Degenerative and Regenerative Actin Cytoskeleton Rearrangements, Cell Death, and Paradoxical Proliferation in the Gills of Pearl Gourami (Trichogaster leerii) Exposed to Suspended Soot Microparticles
Abstract
:1. Introduction
2. Results and Discussion
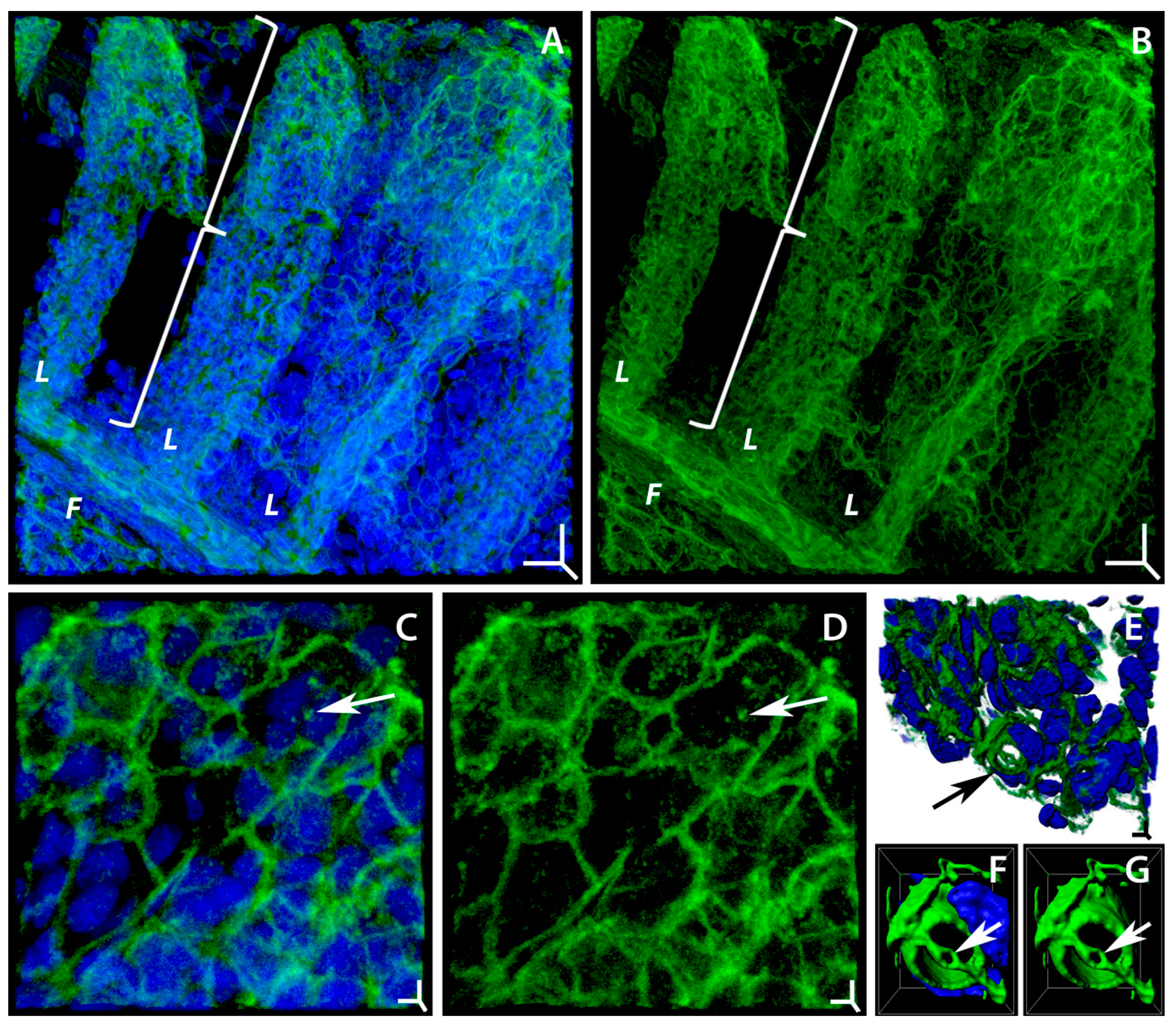
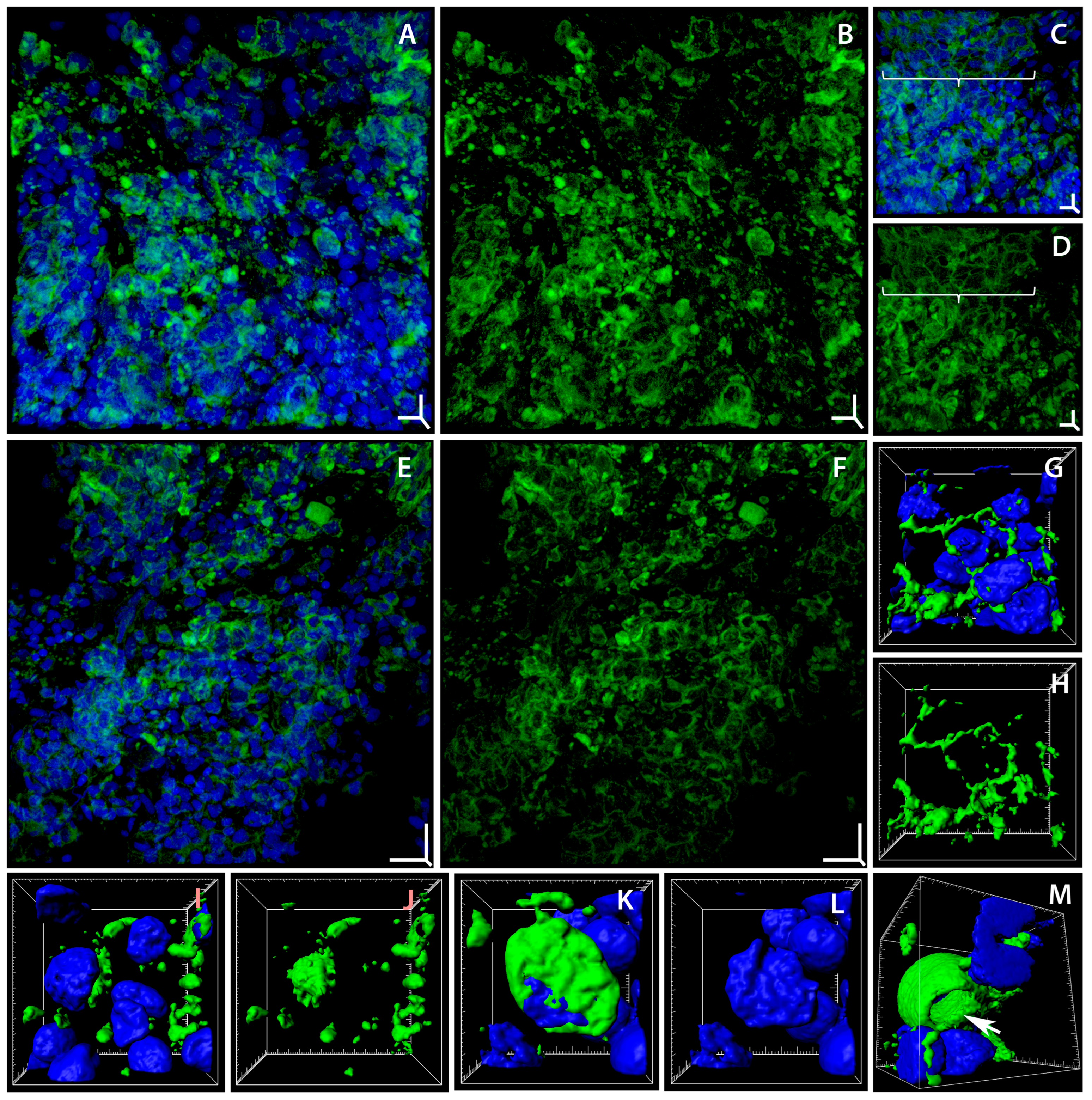
2.1. Degenerative and Adaptive Rearrangements of Actin Microfilaments in Gill Epithelium under the Influence of Soot
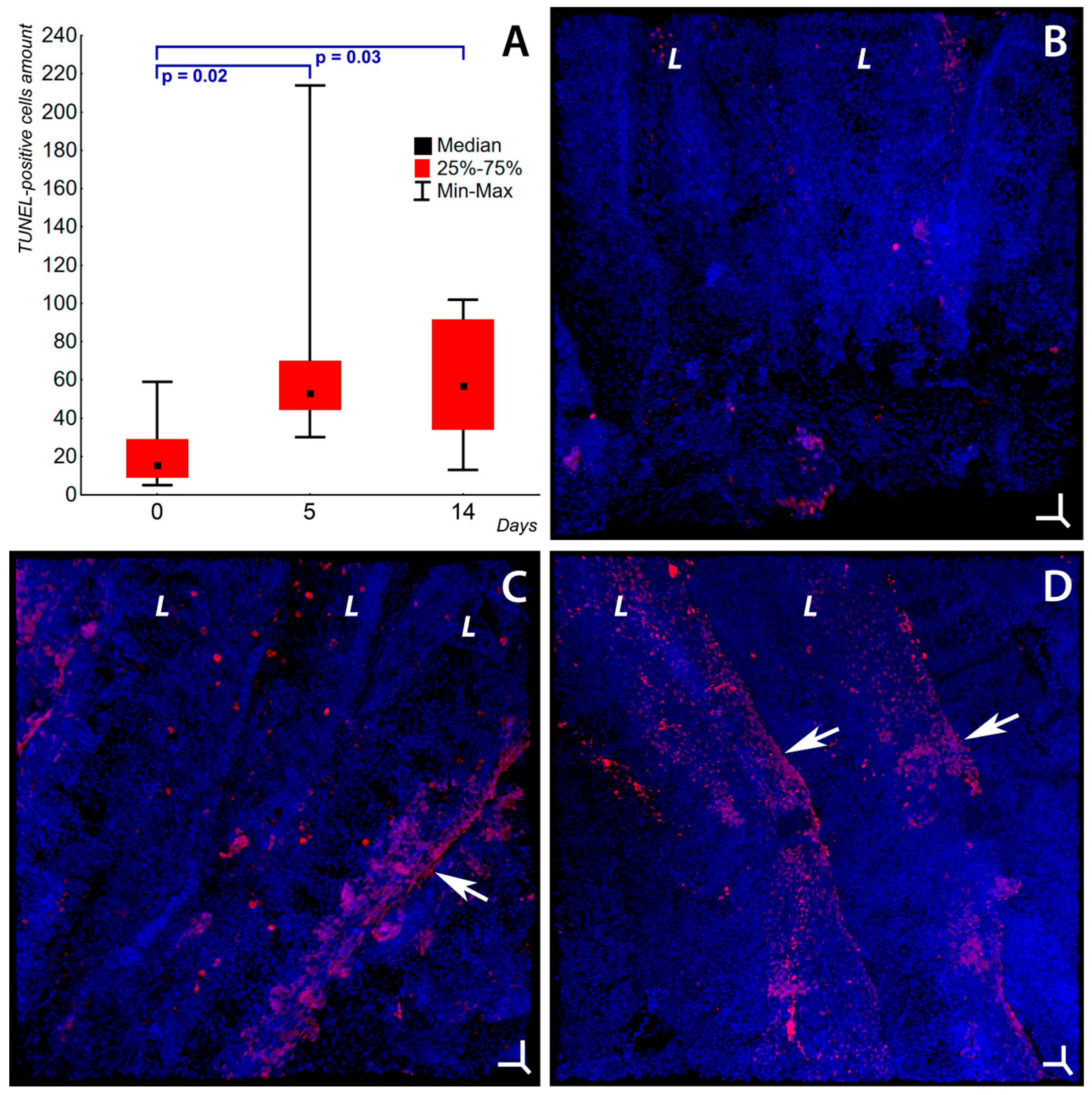
