The Responses of Sucrose Metabolism and Carbon Translocation in Tomato Seedlings under Different Light Spectra
Abstract
:1. Introduction
2. Results
2.1. Effects of Light Spectra on the Growth of Tomato Seedlings
2.2. The Responses of Photosynthetic Performance of Tomato Seedlings under Different Light Spectra
2.3. Carbohydrate Contents in Plants under Different Light Spectra
2.4. Organic Carbon and Isotope Composition of Carbon in Plants under Different Light Spectra
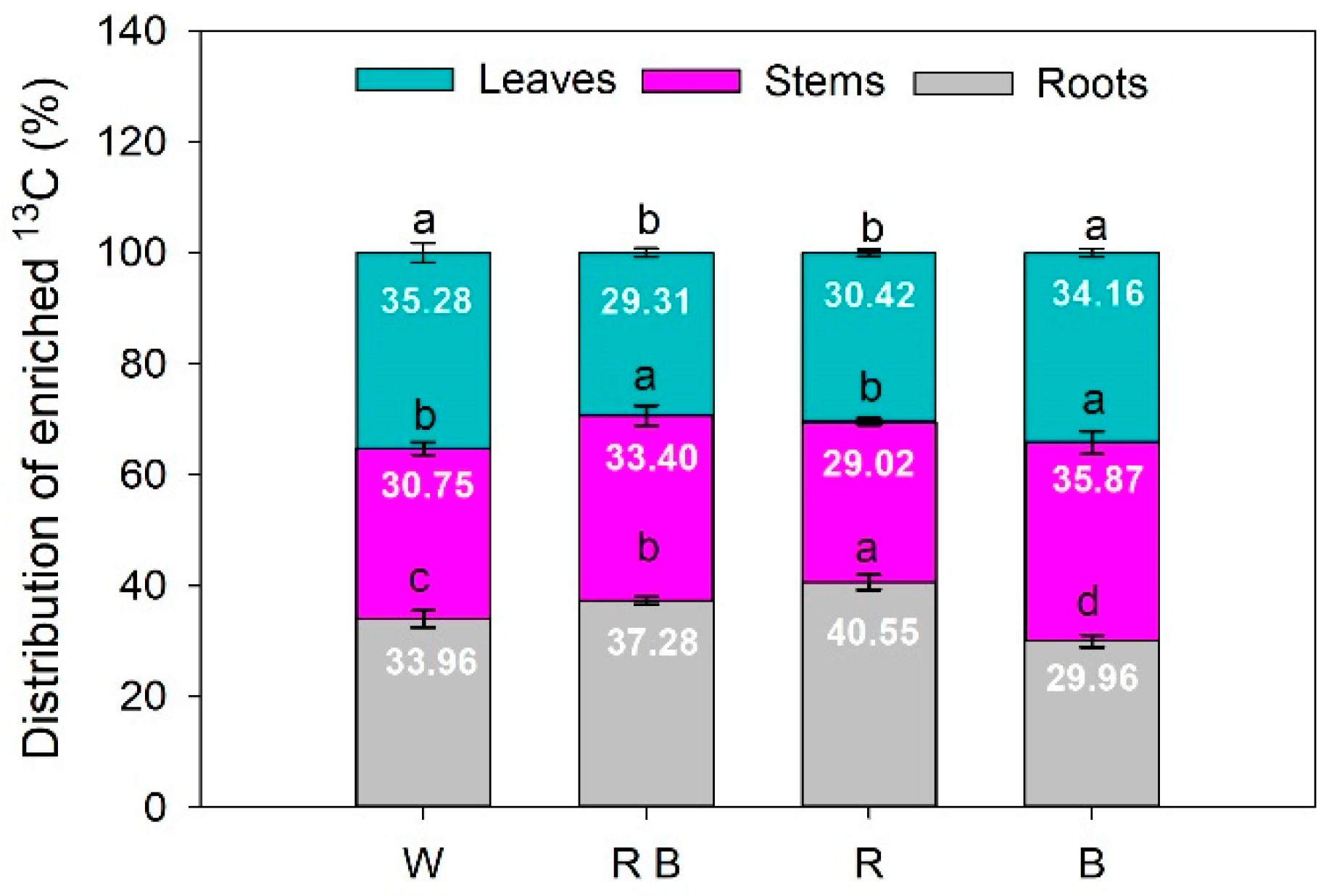
2.5. The Distribution of Photosynthetic Products under Different Light Spectra
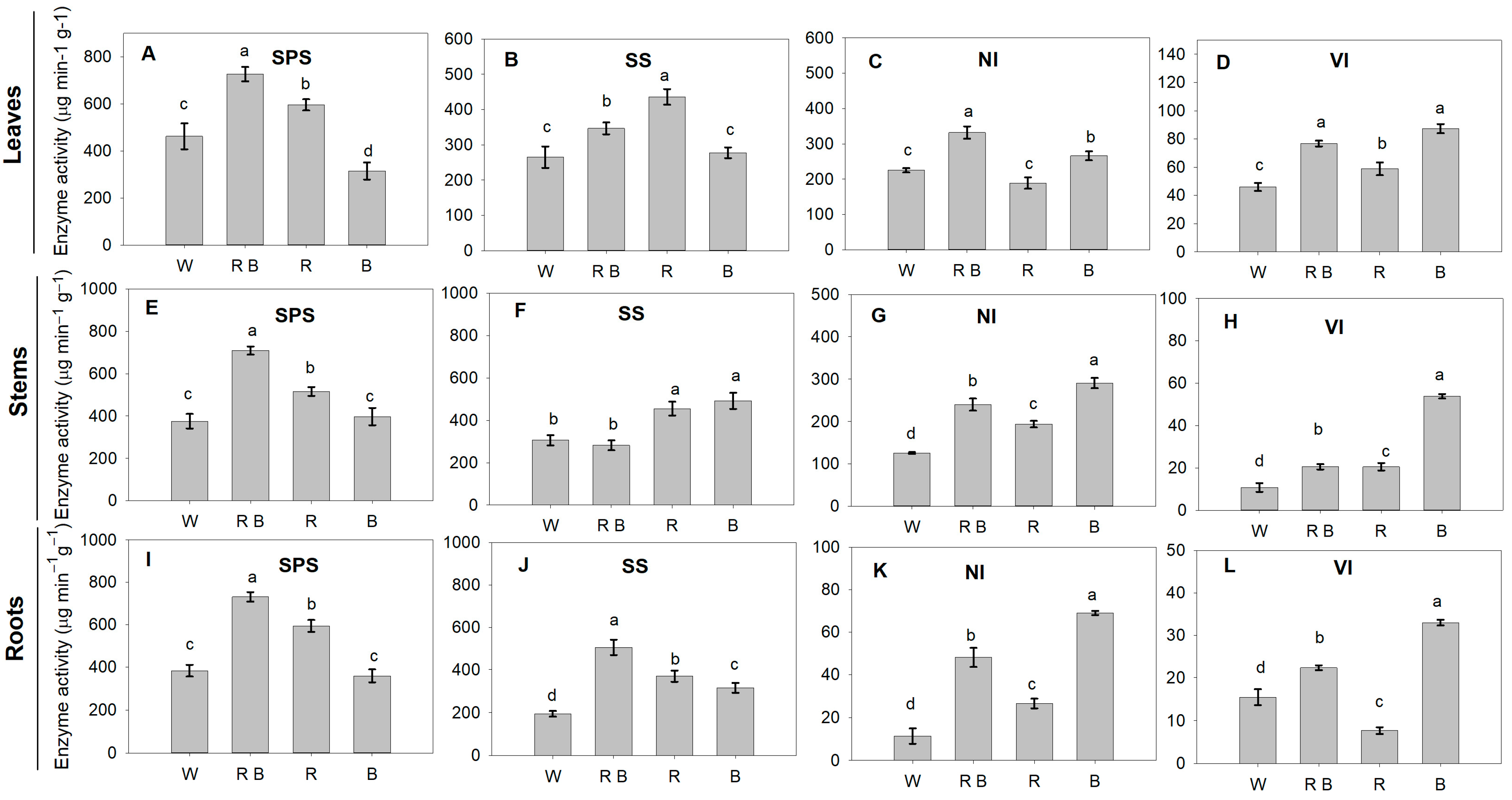
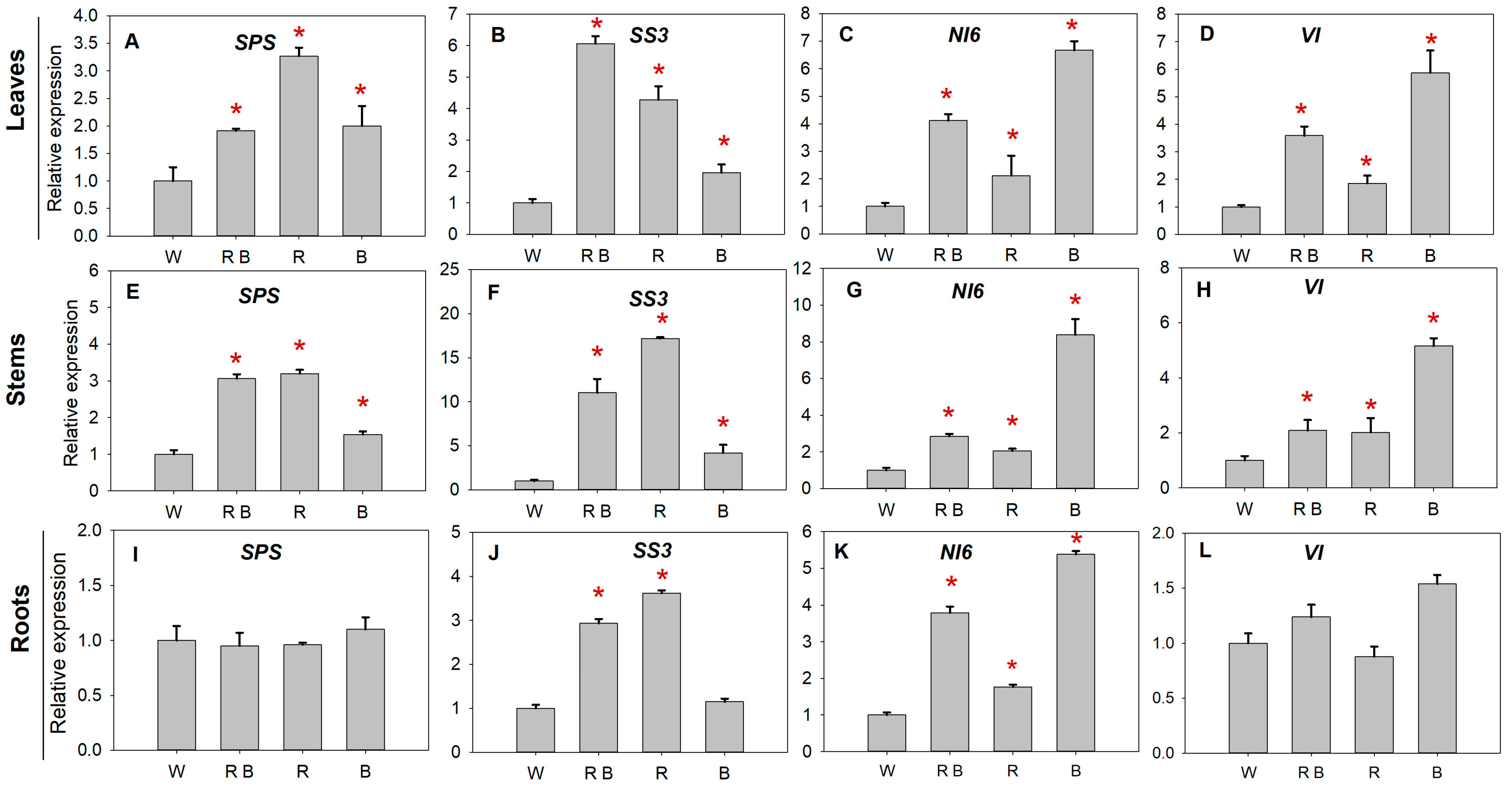
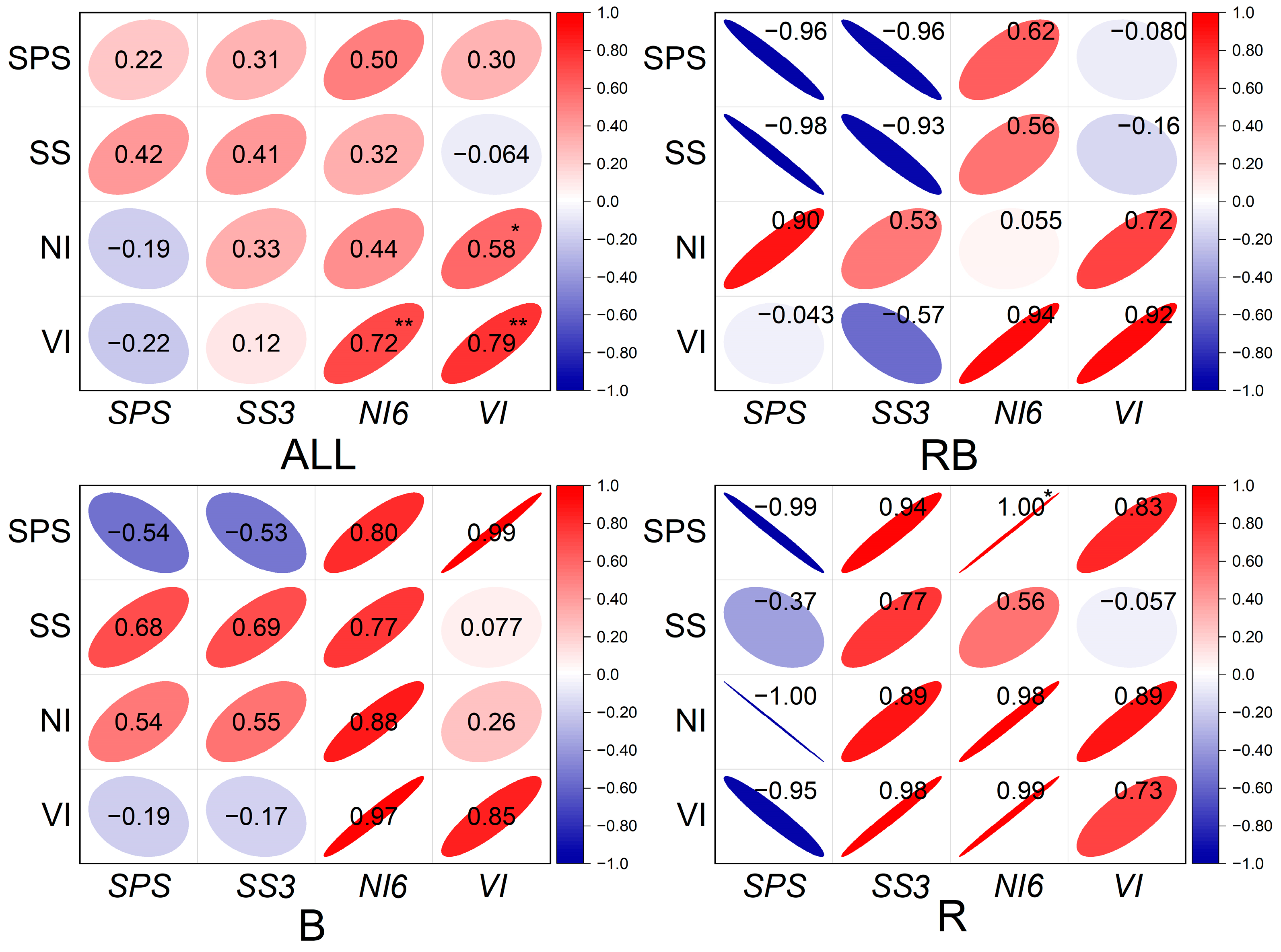
2.6. Sucrose Metabolism Enzyme Activities and Related Gene Expression
3. Discussion
4. Materials and Methods
4.1. Materials and Growth Conditions
4.2. Light Treatment
4.3. Gas Exchange Measurement and Sample Collection
4.4. Biomass and Plant Growth Determination
4.5. Carbohydrate Metabolism Enzyme Activity Measurement
4.6. Carbohydrate Content Measurement
4.7. Pulse-Labeling Experiments and Analysis of Stable Carbon Isotope Composition
4.8. Total RNA Extraction and qPCR Analysis
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| B | R | RB | W | |
|---|---|---|---|---|
| UV-A light (380–400 nm) | 0 | 0 | 0 | 5.47 ± 0.32 |
| Blue light (401–500 nm) | 300 ± 8.79 | 0 | 150 ± 4.16 | 65.42 ± 3.34 |
| Green light (501–600 nm) | 0 | 0 | 0 | 110.82 ± 4.17 |
| Red light (601–700 nm) | 0 | 300 ± 10.42 | 150 ± 6.31 | 127.76 ± 6.45 |
| Far-red light (701–780 nm) | 0 | 0 | 0 | 5.29 ± 0.27 |
| PPFD (400–700 nm) | 300 ± 8.79 | 300 ± 10.42 | 300 ± 11.23 | 300 ± 13.10 |
| Total light intensity | 300 ± 8.79 | 300 ± 10.42 | 300 ± 11.23 | 314.76 ± 14.54 |
