Changes in Serum Protein–Peptide Patterns in Atopic Children Allergic to Plant Storage Proteins
Abstract
1. Introduction
- (1)
- To analyse sensitisation to plant storage proteins in a group of children aged 0–5 years with chronic symptoms of atopic dermatitis.
- (2)
- To discover changes in serum protein–peptide patterns in atopic children allergic to plant storage proteins.
2. Results
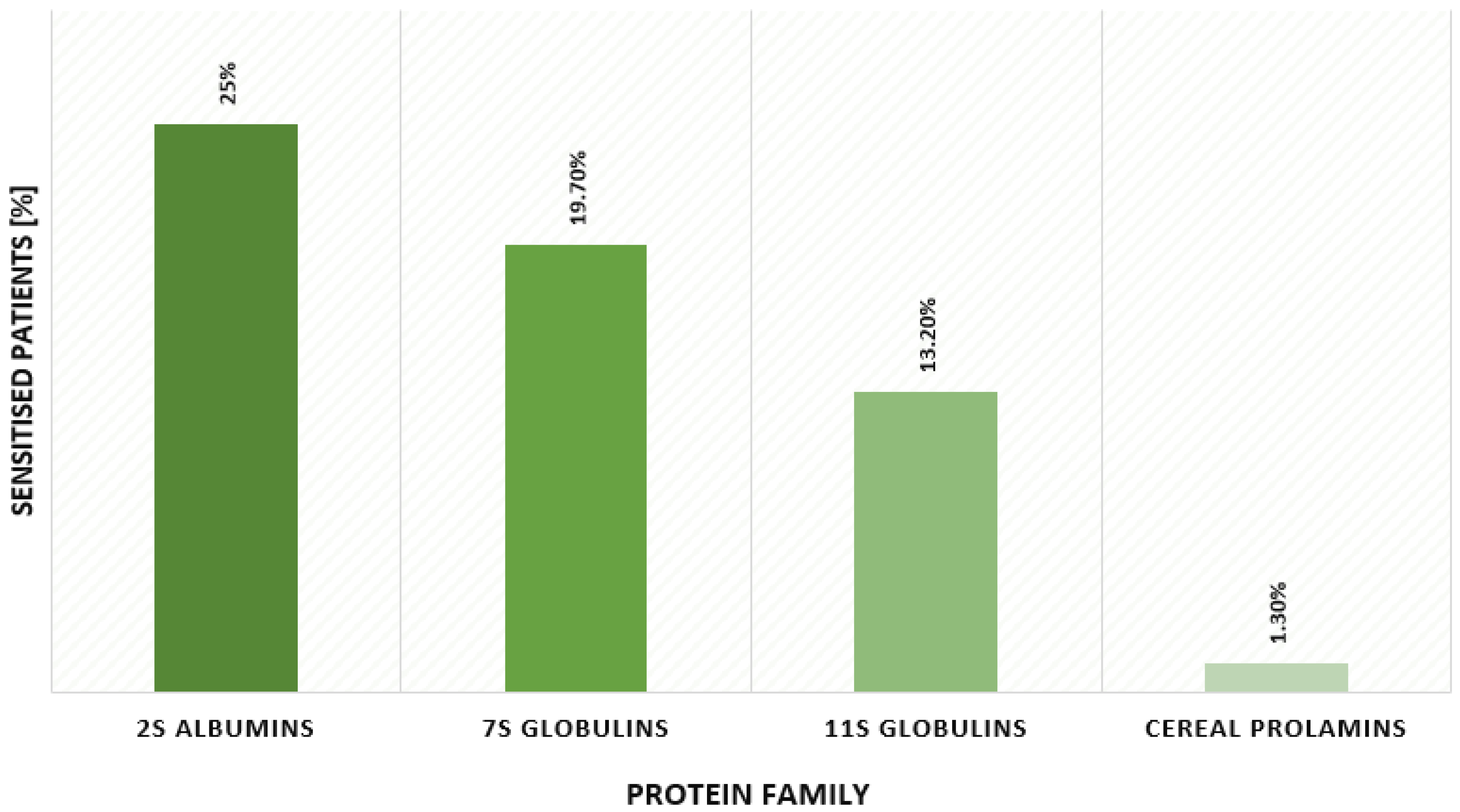
2.1. Allergy to Plant Storage Proteins—Sensitisation Patterns in Atopic Children
2.2. Proteomic Features Characterization of Allergy to Plant Storage Proteins
2.2.1. Protein–Peptide Profiling
2.2.2. Identification of the Discriminatory Features
3. Discussion
3.1. Allergy to Plant Storage Proteins-Sensitisation Pattern in Atopic Children
3.2. Proteomic Features Characterization of Allergy to Plant Storage Proteins
4. Materials and Methods
4.1. Study Group and Sample Collection
4.2. Measurement of sIgE Serum Levels
4.3. Pre-Treatment of the Serum Samples
4.4. MALDI-TOF-MS Protein and Peptide Profiling
4.5. NanoLC-MALDI-TOF/TOF MS Identification of Discriminative Peaks
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgess, J.A.; Dharmage, S.C.; Allen, K.; Koplin, J.; Garcia-Larsen, V.; Boyle, R.; Waidyatillake, N.; Lodge, C.J. Age at introduction to complementary solid food and food allergy and sensitization: A systematic review and meta-analysis. Clin. Exp. Allergy 2019, 49, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Mezawa, H.; Okuyama, M.; Urashima, T.; Hirano, D.; Gocho, N.; Tachimoto, H. Primary Prevention of Cow’s Milk Sensitization and Food Allergy by Avoiding Supplementation with Cow’s Milk Formula at Birth: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Packi, K.; Matysiak, J.; Klimczak, S.; Matuszewska, E.; Bręborowicz, A.; Pietkiewicz, D.; Matysiak, J. Analysis of the Serum Profile of Cytokines Involved in the T-Helper Cell Type 17 Immune Response Pathway in Atopic Children with Food Allergy. Int. J. Environ. Res. Public Health 2022, 19, 7877. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, K.E.C.; Roberts, G.; Selby, A.; Reich, A.; Butiene, I.; Clausen, M.; Dubakiene, R.; Fiandor, A.; Fiocchi, A.; Grabenhenrich, L.; et al. Risk Factors for Hen’s Egg Allergy in Europe: EuroPrevall Birth Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1341–1348.e5. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E.; Eckert, J.K.; Koplin, J.; Lowe, A.; Gurrin, L.; Dharmage, S.; Vuillermin, P.; Tang, M.L.K.; Ponsonby, A.-L.; Matheson, M.; et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin. Exp. Allergy 2015, 45, 255–264. [Google Scholar] [CrossRef]
