Influence of Lanthanum Precursor on the Activity of Nickel Catalysts in the Mixed-Methane Reforming Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity Measurements
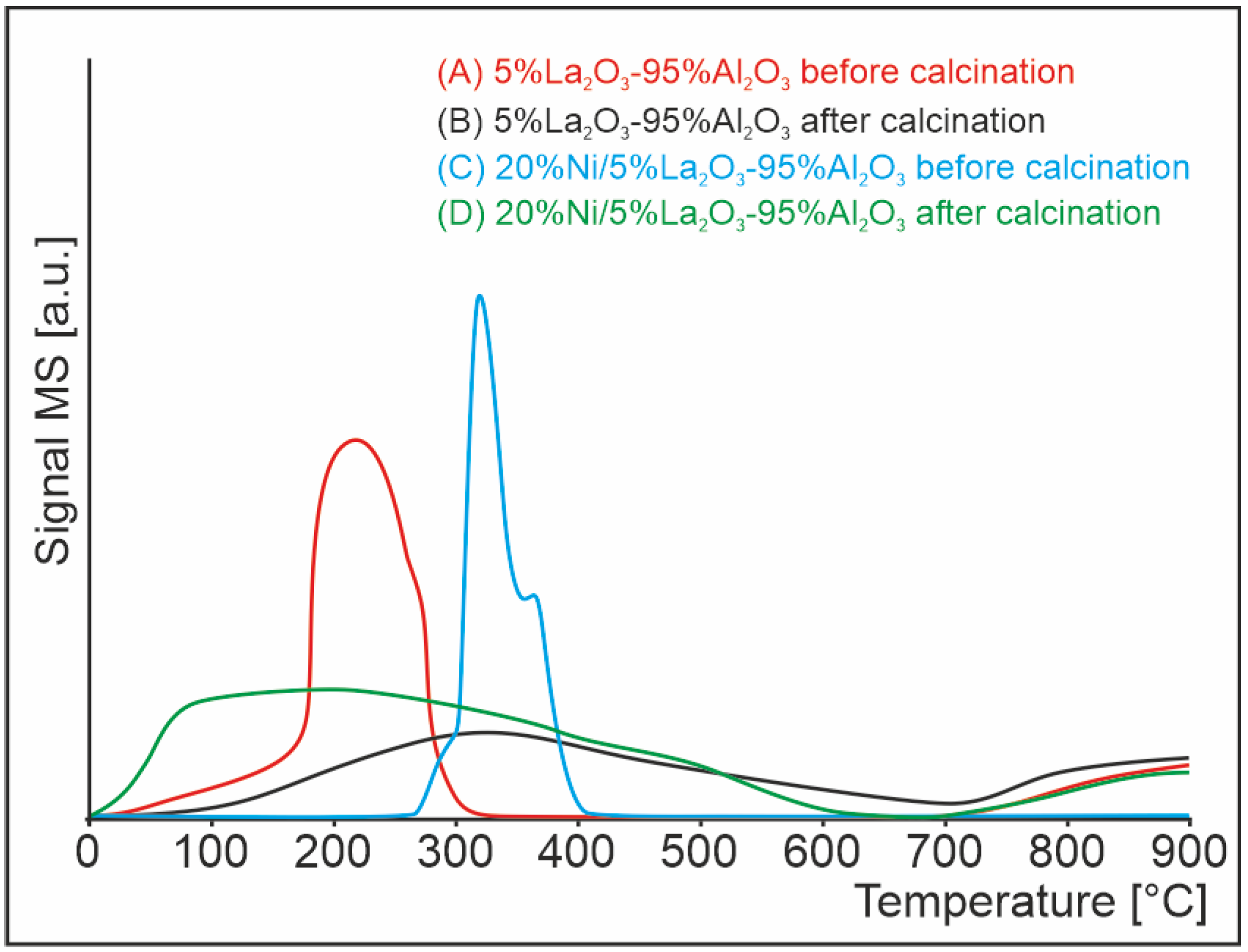
2.2. Temperature-Programmed Hydrogen Chloride Desorption
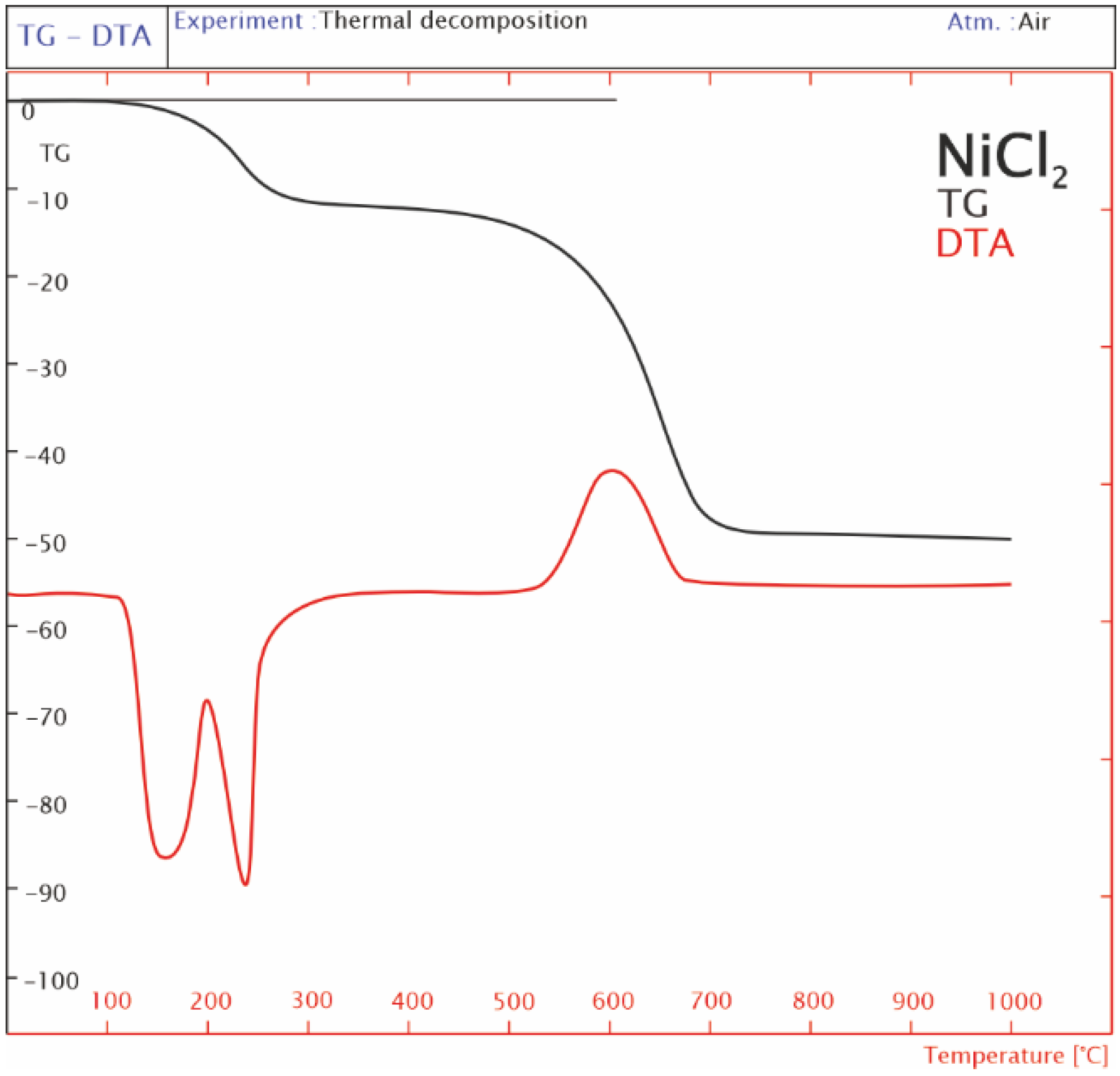
2.3. Thermogravimetric TG-DTA Analysis
- (i)
- (a) LaCl3 × 7H2O => LaCl3 + 7H2O
- (ii)
- NiCl2 × 6H2O => NiCl2 + 6H2O (theoretical reaction)
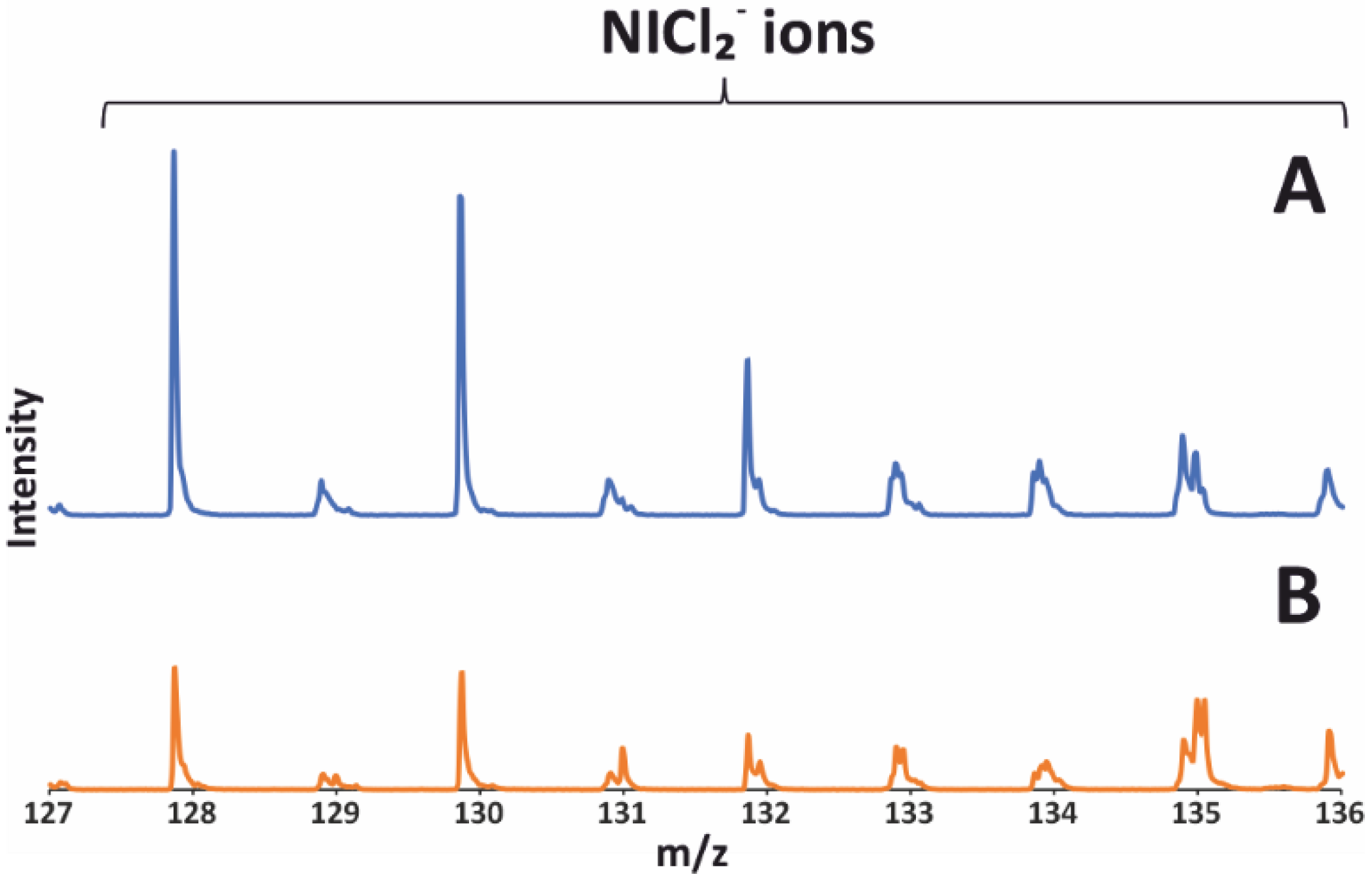
2.4. TOF SIMS Analysis
3. Material and Method
3.1. Catalyst Preparation
3.2. Method and Instruments
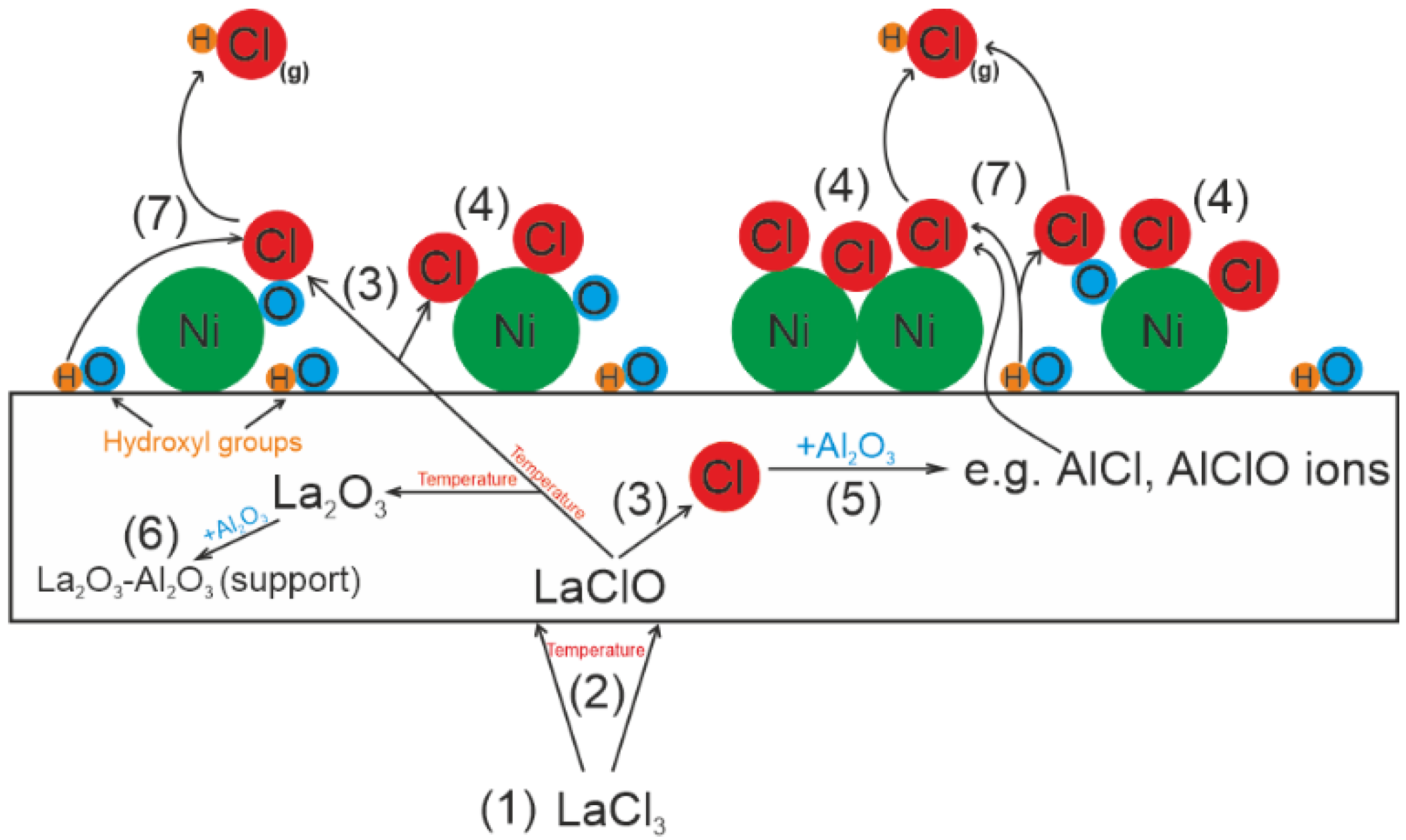
4. Proposed Mechanism of Poisoning the Catalyst with Chlorine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rostrupnielsen, J.R.; Hansen, J.H.B. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Edwards, J.H.; Maitra, A.M. The Chemistry of Methane Reforming with Carbon Dioxide and Its Current and Potential Applications. Fuel Process. Technol. 1995, 42, 269–289. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Choudhary, V.R. Energy-Efficient Syngas Production through Catalytic Oxy-Methane Reforming Reactions. Angew. Chem. Int. Ed. 2008, 47, 1828–1847. [Google Scholar] [CrossRef]