| Gene Name | Gene ID: | Sequences |
|---|---|---|
| SPS | AB051216 | Forward: GCTTGGACGGGATTCTGATA |
| Reverse: TCTCCCCCATCTCACTCATC | ||
| SS3 | LOC543961 | Forward: GGATTGAAAGCCACGGAAAAGG |
| Reverse: ACCAGGCCTCAAACGAATAGCA | ||
| VI | AF465612 | Forward: TCCGCCTCTCGTTACACATTACTC |
| Reverse: CGGTGACTGGTTGTTGAGGATAGG | ||
| NI6 | 100134879 | Forward: GGAAGCTGCTCCATCCTTGT |
| Reverse: GACGTGGCGGTGAATGAGT |
References
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-regulated plant growth and development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [PubMed]
- Dong, F.; Wang, C.Z.; Ren, Y.Q.; Zhang, X.Z.; Liu, S.Q. Effects of light qualities on sugar contents, activities and gene expression of related enzymes involved in sugar metabolism of tomato fruit. Plant Physiol. J. 2018, 54, 1507–1515. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, Z.; Li, S.; Chen, X.; Lu, C. Comparative analysis of phenolic compound profiles, antioxidant capacities, and expressions of phenolic biosynthesis-related genes in soybean microgreens grown under different light spectra. J. Agric. Food Chem. 2019, 67, 13577–13588. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, X.; Wang, Y.; Lu, C. Improving drought tolerance by altering the photosynthetic rate and stomatal aperture via green light in tomato (Solanum lycopersicum L.) seedlings under drought conditions. Environ. Exp. Bot. 2019, 167, 103844. [Google Scholar] [CrossRef]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Liu, T.; Yuan, Q.; Wang, Y. Hierarchical optimization control based on crop growth model for greenhouse light environment. Comput. Electron. Agric. 2021, 180, 105854. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F. Adding blue to red supplemental light increases biomass and yield of greenhouse-grown tomatoes, but only to an optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef]
