Protective Effect of Mycosporine-like Amino Acids Isolated from an Antarctic Diatom on UVB-Induced Skin Damage
Abstract
:1. Introduction
2. Results
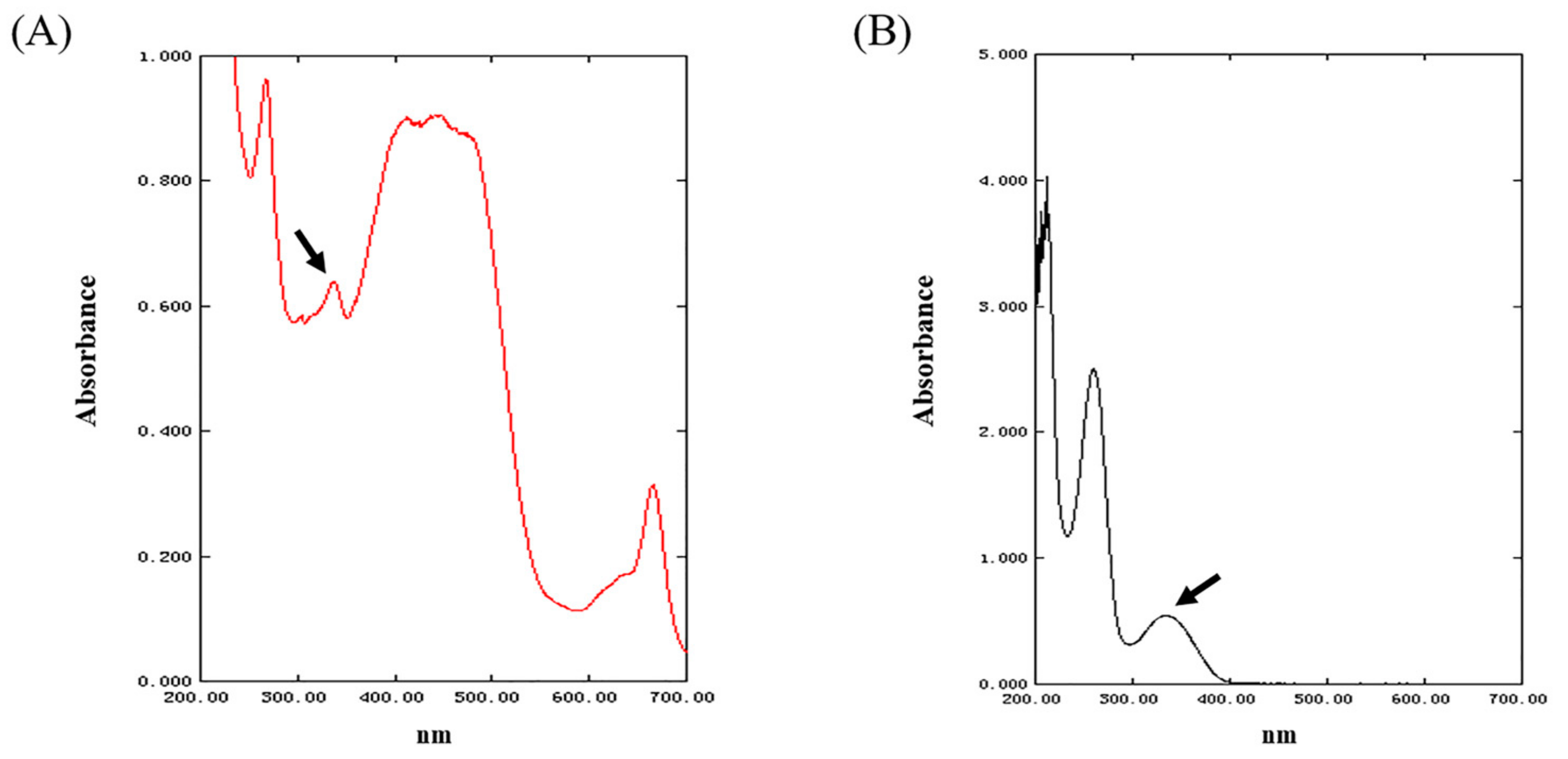
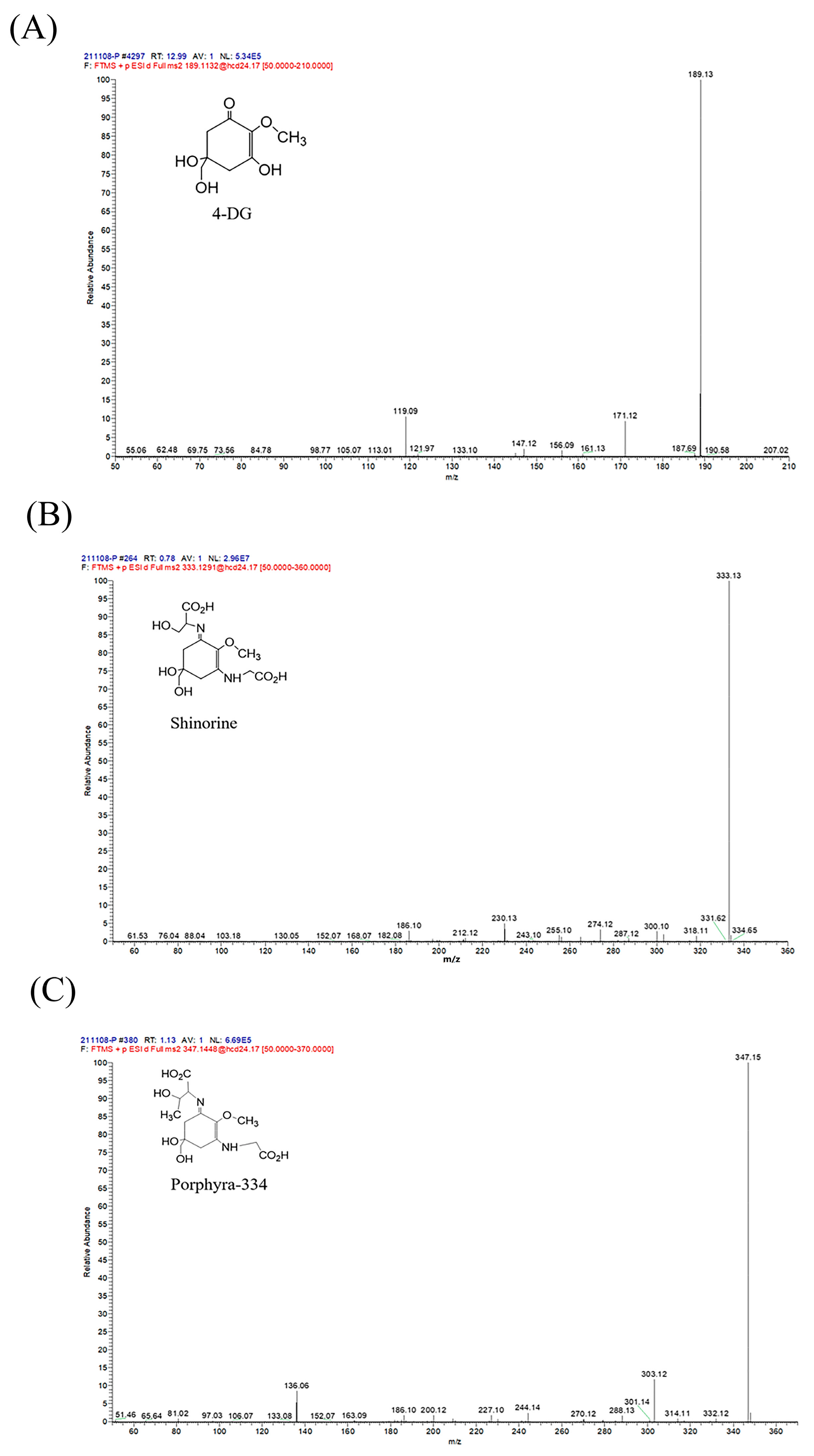
2.1. The Characterization of MAAs Extracted from Phaeodactylum tricornutum ICE-H
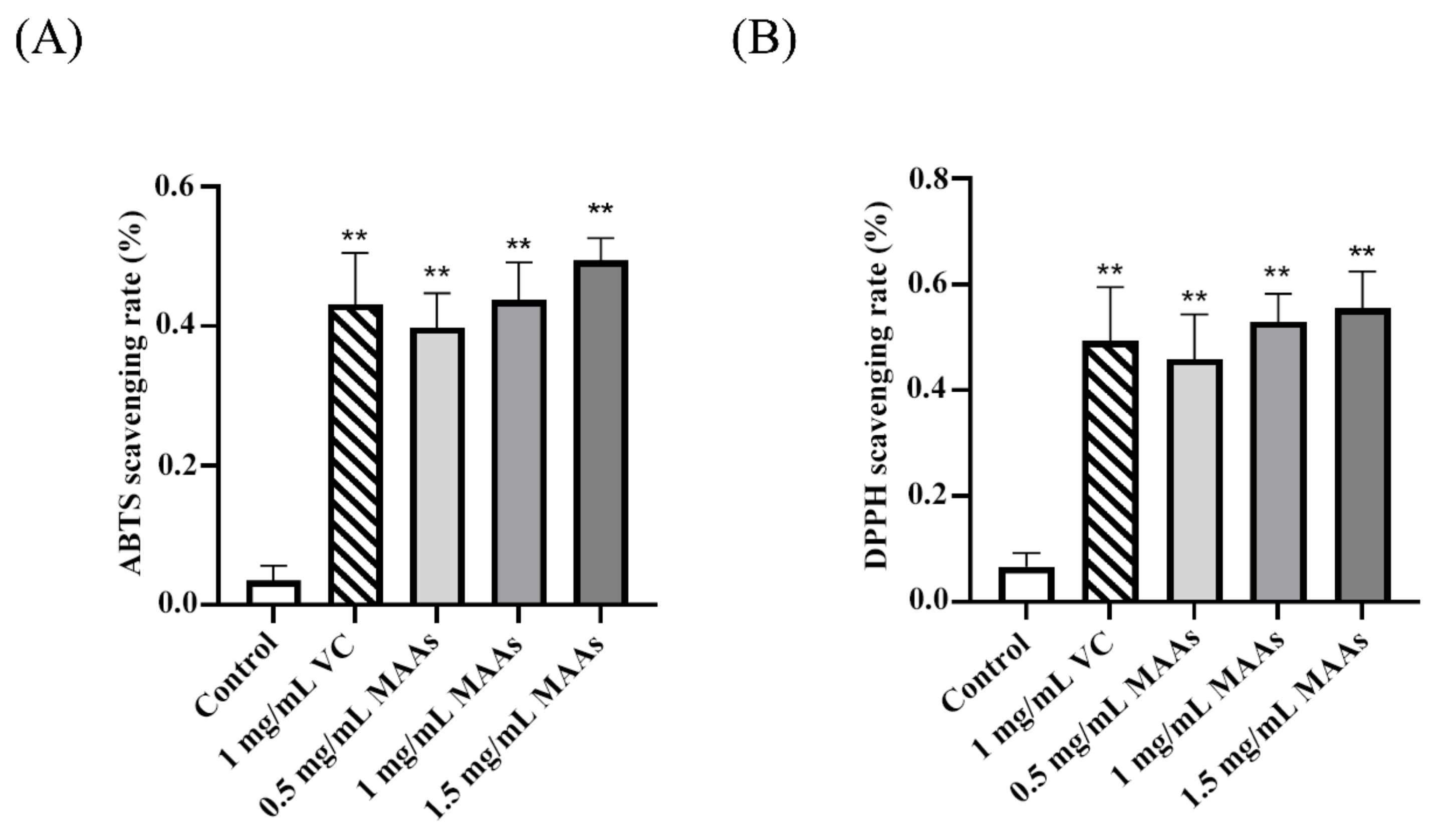
2.2. Antioxidant Levels of MAAs
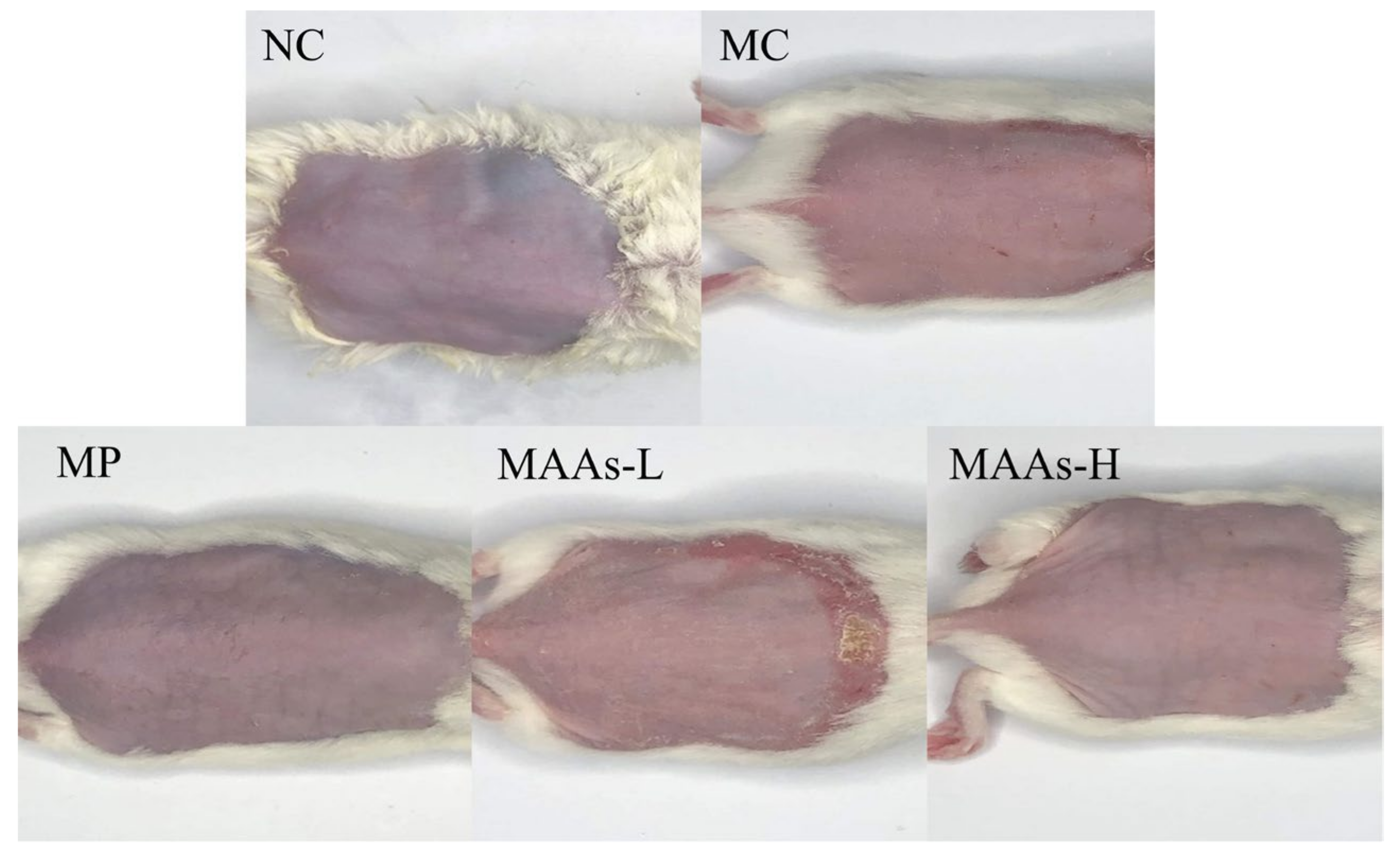
2.3. MAAs Ameliorated Photodamage in Mice Skin
2.4. MAAs Reduced Oxidative Stress Levels in Mice Skin
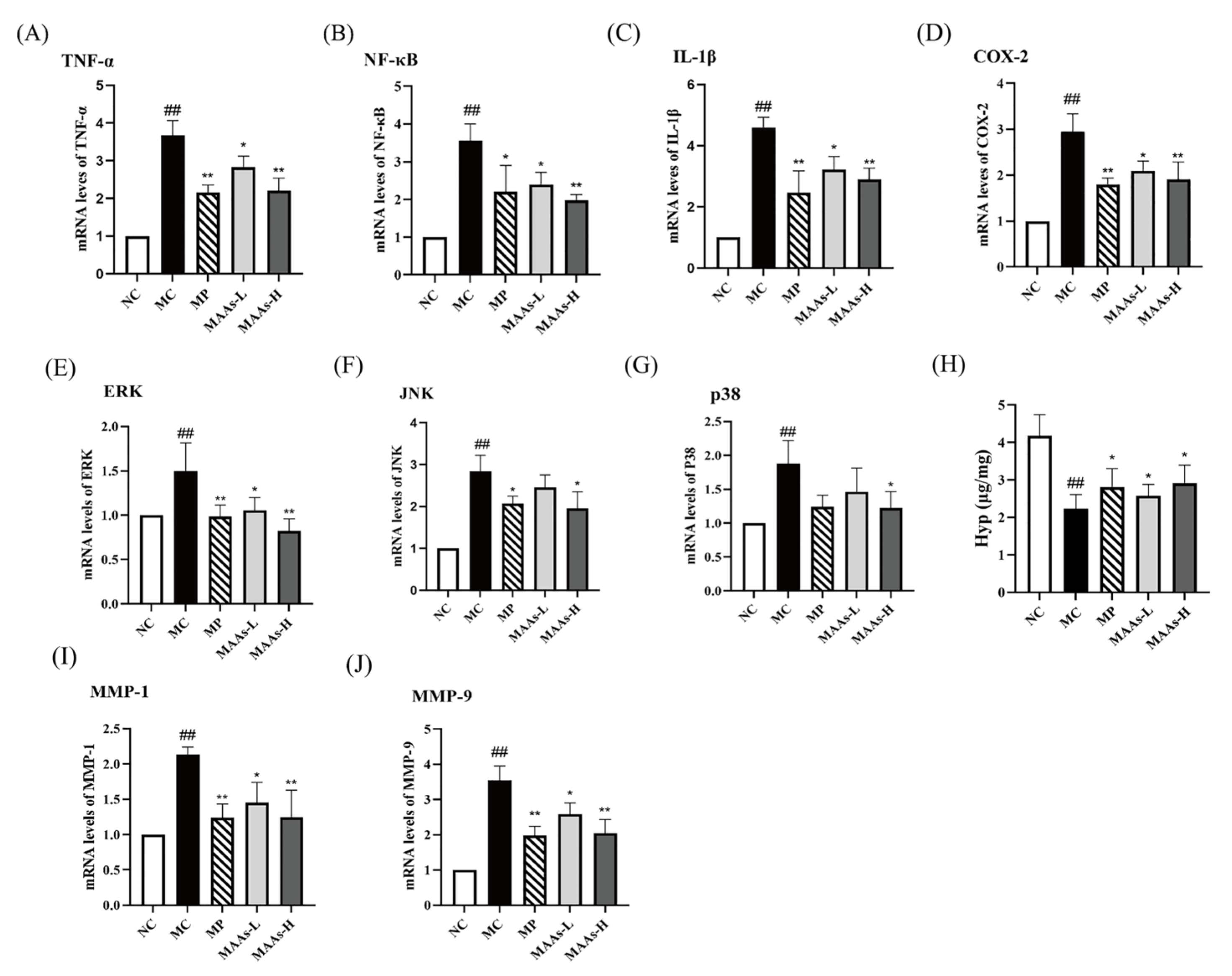
2.5. MAAs Inhibited Skin Inflammation in UVB-Irradiated Mice
2.6. MAAs Reduced Skin Collagen Degradation in UVB-Irradiated Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of MAAs
4.3. Identification and Characterization of MAAs
4.4. Detection of Antioxidant Level of MAAs In Vitro
4.5. Establishment of UVB-Induced Skin Photodamage Model
4.6. Histological Analysis of Skin Tissues
4.7. Biochemical Indicator Analysis
4.8. RNA Extraction and qRT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, K.R.; Alam, M.B.; Park, J.H.; Kim, T.H.; Lee, S.H. Attenuation of UVB-induced photo-aging by polyphenolic-rich spatholobus suberectus stem extract via modulation of MAPK/AP-1/MMPs signaling in human keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef]
