Dental Pulp Inflammation Initiates the Occurrence of Mast Cells Expressing the α1 and β1 Subunits of Soluble Guanylyl Cyclase
Abstract
1. Introduction
2. Results
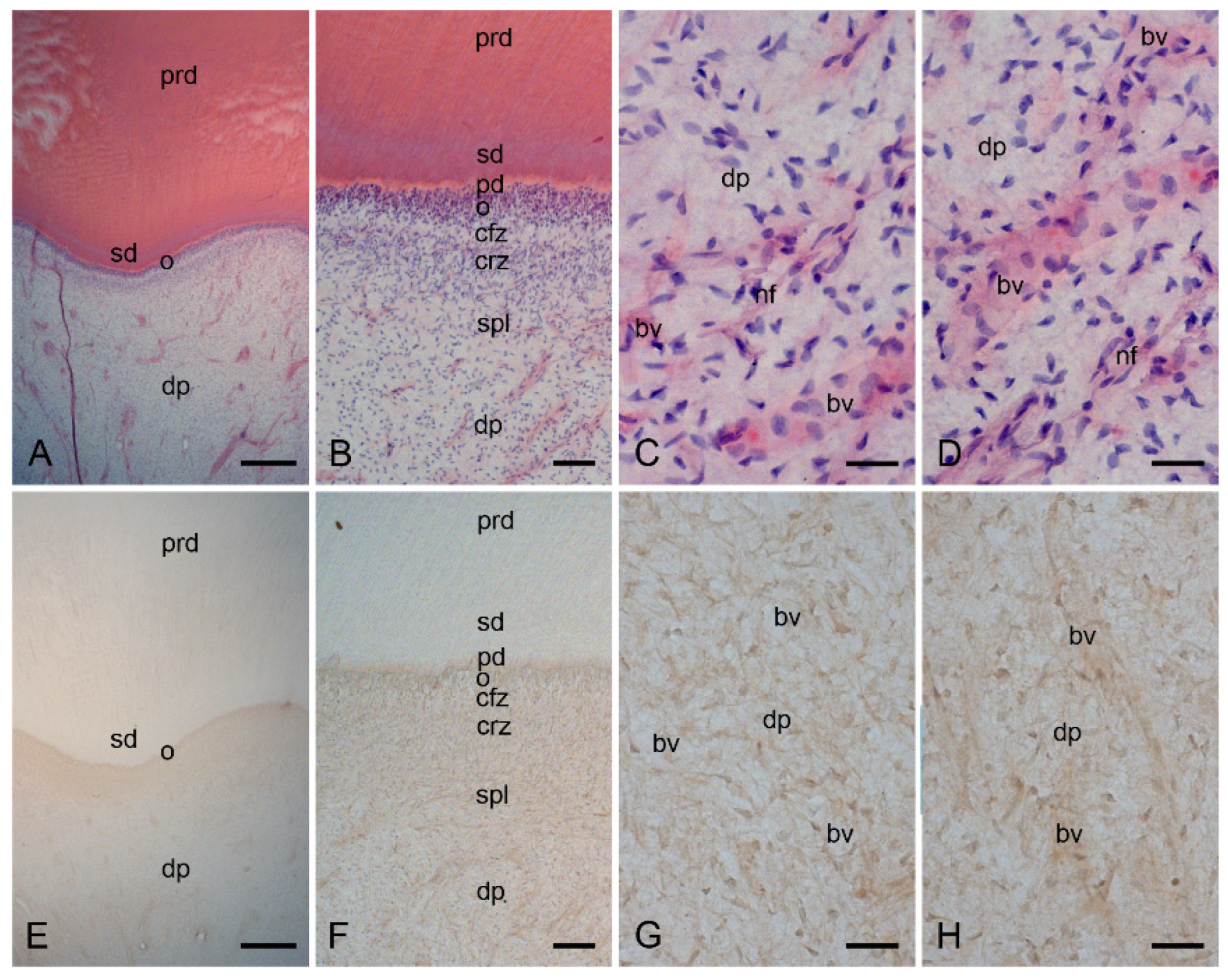
2.1. Characterization of the Healthy Human Dentin–Pulp Complex and the Expression of Mast Cell Tryptase (MCT) in Cells from Healthy Human Dental Pulp
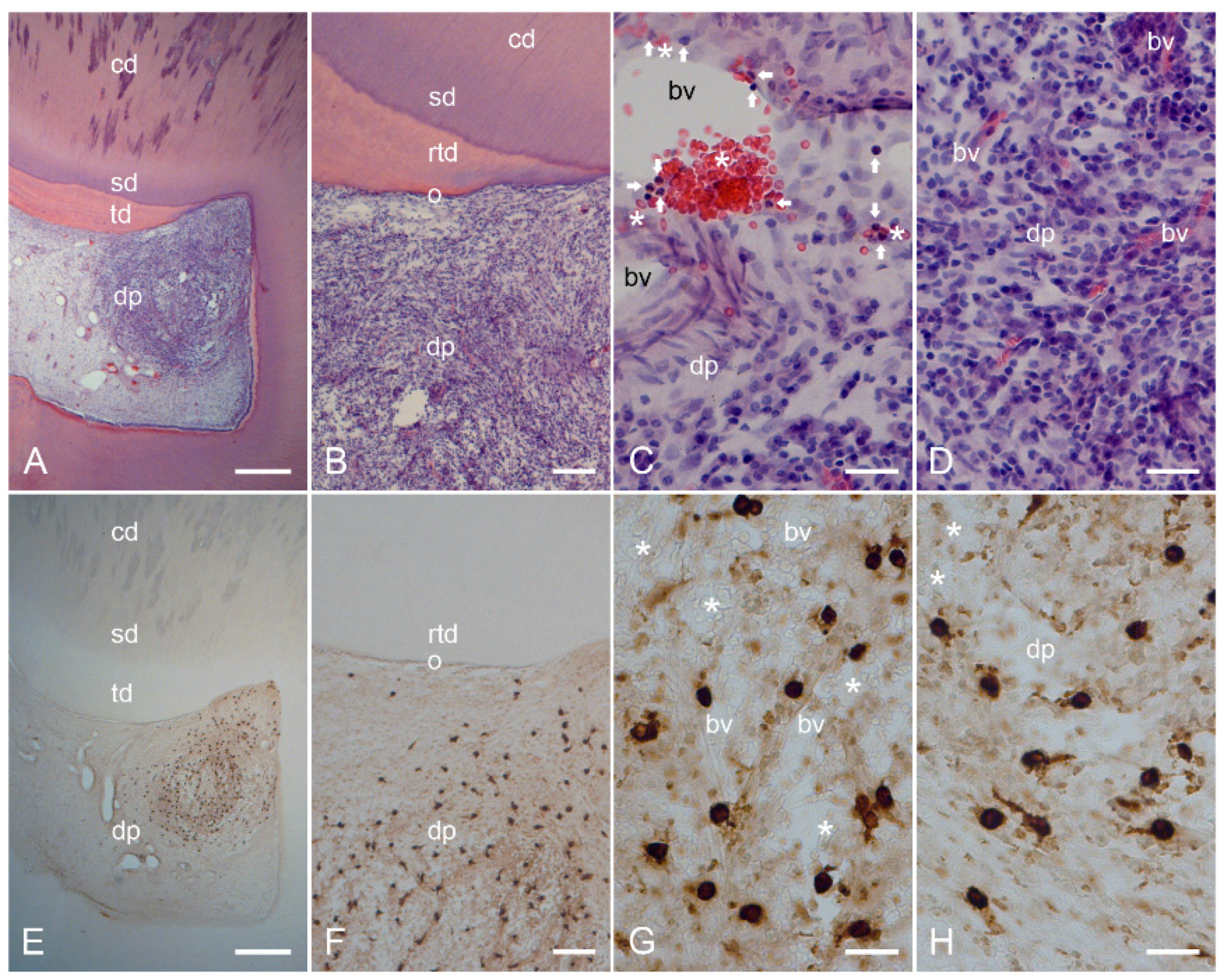
2.2. Characterization of Inflamed Human Dentin–Pulp Complex and the Expression of Mast Cell Tryptase (MCT) in Cells from Inflamed Human Dental Pulp
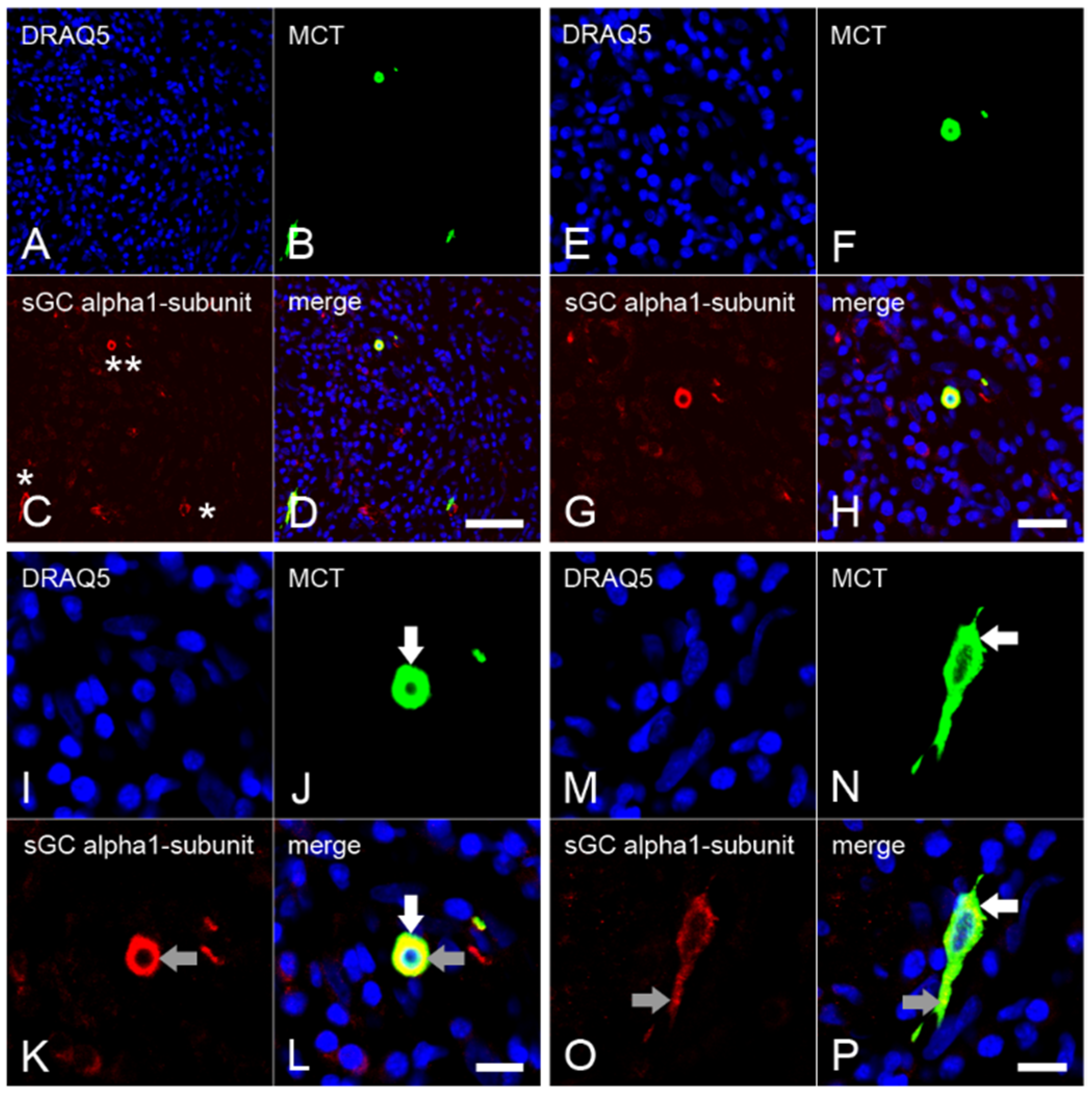
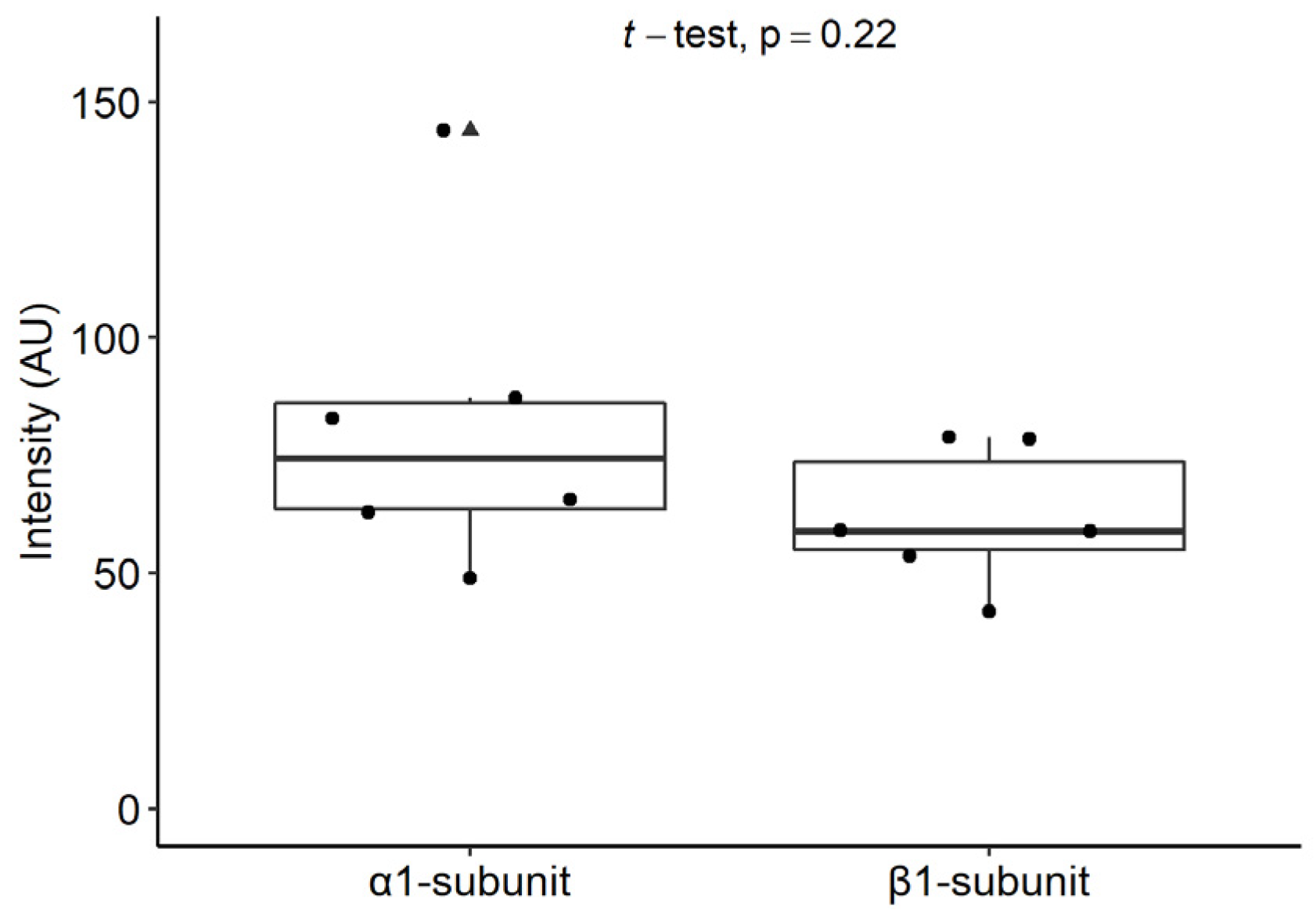
2.3. Expression of the α1 Subunit of sGC in Mast Cells from Inflamed Dental Pulp
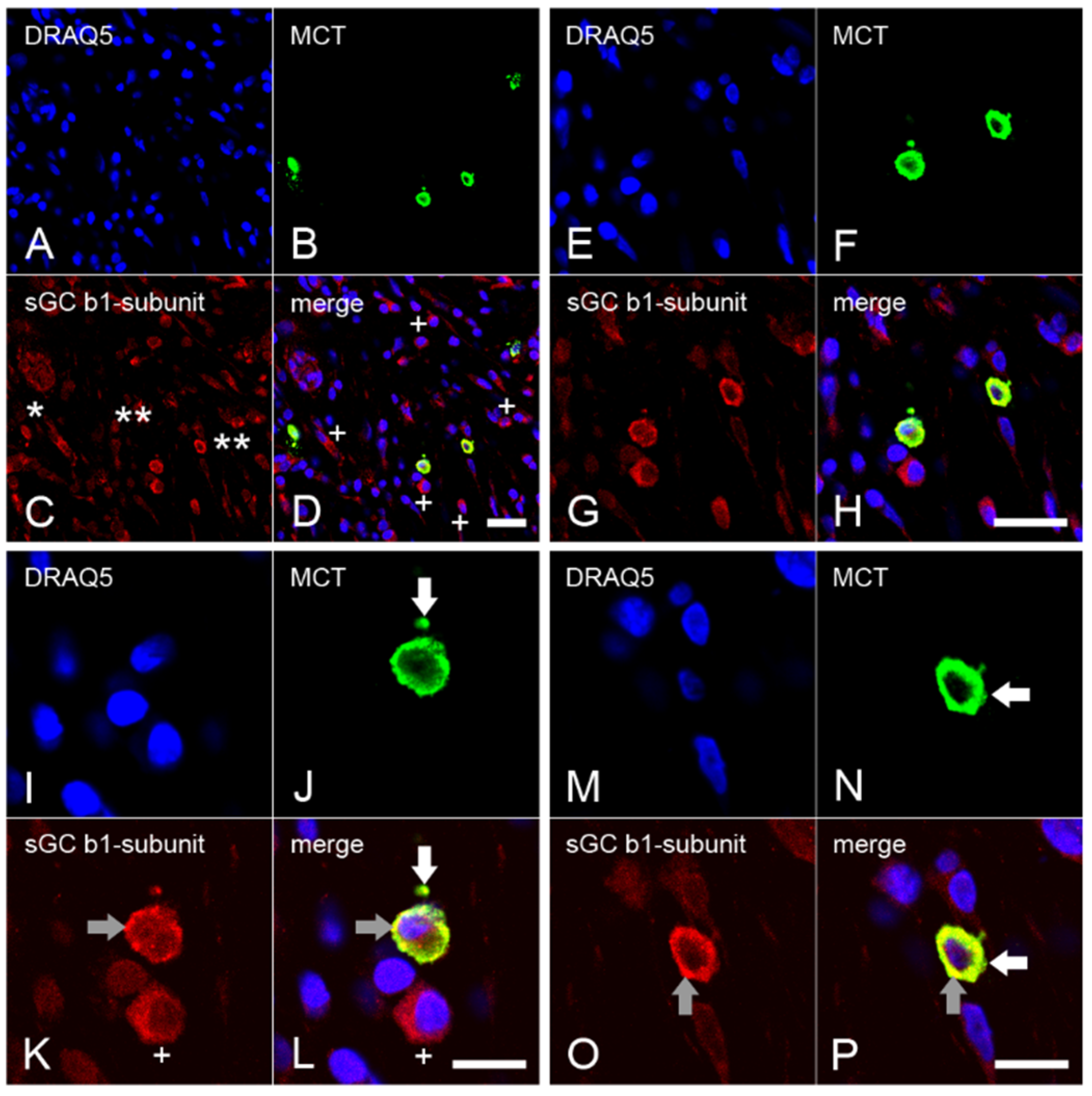
2.4. Expression of the β1 Subunit of sGC in Mast Cells from Inflamed Dental Pulp
2.5. Immunohistochemical Controls
2.6. Quantification of the Data and Statistical Analysis
2.6.1. Counting of Mast Cells in Healthy and Inflamed Dental Pulp
2.6.2. Staining Intensities of the α1 and β1 Subunits of sGC in Mast Cells
3. Discussion
4. Materials and Methods
4.1. Ethics Statement on the Collection of Human Molars
4.2. The Clinical Evaluation of Human Molars
4.3. Tissue Preparation
4.4. Histopathological Evaluation of Healthy and Inflamed Human Dental Pulp
4.5. Specificity of sGC α1 Subunit and β1 Subunit Antibodies
4.6. Immunohistochemical Methods
4.6.1. Avidin–Biotin–Peroxidase Complex Method
4.6.2. Immunofluorescence Double Staining Method
4.7. Quantification of the Data and Statistical Analysis
4.7.1. Counting of Mast Cells in the Inflamed Dental Pulp
4.7.2. Measurement of Immunofluorescence Staining Intensities