- Zhang, J. Effect of moderately-high temperature stress on photosynthesis and carbohydrate metabolism in tomato (Lycopersico esculentum L.) leaves. Afr. J. Agric. Res. 2012, 7, 487–492. [Google Scholar] [CrossRef]
- Rosales, M.A.; Rubio-Wilhelmi, M.M.; Castellano, R.; Castilla, N.; Ruiz, J.M.; Romero, L. Sucrolytic activities in cherry tomato fruits in relation to temperature and solar radiation. Sci. Hortic. 2007, 113, 244–249. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An overview of sucrose synthases in plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, H.; Scofield, G.N.; Badger, M.R.; Chow, W.S.; Furbank, R.T.; Ruan, Y.L. Localization of sucrose synthase in developing seed and siliques of Arabidopsis thaliana reveals diverse roles for SUS during development. J. Exp. Bot. 2008, 59, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- D’Aoust, M.-A.; Yelle, S.; Nguyen-Quoc, B. Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit. Plant Cell 1999, 11, 2407–2418. [Google Scholar] [CrossRef]
- Qin, G.; Zhu, Z.; Wang, W.; Cai, J.; Chen, Y.; Li, L.; Tian, S. A Tomato Vacuolar Invertase Inhibitor Mediates Sucrose Metabolism and Influences Fruit Ripening. Plant Physiol. 2016, 172, 1596–1611. [Google Scholar] [CrossRef]
- Sangu, E.; Tibazarwa, F.I.; Nyomora, A.; Symonds, R.C. Expression of genes for the biosynthesis of compatible solutes during pollen development under heat stress in tomato (Solanum lycopersicum). J. Plant Physiol. 2015, 178, 10–16. [Google Scholar] [CrossRef]