- Yum, H.W.; Kim, S.H.; Kang, J.X.; Surh, Y.J. Amelioration of UVB-induced oxidative stress and inflammation in fat-1 transgenic mouse skin. Biochem. Biophys. Res. Commun. 2018, 502, 1–8. [Google Scholar] [CrossRef]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and treatments of photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Lei, X.; Lei, L.; Zhao, J.; Zeng, K.; Ming, J. Premna microphylla Turcz pectin protected UVB-induced skin aging in BALB/c-nu mice via Nrf2 pathway. Int. J. Biol. Macromol. 2022, 215, 12–22. [Google Scholar] [CrossRef]
- Favre-Bonvin, J.; Bernillon, J.; Salin, N.; Arpin, N. Biosynthesis of mycosporines: Mycosporine glutaminol in Trichothecium roseum. Phytochemistry 1987, 26, 2509–2514. [Google Scholar] [CrossRef]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Sawall, T.; Hanelt, D.; Bischof, K.; Figueroa, F.L.; Flores-Moya, A.; Wiencke, C. An inventory of UV-absorbing mycosporine-like amino acids in macroalgae from polar to warm-temperate regions. Bot. Mar. 1998, 41, 443–454. [Google Scholar] [CrossRef]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef]
- Raj, S.; Kuniyil, A.M.; Sreenikethanam, A.; Gugulothu, P.; Jeyakumar, R.B.; Bajhaiya, A.K. Microalgae as a source of mycosporine-like amino acids (MAAs); Advances and Future Prospects. Int. J. Environ. Res. Public Health 2021, 18, 12402. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, I.M.; Baird, A.H. Ontogenetic change in the abundance of mycosporine-like amino acids in non-zooxanthellate coral larvae. Coral Reefs 2005, 24, 443–452. [Google Scholar] [CrossRef]
