LRP10, PGK1 and RPLP0: Best Reference Genes in Periprostatic Adipose Tissue under Obesity and Prostate Cancer Conditions
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Gene Expression Stability in Periprostatic Adipose Tissue (PPAT) of Patients with Benign Prostate Hyperplasia (BPH) and Prostate Cancer (PCa)
2.2. Influence of BMI (Body Mass Index) and PCa Presence on the Expression of IRGs in PPATs
2.3. Validation of RPLP0, PGK1, and LRP10 as Reference Genes
3. Discussion
4. Materials and Methods
4.1. Subjects and Samples
4.2. Total RNA Isolation and cDNA Synthesis
4.3. Potential Reference Gene Selection and Primer Design
4.4. RT-qPCR and Microfluidic-Based qPCR Array
4.5. Evaluation of Putative IRGs
4.6. Statistical Analyses
4.7. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Reviews. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Lam, F.; Ervik, M.; Soerjomataram, I. Cancer and Obesity: Global Burden of Cancer Attributable to Excess Weight; International Agency for Research on Cancer: Lyon, France, 2015. Available online: http://gco.iarc.fr/obesity (accessed on 1 October 2022).
- Arnold, M.; Pandeya, N.; Byrnes, G.; Renehan, P.A.G.; Stevens, G.A.; Ezzati, P.M.; Ferlay, J.; Miranda, J.J.; Romieu, I.; Dikshit, R.; et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015, 16, 36–46. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Pinthus, J.H.; Duivenvoorden, W.C.M.; Mourtzakis, M. Glucose impairments and insulin resistance in prostate cancer: The role of obesity, nutrition and exercise. Obes. Rev. 2018, 19, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and prostate cancer: Weighing the evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Gómez, E.; Carrasco-Valiente, J.; Campos-Hernández, J.P.; Blanca-Pedregosa, A.M.; Jiménez-Vacas, J.M.; Ruiz-García, J.; Valero-Rosa, J.; Luque, R.M.; Requena-Tapia, M.J. Clinical association of metabolic syndrome, C-reactive protein and testosterone levels with clinically significant prostate cancer. J. Cell. Mol. Med. 2019, 23, 934–942. [Google Scholar] [CrossRef]
- Laurent, V.; Toulet, A.; Attané, C.; Milhas, D.; Dauvillier, S.; Zaidi, F.; Clement, E.; Cinato, M.; Le Gonidec, S.; Guérard, A.; et al. Periprostatic Adipose Tissue Favors Prostate Cancer Cell Invasion in an Obesity-Dependent Manner: Role of Oxidative Stress. Mol. Cancer Res. 2019, 17, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, L.J.; Qi, J.; Yang, Z.G.; Huang, T.; Huo, R.C. Periprostatic adiposity measured on magnetic resonance imaging correlates with prostate cancer aggressiveness. Urol. J. 2014, 11, 1793–1799. [Google Scholar] [CrossRef]