- Coluccio Leskow, C.; Conte, M.; Del Pozo, T.; Bermudez, L.; Lira, B.S.; Gramegna, G.; Baroli, I.; Burgos, E.; Zavallo, D.; Kamenetzky, L.; et al. The cytosolic invertase NI6 affects vegetative growth, flowering, fruit set, and yield in tomato. J. Exp. Bot. 2021, 72, 2525–2543. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Tyagi, P.; Sindhi, V.; Yadavilli, K.S. Invertase and its applications—A brief review. J. Pharm. Res. 2013, 7, 792–797. [Google Scholar] [CrossRef]
- Berger, S.; Papadopoulos, M.; Schreiber, U.; Kaiser, W.; Roitsch, T. Complex regulation of gene expression, photosynthesis and sugar levels by pathogen infection in tomato. Physiol. Plant. 2004, 122, 419–428. [Google Scholar] [CrossRef]
- Logendra, S.; Putman, J.D.; Janes, H.W. The influence of light period on carbon partitioning, translocation and growth in tomato. Sci. Hortic. 1990, 42, 75–83. [Google Scholar] [CrossRef]
- Lanoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of light quality and intensity on diurnal patterns and rates of photo-assimilate translocation and transpiration in tomato leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Carbon assimilation in crops at high temperatures. Plant Cell Env. 2019, 42, 2750–2758. [Google Scholar] [CrossRef]
- Izzo, L.G.; Hay Mele, B.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Fu, G.; Katulanda, P. Induced leaf intercellular CO2, photosynthesis, potassium and nitrate retention and strawberry early fruit formation under macronutrient limitation. Photosynth. Res. 2013, 115, 101–114. [Google Scholar] [CrossRef]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmés, J.; Hallik, L.; Medrano, H.; Ribas-Carbó, M.; Tosens, T.; Vislap, V.; Niinemets, Ü. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: Quantitative limitations and scaling up by models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef]