- Oda, Y.; Zhang, Q.; Matsunaga, S.; Fujita, M.J.; Sakai, R. Two new mycosporine-like amino acids LC-343 and mycosporine-ethanolamine from the micronesian marine sponge Lendenfeldia chondrodes. Chem. Lett. 2017, 46, 1272–1274. [Google Scholar] [CrossRef]
- Adams, N.; Campanale, J.; Gravem, S.; Mallicoat, A.; Mallonee, M. Long-term exposure of adult purple sea urchins, Strongylocentrotus purpuratus, to sunlight protects embryos from ultraviolet radiation. In Proceedings of the Meeting of the Society-for-Integrative-and-Comparative-Biology, Seattle, WA, USA, 3–7 January 2010. [Google Scholar]
- Osborn, A.R.; Almabruk, K.H.; Holzwarth, G.; Asamizu, S.; LaDu, J.; Kean, K.M.; Karplus, P.A.; Tanguay, R.L.; Bakalinsky, A.T.; Mahmud, T. De novo synthesis of a sunscreen compound in vertebrates. eLife 2015, 4, e05919. [Google Scholar] [CrossRef]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like amino acids for skin photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. Experimental study of the excited-state properties and photostability of the mycosporine-like amino acid palythine in aqueous solution. Photochem. Photobiol. Sci. 2007, 6, 669–674. [Google Scholar] [CrossRef]
- Ying, R.; Zhang, Z.; Zhu, H.; Li, B.; Hou, H. The protective effect of mycosporine-like amino acids (MAAs) from Porphyra yezoensis in a mouse model of UV irradiation-induced photoaging. Mar. Drugs 2019, 17, 470. