Structural Modifications of siRNA Improve Its Performance In Vivo
Abstract
:1. Introduction
2. Mechanism of RNA Interference
3. Chemical Modifications
| # | Structure of siRNA—Sense Strand 5’-3’ (S) Above, Antisense Strand 3’-5’ (AS); Below, Pattern of Chemical Modifications 1,2 | In Vitro | In Vivo | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Cells/ Target | Delivery 3 | Efficiency 4 ↑-Increase ↓-Decline | Model/ Target | Dose, Mode of Adm 5 | Efficiency ↑-Increase ↓-Decline | |||
| 1 | ss-siRNA, n/m | HaCaT/F3 | Lf2000 | 87%, mRNA | - | - | - | [41] |
| siRNA, n/m | 90%(↑3%), mRNA | - | - | - | ||||
| 2 | ss-siRNA, (boranophosphate)  | HeLa/EGFP | Oligofectami-ne, 25 nM | 100%, protein | - | - | - | [28] |
| siRNA, n/m | 70%(↓30%), protein | - | - | - | ||||
| 3 | ss-siRNA | HeLa/ PTEN | Lf2000 | IC50 2 nM, mRNA | - | - | - | [42] |
siRNA  | IC50 0.2 nM(↑×10), mRNA | - | - | - | ||||
 R:  | Prim. hepatocytes/ApoC III | Electroporation | IC50 2 mcM, mRNA | Transge-nic mice, liver/ ApoC III | 14 mg/kg, s.c. | 65%, IC50 ~10 mg/kg, mRNA | ||
| 4 | ss-siRNA  | MCF-7/PR | RNAiMAX, 50 nM | ~85%, mRNA | - | - | - | [43] |
| HEK 293/SIN3A | 5%, mRNA | |||||||
siRNA  | MCF-7/PR | ~90%(↑5%), mRNA | - | - | - | |||
| HEK 293/ SIN3A | ~60%(↑55%), mRNA | |||||||
| 5 | ss-siRNA  | Hepa 1–6/CTNNB1 | RNAiMAX | IC50 0.07 nM | Mice, liver/ CTNNB1 | 0.05 mg/kg i.v. with LNP | 50%, mRNA | [44] |
siRNA  | IC50 0.12 nM (↓×1.7) | 65%(↑15%), mRNA | ||||||
4. Single-Stranded siRNAs
5. Segmented siRNAs (sisiRNAs)
| # | Structure of siRNA—Sense Strand 5’-3’ (S); Above, Antisense Strand 3’-5’ (AS); Below, Pattern of Chemical Modifications 1 | In Vitro | In Vivo | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Cells/ Target | Delivery 2 | Efficiency 3 ↑-Increase ↓-Decline | Model/ Target | Dose, Mode of Adm 4 | Efficiency ↑-Increase ↓-Decline | |||
| 6 | siRNA  | MiaPaca-2/EGFP | Lf2000 5 nM | ~62%, mRNA | Xeno-graft MiaPaca−2 in mice/ EGFP | 0.25 mg/kg, s.c. within 1 week, osmotic pump | 50%, mRNA | [22] |
| sisiRNA ss nick  | ~50% (↓12%), mRNA | 50%, mRNA | ||||||
6. Circular Small Interfering RNAs (csiRNA)
7. Dicer Substrates
| # | Structure of siRNA—Sense Strand 5’-3’ (S) Above, Antisense Strand 3’-5’ (AS) Below. Pattern of Chemical Modifications 1,2,3 | In Vitro | In Vivo | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cells/ Target | Delivery 4 | Efficiency 5 ↑-Increase ↓-Decline | Model/ Target | Dose, Mode of Adm 6 | Efficiency ↑-Increase ↓-Decline | ||||
| 7 | siRNA, n/m | HEK 293/EGFP | Lf2000 20 nM | ~47%, IC50 ~30 nM, protein | - | - | - | [57] | |
| DsiRNA (25/27), n/m | ~97%(↑50%), IC50 175 pM (↑×170), protein | - | - | - | |||||
| 8 | Palmitic acid (C16)-siRNA, n/m   | Hela/Rluc | Lf2000 1 nM | 87%, Rluc exp | - | - | - | [58] | |
Palmitic acid (C16)-siRNA (21/23), n/m   | 93% (↑6%), Rluc exp | - | - | - | |||||
Palmitic acid (C16)-DsiRNA (23/25), n/m  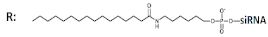 | 91% (↑4%), Rluc exp | - | - | - | |||||
Palmitic acid (C16)-DsiRNA (25/27), n/m  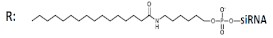 | 92% (↑5%), Rluc exp | - | - | - | |||||
| 9 | DsiRNA (25/27) 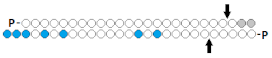 | HEK293/ CCR2 | RNAiMAX 1 nM | 80%, mRNA | Rat, ganglion/CCR2 | 5 mcg, 2× intrathecal/Transductin | 71%, mRNA | [59] | |
| 10 | «RNA aptamer (A-1) to gp120»- DsiRNA 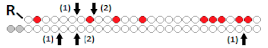 | - | - | - | - | HIV-1 in miceRag2−/−γc−/− | 0.38 mg/kg, 8× i.v. | 90%, mRNA (PBMC), 105 fold (in plasma) | [60] |
| 1 | α- tocopherol -DsiRNA 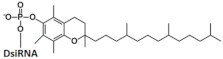  | - | - | - | - | Mice, liver/ApoB | 32 mg/kg, i.v. | 80%, IC50 2 mg/kg, mRNA | [61] |
| 13 | DsiRNA 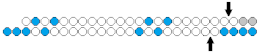 | HeLa/ LDHA | RNAiMax 1 nM | ~96.9%, mRNA | - | - | - | [72] | |
Tetra-looped DsiRNA 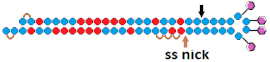  | ~98.5% (↑1.6%), IC50 20 пM, mRNA | Mice, liver/ LDHA | 10 mg/kg, i.v. | 50%, mRNA | |||||
| 14 | siRNA, n/m | Hela/Rluc | Lf2000 50 nM | 48%, Rluc exp | - | - | - | [62] | |