- Almeida-Oliveira, F.; Leandro, J.G.B.; Ausina, P.; Sola-Penna, M.; Majerowicz, D. Reference genes for quantitative PCR in the adipose tissue of mice with metabolic disease. Biomed. Pharmacother. 2017, 88, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Sun, L.; Chen, B.; Han, Y.; Pang, J.; Wu, W.; Qi, R.; Zhang, T.M. Evaluation of candidate reference genes for RT-qPCR studies in three metabolism related tissues of mice after caloric restriction. Sci. Rep. 2016, 6, 38513. [Google Scholar] [CrossRef]
- Zhang, W.X.; Fan, J.; Ma, J.; Rao, Y.S.; Zhang, L.; Yan, Y.E. Selection of Suitable Reference Genes for Quantitative Real-Time PCR Normalization in Three Types of Rat Adipose Tissue. Int. J. Mol. Sci. 2016, 17, 968. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.J.; Rios, L.; Trivedi, P.; D’Souza, K.; Cowie, A.; Nzirorera, C.; Webster, D.; Brunt, K.; Legare, J.F.; Hassan, A.; et al. Validation of optimal reference genes for quantitative real time PCR in muscle and adipose tissue for obesity and diabetes research. Sci. Rep. 2017, 7, 3612. [Google Scholar] [CrossRef] [PubMed]
- Taube, M.; Andersson-Assarsson, J.C.; Lindberg, K.; Pereira, M.J.; Gäbel, M.; Svensson, M.K.; Eriksson, J.W.; Svensson, P.A. Evaluation of reference genes for gene expression studies in human brown adipose tissue. Adipocyte 2015, 4, 280–285. [Google Scholar] [CrossRef]
- Krautgasser, C.; Mandl, M.; Hatzmann, F.M.; Waldegger, P.; Mattesich, M.; Zwerschke, W. Reliable reference genes for expression analysis of proliferating and adipogenically differentiating human adipose stromal cells. Cell. Mol. Biol. Lett. 2019, 24, 14. [Google Scholar] [CrossRef]
- Chang, M.C.; Eslami, Z.; Ennis, M.; Goodwin, P.J. Crown-like structures in breast adipose tissue of breast cancer patients: Associations with CD68 expression, obesity, metabolic factors and prognosis. NPJ Breast Cancer 2021, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int. J. Mol. Sci. 2019, 20, 839. [Google Scholar] [CrossRef]
- Lee, J.O.; Kim, N.; Lee, H.J.; Lee, Y.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci. Rep. 2016, 6, 18923. [Google Scholar] [CrossRef]
- Maalouf, G.E.; El Khoury, D. Exercise-Induced Irisin, the Fat Browning Myokine, as a Potential Anticancer Agent. J. Obes. 2019, 2019, 6561726. [Google Scholar] [CrossRef]
- Rasha, F.; Ramalingam, L.; Gollahon, L.; Rahman, R.L.; Rahman, S.M.; Menikdiwela, K.; Moustaid-Moussa, N. Mechanisms linking the renin-angiotensin system, obesity, and breast cancer. Endocr. Relat. Cancer 2019, 26, R653–R672. [Google Scholar] [CrossRef] [PubMed]