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Zhaohui, Z.; Wenshan, S.; Bafang, L.; Hu, H. Protective effect of MAAs extracted from Porphyra tenera against UV irradiation-induced photoaging in mouse skin. J. Photochem. Photobiol. B Biol. 2019, 192, 26–33. [Google Scholar] [CrossRef] [PubMed]
- De la Coba, F.; Aguilera, J.; de Gálvez, M.V.; Alvarez, M.; Gallego, E.; Figueroa, F.L.; Herrera, E. Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J. Dermatol. Sci. 2009, 55, 161–169. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Wang, X.; Sun, C. Acclimation of Antarctic Chlamydomonas to the sea-ice environment: A transcriptomic analysis. Extremophiles 2016, 20, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.G.; McMinn, A.; Mitchell, K.A.; Trenerry, L. Mycosporine-like amino acids in Antarctic sea ice algae, and their response to UVB radiation. Z. Naturforsch. C J. Biosci. 2002, 57, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.N.; Dieckmann, G.S. Antarctic Sea ice—A habitat for extremophiles. Science 2002, 295, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Lin, S.; Xu, W.; Cheung, P.C.K. Enhancement of the production of bioactive microalgal metabolites by ultraviolet radiation (UVA 365 nm). J. Agric. Food Chem. 2018, 66, 10215–10224. [Google Scholar] [CrossRef] [PubMed]