4.7.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friebe, A.; Koesling, D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ. Res. 2003, 93, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Murad, F. Nitric oxide and cyclic guanosine monophosphate signaling in the eye. Can. J. Ophthalmol. 2008, 43, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.P.; Schmidt, P.M.; Nedvetsky, P.I.; Nedvetskaya, T.Y.; HS, A.K.; Meurer, S.; Deile, M.; Taye, A.; Knorr, A.; Lapp, H.; et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Investig. 2006, 116, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T. Deconstructing endothelial dysfunction: Soluble guanylyl cyclase oxidation and the NO resistance syndrome. J. Clin. Investig. 2006, 116, 2330–2332. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Nguyen, A.T.; Miller, M.P.; Hahn, S.A.; Sparacino-Watkins, C.; Jobbagy, S.; Carew, N.T.; Cantu-Medellin, N.; Wood, K.C.; Baty, C.J.; et al. Cytochrome b5 Reductase 3 Modulates Soluble Guanylate Cyclase Redox State and cGMP Signaling. Circ. Res. 2017, 121, 137–148. [Google Scholar] [CrossRef]
- Shah, R.C.; Sanker, S.; Wood, K.C.; Durgin, B.G.; Straub, A.C. Redox regulation of soluble guanylyl cyclase. Nitric Oxide 2018, 76, 97–104. [Google Scholar] [CrossRef]
- Murad, F. Shattuck Lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef]
- Münzel, T.; Feil, R.; Mülsch, A.; Lohmann, S.M.; Hofmann, F.; Walter, U. Physiology and pathophysiology of vascular signaling controlled by cyclic guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. Circulation 2003, 108, 2172–2183. [Google Scholar] [CrossRef]
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011, 123, 2263–2273. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Foster, P.; Scotland, R.S.; McLean, P.G.; Mathur, A.; Perretti, M.; Moncada, S.; Hobbs, A.J. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of Pselectin expression and leukocyte recruitment. Proc. Natl. Acad. Sci. USA 2004, 101, 1386–1391. [Google Scholar] [CrossRef]