- Cartledge, N.; Chan, S. Atopic Dermatitis and Food Allergy: A Paediatric Approach. Curr. Pediatr. Rev. 2018, 14, 171–179. [Google Scholar] [CrossRef]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef]
- Mavroudi, A.; Karagiannidou, A.; Xinias, I.; Cassimos, D.; Karantaglis, N.; Farmaki, E.; Imvrios, G.; Fotoulaki, M.; Eboriadou, M.; Tsanakas, J. Assessment of IgE-mediated food allergies in children with atopic dermatitis. Allergol. Immunopathol. 2017, 45, 77–81. [Google Scholar] [CrossRef]
- Blom, W.M.; Vlieg-Boerstra, B.J.; Kruizinga, A.G.; van der Heide, S.; Houben, G.F.; Dubois, A.E.J. Threshold dose distributions for 5 major allergenic foods in children. J. Allergy Clin. Immunol. 2013, 131, 172–179. [Google Scholar] [CrossRef]
- Burney, P.G.J.; Potts, J.; Kummeling, I.; Mills, E.N.C.; Clausen, M.; Dubakiene, R.; Barreales, L.; Pérez, C.F.; Fernandez-Rivas, M.; Le, T.-M.; et al. The prevalence and distribution of food sensitization in European adults. Allergy 2014, 69, 365–371. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. S23), 1–250. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Valenta, R.; Puerta, L.; Pomés, A.; Zakzuk, J.; Fernandez-Caldas, E.; Acevedo, N.; Sanchez-Borges, M.; Ansotegui, I.; Zhang, L.; et al. The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources. World Allergy Organ J. 2020, 13, 100118. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, S.; Toda, M.; Vieths, S. What makes an allergen? Clin. Exp. Allergy 2015, 45, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Scheurer, S. Stabile pflanzliche Nahrungsmittelallergene: Lipid-Transfer-Proteine. Allergo J. 2011, 20, 384–386. [Google Scholar] [CrossRef]
- Shewry, P.R.; Napier, J.; Tatham, A. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945–956. [Google Scholar] [CrossRef]
- Rasheed, F.; Markgren, J.; Hedenqvist, M.; Johansson, E. Modeling to Understand Plant Protein Structure-Function Relationships-Implications for Seed Storage Proteins. Molecules 2020, 25, 873. [Google Scholar] [CrossRef]
- Vissers, Y.M.; Blanc, F.; Skov, P.S.; Johnson, P.E.; Rigby, N.M.; Przybylski-Nicaise, L.; Bernard, H.; Wal, J.-M.; Ballmer-Weber, B.; Zuidmeer-Jongejan, L.; et al. Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. PLoS ONE 2011, 6, e23998. [Google Scholar] [CrossRef]