- Carreto, J.I.; Carignan, M.O.; Montoya, N.G. A high-resolution reverse-phase liquid chromatography method for the analysis of mycosporine-like amino acids (MAAs) in marine organisms. Mar. Biol. 2004, 146, 237–252. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, Contents, and types of mycosporine-like amino acids (MAAs) in marine macroalgae and a database for MAAs based on these characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Rubin, G.M.; Jiang, G.; Raad, Z.; Ding, Y. Biosynthesis and heterologous production of mycosporine-like amino acid palythines. J. Org. Chem. 2021, 86, 11160–11168. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N.; Braun, C.; Kvaskoff, D. Extraction and analysis of mycosporine-like amino acids in marine algae. Methods Mol. Biol. 2015, 1308, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The banned sunscreen ingredients and their impact on human health: A systematic review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef]
- Coba, F.D.L.; Aguilera, J.; Figueroa, F.L.; Gálvez, M.V.d.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D.; Incharoensakdi, A. Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res. 2016, 16, 110–118. [Google Scholar] [CrossRef]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef] [PubMed]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y.V. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute configuration of mycosporine-like amino acids, their wound healing properties and in vitro anti-aging effects. Mar. Drugs 2019, 18, 35. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39. [Google Scholar] [CrossRef]
- Peng, J.; Guo, F.; Liu, S.; Fang, H.; Xu, Z.; Wang, T. Recent advances and future prospects of mycosporine-like amino acids. Molecules 2023, 28, 5588. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, R.; Wang, M.; Zhai, L.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J. Ginseng Res. 2022, 46, 115–125. [Google Scholar] [CrossRef]
- Hu, S.; Huang, J.; Pei, S.; Ouyang, Y.; Ding, Y.; Jiang, L.; Lu, J.; Kang, L.; Huang, L.; Xiang, H.; et al. Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J. Cell. Physiol. 2019, 234, 7330–7340. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Fotakis, C.; Nikokavoura, A.; Papadopoulos, K.; Calokerinos, A.C. Modified DPPH and ABTS assays to assess the antioxidant profile of untreated oils. Food Anal. Methods 2014, 8, 1294–1302. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, C.; Zhang, K.; He, Y.; Zhao, X.; Yang, L.; Zheng, Z.; Ma, X.; Wang, X.; Wang, W.; et al. Adaptation to extreme antarctic environments revealed by the genome of a sea ice green alga. Curr. Biol. CB 2020, 30, 3330–3341.e7. [Google Scholar] [CrossRef]
- Adams, A.; De Kimpe, N.; van Boekel, M.A. Modification of casein by the lipid oxidation product malondialdehyde. J. Agric. Food Chem. 2008, 56, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Degitz, K.; Quirling, M.; Jilg, N.; Page, S.; Brand, K. Involvement of NF-kappaB signalling in skin physiology and disease. Cell. Signal. 2003, 15, 1–7. [Google Scholar] [CrossRef]