| DsiRNA (27/27, “blunt” ends), n/m | 83% (↑35%) | - | - | - | |||||
Cholesterol-DsiRNA (27/27, “blunt” ends), n/m  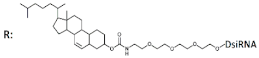 | - 600 nM | 30%, Rluc exp | - | - | - | ||||
| 15 | Palmitic acid (C16)-siRNA, n/m  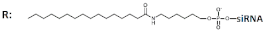 | GCIY- eGFP/ EGFP | RNAiMAX 100 nM | 75%, mRNA | Xenograft GCIY- eGFP in nude mice/ EGFP | 3 nmol, i.t./Invivofectamine 2.0 | 62% | [63] | |
Palmitic acid (C16)-DsiRNA (27/27, “blunt” ends), n/m   | 77%(↑2%), mRNA | 70%(↑8%) | |||||||
8. Multimeric siRNAs
8.1. Linear Multimeric siRNA Structures
| # | Structure of siRNA—Sense Strand 5’-3’ (S) Above, Antisense Strand 3’-5’ (AS) Below. Pattern of Chemical Modifications 1,2,3 | In Vitro | In Vivo | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Cells/ Target | Delivery 4 | Efficiency 5 ↑-Increase ↓-Decline | Model/ Target | Dose, mode of Adm 6 | Cells/ Target | |||
| 16 | siRNA mix 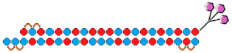  | Prim. hepatocytes/TTR/FVII | - | IC50 0.1 nM (TTR) IC50 ~0.25 nM (FVII) | Mice liver/ TTR/ FVII | 3 mg/kg, s.c. | ~98%/~98% | [76] |
Dimeric siRNA 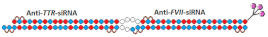 | IC50 0.4 nM (↓×4)/~1 nM(↓×4) | 3 mg/kg, s.c. | 92%(↓6%)/92% (↓6%) | |||||
Dimeric siRNA 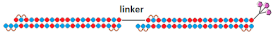 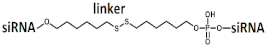 | IC50 1 nM (↓×10)/IC50 1 nM (↓×4) | 3 mg/kg, s.c. | 73%(↓25%)/~82%(↓16%) | |||||
| 17 | siRNA 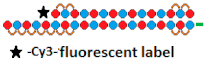 | HeLa/HTT | RNAiMax 0.1 nM | 70%, IC50 ~25 pM, mRNA | - | - | - | [77] |
Dimeric siRNA 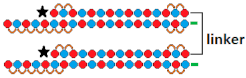  | 80%, (↑10%) IC50 ~10 pM (↑×2.5), mRNA | Mice, brain/ HTT | 23.75 mg/kg, i.c.v. bilaterally | >90%, protein in the hippocampus | ||||
| >70%, protein, in the cortex | ||||||||
| M.Cynomolgus brain | 0.017 mg/kg, i.c.v. | >90%, protein, in the cortex | ||||||
| 18 | GalNAc-siRNA  | - | - | - | Mice, liver/FVII | 50 mg/kg, s.c. | 85%, protein | [82] |
GalNAc-dimeric siRNA  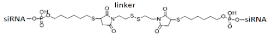 | - | - | - | 75%(↓10%), protein | ||||
| 19 | GalNAc-siRNA mix | - | - | - | Mice, liver/FVII/ApoB/TTR | 50 mg/kg, s.c. | 50%/30%/97% | |
GalNAc-trimeric siRNA 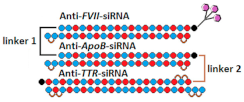  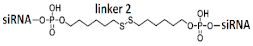 | - | - | - | 62% (↑12%)/30%/97% | ||||
| 20 | siRNA  | T24/BIRC5/BCL2 | Lf2000 10 nM | 55%/85%,mRNA | - | - | - | [78] |
Dimeric siRNA  | 66% (↑11%)/83% (↓2%), mRNA | - | - | - | ||||
| 21 | siRNA mix | Hela/LMNA/ TIG3 | Lf2000 10 nM | 90%/98%, mRNA | - | - | - | [79] |
Dimeric siRNA (34 bp), n/m  | 98% (↑8%)/96% (↓2%), mRNA | - | - | - | ||||
| 22 | siRNA  | KB-8-5/MDR1 | Oligofectamine | IC50 23 nM | - | - | - | [80] |
Dimeric siRNA  | IC50 10 nM (↑×2.3) | - | - | - | ||||
Trimeric siRNA  | MDR1, IC50 5 nM (↑×4.6) | - | - | - | ||||
| 23 | Cholesterol-siRNA   | KB-8-5-MDR1-GFP/MDR1 | Lf2000 | IC50 29 nM | Xenograft KB-8-5 in SCID mice/MDR1 | 8.5 mg/kg i.v. | 60%, protein | [8] |
| - 5 mcM | 48%, protein | |||||||
Cholesterol—trimeric siRNA  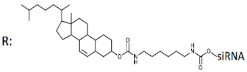 | Lf2000 | IC50 16 nM (↑×1.8) | 0% (↓60%), protein | |||||
| - 5 mcM | 2%(↓46%), protein | |||||||
8.2. Branched Multimeric siRNA Structures
| # | Structure of siRNA—Sense Strand 5’-3’ (S) Above, Antisense Strand 3’-5’ (AS) Below, Pattern of Chemical Modifications 1,2,3 | In Vitro | In Vivo | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Cells/ Target | Delivery 4 | Efficiency 5 ↑-Increase ↓-Decline | Model/ Target | Dose, Mode of Adm 6 | Cells/ Target | |||
| 24 | siRNA, n/m | Hela/ BIRC5 | Lf2000 | IC50 3.89 nM, mRNA | - | - | - | [83] |
Branched siRNA (36 n.), n/m  | IC50 0.83 nM (↑×4.7), mRNA | - | - | - | ||||
| 25 | siRNA mix | HeLa/LMNA/ DBP/TIG3 | Lf2000 | 60%/47%/82%, Rluc exp | - | - | - | [84] |
Branched siRNA (38 n.) 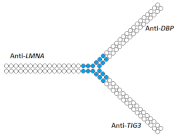 | 68% (↑8%)/62% (↑15%)/85% (↑3%) | - | - | - | ||||
| 26 | siRNA mix | Hela/BIRC5/CTNNB/MET | Lf2000 | 76%/77%/90%, Rluc exp | - | - | - | [85] |