- Sturtz, L.A.; Deyarmin, B.; van Laar, R.; Yarina, W.; Shriver, C.D.; Ellsworth, R.E. Gene expression differences in adipose tissue associated with breast tumorigenesis. Adipocyte 2014, 3, 107–114. [Google Scholar] [CrossRef]
- Ribeiro, R.; Monteiro, C.; Catalán, V.; Hu, P.; Cunha, V.; Rodríguez, A.; Gómez-Ambrosi, J.; Fraga, A.; Príncipe, P.; Lobato, C.; et al. Obesity and prostate cancer: Gene expression signature of human periprostatic adipose tissue. BMC Med. 2012, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Monteiro, C.; Cunha, V.; Oliveira, M.J.; Freitas, M.; Fraga, A.; Príncipe, P.; Lobato, C.; Lobo, F.; Morais, A.; et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J. Exp. Clin. Cancer Res. 2012, 31, 32. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Choi, S.; Oh, J.; Lee, S.-G.; Choi, S.Y.; Kim, K.K.; Park, C. Conventionally used reference genes are not outstanding for normalization of gene expression in human cancer research. BMC Bioinform. 2019, 20, 245. [Google Scholar] [CrossRef] [PubMed]
- Bandini, M.; Gandaglia, G.; Briganti, A. Obesity and prostate cancer. Curr. Opin. Urol. 2017, 27, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Finley, D.S.; Calvert, V.S.; Inokuchi, J.; Lau, A.; Narula, N.; Petricoin, E.F.; Zaldivar, F.; Santos, R.; Tyson, D.R.; Ornstein, D.K. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J. Urol. 2009, 182, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cen, Z.; Zheng, X.; Pan, Q.; Chen, X.; Zhu, L.; Chen, S.; Wu, H.; Xie, F.; Wang, H.; et al. LRP10 in autosomal-dominant Parkinson’s disease. Mov. Disord. 2019, 34, 912–916. [Google Scholar] [CrossRef]
- Gabrielsson, B.G.; Olofsson, L.E.; Sjögren, A.; Jernås, M.; Elander, A.; Lönn, M.; Rudemo, M.; Carlsson, L.M. Evaluation of reference genes for studies of gene expression in human adipose tissue. Obes. Res. 2005, 13, 649–652. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, W.; Qin, J.; Chen, M.; Gong, L.; Li, L.; Liu, X.; Tao, Y.; Yin, H.; Zhou, H.; et al. Acetylation of PGK1 promotes liver cancer cell proliferation and tumorigenesis. Hepatology 2017, 65, 515–528. [Google Scholar] [CrossRef]
- Lu, W.; Gao, J.; Yang, J.; Cao, Y.; Jiang, L.; Li, M.; Zhang, Y.; Zhou, J.; Liu, Y. Down-Regulated Phosphoglycerate Kinase 1 Expression Is Associated With Poor Prognosis in Patients With Gallbladder Cancer. Medicine 2015, 94, e2244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, G.; Chu, H.; Wang, X.; Xiong, L.; Cai, G.; Liu, R.; Gao, H.; Tao, B.; Li, W.; et al. Macrophage-Associated PGK1 Phosphorylation Promotes Aerobic Glycolysis and Tumorigenesis. Mol. Cell 2018, 71, 201–215.e7. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, V.R.; Whistler, T.; Murray, J.R.; Unger, E.R.; Rajeevan, M.S. Identification of Phosphoglycerate Kinase 1 (PGK1) as a reference gene for quantitative gene expression measurements in human blood RNA. BMC Res. Notes 2011, 4, 324. [Google Scholar] [CrossRef]