- Beyer, K.; Morrowa, E.; Li, X.-M.; Bardina, L.; Bannon, G.A.; Burks, A.W.; Sampson, H.A. Effects of cooking methods on peanut allergenicity. J. Allergy Clin. Immunol. 2001, 107, 1077–1081. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, X.; Tang, Y.; Zhang, Y.; Yuan, J.; Li, X.; Yang, A.; Tong, P.; Wu, Z.; Chen, H. Effect of Processing on the Structure and Allergenicity of Peanut Allergen Ara h 2 Roasted in a Matrix. J. Agric. Food Chem. 2022, 70, 626–633. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Evolutionary Biology of Plant Food Allergens—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17689599/ (accessed on 14 December 2022).
- Allergens of the Cupin Superfamily—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12440948/ (accessed on 14 December 2022).
- ABC Diagnostyki Molekularnej w Alergologii—Łukasz Błażowski|Książka w Tezeusz.pl Książki Promocje, Używane Książki, Nowości Wydawnicze. Available online: https://tezeusz.pl/abc-diagnostyki-molekularnej-w-alergologii-lukasz-blazowski-3950636 (accessed on 26 November 2022).
- Molecular Allergy Diagnostics|SpringerLink. Available online: https://link.springer.com/book/10.1007/978-3-319-42499-6 (accessed on 26 November 2022).
- Koid, A.E.; Chapman, M.D.; Hamilton, R.G.; van Ree, R.; Versteeg, S.A.; Dreskin, S.C.; Koppelman, S.J.; Wünschmann, S. Ara h 6 Complements Ara h 2 as an Important Marker for IgE Reactivity to Peanut. J. Agric. Food Chem. 2014, 62, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Ballmer-Weber, B.K.; Lidholm, J.; Fernández-Rivas, M.; Seneviratne, S.; Hanschmann, K.-M.; Vogel, L.; Bures, P.; Fritsche, P.; Summers, C.; Knulst, A.C.; et al. IgE recognition patterns in peanut allergy are age dependent: Perspectives of the EuroPrevall study. Allergy 2015, 70, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Klemans, R.J.B.; Van Os-Medendorp, H.; Blankestijn, M.; Bruijnzeel-Koomen, C.A.F.M.; Knol, E.; Knulst, A.C. Diagnostic accuracy of specific IgE to components in diagnosing peanut allergy: A systematic review. Clin. Exp. Allergy 2015, 45, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Klemans, R.J.B.; Otte, D.; Knol, M.; Knol, E.F.; Meijer, Y.; Gmelig-Meyling, F.H.; Bruijnzeel-Koomen, C.A.; Knulst, A.C.; Pasmans, S.G. The diagnostic value of specific IgE to Ara h 2 to predict peanut allergy in children is comparable to a validated and updated diagnostic prediction model. J. Allergy Clin. Immunol. 2013, 131, 157–163. [Google Scholar] [CrossRef]
- Bublin, M.; Breiteneder, H. Cross-reactivity of peanut allergens. Curr. Allergy Asthma Rep. 2014, 14, 426. [Google Scholar] [CrossRef]
- Detailed Characterization of Act d 12 and Act d 13 from Kiwi Seeds: Implication in IgE Cross-Reactivity with Peanut and Tree Nuts–Sirvent–2014–Allergy—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/all.12486 (accessed on 29 November 2022).