Branched siRNA (38 n.), n/m  | 85% (↑9%)/45% (↑32%)/77% (↓13%)%, Rluc exp | - | - | - | ||||
| 27 | siRNA, non-modified | Hepa 1–6/ApoB | PEI-Gal 100 nM | 50%, mRNA | Mice, liver/ApoB | 6 mg/kg, i.v. with PEI-Gal | 33%, mRNA | [86] |
Branched siRNA (32 n.), n/m 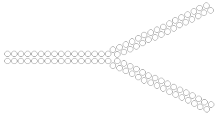 | ApoB 70% (↑20%), mRNA | 63% (↑30%), mRNA | ||||||
| 28 | siRNA, n/m | Hela/Rluc | RNAiMax | 78%, Rluc exp | - | - | - | [87] |
Branched siRNA (21 n.), n/m 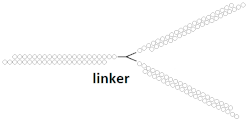 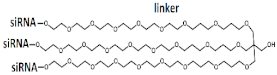 | 81% (↑3%), Rluc exp | - | - | - | ||||
| 29 | siRNA mix | Hela/BIRC5/CTNNB/ STAT3/ MET | PEI 25 nM | 39%/30%/40%/30%, mRNA, 1 d. | - | - | - | [88] |
Branched siRNA (38 n.)  | 61% (↑22%)/40% (↑10%)/70% (↑30%)/62% (↑32%), mRNA | - | - | - | ||||
| 30 | siRNA, n/m | SMMC-7721/ BIRC5/BCL2 | Lf2000 | 80%/70%, protein, 2 d. | - | - | - | [89] |
Dimeric siRNA 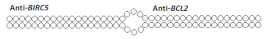 | 48% (↓32%)/45% (↓25%), protein | - | - | - | ||||
Dimeric siRNA 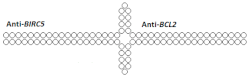 | 46% (↓34%)/57% (↓13%), protein | - | - | - | ||||
Trimeric siRNA 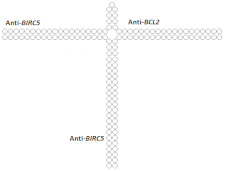 | 68% (↓12%)/48% (↓22%), protein | - | - | - | ||||
9. Conclusions
10. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Inclisiran: First Approval. Drugs 2021, 81, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Vutrisiran: First Approval. Drugs 2022, 82, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, X.; Li, S.; Wang, P.; Fang, J. Liver-Targeted Delivery of Oligonucleotides with N-Acetylgalactosamine Conjugation. ACS Omega 2021, 6, 16259–16265. [Google Scholar] [CrossRef]
- Springer, A.D.; Dowdy, S.F. GalNAc-SiRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Chernikov, I.V.; Vlassov, V.V.; Chernolovskaya, E.L. Current Development of SiRNA Bioconjugates: From Research to the Clinic. Front. Pharmacol. 2019, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Benizri, S.; Gissot, A.; Martin, A.; Vialet, B.; Grinstaff, M.W.; Barthélémy, P. Bioconjugated Oligonucleotides: Recent Developments and Therapeutic Applications. Bioconjug. Chem. 2019, 30, 366–383. [Google Scholar] [CrossRef]
- Chernikov, I.V.; Gladkikh, D.V.; Karelina, U.A.; Meschaninova, M.I.; Ven’yaminova, A.G.; Vlassov, V.V.; Chernolovskaya, E.L. Trimeric Small Interfering RNAs and Their Cholesterol-Containing Conjugates Exhibit Improved Accumulation in Tumors, but Dramatically Reduced Silencing Activity. Molecules 2020, 25, 1877. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-Nucleotide RNAs Mediate RNA Interference in Cultured Mammalian Cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Song, M.; Rossi, J.J. The Effect of Dicer Knockout on RNA Interference Using Various Dicer Substrate Interfering RNA Structures. bioRxiv Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Tomari, Y.; Matranga, C.; Haley, B.; Martinez, N.; Zamore, P.D. A Protein Sensor for SiRNA Asymmetry. Science 2004, 306, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Kini, H.K.; Walton, S.P. Effect of SiRNA Terminal Mismatches on TRBP and Dicer Binding and Silencing Efficacy. FEBS J. 2009, 276, 6576–6585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, F.; Sonenberg, N.; Nagar, B. Structural Basis for 5′-Nucleotide Base-Specific Recognition of Guide RNA by Human AGO2. Nature 2010, 465, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.S.; Yan, S.; Farooq, A.; Han, A.; Zeng, L.; Zhou, M.M. Structure and Conserved RNA Binding of the PAZ Domain. Nature 2003, 426, 468–474. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Hutvágner, G.; Du, T.; Xu, Z.; Aronin, N.; Zamore, P.D. Asymmetry in the Assembly of the RNAi Enzyme Complex. Cell 2003, 115, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.I.; Katsura, A.; Yasuda, T.; Ueno, T.; Mano, H.; Sugimoto, K.; Miyazono, K. Small-RNA Asymmetry Is Directly Driven by Mammalian Argonautes. Nat. Struct. Mol. Biol. 2015, 22, 512–521. [Google Scholar] [CrossRef]
