12814. [Google Scholar] [CrossRef]
- Wu, S.N.; Lo, Y.C.; Shen, A.Y.; Chen, B.S. Contribution of non-inactivating Na+ current induced by oxidizing agents to the firing behavior of neuronal action potentials: Experimental and theoretical studies from NG108-15 neuronal cells. Chin. J. Physiol. 2011, 54, 19–29. [Google Scholar] [CrossRef]
- Tryba, A.K.; Ramirez, J.M. Background sodium current stabilizes bursting in respiratory pacemaker neurons. J. Neurobiol. 2004, 60, 481–489. [Google Scholar] [CrossRef]
- Kovalsky, Y.; Amir, R.; Devor, M. Simulation in sensory neurons reveals a key role for delayed Na+ current in subthreshold oscillations and ectopic discharge: Implications for neuropathic pain. J. Neurophysiol. 2009, 102, 1430–1442. [Google Scholar] [CrossRef]
- Khaliq, Z.M.; Bean, B.P. Pacemaking in dopaminergic ventral tegmental area neurons: Depolarizing drive from background and voltage-dependent sodium conductances. J. Neurosci. 2010, 30, 7401–7413. [Google Scholar] [CrossRef]
- Zheng, H.; Drumm, B.T.; Zhu, M.H.; Xie, Y.; O’Driscoll, K.E.; Baker, S.A.; Perrino, B.A.; Koh, S.D.; Sanders, K.M. Na(+)/Ca(2 +) Exchange and Pacemaker Activity of Interstitial Cells of Cajal. Front. Physiol. 2020, 11, 230. [Google Scholar] [CrossRef]
- Mino, H. Information Rate of Neural Spike Trains in Response to Sinusoidal Electric Stimuli in the Presence of a Pseudo-spontaneous Activity. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2005, 2006, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.; de Groat, W.C.; Roppolo, J.R. Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Farkas, B.; Balogh, A.; Farkas, A.; Marosi, G.; Nagy, Z.K. Frequency and waveform dependence of alternating current electrospinning and their uses for drug dissolution enhancement. Int. J. Pharm. 2020, 586, 119593. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Wei, W.Y.; Ho, P.C. Short-Term Cortical Electrical Stimulation during the Acute Stage of Traumatic Brain Injury Improves Functional Recovery. Biomedicines 2022, 10, 1965. [Google Scholar] [CrossRef]
- Bassetti, D.; Hammann, J.; Luhmann, H.J.; White, R.; Kirischuk, S. Ryanodine receptor- and sodium-calcium exchanger-mediated spontaneous calcium activity in immature oligodendrocytes in cultures. Neurosci. Lett. 2020, 732, 134913. [Google Scholar] [CrossRef]
- Chan, C.S.; Lin, Y.S.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Hsu, C.C.; Chen, S.A.; Chen, Y.J. Atrial arrhythmogenesis in a rabbit model of chronic obstructive pulmonary disease. Transl. Res. 2020, 223, 25–39. [Google Scholar] [CrossRef]
- Liu, C.M.; Lin, F.Z.; Chen, Y.C.; Lin, Y.K.; Lu, Y.Y.; Wu, C.I.; Higa, S.; Chen, S.A.; Chen, Y.J. Concurrent increases in post-pacing action potential duration and contractility predict occurrence of ventricular arrhythmia. Pflugers Arch. 2020, 472, 1783–1791. [Google Scholar] [CrossRef]
- Choi, K.J.; Hwang, J.W.; Kim, S.H.; Park, H.S. Ca(2+) entry through reverse Na+/Ca(2+) exchanger in NCI-H716, glucagon-like peptide-1 secreting cells. Korean J. Physiol. Pharmacol. 2022, 26, 219–225. [Google Scholar] [CrossRef]
- Li, X.; Xu, F.; Xu, H.; Zhang, S.; Gao, Y.; Zhang, H.; Dong, Y.; Zheng, Y.; Yang, B.; Sun, J.; et al. Structural basis for modulation of human Na(V)1.3 by clinical drug and selective antagonist. Nat. Commun. 2022, 13, 1286. [Google Scholar] [CrossRef]
- Boucher, P.A.; Joós, B.; Morris, C.E. Coupled left-shift of Nav channels: Modeling the Na⁺-loading and dysfunctional excitability of damaged axons. J. Comput. Neurosci. 2012, 33, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kushmerick, C.; von Gersdorff, H. Presynaptic resurgent Na+ currents sculpt the action potential waveform and increase firing reliability at a CNS nerve terminal. J. Neurosci. 2010, 30, 15479–15490. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cheng, Q.; Ma, X.; Song, M. Suppressing Effect of Na(+)/Ca(2+) Exchanger (NCX) Inhibitors on the Growth of Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 901. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Liu, P.Y.; Gao, Z.H.; Lee, S.W.; Lee, W.K.; Wu, S.N. Evidence for the Effectiveness of Remdesivir (GS-5734), a Nucleoside-Analog Antiviral Drug in the Inhibition of I (K(M)) or I (K(DR)) and in the Stimulation of I (MEP). Front. Pharmacol. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-N.; Yu, M.-C. Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process. Int. J. Mol. Sci. 2023, 24, 1805. https://doi.org/10.3390/ijms24021805
Wu S-N, Yu M-C. Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process. International Journal of Molecular Sciences. 2023; 24(2):1805. https://doi.org/10.3390/ijms24021805
Chicago/Turabian StyleWu, Sheng-Nan, and Meng-Cheng Yu. 2023. "Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process" International Journal of Molecular Sciences 24, no. 2: 1805. https://doi.org/10.3390/ijms24021805
APA StyleWu, S.-N., & Yu, M.-C. (2023). Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process. International Journal of Molecular Sciences, 24(2), 1805. https://doi.org/10.3390/ijms24021805