- Navarro, R.M.; Peña, M.A.; Fierro, J.L.G. Hydrogen Production Reactions from Carbon Feedstocks: Fossil Fuels and Biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef]
- Freni, S.; Calogero, G.; Cavallaro, S. Hydrogen Production from Methane through Catalytic Partial Oxidation Reactions. J. Power Sources 2000, 87, 28–38. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Bai, P.; Liu, X.; Yan, Z.-F. CO2 Reforming of CH4 over Nanocrystalline Zirconia-Supported Nickel Catalysts. Appl. Catal. B 2008, 77, 346–354. [Google Scholar] [CrossRef]
- Daza, C.E.; Gallego, J.; Moreno, J.A.; Mondragón, F.; Moreno, S.; Molina, R. CO2 Reforming of Methane over Ni/Mg/Al/Ce Mixed Oxides. Catal. Today 2008, 133–135, 357–366. [Google Scholar] [CrossRef]
- Cao, W.; Zheng, D. Thermodynamic Performance of O2/CO2 Gas Turbine Cycle with Chemical Recuperation by CO2-Reforming of Methane. Fuel 2007, 86, 2864–2870. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Effects of Metal Content on Activity and Stability of Ni-Co Bimetallic Catalysts for CO2 Reforming of CH4. Appl. Catal. A Gen. 2008, 339, 121–129. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Zhang, J.; Zhang, J. Effect of Preparation Methods on Structure and Performance of Ni/Ce0.75Zr0.25O2 Catalysts for CH4–CO2 Reforming. Fuel 2008, 87, 2901–2907. [Google Scholar] [CrossRef]