- Geiselhart, S.; Hoffmann-Sommergruber, K.; Bublin, M. Tree nut allergens. Mol. Immunol. 2018, 100, 71–81. [Google Scholar] [CrossRef]
- De Leon, M.; Drew, A.; Glaspole, I.; Suphioglu, C.; O’Hehir, R.; Rolland, J. IgE cross-reactivity between the major peanut allergen Ara h 2 and tree nut allergens. Mol. Immunol. 2007, 44, 463–471. [Google Scholar] [CrossRef]
- Lange, L.; Lasota, L.; Finger, A.; Vlajnic, D.; Büsing, S.; Meister, J.; Broekaert, I.; Pfannenstiel, C.; Friedrichs, F.; Price, M.; et al. Ana o 3-specific IgE is a good predictor for clinically relevant cashew allergy in children. Allergy 2017, 72, 598–603. [Google Scholar] [CrossRef]
- Villalta, D.; Conte, M.; Asero, R.; Da Re, M.; Stella, S.; Martelli, P. Isolated IgE reactivity to native walnut vicilin-like protein (nJug r 2) on ISACTM microarray is due to cross-reactive carbohydrate epitopes. Clin. Chem. Lab. Med. 2013, 51, 1991–1995. [Google Scholar] [CrossRef]
- Willison, L.N.; Tawde, P.; Robotham, J.M.; Penney, R.M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Pistachio vicilin, Pis v 3, is immunoglobulin E-reactive and cross-reacts with the homologous cashew allergen, Ana o 1. Clin. Exp. Allergy 2008, 38, 1229–1238. [Google Scholar] [CrossRef]
- Cabanillas, B.; Jappe, U.; Novak, N. Allergy to Peanut, Soybean, and Other Legumes: Recent Advances in Allergen Characterization, Stability to Processing and IgE Cross-Reactivity. Mol. Nutr. Food Res. 2018, 62, 1700446. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Kostadinova, M.; Radauer, C.; Hafner, C.; Szépfalusi, Z.; Varga, E.-M.; Maleki, S.J.; Hoffmann-Sommergruber, K.; Breiteneder, H. IgE cross-reactivity between the major peanut allergen Ara h 2 and the nonhomologous allergens Ara h 1 and Ara h 3. J. Allergy Clin. Immunol. 2013, 132, 118–124. [Google Scholar] [CrossRef] [PubMed]
- High-Affinity Allergen-Specific Human Antibodies Cloned from Single IgE B Cell Transcriptomes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30545888/ (accessed on 5 December 2022).
- Masthoff, L.J.N.; Mattsson, L.; Zuidmeer-Jongejan, L.; Lidholm, J.; Andersson, K.; Akkerdaas, J.H.; Versteeg, S.A.; Garino, C.; Meijer, Y.; Kentie, P.; et al. Sensitization to Cor a 9 and Cor a 14 is highly specific for a hazelnut allergy with objective symptoms in Dutch children and adults. J. Allergy Clin. Immunol. 2013, 132, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Villalta, D.; Scala, E.; Mistrello, G.; Amato, S.; Asero, R. Evidence of Cross-Reactivity between Different Seed Storage Proteins from Hazelnut (Corylus avellana) and Walnut (Juglans regia) Using Recombinant Allergen Proteins. Int. Arch. Allergy Immunol. 2019, 178, 89–92. [Google Scholar] [CrossRef]
- The 11S Globulin Sin a 2 from Yellow Mustard Seeds Shows IgE Cross-Reactivity with Homologous Counterparts from Tree Nuts and Peanut—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3583068/ (accessed on 29 November 2022).