- Loreto, F.; Tsonev, T.; Centritto, M. The impact of blue light on leaf mesophyll conductance. J. Exp. Bot. 2009, 60, 2283–2290. [Google Scholar] [CrossRef]
- Pang, Y.; Liao, Q.; Peng, H.; Qian, C.; Wang, F. CO2 mesophyll conductance regulated by light: A review. Planta 2023, 258, 11. [Google Scholar] [CrossRef]
- Castillo-Argaez, R.; Schaffer, B.; Vazquez, A.; Sternberg, L.D.S.L. Leaf gas exchange and stable carbon isotope composition of redbay and avocado trees in response to laurel wilt or drought stress. Environ. Exp. Bot. 2020, 171, 103948. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, L.C.; Li, T.; Yang, Q.C.; Guo, W.Z. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Zhang, K.; Gong, X.-C.; Wang, H.-Y.; Gao, Y.-H.; Wang, X.-Q.; Zeng, Z.-H.; Hu, Y.-G. Effects of different LEDs light spectrum on the growth, leaf anatomy, and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse. J. Integr. Agric. 2020, 19, 108–119. [Google Scholar] [CrossRef]
- Yu, S.M.; Lo, S.F.; Ho, T.D. Source-Sink Communication: Regulated by Hormone, Nutrient, and Stress Cross-Signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2-R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Matsui, T.; Yoshida, Y. Carbohydrate content and the activities of sucrose synthase, sucrose phosphate synthase and acid invertase in different tomato cultivars during fruit development. Sci. Hortic. 1996, 65, 125–136. [Google Scholar] [CrossRef]
- Ruan, Y.L. Signaling role of sucrose metabolism in development. Mol. Plant 2012, 5, 763–765. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T.J.S.h. Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Shi, Q.; Yang, F.; Wei, M. Mixed red and blue light promotes tomato seedlings growth by influencing leaf anatomy, photosynthesis, CO2 assimilation and endogenous hormones. Sci. Hortic. 