- Herrera-Carrillo, E.; Berkhout, B. Dicer-Independent Processing of Small RNA Duplexes: Mechanistic Insights and Applications. Nucleic Acids Res. 2017, 45, 10369–10379. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.P.; Karg, M.; Harwig, A.; Herrera-Carrillo, E.; Jongejan, A.; Van Kampen, A.; Berkhout, B. Mechanistic Insights on the Dicer-Independent AGO2-Mediated Processing of AgoshRNAs. RNA Biol. 2015, 12, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Deleavey, G.F.; Damha, M.J. Designing Chemically Modified Oligonucleotides for Targeted Gene Silencing. Chem. Biol. 2012, 19, 937–954. [Google Scholar] [CrossRef] [Green Version]
- Khvorova, A.; Watts, J.K. The Chemical Evolution of Oligonucleotide Therapies of Clinical Utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef]
- Bramsen, J.B.; Laursen, M.B.; Damgaard, C.K.; Lena, S.W.; Ravindra Babu, B.; Wengel, J.; Kjems, J. Improved Silencing Properties Using Small Internally Segmented Interfering RNAs. Nucleic Acids Res. 2007, 35, 5886–5897. [Google Scholar] [CrossRef]
- Mook, O.R.; Vreijling, J.; Wengel, S.; Wengel, J.; Zhou, C.; Chattopadhyaya, J.; Baas, F.; Fluiter, K. In Vivo Efficacy and Off-Target Effects of Locked Nucleic Acid (LNA) and Unlocked Nucleic Acid (UNA) Modified SiRNA and Small Internally Segmented Interfering RNA (SisiRNA) in Mice Bearing Human Tumor Xenografts. Artif. DNA PNA XNA 2010, 1, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Dande, P.; Prakash, T.P.; Sioufi, N.; Gaus, H.; Jarres, R.; Berdeja, A.; Swayze, E.E.; Griffey, R.H.; Bhat, B. Improving RNA Interference in Mammalian Cells by 4′-Thio-Modified Small Interfering RNA (SiRNA): Effect on SiRNA Activity and Nuclease Stability When Used in Combination with 2′-O-Alkyl Modifications. J. Med. Chem. 2006, 49, 1624–1634. [Google Scholar] [CrossRef]
- Laursen, M.B.; Pakula, M.M.; Gao, S.; Fluiter, K.; Mook, O.R.; Baas, F.; Langklær, N.; Wengel, S.L.; Wengel, J.; Kjems, J.; et al. Utilization of Unlocked Nucleic Acid (UNA) to Enhance SiRNA Performance in Vitro and in Vivo. Mol. Biosyst. 2010, 6, 862–870. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wang, X.; Ma, Y.; Liang, Z.; Yang, Z.; Cao, H. Site-Specific Modification Using the 2′-Methoxyethyl Group Improves the Specificity and Activity of SiRNAs. Mol. Ther.-Nucleic Acids 2017, 9, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Nauwelaerts, K.; Fisher, M.; Froeyen, M.; Lescrinier, E.; Van Aerschot, A.; Xu, D.; DeLong, R.; Kang, H.; Juliano, R.L.; Herdewijn, P. Structural Characterization and Biological Evaluation of Small Interfering RNAs Containing Cyclohexenyl Nucleosides. J. Am. Chem. Soc. 2007, 129, 9340–9348. [Google Scholar] [CrossRef]
- Takahashi, M.; Nagai, C.; Hatakeyama, H.; Minakawa, N.; Harashima, H.; Matsuda, A. Intracellular Stability of 2′-OMe-4′-Thioribonucleoside Modified SiRNA Leads to Long-Term RNAi Effect. Nucleic Acids Res. 2012, 40, 5787–5793. [Google Scholar] [CrossRef]
- Hall, A.H.S.; Wan, J.; Spesock, A.; Sergueeva, Z.; Shaw, B.R.; Alexander, K.A. High Potency Silencing by Single-Stranded Boranophosphate SiRNA. Nucleic Acids Res. 2006, 34, 2773–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, D.; Hagopian, J.C.; Springer, A.D.; Eguchi, A.; Kacsinta, A.D. Neutralizing Phosphotriester Backbone Modifications. Nat Biotechnol. 2015, 32, 1256–1261. [Google Scholar]
- Wu, S.Y.; Yang, X.; Gharpure, K.M.; Hatakeyama, H.; Egli, M.; McGuire, M.H.; Nagaraja, A.S.; Miyake, T.M.; Rupaimoole, R.; Pecot, C.V.; et al. 2′-OMe-Phosphorodithioate-Modified SiRNAs Show Increased Loading into the RISC Complex and Enhanced Anti-Tumour Activity. Nat. Commun. 2014, 5, 3459. [Google Scholar] [CrossRef] [Green Version]
- Sipa, K.; Sochacka, E.; Kazmierczak-Baranska, J.; Maszewska, M.; Janicka, M.; Nowak, G.; Nawrot, B. Effect of Base Modifications on Structure, Thermodynamic Stability, and Gene Silencing Activity of Short Interfering RNA. RNA 2007, 13, 1301–1316. [Google Scholar] [CrossRef]