- Friebe, A.; Koesling, D. The function of NO-sensitive guanylyl cyclase: What we can learn from genetic mouse models. Nitric Oxide 2009, 21, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Koesling, D.; Mergia, E.; Russwurm, M. Physiological Functions of NO-Sensitive Guanylyl Cyclase Isoforms. Curr. Med. Chem. 2016, 23, 2653–2665. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160–1171.e5. [Google Scholar] [CrossRef] [PubMed]
- St John, A.L.; Rathore, A.P.S.; Ginhoux, F. New perspectives on the origins and heterogeneity of mast cells. Nat. Rev. Immunol. 2022, 23, 55–68. [Google Scholar] [CrossRef]
- Voehringer, D. Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 2013, 13, 362–375. [Google Scholar] [CrossRef]
- Palker, T.J.; Dong, G.; Leitner, W.W. Mast cells in innate and adaptive immunity to infection. Eur. J. Immunol. 2010, 40, 13–18. [Google Scholar] [CrossRef]
- Piliponsky, A.M.; Romani, L. The contribution of mast cells to bacterial and fungal infection immunity. Immunol. Rev. 2018, 282, 188–197. [Google Scholar] [CrossRef]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef]
- Abraham, S.N.; St John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.Y.; Tergaonkar, V.; Kumar, A.P.; Ahn, K.S. Mast cells: Therapeutic targets for COVID-19 and beyond. IUBMB Life. 2021, 73, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef]
- Forsythe, P.; Gilchrist, M.; Kulka, M.; Befus, A.D. Mast cells and nitric oxide: Control of production, mechanisms of response. Int. Immunopharmacol. 2001, 1, 1525–1541. [Google Scholar] [CrossRef]
- Gilchrist, M.; McCauley, S.D.; Befus, A.D. Expression, localization, and regulation of NOS in human mast cell lines: Effects on leukotriene production. Blood 2004, 104, 462–469. [Google Scholar] [CrossRef]
- Bidri, M.; Féger, F.; Varadaradjalou, S.; Ben Hamouda, N.; Guillosson, J.J.; Arock, M. Mast cells as a source and target for nitric oxide. Int. Immunopharmacol. 2001, 1, 1543–1558. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide: A regulator of mast cell activation and mast cell-mediated inflammation. Clin. Exp. Immunol. 2002, 129, 4–10. [Google Scholar] [CrossRef]