2021, 290, 110500. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, A.H.; Jiang, J. Gene expression patterns of invertase gene families and modulation of the inhibitor gene in tomato sucrose metabolism. Genet. Mol. Res. 2013, 12, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, L.; Zhang, H.; Zhou, Q.; Su, X.; Cui, X.; Bu, D.; Gong, X.; Poetsch, A.; Ning, K. Bi-clustering interpretation and prediction of correlation between gene expression and protein abundance. bioRxiv 2018, 270393, 1–33. [Google Scholar] [CrossRef]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef]
- Fischer, R.A.; Rees, D.; Sayre, K.D.; Lu, Z.M.; Condon, A.G.; Saavedra, A.L. Wheat Yield Progress Associated with Higher Stomatal Conductance and Photosynthetic Rate, and Cooler Canopies. Crop Sci. 1998, 38, 1467–1475. [Google Scholar] [CrossRef]
- Doehlert, D.C.; Kuo, T.M.; Felker, F.C. Enzymes of sucrose and hexose metabolism in developing kernels of two inbreds of maize. Plant Physiol. 1988, 86, 1013–1019. [Google Scholar] [CrossRef]
- Wardlaw, I.F.; Willenbrink, J. Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: The relation to sucrose synthase and sucrose-phosphate synthase. Funct. Plant Biol. 1994, 21, 255–271. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Li, W.; He, B.-J.; Gao, Y.-H.; Lu, J.-X. Sucrose metabolism in grape (Vitis vinifera L.) branches under low temperature during overwintering covered with soil. Plant Growth Regul. 2014, 72, 229–238. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Pump, J.; Conrad, R. Rice biomass production and carbon cycling in 13 CO2 pulse-labeled microcosms with different soils under submerged conditions. Plant Soil 2014, 384, 213–229. [Google Scholar] [CrossRef]