- Panina, Y.; Germond, A.; Masui, S.; Watanabe, T.M. Validation of Common Housekeeping Genes as Reference for qPCR Gene Expression Analysis During iPS Reprogramming Process. Sci. Rep. 2018, 8, 8716. [Google Scholar] [CrossRef]
- Neville, M.J.; Collins, J.M.; Gloyn, A.L.; McCarthy, M.I.; Karpe, F. Comprehensive human adipose tissue mRNA and microRNA endogenous control selection for quantitative real-time-PCR normalization. Obesity 2011, 19, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Fuentes-Fayos, A.C.; Vázquez-Borrego, M.C.; Jiménez-Vacas, J.M.; Bejarano, L.; Pedraza-Arévalo, S.; L.-López, F.; Blanco-Acevedo, C.; Sánchez-Sánchez, R.; Reyes, O.; Ventura, S.; et al. Splicing machinery dysregulation drives glioblastoma development/aggressiveness: Oncogenic role of SRSF3. Brain 2020, 143, 3273–3293. [Google Scholar] [CrossRef]
- Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Montero-Hidalgo, A.J.; Gómez-Gómez, E.; Fuentes-Fayos, A.C.; León-González, A.J.; Sáez-Martínez, P.; Alors-Pérez, E.; Pedraza-Arévalo, S.; González-Serrano, T.; et al. Dysregulation of the splicing machinery is directly associated to aggressiveness of prostate cancer. EBioMedicine 2020, 51, 102547. [Google Scholar] [CrossRef]
- Del Río-Moreno, M.; Alors-Pérez, E.; González-Rubio, S.; Ferrín, G.; Reyes, O.; Rodríguez-Perálvarez, M.; Sánchez-Frías, M.E.; Sánchez-Sánchez, R.; Ventura, S.; López-Miranda, J.; et al. Dysregulation of the Splicing Machinery Is Associated to the Development of Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2019, 104, 3389–3402. [Google Scholar] [CrossRef]
- Gahete, M.D.; Del Rio-Moreno, M.; Camargo, A.; Alcala-Diaz, J.F.; Alors-Perez, E.; Delgado-Lista, J.; Reyes, O.; Ventura, S.; Perez-Martínez, P.; Castaño, J.P.; et al. Changes in Splicing Machinery Components Influence, Precede, and Early Predict the Development of Type 2 Diabetes: From the CORDIOPREV Study. EBioMedicine 2018, 37, 356–365. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genom. 2023, 23, 125. [Google Scholar] [CrossRef] [PubMed]
- Boos, D.D. Introduction to the Bootstrap World. Stat. Sci. 2003, 18, 168–174. [Google Scholar] [CrossRef]