- Cummins, L.L.; Owens, S.R.; Risen, L.M.; Lesnik, E.A.; Freier, S.M.; Mc Gee, D.; Cook, C.J.; Cook, P.D. Characterization of Fully 2’-Modified Oligoribonucleotide Hetero-and Homoduplex Hybridization Andnuclease Sensitivity. Nucleic Acids Res. 1995, 23, 2019–2024. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Minakawa, N.; Matsuda, A. Synthesis and Characterization of 2′-Modified-4′-ThioRNA: A Comprehensive Comparison of Nuclease Stability. Nucleic Acids Res. 2009, 37, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Foster, D.J.; Brown, C.R.; Shaikh, S.; Trapp, C.; Schlegel, M.K.; Qian, K.; Sehgal, A.; Rajeev, K.G.; Jadhav, V.; Manoharan, M.; et al. Advanced SiRNA Designs Further Improve In Vivo Performance of GalNAc-SiRNA Conjugates. Mol. Ther. 2018, 26, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Khvorova, A. Oligonucleotide Therapeutics—A New Class of Cholesterol-Lowering Drugs. N. Engl. J. Med. 2017, 376, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Berk, C.; Civenni, G.; Wang, Y.; Steuer, C.; Catapano, C.V.; Hall, J. Pharmacodynamic and Pharmacokinetic Properties of Full Phosphorothioate Small Interfering RNAs for Gene Silencing In Vivo. Nucleic Acid Ther. 2020, 31, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, K.; White, S.; Borodovsky, A.; Bettencourt, B.R.; Strahs, A.; Clausen, V.; Wijngaard, P.; Horton, J.D.; Taubel, J.; Brooks, A.; et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N. Engl. J. Med. 2017, 376, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.M.; Hariharan, V.N.; Lo, A.; Turanov, A.A.; Echeverria, D.; Sousa, J.; McHugh, N.; Biscans, A.; Alterman, J.F.; Karumanchi, S.A.; et al. Chemical Optimization of SiRNA for Safe and Efficient Silencing of Placental SFLT1. Mol. Ther.-Nucleic Acids 2022, 29, 135–149. [Google Scholar] [CrossRef]
- Shmushkovich, T.; Monopoli, K.R.; Homsy, D.; Leyfer, D.; Betancur-Boissel, M.; Khvorova, A.; Wolfson, A.D. Functional Features Defining the Efficacy of Cholesterol-Conjugated, Self-Deliverable, Chemically Modified SiRNAs. Nucleic Acids Res. 2018, 46, 10905–10916. [Google Scholar] [CrossRef] [Green Version]
- Lamontagne, B.; Hannoush, R.N.; Damha, M.J.; Elela, S.A. Molecular Requirements for Duplex Recognition and Cleavage by Eukaryotic RNase III: Discovery of an RNA-Dependent DNA Cleavage Activity of Yeast Rnt1p. J. Mol. Biol. 2004, 338, 401–418. [Google Scholar] [CrossRef]
- Holen, T. Similar Behaviour of Single-Strand and Double-Strand SiRNAs Suggests They Act through a Common RNAi Pathway. Nucleic Acids Res. 2003, 31, 2401–2407. [Google Scholar] [CrossRef]
- Prakash, T.P.; Lima, W.F.; Murray, H.M.; Li, W.; Kinberger, G.A.; Chappell, A.E.; Gaus, H.; Seth, P.P.; Bhat, B.; Crooke, S.T.; et al. Identification of Metabolically Stable 5′-Phosphate Analogs That Support Single-Stranded SiRNA Activity. Nucleic Acids Res. 2015, 43, 2993–3011. [Google Scholar] [CrossRef] [Green Version]
- Pendergraff, H.M.; Debacker, A.J.; Watts, J.K. Single-Stranded Silencing RNAs: Hit Rate and Chemical Modification. Nucleic Acid Ther. 2016, 26, 216–222. [Google Scholar] [CrossRef]
- Chang, W.; Pei, Y.; Guidry, E.N.; Zewge, D.; Parish, C.A.; Sherer, E.C.; Dimuzio, J.; Zhang, H.; South, V.J.; Strapps, W.R.; et al. Bioorganic & Medicinal Chemistry Letters Systematic Chemical Modifications of Single Stranded SiRNAs Significantly Improved CTNNB1 MRNA Silencing. Bioorg. Med. Chem. Lett. 2016, 26, 4513–4517. [Google Scholar] [CrossRef]
- Lima, W.F.; Prakash, T.P.; Murray, H.M.; Kinberger, G.A.; Li, W.; Chappell, A.E.; Li, C.S.; Murray, S.F.; Gaus, H.; Seth, P.P.; et al. Single-Stranded SiRNAs Activate RNAi in Animals. Cell 2012, 150, 883–894. [Google Scholar] [CrossRef] [Green Version]
- Crooke, S.T. Antisense Drug Technology: Principles, Strategies, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781439832509. [Google Scholar]