- Surowiak, P.; Gansukh, T.; Donizy, P.; Halon, A.; Rybak, Z. Increase in cyclooxygenase-2 (COX-2) expression in keratinocytes and dermal fibroblasts in photoaged skin. J. Cosmet. Dermatol. 2014, 13, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Nakagawa, K.; Kawakami, Y.; Tsuzuki, T.; Miyazawa, T. Suppression of gamma-tocotrienol on UVB induced inflammation in HaCaT keratinocytes and HR-1 hairless mice via inflammatory mediators multiple signaling. J. Agric. Food Chem. 2010, 58, 7013–7020. [Google Scholar] [CrossRef]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef]
- Çam, S.T.; Seyhan, N.; Kavaklı, C.; Çelikbıçak, Ö. Effects of 900 MHz radiofrequency radiation on skin hydroxyproline contents. Cell Biochem. Biophys. 2014, 70, 643–649. [Google Scholar] [CrossRef]
- Lee, J.K.; Ko, S.H.; Ye, S.K.; Chung, M.H. 8-Oxo-2′-deoxyguanosine ameliorates UVB-induced skin damage in hairless mice by scavenging reactive oxygen species and inhibiting MMP expression. J. Dermatol. Sci. 2013, 70, 49–57. [Google Scholar] [CrossRef]
- Roh, E.; Kim, J.E.; Kwon, J.Y.; Park, J.S.; Bode, A.M.; Dong, Z.; Lee, K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food Sci. Nutr. 2017, 57, 1631–1637. [Google Scholar] [CrossRef]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric Skin Aging-Contributors and Inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2004, 3, 960–967. [Google Scholar] [CrossRef]