- Serret, M.D.; Yousfi, S.; Vicente, R.; Piñero, M.C.; Otálora-Alcón, G.; del Amor, F.M.; Araus, J.L. Interactive effects of CO2 concentration and water regime on stable isotope signatures, nitrogen assimilation and growth in sweet pepper. Front. Plant Sci. 2018, 8, 2180. [Google Scholar] [CrossRef] [PubMed]

| Leaf Area (cm−2) | Dry Weight (DW, g) | Biomass Distribution Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Roots | ||||||
| W | 325.62 ± 46.89 b | 1.11 ± 0.10 b | 53.31 ± 0.80% a | 37.09 ± 0.64% d | 9.60 ± 0.51% b | |||
| RB | 476.09 ± 53.4 1 a | 1.79 ± 0.26 a | 43.78 ± 2.1% c | −9.53% | 44.01 ± 1.1% a | 6.92% | 12.21 ± 0.53% a | 2.61% |
| R | 266.01 ± 38.25 bc | 0.93 ± 0.04 c | 47.74 ± 2.6% b | −7.57% | 40.80 ± 0.40% c | 3.71% | 11.46 ± 1.2% ab | 1.86% |
| B | 205.86 ± 20.17 c | 0.87 ± 0.08 c | 48.30 ± 1.1% b | −5.01% | 41.96 ± 0.61% b | 4.87% | 9.74 ± 1.3% b | 0.1% |
| W | RB | R | B | |
|---|---|---|---|---|
| Leaves (mg g−1) | ||||
| Glucose | 7.33 ± 0.22 d | 18.69 ± 0.35 a | 10.62 ± 0.30 b | 8.35 ± 0.41 c |
| Sucrose | 6.40 ± 0.10 c | 8.34 ± 0.31 a | 7.64 ± 0.32 b | 4.88 ± 0.26 d |
| Starch | 5.08 ± 0.16 b | 8.40 ± 0.09 a | 5.34 ± 0.20 b | 4.56 ± 0.23 c |
| Stems (mg g−1) | ||||
| Glucose | 11.34 ± 0.24 b | 20.86 ± 0.55 a | 11.19 ± 0.28 b | 8.05 ± 0.31 c |
| Sucrose | 1.70 ± 0.20 c | 3.31 ± 0.07 a | 2.03 ± 0.01 b | 1.71 ± 0.09 c |
| Starch | 4.12 ± 0.04 b | 6.88 ± 0.28 a | 3.33 ± 0.05 d | 3.69 ± 0.02 c |
| Roots (mg g−1) | ||||
| Glucose | 8.69 ± 0.23 b | 9.50 ± 0.36 a | 8.61 ± 0.35 b | 8.91 ± 0.03 b |
| Sucrose | 5.26 ± 0.11 a | 3.44 ± 0.11 b | 3.20 ± 0.07 b | 2.18 ± 0.09 d |
| Starch | 4.33 ± 0.09 a | 3.13 ± 0.94 bc | 3.55 ± 0.05 b | 3.03 ± 0.06 c |