- Yu, D.; Pendergraff, H.; Liu, J.; Kordasiewicz, H.B.; Cleveland, D.W.; Swayze, E.E.; Lima, W.F.; Crooke, S.T.; Prakash, T.P.; Corey, D.R. Single-Stranded RNAs Use RNAi to Potently and Allele-Selectively Inhibit Mutant Huntingtin Expression. Cell 2012, 150, 895–908. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.A.; Nam, Y.S. Reducible Dimeric Conjugates of Small Internally Segment Interfering RNA for Efficient Gene Silencing. Macromol. Biosci. 2016, 16, 1442–1449. [Google Scholar] [CrossRef]
- Tang, X.; Ren, H.; Guo, M.; Qian, J.; Yang, Y.; Gu, C. Review on Circular RNAs and New Insights into Their Roles in Cancer. Comput. Struct. Biotechnol. J. 2021, 19, 910–928. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, Y.; Wang, X.; Shen, J.; An, W. Advances in Circular RNA and Its Applications. Int. J. Med. Sci. 2022, 19, 975–985. [Google Scholar] [CrossRef]
- Yu, J.; Xie, D.; Huang, N.; Zhou, Q. Circular RNAs as Novel Diagnostic Biomarkers and Therapeutic Targets in Kidney Disease. Front. Med. 2021, 8, 8–10. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, D.; Wang, Y.; Li, D.; Zhang, J.; Wu, L.; Feng, M.; Yi, F.; Xu, L.; Lei, L.; et al. Caged Circular SiRNAs for Photomodulation of Gene Expression in Cells and Mice. Chem. Sci. 2017, 9, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, D.; Chen, C.; Wang, Y.; Amu, G.; Yang, J.; Yu, L.; Dmochowski, I.J.; Tang, X. Circular SiRNAs for Reducing Off-Target Effects and Enhancing Long-Term Gene Silencing in Cells and Mice. Mol. Ther.-Nucleic Acids 2018, 10, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Shu, Z.; Ito, M.; Abe, N.; Nakamoto, K.; Tomoike, F.; Shuto, S.; Ito, Y.; Abe, H. Intracellular Build-up RNAi with Single-Strand Circular RNAs as SiRNA Precursors. Chem. Commun. 2020, 56, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Abe, H.; Ohshiro, T.; Nakashima, Y.; Maeda, M.; Ito, Y. Synthesis and Characterization of Small Circular Double-Stranded RNAs. Chem. Commun. 2011, 47, 2125–2127. [Google Scholar] [CrossRef] [PubMed]
- Jahns, H.; Degaonkar, R.; Podbevsek, P.; Gupta, S.; Bisbe, A.; Aluri, K.; Szeto, J.; Kumar, P.; LeBlanc, S.; Racie, T.; et al. Small Circular Interfering RNAs (SciRNAs) as a Potent Therapeutic Platform for Gene-Silencing. Nucleic Acids Res. 2021, 49, 10250–10264. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Behlke, M.A.; Rose, S.D.; Chang, M.-S.; Choi, S.; Rossi, J.J. Synthetic DsRNA Dicer Substrates Enhance RNAi Potency and Efficacy. Nat. Biotechnol. 2005, 23, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Kubo, T.; Yanagihara, K.; Sato, Y.; Nishimura, Y.; Kondo, S.; Seyama, T. Gene-Silencing Potency of Symmetric and Asymmetric Lipid-Conjugated Sirnas and Its Correlation with Dicer Recognition. Bioconjug. Chem. 2013, 24, 2045–2057. [Google Scholar] [CrossRef]
- Bégin-Lavallée, V.; Midavaine, É.; Dansereau, M.-A.; Tétreault, P.; Longpré, J.-M.; Jacobi, A.M.; Rose, S.D.; Behlke, M.A.; Beaudet, N.; Sarret, P. Functional Inhibition of Chemokine Receptor CCR2 by Dicer-Substrate-SiRNA Prevents Pain Development. Mol. Pain 2016, 12, 174480691665396. [Google Scholar] [CrossRef] [Green Version]
- Neff, C.P.; Zhou, J.; Remling, L.; Kuruvilla, J.; Zhang, J.; Li, H.; Smith, D.D.; Swiderski, P.; Rossi, J.J.; Akkina, R. An Aptamer-SiRNA Chimera Suppresses HIV-1 Viral Loads and Protects from Helper CD4+ T Cell Decline in Humanized Mice. Sci. Transl. Med. 2011, 3, 66ra6. [Google Scholar] [CrossRef] [Green Version]
- Alterman, J.F.; Hall, L.M.; Coles, A.H.; Hassler, M.R.; Didiot, M.-C.; Chase, K.; Abraham, J.; Sottosanti, E.; Johnson, E.; Sapp, E.; et al. Hydrophobically Modified SiRNAs Silence Huntingtin MRNA in Primary Neurons and Mouse Brain. Mol. Ther.-Nucleic Acids 2015, 4, e266. [Google Scholar] [CrossRef]