- Altuna-Coy, A.; Ruiz-Plazas, X.; Sánchez-Martin, S.; Ascaso-Til, H.; Prados-Saavedra, M.; Alves-Santiago, M.; Bernal-Escoté, X.; Segarra-Tomás, J.; Chacón, M.R. The lipidomic profile of the tumoral periprostatic adipose tissue reveals alterations in tumor cell’s metabolic crosstalk. BMC Med. 2022, 20, 255. [Google Scholar] [CrossRef]
- Roumiguié, M.; Estève, D.; Manceau, C.; Toulet, A.; Gilleron, J.; Belles, C.; Jia, Y.; Houël, C.; Pericart, S.; LeGonidec, S.; et al. Periprostatic Adipose Tissue Displays a Chronic Hypoxic State that Limits Its Expandability. Am. J. Pathol. 2022, 192, 926–942. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, L.J.; Yang, Z.G.; Zhang, G.M.; Huo, R.C. Influence of adipocytokines in periprostatic adipose tissue on prostate cancer aggressiveness. Cytokine 2016, 85, 148–156. [Google Scholar] [CrossRef]
- Cahyadi, D.D.; Warita, T.; Irie, N.; Mizoguchi, K.; Tashiro, J.; Hosaka, Y.Z.; Warita, K. Housekeeping gene expression variability in differentiating and non-differentiating 3T3-L1 cells. Adipocyte 2023, 12, 2235081. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Hollifield, G. Glucose-6-phosphate dehydrogenase activity in adipose tissue from two types of obese mice during starvation. Metabolism 1966, 15, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.H.; Lauritzen, L.; Hellgren, L.I. Fish oil combined with SCFA synergistically prevent tissue accumulation of NEFA during weight loss in obese mice. Br. J. Nutr. 2011, 106, 1449–1456. [Google Scholar] [CrossRef]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Barrett, P.; Bashir, S.; Miller, J.D. Inhibin betaB expression in murine adipose tissue and its regulation by leptin, insulin and dexamethasone. J. Mol. Endocrinol. 2009, 43, 171–177. [Google Scholar] [CrossRef]
- Fromm-Dornieden, C.; Lytovchenko, O.; von der Heyde, S.; Behnke, N.; Hogl, S.; Berghoff, J.; Köpper, F.; Opitz, L.; Renne, U.; Hoeflich, A.; et al. Extrinsic and intrinsic regulation of DOR/TP53INP2 expression in mice: Effects of dietary fat content, tissue type and sex in adipose and muscle tissues. Nutr. Metab. 2012, 9, 86. [Google Scholar] [CrossRef]
- Wu, Y.; Kim, J.Y.; Zhou, S.; Smas, C.M. Differential screening identifies transcripts with depot-dependent expression in white adipose tissues. BMC Genom. 2008, 9, 397. [Google Scholar] [CrossRef]