- An, M.; Qu, C.; Miao, J.; Sha, Z. Two class II CPD photolyases, PiPhr1 and PiPhr2, with CPD repair activity from the Antarctic diatom Phaeodactylum tricornutum ICE-H. 3 Biotech 2021, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.H.; Khan, N.; Zaid, M.A.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Deng, Y.; He, Y.; Cao, J.; Zhang, L.; Qin, L.; Qu, C.; Li, H.; Miao, J. Protective Effect of Mycosporine-like Amino Acids Isolated from an Antarctic Diatom on UVB-Induced Skin Damage. Int. J. Mol. Sci. 2023, 24, 15055. https://doi.org/10.3390/ijms242015055
Wang K, Deng Y, He Y, Cao J, Zhang L, Qin L, Qu C, Li H, Miao J. Protective Effect of Mycosporine-like Amino Acids Isolated from an Antarctic Diatom on UVB-Induced Skin Damage. International Journal of Molecular Sciences. 2023; 24(20):15055. https://doi.org/10.3390/ijms242015055
Chicago/Turabian StyleWang, Kai, Yashan Deng, Yingying He, Junhan Cao, Liping Zhang, Ling Qin, Changfeng Qu, Hongmei Li, and Jinlai Miao. 2023. "Protective Effect of Mycosporine-like Amino Acids Isolated from an Antarctic Diatom on UVB-Induced Skin Damage" International Journal of Molecular Sciences 24, no. 20: 15055. https://doi.org/10.3390/ijms242015055
APA StyleWang, K., Deng, Y., He, Y., Cao, J., Zhang, L., Qin, L., Qu, C., Li, H., & Miao, J. (2023). Protective Effect of Mycosporine-like Amino Acids Isolated from an Antarctic Diatom on UVB-Induced Skin Damage. International Journal of Molecular Sciences, 24(20), 15055. https://doi.org/10.3390/ijms242015055