- Varga, E.-M.; Kollmann, D.; Zach, M.; Bohle, B. Anaphylaxis to buckwheat in an atopic child: A risk factor for severe allergy to nuts and seeds? Int. Arch. Allergy Immunol. 2011, 156, 112–116. [Google Scholar] [CrossRef]
- Barre, A.; Jacquet, G.; Sordet, C.; Culerrier, R.; Rougé, P. Homology modelling and conformational analysis of IgE-binding epitopes of Ara h 3 and other legumin allergens with a cupin fold from tree nuts. Mol. Immunol. 2007, 44, 3243–3255. [Google Scholar] [CrossRef]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat Allergy in Children: A Comprehensive Update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef]
- Proteomic Features Characterization of Hymenoptera Venom Allergy|Allergy, Asthma & Clinical Immunology|Full Text. Available online: https://aacijournal.biomedcentral.com/articles/10.1186/s13223-019-0387-5 (accessed on 7 December 2022).
- Pavel, A.B.; Zhou, L.; Diaz, A.; Ungar, B.; Dan, J.; He, H.; Estrada, Y.D.; Xu, H.; Fernandes, M.; Renert-Yuval, Y.; et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J. Am. Acad. Dermatol. 2020, 82, 690–699. [Google Scholar] [CrossRef]
- Yin, J.; Wen, Y.; Zeng, J.; Zhang, Y.; Chen, J.; Zhang, Y.; Han, T.; Li, X.; Huang, H.; Cai, Y.; et al. CDC50A might be a novel biomarker of epithelial ovarian cancer-initiating cells. BMC Cancer 2022, 22, 903. [Google Scholar] [CrossRef]
- Codreanu, F.; Collignon, O.; Roitel, O.; Thouvenot, B.; Sauvage, C.; Vilain, A.-C.; Cousin, M.-O.; Decoster, A.; Renaudin, J.-M.; Astier, C.; et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int. Arch. Allergy Immunol. 2011, 154, 216–226. [Google Scholar] [CrossRef]
- Andersen, J.P.; Vestergaard, A.L.; Mikkelsen, S.A.; Mogensen, L.S.; Chalat, M.; Molday, R.S. P4-ATPases as Phospholipid Flippases—Structure, Function, and Enigmas. Front. Physiol. 2016, 7, 275. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2016.00275 (accessed on 12 December 2022). [CrossRef] [PubMed]
- Caspase-Mediated Cleavage of Phospholipid Flippase for Apoptotic Phosphatidylserine Exposure—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24904167/ (accessed on 12 December 2022).
- TMEM30A Deficiency in Endothelial Cells Impairs Cell Proliferation and Angiogenesis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30814335/ (accessed on 12 December 2022).
- Role for Phospholipid Flippase Complex of ATP8A1 and CDC50A Proteins in Cell Migration—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23269685/ (accessed on 12 December 2022).
- The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18485877/ (accessed on 12 December 2022).
- Yoshimura, S.-I.; Gerondopoulos, A.; Linford, A.; Rigden, D.; Barr, F.A. Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors. J. Cell Biol. 2010, 191, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Recacha, R.; Boulet, A.; Jollivet, F.; Monier, S.; Houdusse, A.; Goud, B.; Khan, A.R. Structural basis for recruitment of Rab6-interacting protein 1 to Golgi via a RUN domain. Structure 2009, 17, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Epileptic Encephalopathy Caused by Mutations in the Guanine Nucleotide Exchange Factor DENND5A—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27866705/ (accessed on 13 December 2022).
- Arndt, S.; Poser, I.; Schubert, T.; Moser, M.; Bosserhoff, A.-K. Cloning and functional characterization of a new Ski homolog, Fussel-18, specifically expressed in neuronal tissues. Lab. Investig. 2005, 85, 1330–1341. [Google Scholar] [CrossRef]
- SKI Pathways Inducing Progression of Human Melanoma|SpringerLink. Available online: https://link.springer.com/article/10.1007/s10555-005-1576-x (accessed on 13 December 2022).