- Gallego, G.S.; Batiot-Dupeyrat, C.; Barrault, J.; Florez, E.; Mondragón, F. Dry Reforming of Methane over LaNi1−yByO3±δ (B = Mg, Co) Perovskites Used as Catalyst Precursor. Appl. Catal. A Gen. 2008, 334, 251–258. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Huerta, M.V.M.; Fierro, J.L.G. The Effect of CeO2 on the Surface and Catalytic Properties of Pt/CeO2–ZrO2 Catalysts for Methane Dry Reforming. Appl. Catal. B 2009, 89, 149–159. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; Goldwasser, M.R. Dry Reforming of CH4 over Solid Solutions of LaNi1−xCoxO3. Catal. Today 2008, 133–135, 142–148. [Google Scholar] [CrossRef]
- Sierra Gallego, G.; Mondragón, F.; Tatibouët, J.-M.; Barrault, J.; Batiot-Dupeyrat, C. Carbon Dioxide Reforming of Methane over La2NiO4 as Catalyst Precursor—Characterization of Carbon Deposition. Catal. Today 2008, 133–135, 200–209. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Chadwick, D.; Assabumrungrat, S. Effect of High Surface Area CeO2 and Ce-ZrO2 Supports over Ni Catalyst on CH4 Reforming with H2O in the Presence of O2, H2, and CO2. Chem. Eng. J. 2008, 138, 264–273. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.-F. A Highly Stable Catalyst in Methane Reforming with Carbon Dioxide. Scr. Mater. 2009, 61, 173–176. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, Z.; Hong, X.; Liu, Y. Oxidative Reformings of Methane to Syngas with Steam and CO2 Catalyzed by Metallic Ni Based Monolithic Catalysts. Catal. Commun. 2008, 9, 1040–1044. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Binary MgO-Based Solid Solution Catalysts For Methane Conversion To Syngas. Catal. Rev. 2002, 44, 423–453. [Google Scholar] [CrossRef]
- Li, L.; Liu, B.S.; Leung, J.W.H.; Au, C.T.; Cheung, A.S.-C. CH4/CO2 Reforming over La2NiO4 and 10%NiO/CeO2–La2O3 Catalysts under the Condition of Supersonic Jet Expansion via Cavity Ring-down Spectroscopic Analysis. Catal. Today 2008, 131, 533–540. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-Based Ceria, Zirconia, and Ceria–Zirconia Catalytic Systems for Low-Temperature Carbon Dioxide Reforming of Methane. Energy Fuels 2007, 21, 3113–3123. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Catalytic Conversion of Methane to Synthesis Gas by Partial Oxidation and CO2 Reforming. Adv. Catal. 2004, 48, 297–345. [Google Scholar]
- Yu, D.; Xingyi, W.; Dao, L.; Qiguang, D. Catalytic Combustion of Chlorobenzene over Mn-Ce-La-O Mixed Oxide Catalysts. J. Hazard. Mater. 2011, 188, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Marceau, E.; Che, M.; Saint-Just, J.; Tatibouët, J.M. Influence of Chlorine Ions in Pt/A12O3 Catalysts for Methane Total Oxidation. Catal. Today 1996, 29, 415–419. [Google Scholar] [CrossRef]