- Wargent, E.T.; O’Dowd, J.F.; Zaibi, M.S.; Gao, D.; Bing, C.; Trayhurn, P.; Cawthorne, M.A.; Arch, J.R.; Stocker, C.J. Contrasts between the effects of zinc-α2-glycoprotein, a putative β3/2-adrenoceptor agonist and the β3/2-adrenoceptor agonist BRL35135 in C57Bl/6 (ob/ob) mice. J. Endocrinol. 2013, 216, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Gelinas, Y.; Mathieu, P.; Deshaies, Y.; Richard, D. Validation of reference genes for the relative quantification of gene expression in human epicardial adipose tissue. PLoS ONE 2012, 7, e32265. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yao, H.; Liu, X.; Shi, Q.; Lv, L.; Li, P.; Wang, R.; Tang, T.; Qi, K. High-Fat Diet Alters the Expression of Reference Genes in Male Mice. Front. Nutr. 2020, 7, 589771. [Google Scholar] [CrossRef]
- Hurtado del Pozo, C.; Calvo, R.M.; Vesperinas-García, G.; Gómez-Ambrosi, J.; Frühbeck, G.; Corripio-Sánchez, R.; Rubio, M.A.; Obregon, M.J. IPO8 and FBXL10: New reference genes for gene expression studies in human adipose tissue. Obesity 2010, 18, 897–903. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rotellar, F.; Silva, C.; Rodríguez, A.; Salvador, J.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Validation of endogenous control genes in human adipose tissue: Relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm. Metab. Res. 2007, 39, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.C.; Yogarajah, T.; Lim, S.K.; Tee, G.B.Y.; Khoo, B.Y. Pilot study and bioinformatics analysis of differentially expressed genes in adipose tissues of rats with excess dietary intake. Mol. Med. Rep. 2020, 21, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Shah, S.G.; Natu, A.; Verma, T.; Rauniyar, S.; Gera, P.B.; Gupta, S. RPS13, a potential universal reference gene for normalisation of gene expression in multiple human normal and cancer tissue samples. Mol. Biol. Rep. 2021, 48, 7967–7974. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, H.J.; Fehrmann, R.S.; de Bont, E.S.; Hofstra, R.M.; Gerbens, F.; Kamps, W.A.; de Vries, E.G.; van der Zee, A.G.; Meerman, G.J.T.; Elst, A.T. Evidence based selection of housekeeping genes. PLoS ONE 2007, 2, e898. [Google Scholar] [CrossRef] [PubMed]
- Sharan, R.N.; Vaiphei, S.T.; Nongrum, S.; Keppen, J.; Ksoo, M. Consensus reference gene(s) for gene expression studies in human cancers: End of the tunnel visible? Cell Oncol. 2015, 38, 419–431. [Google Scholar] [CrossRef]
- Caracausi, M.; Piovesan, A.; Antonaros, F.; Strippoli, P.; Vitale, L.; Pelleri, M.C. Systematic identification of human housekeeping genes possibly useful as references in gene expression studies. Mol. Med. Rep. 2017, 16, 2397–2410. [Google Scholar] [CrossRef]


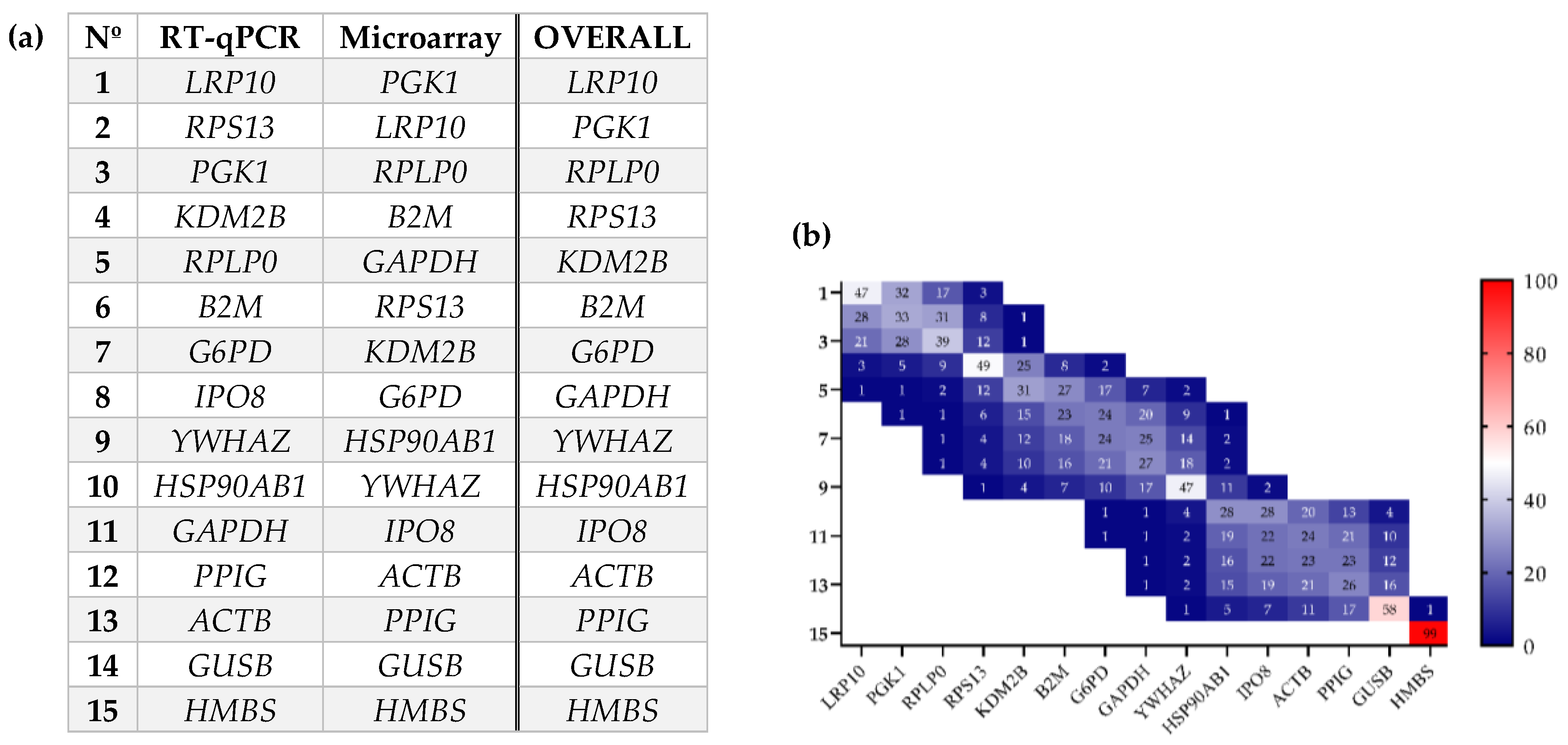
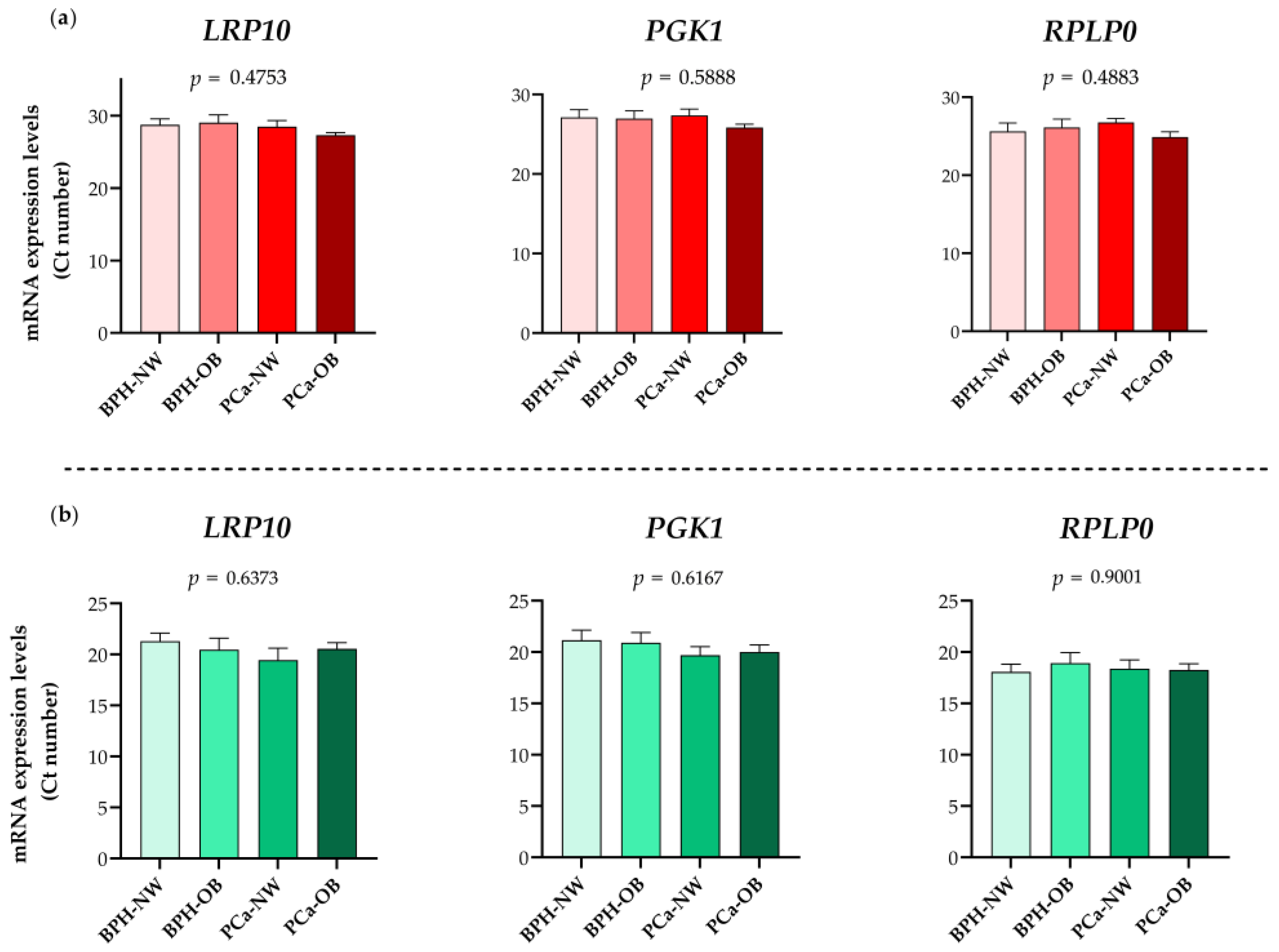

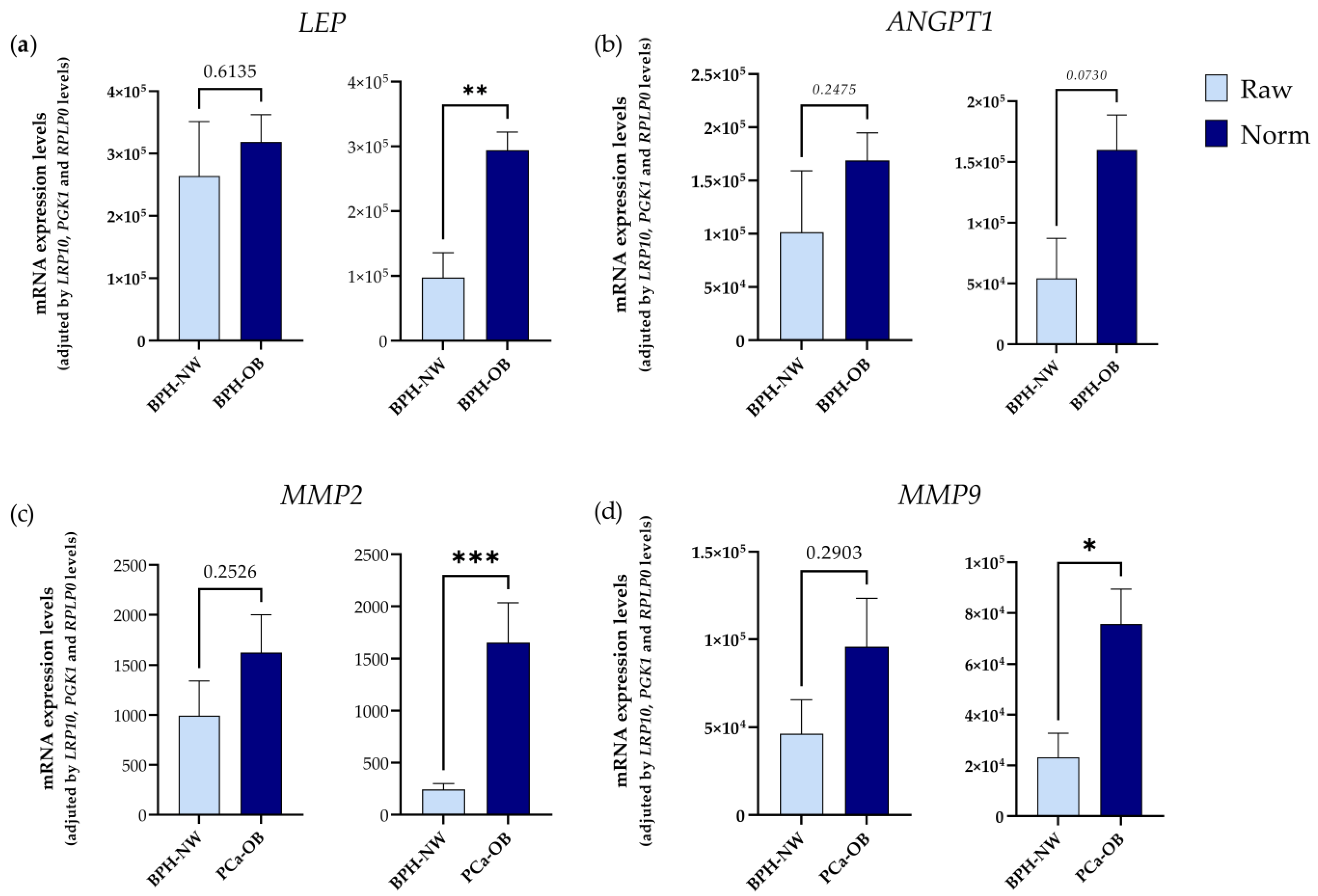
| Methodology | BMI | Parameter | BPH Group | PCa Group |
|---|---|---|---|---|
| RT-qPCR Cohort 1 (n = 20) | Normo-weight | n | 5 | 5 |