- Competition between Ski and CREB-binding Protein for Binding to Smad Proteins in Transforming Growth Factor-β Signaling *. J. Biol. Chem. 2007, 282, 11365–11376. Available online: https://www.jbc.org/article/S0021-9258(20)76716-5/fulltext (accessed on 13 December 2022). [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; I Hurwitz, D.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.-H.; Chung, T.-G.; Kim, H.-J.; Yoon, T.-K.; Kwak, I.-P.; Park, S.-H.; Cha, W.-T.; Cho, S.-W.; Cha, K.-Y. Molecular and cytogenetic characterization of two azoospermic patients with X-autosome translocation. J. Assist. Reprod. Genet. 2003, 20, 385–389. [Google Scholar] [CrossRef]
- Alvaro, C.G.; Braz, J.M.; Bernstein, M.; Hamel, K.A.; Craik, V.; Yamanaka, H.; Basbaum, A.I. Hippocalcin-like 4, a neural calcium sensor, has a limited contribution to pain and itch processing. PLoS ONE 2020, 15, e0226289. [Google Scholar] [CrossRef]
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef]
- Swiatly, A.; Horala, A.; Hajduk, J.; Matysiak, J.; Nowak-Markwitz, E.; Kokot, Z.J. MALDI-TOF-MS analysis in discovery and identification of serum proteomic patterns of ovarian cancer. BMC Cancer 2017, 17, 472. [Google Scholar] [CrossRef]
- Klupczynska, A.; Swiatly, A.; Hajduk, J.; Matysiak, J.; Dyszkiewicz, W.; Pawlak, K.; Kokot, Z.J. Identification of Serum Peptidome Signatures of Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2016, 17, 410. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of a New Multiplex Assay for Allergy Diagnosis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30822401/ (accessed on 5 December 2022).
- Blazowski, L.; Majak, P.; Kurzawa, R.; Kuna, P.; Jerzynska, J. Food Allergy Endotype with High Risk of Severe Anaphylaxis in Children—Monosensitization to Cashew 2S Albumin Ana o 3. Allergy 2019, 74, 1945–1955. [Google Scholar] [CrossRef]





| Superfamily | Family | Allergen | Allergen Source | Concentration (kUA/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Population | Allergy Group | ||||||||
| Mean | SD | Median | Mean | SD | Median | ||||
| Cupins | Vicilins/7S globulins | nAra h 1 | Peanut | 2.104 | 7.669 | 0.1 | 10.93 | 14.959 | 3.165 |
| nCor a 11 | Hazelnut | 0.210 | 0.413 | 0.1 | 1.592 | 0.727 | 1.6 | ||
| nJug r 6 | Walnut | 0.146 | 0.189 | 0.1 | 0.838 | 0.399 | 0.705 | ||
| nJug r 2 | Walnut | 0.187 | 0.474 | 0.1 | 2.223 | 1.169 | 2.43 | ||
| nPis v 3 | Pistachio | 0.106 | 0.039 | 0.1 | 0.44 | 0.0 | 0.44 | ||
| rGly m 5 | Soy | - | - | - | - | - | - | ||
| Legumins/11S globulins | nAra h 3 | Peanut | 1.160 | 4.820 | 0.1 | 9.038 | 11.216 | 3.59 | |
| nCor a 9 | Hazelnut | 0.342 | 1.113 | 0.1 | 3.157 | 2.662 | 2.695 | ||
| nJug r 4 | Walnut | 0.206 | 0.516 | 0.1 | 1.662 | 1.332 | 1.47 | ||
| rGly m 6 | Soy | 0.391 | 1.652 | 0.1 | 5.623 | 4.792 | 4.455 | ||
| rAna o 2 | Cashew nut | - | - | - | - | - | - | ||
| nPis v 2 | Pistachio | - | - | - | - | - | - | ||
| Prolamins | 2S albumins | nAra h 2 | Peanut | 2.553 | 8.335 | 0.1 | 14.434 | 15.359 | 9.81 |
| nAra h 6 | Peanut | 2.376 | 8.052 | 0.1 | 14.513 | 15.353 | 7.975 | ||