- Kubo, T.; Zhelev, Z.; Ohba, H.; Bakalova, R. Modified 27-Nt DsRNAs with Dramatically Enhanced Stability in Serum and Long-Term RNAi Activity. Oligonucleotides 2007, 17, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Yanagihara, K.; Seyama, T. In Vivo RNAi Efficacy of Palmitic Acid-Conjugated Dicer-Substrate SiRNA in a Subcutaneous Tumor Mouse Model. Chem. Biol. Drug Des. 2016, 87, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.J. Expression Strategies for Short Hairpin RNA Interference Triggers. Hum. Gene Ther. 2008, 19, 313–317. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, A.P.; Meuse, L.; Pham, T.-T.T.; Conklin, D.S.; Hannon, G.J.; Kay, M.A. RNA Interference in Adult Mice. Nature 2002, 418, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Brummelkamp, T.R. A System for Stable Expression of Short Interfering RNAs in Mammalian Cells. Science 2002, 296, 550–553. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, J.; Cheng, H.; Ke, X.; Sun, L.; Zhang, Q.C.; Wang, H.W. Cryo-EM Structure of Human Dicer and Its Complexes with a Pre-MiRNA Substrate. Cell 2018, 173, 1191–1203. [Google Scholar] [CrossRef] [Green Version]
- Pokornowska, M.; Milewski, M.C.; Ciechanowska, K.; Szczepańska, A.; Wojnicka, M.; Radogostowicz, Z.; Figlerowicz, M.; Kurzynska-Kokorniak, A. The RNA–RNA Base Pairing Potential of Human Dicer and Ago2 Proteins. Cell. Mol. Life Sci. 2020, 77, 3231–3244. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.R.; Aderounmu, A.M.; Donelick, H.M.; Bass, B.L. Dicer’s Helicase Domain: A Meeting Place for Regulatory Proteins. Cold Spring Harb. Symp. Quant. Biol. 2019, 84, 185–193. [Google Scholar] [CrossRef]
- Park, J.-E.; Heo, I.; Tian, Y.; Simanshu, D.K.; Chang, H.; Jee, D.; Patel, D.J.; Kim, V.N. Dicer Recognizes the 5′ End of RNA for Efficient and Accurate Processing. Nature 2011, 475, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, S.; Sternberg, S.H.; Kellenberger, C.A.; Doudna, J.A. Substrate-Specific Kinetics of Dicer-Catalyzed RNA Processing. J. Mol. Biol. 2010, 404, 392–402. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.; Dudek, H.; Lai, C. Therapeutic Inhibition of Lactate Dehydrogenase and Agents Therefor. United States Patent U.S. 10,351,854, 16 July 2019. [Google Scholar]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Pan, R.; Zhang, B.; Qu, M.; Lian, B.; Jiang, H.; Gao, Z.; Wu, J. Liver-Targeted Combination Therapy Basing on Glycyrrhizic Acid-Modified DSPE-PEG-PEI Nanoparticles for Co-Delivery of Doxorubicin and Bcl-2 SiRNA. Front. Pharmacol. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborn, M.F.; Coles, A.H.; Biscans, A.; Haraszti, R.A.; Roux, L.; Davis, S.; Ly, S.; Echeverria, D.; Hassler, M.R.; Godinho, B.M.D.C.; et al. Hydrophobicity Drives the Systemic Distribution of Lipid-Conjugated SiRNAs via Lipid Transport Pathways. Nucleic Acids Res. 2019, 47, 1070–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theile, A.C.S.; Zlatev, I.; Castoreno, A.; Bisbe, A.; Williams, S.; Nguyen, T.; Schlegel, M.K.; Waldron, S.; Qin, J.; Fishman, S.; et al. Bis-RNAiTM Conjugates for Simultaneous Silencing of Two Different Gene Transcripts. In Proceedings of the 14th Annual Meeting of the Oligonucleotide Therapeutics Society, Seattle, WA, USA, 30 September–3 October 2018; p. 75. [Google Scholar]
- Alterman, J.F.; Godinho, B.M.D.C.; Hassler, M.R.; Ferguson, C.M.; Echeverria, D.; Sapp, E.; Haraszti, R.A.; Coles, A.H.; Conroy, F.; Miller, R.; et al. A Divalent SiRNA Chemical Scaffold for Potent and Sustained Modulation of Gene Expression throughout the Central Nervous System. Nat. Biotechnol. 2019, 37, 884–894. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Chen, J.; Qin, Y.; Yang, Z.; Zhu, Y.Y. Long Double-Stranded Multiplex SiRNAs for Dual Genes Silencing. Nucleic Acid Ther. 2013, 23, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.I.; Kang, H.S.; Ban, C.; Kim, S.; Lee, D.K. Dual-Target Gene Silencing by Using Long, Synthetic SiRNA Duplexes without Triggering Antiviral Responses. Mol. Cells 2009, 27, 689–695. [Google Scholar] [CrossRef]