- Davis, B.J.; Flanagan, B.F.; Gilfillan, A.M.; Metcalfe, D.D.; Coleman, J.W. Nitric oxide inhibits IgE-dependent cytokine production and Fos and Jun activation in mast cells. J. Immunol. 2004, 173, 6914–6920. [Google Scholar] [CrossRef]
- Yip, K.H.; Huang, Y.; Leung, F.P.; Lau, H.Y. Cyclic guanosine monophosphate dependent pathway contributes to human mast cell inhibitory actions of the nitric oxide donor, diethylamine NONOate. Eur. J. Pharmacol. 2010, 632, 86–92. [Google Scholar] [CrossRef]
- Stasch, J.P.; Schmidt, P.; Alonso-Alija, C.; Apeler, H.; Dembowsky, K.; Haerter, M.; Heil, M.; Minuth, T.; Perzborn, E.; Pleiss, U.; et al. NO- and haem-independent activation of soluble guanylyl cyclase: Molecular basis and cardiovascular implications of a new pharmacological principle. Br. J. Pharmacol. 2002, 136, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Krieg, T.; Liu, Y.; Rütz, T.; Methner, C.; Yang, X.M.; Dost, T.; Felix, S.B.; Stasch, J.P.; Cohen, M.V.; Downey, J.M. BAY 58-2667, a nitric oxide-independent guanylyl cyclase activator, pharmacologically post-conditions rabbit and rat hearts. Eur. Heart J. 2009, 30, 1607–1613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Follmann, M.; Griebenow, N.; Hahn, M.G.; Hartung, I.; Mais, F.J.; Mittendorf, J.; Schäfer, M.; Schirok, H.; Stasch, J.P.; Stoll, F.; et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew. Chem. Int. Ed. Engl. 2013, 52, 9442–9462. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P.; Follmann, M.; Becker-Pelster, E.; Hahn, M.G.; Meier, C.; Freitas, C.; Roessig, L.; Stasch, J.P. Soluble GC stimulators and activators: Past, present and future. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Jenkinson, H.F. Beyond the oral microbiome. Environ. Microbiol. 2011, 13, 3077–3087. [Google Scholar] [CrossRef]