| BMI (kg/cm2) | 23.76 ± 1.71 | 23.47 ± 1.39 | ||
| PSA (ng/mL) | 4.28 ± 5.67 | 6.73 ± 4.28 | ||
| Gleason score | - | 7 ± 0 | ||
| Age | 69.9 ± 5.59 | 60.8 ± 4.96 | ||
| PI-RADS | 2.67 ± 1.15 | 4.25 ± 0.50 | ||
| Obesity | n | 5 | 5 | |
| BMI (kg/cm2) | 31.05 ± 2.09 | 33.63 ± 1.25 | ||
| PSA (ng/mL) | 9.60 ± 17.04 | 14.29 ± 8.14 | ||
| Gleason score | - | 7 ± 0 | ||
| Age | 69.4 ± 7.66 | 62.2 ± 3.42 | ||
| PI-RADS | 2 ± 0 | 4.20 ± 1.30 | ||
| Microfluidic-based qPCR array Cohort 2 (n = 48) | Normo-weight | n | 6 | 14 |
| BMI (kg/cm2) | 24.161 3.20 | 23.80 1.12 | ||
| PSA (ng/mL) | 3.99 5.12 | 8.43 7.35 | ||
| Gleason score | - | 7 | ||
| Age | 69.33 ± 5.04 | 59.45 ± 5.15 | ||
| PI-RADS | 2.33 ± 0.81 | 4.51 ± 0.53 | ||
| Obesity | n | 14 | 14 | |
| BMI (kg/cm2) | 31.26 ± 2.07 | 31.13 ± 1.01 | ||
| PSA (ng/mL) | 6.99 ± 10.31 | 7 ± 4.95 | ||
| Gleason score | - | 6.85 ± 0.37 | ||
| Age | 69.22 ± 6.62 | 58.85 ± 5.33 | ||
| PI-RADS | 2 ± 0 | 4.27 ± 0.90 |
| Gene | Sequence | A.S (bp) | Official Name | Function |
|---|---|---|---|---|
| ACTB | ACTCTTCCAGCCTTCCTTCCT | 176 | Actin Beta | Highly conserved proteins involved in cell motility, structure, and integrity |
| CAGTGATCTCCTTCTGCATCCT | ||||
| B2M | GCTCGCGCTACTCTCTCTTT | 88 | Beta-2-Microglobulin | MHC class I associated protein that is found on nearly all cellular surfaces |
| TCCATTCTCTGCTGGATGAC | ||||
| G6PD | GCAAACAGAGTGAGCCCTTC | 89 | Glucose-6-Phosphate Dehydrogenase | Cytosolic enzyme whose main function is to produce NADPH using glucose |
| GCCAGCCACATAGGAGTTG | ||||
| GAPDH | GCCTCAAGATCATCAGCAATG | 90 | Glyceraldehyde-3-Phosphate Dehydrogenase | Enzyme related to carbohydrate metabolism and DNA glycosylation |
| CTTCCACGATACCAAAGTTGT | ||||
| GUSB | TGACCGCTATGGGATTGT | 120 | Glucuronidase Beta | Enzyme able to hydrolyze glycosaminoglycans |
| CTACGCACCACTTCTTCCA | ||||
| HMBS | TTGCTATGTCCACCACAGG | 117 | Hydroxymethylbilane Synthase | Enzyme involved in the condensation of porphobilinogen |
| CCAGGTCCACTTCATTCTTCT | ||||
| HSP90AB1 | ATGGAAGAGAGCAAGGCAAAG | 114 | Heat Shock Protein 90α Family Class B Member 1 | Constitutive form of the cytosolic 90 kDa heat-shock protein |
| GCAGCAAGGTGAAGACACAA | ||||
| IPO8 | CCAAGGGGTGGTTCATTCT | 120 | Importin 8 | GTPase involved in the nuclear import of proteins |
| TCTTGCCACAGCTCTTCATC | ||||
| KDM2B | TGGAGGGCAAAGATTTCAAC | 86 | Lysine Demethylase 2B | Component of SCFs complex involved in ubiquitination |
| TCCCAGTCCATCCTTTTCTC | ||||
| LRP10 | GGCAACGTCACCATCACTT | 82 | LDL Receptor Related Protein 10 | Encodes for a low-density lipoprotein receptor family protein |