- Gvozdeva, O.V.; Dovydenko, I.S.; Venyaminova, A.G.; Zenkova, M.A.; Vlassov, V.V.; Chernolovskaya, E.L. 42- and 63-Bp Anti-MDR1-SiRNAs Bearing 2′-OMe Modifications in Nuclease-Sensitive Sites Induce Specific and Potent Gene Silencing. FEBS Lett. 2014, 588, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Gvozdeva, O.V.; Gladkih, D.V.; Chernikov, I.V.; Meschaninova, M.I.; Venyaminova, A.G.; Zenkova, M.A.; Vlassov, V.V.; Chernolovskaya, E.L. Nuclease-Resistant 63-Bp Trimeric SiRNAs Simultaneously Silence Three Different Genes in Tumor Cells. FEBS Lett. 2018, 592, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.M.; Dahlman, J.E.; Neuman, K.K.; Prata, C.A.H.; Krampert, M.C.; Hadwiger, P.M.; Vornlocher, H.-P. Ligand Conjugated Multimeric SiRNAs Enable Enhanced Uptake and Multiplexed Gene Silencing. Nucleic Acid Ther. 2019, 29, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.I.; Lee, T.Y.; Kim, S.; Sun, X.; Hong, S.W.; Yoo, J.W.; Dua, P.; Kang, H.S.; Kim, S.; Li, C.J.; et al. Enhanced Intracellular Delivery and Multi-Target Gene Silencing Triggered by Tripodal RNA Structures. J. Gene Med. 2012, 14, 138–146. [Google Scholar] [CrossRef]
- Chang, C.I.; Lee, T.Y.; Yoo, J.W.; Shin, D.; Kim, M.; Kim, S.; Lee, D. Branched, Tripartite-Interfering RNAs Silence Multiple Target Genes with Long Guide Strands. Nucleic Acid Ther. 2012, 22, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, Y.G.; Choe, J.Y.; Lee, D.; Shin, C.; Hong, S.W.; Lee, D.K. RNA Interference-Mediated Gene Silencing by Branched Tripodal RNAs Does Not Require Dicer Processing. Nucleic Acid Ther. 2018, 28, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, S.; Lee, T.Y.; Kim, J.K.; Son, D.S.; Hong, S.W.; Kim, S.; Yun, W.S.; Kim, S.; Chang, C.; Li, C.; et al. Efficient Intracellular Delivery and Multiple-Target Gene Silencing Triggered by Tripodal RNA Based Nanoparticles: A Promising Approach in Liver-Specific RNAi Delivery. J. Control. Release 2014, 196, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Chandela, A.; Ueno, Y. Design, Synthesis and Evaluation of Novel, Branched Trident Small Interfering RNA Nanostructures for Sequence-Specific RNAi Activity. RSC Adv. 2019, 9, 34166–34171. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.Y.; Chang, C.I.; Lee, D.; Hong, S.W.; Shin, C.; Li, C.J.; Kim, S.; Haussecker, D.; Lee, D.K. RNA Interference-Mediated Simultaneous Silencing of Four Genes Using Cross-Shaped RNA. Mol. Cells 2013, 35, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Zhu, Y.Y.; Ji, Y.; Zhou, S. Interfering RNA with Multi-Targets for Efficient Gene Suppression in HCC Cells. Int. J. Mol. Med. 2018, 41, 3604–3610. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.M.; Nair, J.K.; Janas, M.M.; Anglero-Rodriguez, Y.I.; Dang, L.T.H.; Peng, H.; Theile, C.S.; Castellanos-Rizaldos, E.; Brown, C.; Foster, D.; et al. Expanding RNAi Therapeutics to Extrahepatic Tissues with Lipophilic Conjugates. Nat. Biotechnol. 2022, 40, 1500–1508. [Google Scholar] [CrossRef]
- Engelbeen, S.; Pasteuning-Vuhman, S.; Boertje-van der Meulen, J.; Parmar, R.; Charisse, K.; Sepp-Lorenzino, L.; Manoharan, M.; Aartsma-Rus, A.; van Putten, M. Efficient Downregulation of Alk4 in Skeletal Muscle After Systemic Treatment with Conjugated SiRNAs in a Mouse Model for Duchenne Muscular Dystrophy. Nucleic Acid Ther. 2022. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernikov, I.V.; Ponomareva, U.A.; Chernolovskaya, E.L. Structural Modifications of siRNA Improve Its Performance In Vivo. Int. J. Mol. Sci. 2023, 24, 956. https://doi.org/10.3390/ijms24020956
Chernikov IV, Ponomareva UA, Chernolovskaya EL. Structural Modifications of siRNA Improve Its Performance In Vivo. International Journal of Molecular Sciences. 2023; 24(2):956. https://doi.org/10.3390/ijms24020956
Chicago/Turabian StyleChernikov, Ivan V., Ulyana A. Ponomareva, and Elena L. Chernolovskaya. 2023. "Structural Modifications of siRNA Improve Its Performance In Vivo" International Journal of Molecular Sciences 24, no. 2: 956. https://doi.org/10.3390/ijms24020956
APA StyleChernikov, I. V., Ponomareva, U. A., & Chernolovskaya, E. L. (2023). Structural Modifications of siRNA Improve Its Performance In Vivo. International Journal of Molecular Sciences, 24(2), 956. https://doi.org/10.3390/ijms24020956