- Farges, J.C.; Alliot-Licht, B.; Baudouin, C.; Msika, P.; Bleicher, F.; Carrouel, F. Odontoblast control of dental pulp inflammation triggered by cariogenic bacteria. Front. Physiol. 2013, 4, 326. [Google Scholar] [CrossRef]
- Kim, D.; Barraza, J.P.; Arthur, R.A.; Hara, A.; Lewis, K.; Liu, Y.; Scisci, E.L.; Hajishengallis, E.; Whiteley, M.; Koo, H. Spatial mapping of polymicrobial communities reveals a precise biogeography associated with human dental caries. Proc. Natl. Acad. Sci. USA 2020, 117, 12375–12386. [Google Scholar] [CrossRef]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory response mechanisms of the dentine-pulp complex and the periapical tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef] [PubMed]
- Love, R.M.; Jenkinson, H.F. Invasion of dentinal tubules by oral bacteria. Crit. Rev. Oral Biol. Med. 2002, 13, 171–183. [Google Scholar] [CrossRef]
- Hahn, C.L.; Liewehr, F.R. Relationships between caries bacteria, host responses, and clinical signs and symptoms of pulpitis. J. Endod. 2007, 33, 213–219. [Google Scholar] [CrossRef]
- Jontell, M.; Okiji, T.; Dahlgren, U.; Bergenholtz, G. Immune defense mechanisms of the dental pulp. Crit. Rev. Oral Biol. Med. 1998, 9, 179–200. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Farges, J.C.; Lacerda-Pinheiro, S.; Six, N.; Jegat, N.; Decup, F.; Septier, D.; Carrouel, F.; Durand, S.; Chaussain-Miller, C.; et al. Inflammatory and immunological aspects of dental pulp repair. Pharmacol. Res. 2008, 58, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Smith, A.J. Molecular mediators of pulp inflammation and regeneration. Endod. Top. 2013, 28, 90–105. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Krämer, S. Human mast cells, bacteria, and intestinal immunity. Immunol. Rev. 2007, 217, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.Y.; St John, A.L.; Abraham, S.N. Plasticity in mast cell responses during bacterial infections. Curr. Opin. Microbiol. 2012, 15, 78–84. [Google Scholar] [CrossRef] [PubMed]