- Cant, N.W.; Angove, D.E.; Patterson, M.J. The Effects of Residual Chlorine on the Behaviour of Platinum Group Metals for Oxidation of Different Hydrocarbons. Catal. Today 1998, 44, 93–99. [Google Scholar] [CrossRef]
- Roth, D.; Gélin, P.; Primet, M.; Tena, E. Catalytic Behaviour of Cl-Free and Cl-Containing Pd/Al2O3 Catalysts in the Total Oxidation of Methane at Low Temperature. Appl. Catal. A Gen. 2000, 203, 37–45. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Shtyka, O.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Arcab, N.; Maniecki, T. Effect of Ruthenium and Cerium Oxide (IV) Promotors on the Removal of Carbon Deposit Formed during the Mixed Methane Reforming Process. Materials 2021, 14, 7581. [Google Scholar] [CrossRef]
- Marsal, A.; Dezanneau, G.; Cornet, A.; Morante, J.R. A New CO2 Gas Sensing Material. Sens. Actuators B Chem. 2003, 95, 266–270. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kanungo, S.B. Thermal Dehydration and Decomposition of Nickel Chloride Hydrate (NiCl2·xH2O). J. Therm. Anal. 1992, 38, 2417–2436. [Google Scholar] [CrossRef]
- Shtyka, O.; Zakrzewski, M.; Ciesielski, R.; Kedziora, A.; Dubkov, S.; Ryazanov, R.; Szynkowska, M.; Maniecki, T. Efficient Removal of the Carbon Deposits Formed during the Mixed Methane Reforming over Ni/Al2O3. Korean J. Chem. Eng. 2020, 37, 209–215. [Google Scholar] [CrossRef]