| nCor a 14 | Hazelnut | 0.315 | 0.926 | 0.1 | 2.767 | 2.081 | 2.21 | ||
| nSes i 1 | Sesame | 0.202 | 0.443 | 0.1 | 1.56 | 0.997 | 1.19 | ||
| nSin a 1 | Mustard | 0.298 | 1.193 | 0.1 | 5.047 | 3.549 | 3.65 | ||
| nJug r 1 | Walnut | 0.425 | 2.514 | 0.1 | 8.287 | 9.787 | 2.38 | ||
| rAna o 3 | Cashew nut | 0.141 | 0.283 | 0.1 | 1.127 | 1.011 | 0.53 | ||
| nMac i | Macadamia | 0.149 | 0.395 | 0.1 | 1.94 | 1.62 | 1.94 | ||
| nBer e 1 | Brazil nut | 0.107 | 0.062 | 0.1 | 0.64 | 0.0 | 0.64 | ||
| rPis v 1 | Pistachio | 0.121 | 0.179 | 0.1 | 1.67 | 0.0 | 1.67 | ||
| nPap s | Poppy seeds | 0.107 | 0.062 | 0.1 | 0.64 | 0.0 | 0.64 | ||
| nFag e 2 | Buckwheat | - | - | - | - | - | - | ||
| rGly m 8 | Soy | - | - | - | - | - | - | ||
| Cereal prolamins | rTri a 19 (gliadin) | Wheat | 0.113 | 0.037 | 0.1 | 0.3 | 0.0 | 0.3 | |
| GA | QC | SNN |
|---|---|---|
| 1077.88 1219.27 1419.92 1897.85 2661.39 3159.56 3198.01 3689.35 4287.68 4396.62 4645.8 4964.58 7922.04 8126.66 8866.89 | 1277.39 1773.08 1795.39 1945.54 2012.59 2016.56 2144.89 2269.28 2474.89 2601.66 2726.71 3689.35 4964.58 5004.83 5017.61 5044.33 6065.66 8866.89 | 1450.43 1795.39 4151.16 5352.47 5905.01 6221.79 8601.73 9135.64 |
| GA | QC | SNN | |
|---|---|---|---|
| Cross-validation (%) | 94.91 | 67.56 | 62.29 |
| Recognition capability (%) | 99.25 | 68.61 | 56.66 |
| Correct classified (%): | |||
| 42.7 | 54.5 | 0 |
| 94.4 | 81.5 | 96.3 |
| Precursor Ion m/z | p-Value | UniProtKB-ID | Peptide Sequence | Protein Name |
|---|---|---|---|---|
| 1077.88 | 0.0158 | CC50A_HUMAN | R.NSSNTADITI.- | Cell cycle control protein 50A |
| 1419.92 | 0.0139 | TX13B_HUMAN | K.EACTWGSLALGVR.F | Testis-expressed sequence 13B |
| 2012.59 | 0.00467 | DEN5A_HUMAN | K.LNTGQIQESIGEAVNGIVK.H | DENN domain-containing protein 5A |
| 2601.66 | 0.00194 | SKOR2_HUMAN | K.AGGGSYHHSSAFRPVGGKDDAESLAK.L | SKI family transcriptional corepressor 2 |
| 2661.39 | 0.532 | SPAG6_HUMAN | R.LPGIMMLGYVAAHSENLAMAVIISK.G | Sperm-associated antigen 6 |
| Allergen Characteristics | Ara h 1 | Ara h 2 | Ara h 3 |
|---|---|---|---|
| Source | peanut | peanut | peanut |
| Protein family | 7S globulins | 2S albumins | 11S globulins |
| % allergy suffers in the study group | 18.4% | 17.1% | 11.8% |
| Biological function | seed storage protein | seed storage protein | seed storage protein |
| Molecular structure | 2 β-barrels surrounded by α-helical and unstructured loops  monomer  trimer | 5 helix bundle held together by 4 disulphide bonds monomer  maltose-binding protein (MBP)-Ara h 2 fusion system | 2 β-barrels surrounded by α-helical and unstructured loops, composed of 2 disulphide-linked chains; each subunit is composed of an acidic and a basic chain derived from a single precursor and linked by a disulphide bond monomer  hexamer |
| Molecular weight | 64 kDa | 17 kDa | 60 kDa, 37 kDa (fragment) |
| Length | 626 aa | 157 aa | 512 aa |
| Ligand binding | Small molecular ligands, e.g., Ca2+ | No | Metal ions, e.g., Mg2+ |
| Oligomerization | Trimers | No | Hexamers |
| Glycosylation | Yes | No | No |