- West, P.W.; Bulfone-Paus, S. Mast cell tissue heterogeneity and specificity of immune cell recruitment. Front. Immunol. 2022, 13, 932090. [Google Scholar] [CrossRef] [PubMed]
- Zachrisson, B.U. Mast cells in human dental pulp. Arch. Oral Biol. 1971, 16, 555–556. [Google Scholar] [CrossRef]
- Miller, G.S.; Sternberg, R.N.; Piliero, S.J.; Rosenberg, P.A. Histologic identification of mast cells in human dental pulp. Oral Surg. Oral Med. Oral Pathol. 1978, 46, 559–566. [Google Scholar] [CrossRef]
- Erdek, Ö.; Bloch, W.; Rink-Notzon, S.; Roggendorf, H.C.; Uzun, S.; Meul, B.; Koch, M.; Neugebauer, J.; Deschner, J.; Korkmaz, Y. Inflammation of the Human Dental Pulp Induces Phosphorylation of eNOS at Thr495 in Blood Vessels. Biomedicines 2022, 10, 1586. [Google Scholar] [CrossRef]
- Korkmaz, Y.; Lang, H.; Beikler, T.; Cho, B.; Behrends, S.; Bloch, W.; Addicks, K.; Raab, W.H. Irreversible inflammation is associated with decreased levels of the α1-, β1- and α2-subunit of sGC in human odontoblasts. J. Dent. Res. 2011, 90, 517–522. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Galdiero, M.R.; Del Prete, D.; Cassatella, M.A.; Garlanda, C.; Mantovani, A. Neutrophils in innate and adaptive immunity. Semin. Immunopathol. 2013, 35, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophils at the crossroads of innate and adaptive immunity. J. Leukoc. Biol. 2020, 108, 377–396. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Dudeck, J.; Kotrba, J.; Immler, R.; Hoffmann, A.; Voss, M.; Alexaki, V.I.; Morton, L.; Jahn, S.R.; Katsoulis-Dimitriou, K.; Winzer, S.; et al. Directional mast cell degranulation of tumor necrosis factor into blood vessels primes neutrophil extravasation. Immunity 2021, 54, 468–483. [Google Scholar] [CrossRef]
- He, S.; Peng, Q.; Walls, A.F. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: Selective enhancement of eosinophil recruitment by histamine. J. Immunol. 1997, 159, 6216–6225. [Google Scholar] [CrossRef]