| AATCTTGGCTGTAGGAGAGCA | ||||
| PGK1 | GGTGGAATGGCTTTTACCTT | 83 | Phosphoglycerate Kinase 1 | Glycolytic enzyme that catalyzes the conversion of 1,3-diphosphoglycerate |
| ATCTTGGCTCCCTCTTCATC | ||||
| PPIG | TTGCGGAGGTACGGATACT | 104 | Peptidylprolyl Isomerase G | Protein involved in protein peptidyl-prolyl isomerization |
| TGAGGAGGAGGAAGATGATG | ||||
| RPLP0 | AACTCTGCATTCTCGCTTCC | 119 | Ribosomal Protein Lateral Stalk Subunit P0 | Ribosomal protein that is a component of the 60S subunit |
| GGACTCGTTTGTACCCGTTG | ||||
| RPS13 | CCCACTTGGTTGAAGTTGAC | 86 | Ribosomal Protein S13 | Ribosomal protein that is a component of the 40S subunit |
| CCGATCTGTGAAGGAGTAAGG | ||||
| YWHAZ | TACCGTTACTTGGCTGAGGTT | 118 | Y3-Monooxygenase/W5-M.oxygenase Act. Prot. Z | Signal transduction mediation by binding to phosphoserine-containing proteins |
| GATGTGTTGGTTGCATTTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Gómez, J.M.; Porcel-Pastrana, F.; De La Luz-Borrero, M.; Montero-Hidalgo, A.J.; Gómez-Gómez, E.; Herrera-Martínez, A.D.; Guzmán-Ruiz, R.; Malagón, M.M.; Gahete, M.D.; Luque, R.M. LRP10, PGK1 and RPLP0: Best Reference Genes in Periprostatic Adipose Tissue under Obesity and Prostate Cancer Conditions. Int. J. Mol. Sci. 2023, 24, 15140. https://doi.org/10.3390/ijms242015140
Pérez-Gómez JM, Porcel-Pastrana F, De La Luz-Borrero M, Montero-Hidalgo AJ, Gómez-Gómez E, Herrera-Martínez AD, Guzmán-Ruiz R, Malagón MM, Gahete MD, Luque RM. LRP10, PGK1 and RPLP0: Best Reference Genes in Periprostatic Adipose Tissue under Obesity and Prostate Cancer Conditions. International Journal of Molecular Sciences. 2023; 24(20):15140. https://doi.org/10.3390/ijms242015140
Chicago/Turabian StylePérez-Gómez, Jesús M., Francisco Porcel-Pastrana, Marina De La Luz-Borrero, Antonio J. Montero-Hidalgo, Enrique Gómez-Gómez, Aura D. Herrera-Martínez, Rocío Guzmán-Ruiz, María M. Malagón, Manuel D. Gahete, and Raúl M. Luque. 2023. "LRP10, PGK1 and RPLP0: Best Reference Genes in Periprostatic Adipose Tissue under Obesity and Prostate Cancer Conditions" International Journal of Molecular Sciences 24, no. 20: 15140. https://doi.org/10.3390/ijms242015140
APA StylePérez-Gómez, J. M., Porcel-Pastrana, F., De La Luz-Borrero, M., Montero-Hidalgo, A. J., Gómez-Gómez, E., Herrera-Martínez, A. D., Guzmán-Ruiz, R., Malagón, M. M., Gahete, M. D., & Luque, R. M. (2023). LRP10, PGK1 and RPLP0: Best Reference Genes in Periprostatic Adipose Tissue under Obesity and Prostate Cancer Conditions. International Journal of Molecular Sciences, 24(20), 15140. https://doi.org/10.3390/ijms242015140