| Treatments | Organic Carbon (g g−1) | δ13C (‰) | ||||
|---|---|---|---|---|---|---|
| Leaves | Stems | Roots | Leaves | Stems | Roots | |
| W | 0.72 ± 0.04 a | 0.59 ± 0.05 c | 0.62 ± 0.02 b | −41.61 ± 0.78 a | −42.43 ± 1.21 d | −31.99 ± 0.78 b |
| RB | 0.69 ± 0.07 a | 0.66 ± 0.02 a | 0.80 ± 0.04 a | −41.64 ±1.53 a | −31.14 ± 0.91 b | −36.50 ± 0.45 c |
| R | 0.79 ± 0.08 a | 0.64 ± 0.07 b | 0.69 ± 0.05 b | −41.27 ± 0.54 a | −27.86 ±1.08 a | −16.93 ± 0.19 a |
| B | 0.67 ± 0.06 a | 0.61 ± 0.05 b | 0.71 ± 0.07 b | −49.53 ± 0.24 b | −35.50 ± 2.13 c | −45.53 ± 0.31 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, X.; Yang, Q.; Wang, S.; Wang, Y.; Fan, X.; Bian, Z. The Responses of Sucrose Metabolism and Carbon Translocation in Tomato Seedlings under Different Light Spectra. Int. J. Mol. Sci. 2023, 24, 15054. https://doi.org/10.3390/ijms242015054
Zhan X, Yang Q, Wang S, Wang Y, Fan X, Bian Z. The Responses of Sucrose Metabolism and Carbon Translocation in Tomato Seedlings under Different Light Spectra. International Journal of Molecular Sciences. 2023; 24(20):15054. https://doi.org/10.3390/ijms242015054
Chicago/Turabian StyleZhan, Xiaoxu, Qichang Yang, Sen Wang, Yu Wang, Xiaoxue Fan, and Zhonghua Bian. 2023. "The Responses of Sucrose Metabolism and Carbon Translocation in Tomato Seedlings under Different Light Spectra" International Journal of Molecular Sciences 24, no. 20: 15054. https://doi.org/10.3390/ijms242015054
APA StyleZhan, X., Yang, Q., Wang, S., Wang, Y., Fan, X., & Bian, Z. (2023). The Responses of Sucrose Metabolism and Carbon Translocation in Tomato Seedlings under Different Light Spectra. International Journal of Molecular Sciences, 24(20), 15054. https://doi.org/10.3390/ijms242015054