- Huang, C.; Friend, D.S.; Qiu, W.T.; Wong, G.W.; Morales, G.; Hunt, J.; Stevens, R.L. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 1998, 160, 1910–1919. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Clermont, G.; Vodovotz, Y.; Chow, C.C. The dynamics of acute inflammation. J. Theor. Biol. 2004, 230, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Abudukelimu, A.; Barberis, M.; Redegeld, F.A.; Sahin, N.; Westerhoff, H.V. Predictable Irreversible Switching Between Acute and Chronic Inflammation. Front. Immunol. 2018, 9, 1596. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.B.; Storkus, W.J. Chronic inflammation and immunologic-based constraints in malignant disease. Immunotherapy 2011, 3, 1265–1274. [Google Scholar] [CrossRef]
- Rodewald, H.R.; Feyerabend, T.B. Widespread immunological functions of mast cells: Fact or fiction? Immunity 2012, 37, 13–24. [Google Scholar] [CrossRef]
- St John, A.L.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef]
- Li, H.; Förstermann, U. Pharmacological prevention of eNOS uncoupling. Curr. Pharm. Des. 2014, 20, 3595–3606. [Google Scholar] [CrossRef] [PubMed]
- Karbach, S.; Wenzel, P.; Waisman, A.; Munzel, T.; Daiber, A. eNOS uncoupling in cardiovascular diseases-the role of oxidative stress and inflammation. Curr. Pharm. Des. 2014, 20, 3579–3594. [Google Scholar] [CrossRef]
- Swindle, E.J.; Metcalfe, D.D. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol. Rev. 2007, 217, 186–205. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Faul, E.M.; Ghosh, A.; Stuehr, D.J. NO rapidly mobilizes cellular heme to trigger assembly of its own receptor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115774119. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, Y.; Puladi, B.; Galler, K.; Kämmerer, P.W.; Schröder, A.; Gölz, L.; Sparwasser, T.; Bloch, W.; Friebe, A.; Deschner, J. Inflammation in the Human Periodontium Induces Downregulation of the α1- and β1-Subunits of the sGC in Cementoclasts. Int. J. Mol. Sci. 2021, 22, 539. [Google Scholar] [CrossRef]