| Catalytic System/Support Precursor | Conversion of CH4 after 3 h Reaction | Conversion of CO2 after 3 h Reaction |
|---|---|---|
| 20%Ni/5%La2O3–95%Al2O3/Lanthanum (III) nitrate | 71% | 68% |
| 20%Ni/5%La2O3–95%Al2O3/Lanthanum (III) chloride | 49% | 56% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, M.; Shtyka, O.; Rogowski, J.; Ciesielski, R.; Kedziora, A.; Maniecki, T. Influence of Lanthanum Precursor on the Activity of Nickel Catalysts in the Mixed-Methane Reforming Process. Int. J. Mol. Sci. 2023, 24, 975. https://doi.org/10.3390/ijms24020975
Zakrzewski M, Shtyka O, Rogowski J, Ciesielski R, Kedziora A, Maniecki T. Influence of Lanthanum Precursor on the Activity of Nickel Catalysts in the Mixed-Methane Reforming Process. International Journal of Molecular Sciences. 2023; 24(2):975. https://doi.org/10.3390/ijms24020975
Chicago/Turabian StyleZakrzewski, Mateusz, Oleksandr Shtyka, Jacek Rogowski, Radoslaw Ciesielski, Adam Kedziora, and Tomasz Maniecki. 2023. "Influence of Lanthanum Precursor on the Activity of Nickel Catalysts in the Mixed-Methane Reforming Process" International Journal of Molecular Sciences 24, no. 2: 975. https://doi.org/10.3390/ijms24020975