| Disulphide bonds | No | 4 | 2 |
| Isoelectric point | 4.55 | 5.2 | 5.5 |
| Heat stability | Yes | Yes | Yes |
| Denaturation temperature | 88.3 °C | 110 °C | 95 °C |
| Characteristics of the Participants | |
|---|---|
| No. of subjects | 76 |
| Sex | |
| Male | 44 (57.9%) |
| Female | 32 (42.1%) |
| Age (months) | |
| Median | 19 |
| Mean | 23, 24 |
| Range | 2–60 |
| Eczema (for the past 6 weeks or more) | 76 |
| Patient age on the onset of eczema (months) | |
| Median | 3 |
| Mean | 5, 12 |
| Range | 1–36 |
| Atopic dermatitis (L20) | 76 |
| Association with foods: | |
| Milk | 51 |
| Egg | 16 |
| Egg white | 2 |
| Egg yolk | 1 |
| Cocoa | 8 |
| Chocolate | 2 |
| Oatmeal | 1 |
| Flour | 1 |
| Wheat | 5 |
| Gluten | 4 |
| Rye bread | 1 |
| Ketchup | 1 |
| Sweets | 1 |
| Nuts | 15 |
| Coconut | 2 |
| Fruits | 15 |
| Banana | 2 |
| Strawberry | 5 |
| Apple | 3 |
| Peach | 1 |
| Citrus | 3 |
| Juice | 2 |
| Carrot | 2 |
| Tomato | 1 |
| Potato | 1 |
| Soy | 1 |
| Chickpeas | 1 |
| Silage | 1 |
| Allergic Urticaria (L50) | 10 |
| Angioedema (T78.3) | 5 |
| The causative food: | |
| Hazelnut | 1 |
| Milk | 2 |
| Egg | 3 |
| Egg white | 1 |
| Peanut | 2 |
| White fish | 1 |
| Raisins | 1 |
| Gluten | 1 |
| Cauliflower | 1 |
| Anaphylactic shock (T78.0) | 5 |
| Symptoms of a generalized reaction: | |
| Dyspnoea | 5 |
| Vascular oedema | 2 |
| Hives | 1 |
| Adrenaline | 0 |
| The causative food: | |
| Hazelnut | 1 |
| Milk | 1 |
| Peanut | 1 |
| Egg | 1 |
| Fish (cod) | 1 |
| Chronic symptoms of the digestive system: | |
| Colic | 6 |
| Abdominal pain | 5 |
| Abdominal gas | 3 |
| Vomiting, Downpouring | 3 |
| Diarrhoea | 6 |
| Constipation | 3 |
| Mucus in the stool | 2 |
| Blood in the stool | 0 |
| Chronic diseases: | |
| Early childhood asthma | 8 |
| Recurrent bronchitis | 0 |
| Allergic rhinitis | 20 |
| Recurrent upper respiratory tract infections | 1 |
| Neurogenic bladder | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Packi, K.; Matysiak, J.; Matuszewska, E.; Bręborowicz, A.; Matysiak, J. Changes in Serum Protein–Peptide Patterns in Atopic Children Allergic to Plant Storage Proteins. Int. J. Mol. Sci. 2023, 24, 1804. https://doi.org/10.3390/ijms24021804
Packi K, Matysiak J, Matuszewska E, Bręborowicz A, Matysiak J. Changes in Serum Protein–Peptide Patterns in Atopic Children Allergic to Plant Storage Proteins. International Journal of Molecular Sciences. 2023; 24(2):1804. https://doi.org/10.3390/ijms24021804
Chicago/Turabian StylePacki, Kacper, Joanna Matysiak, Eliza Matuszewska, Anna Bręborowicz, and Jan Matysiak. 2023. "Changes in Serum Protein–Peptide Patterns in Atopic Children Allergic to Plant Storage Proteins" International Journal of Molecular Sciences 24, no. 2: 1804. https://doi.org/10.3390/ijms24021804
APA StylePacki, K., Matysiak, J., Matuszewska, E., Bręborowicz, A., & Matysiak, J. (2023). Changes in Serum Protein–Peptide Patterns in Atopic Children Allergic to Plant Storage Proteins. International Journal of Molecular Sciences, 24(2), 1804. https://doi.org/10.3390/ijms24021804