- Korkmaz, Y.; Roggendorf, H.C.; Siefer, O.G.; Seehawer, J.; Imhof, T.; Plomann, M.; Bloch, W.; Friebe, A.; Huebbers, C.U. Downregulation of the α1- and β1-subunit of sGC in Arterial Smooth Muscle Cells of OPSCC Is HPV-Independent. J. Dent. Res. 2018, 97, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Mergia, E.; Dangel, O.; Lange, A.; Koesling, D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc. Natl. Acad. Sci. USA 2007, 104, 7699–7704. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz, Y.; Plomann, M.; Puladi, B.; Demirbas, A.; Bloch, W.; Deschner, J. Dental Pulp Inflammation Initiates the Occurrence of Mast Cells Expressing the α1 and β1 Subunits of Soluble Guanylyl Cyclase. Int. J. Mol. Sci. 2023, 24, 901. https://doi.org/10.3390/ijms24020901
Korkmaz Y, Plomann M, Puladi B, Demirbas A, Bloch W, Deschner J. Dental Pulp Inflammation Initiates the Occurrence of Mast Cells Expressing the α1 and β1 Subunits of Soluble Guanylyl Cyclase. International Journal of Molecular Sciences. 2023; 24(2):901. https://doi.org/10.3390/ijms24020901
Chicago/Turabian StyleKorkmaz, Yüksel, Markus Plomann, Behrus Puladi, Aysegül Demirbas, Wilhelm Bloch, and James Deschner. 2023. "Dental Pulp Inflammation Initiates the Occurrence of Mast Cells Expressing the α1 and β1 Subunits of Soluble Guanylyl Cyclase" International Journal of Molecular Sciences 24, no. 2: 901. https://doi.org/10.3390/ijms24020901
APA StyleKorkmaz, Y., Plomann, M., Puladi, B., Demirbas, A., Bloch, W., & Deschner, J. (2023). Dental Pulp Inflammation Initiates the Occurrence of Mast Cells Expressing the α1 and β1 Subunits of Soluble Guanylyl Cyclase. International Journal of Molecular Sciences, 24(2), 901. https://doi.org/10.3390/ijms24020901