2.2. Programmed Cell Death in Gill Epithelium under the Influence of Soot
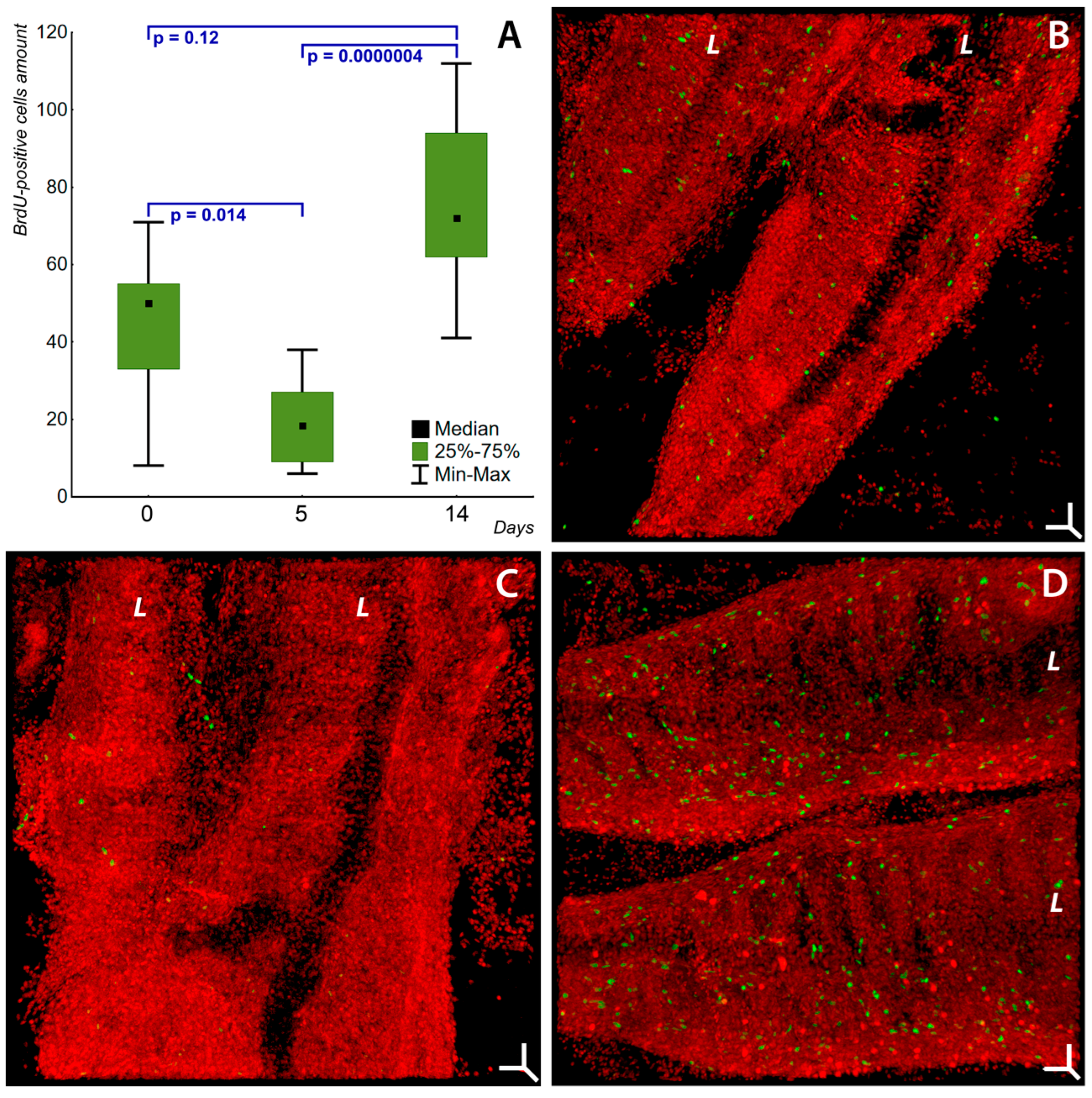
2.3. Paradoxical Effect of Soot Microparticles on Cell Proliferation in Fish Gill Epithelium
3. Material and Methods
3.1. Animals and Design of Experiment
3.2. Laser Confocal Microscopy
3.2.1. F-actin Staining
3.2.2. Programmed Cell Death Analysis
3.2.3. Cell Proliferation Assay
3.2.4. Microscopy and Image Analysis
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Liu, C.; Ma, J.; Ma, Q.; He, H. Structural and hygroscopic changes of soot during heterogeneous reaction with O(3). Phys. Chem. Chem. Phys. 2010, 12, 10896–10903. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nakane, M.; Mori, Y.; Nishioka, J.; Ogawa, H. Fate of dissolved black carbon in the deep Pacific Ocean. Nat. Commun. 2022, 13, 307. [Google Scholar] [CrossRef]
- Rajesh, T.A.; Ramachandran, S. Characteristics and source apportionment of black carbon aerosols over an urban site. Environ. Sci. Pollut. Res. Int. 2017, 24, 8411–8424. [Google Scholar] [CrossRef] [PubMed]
- Lammel, G.; Novakov, T. Water nucleation properties of carbon black and diesel soot particles. Atmos. Environ. 1995, 29, 813–823. [Google Scholar] [CrossRef]
- Coppola, A.I.; Druffel, E.R.M. Cycling of black carbon in the ocean. Geophys. Res. Lett. 2016, 43, 4477–4482. [Google Scholar] [CrossRef]
- Ziolkowski, L.A.; Druffel, E.R.M. Aged black carbon identified in marine dissolved organic carbon. Geophys. Res. Lett. 2010, 37, L16601. [Google Scholar] [CrossRef]
- Neff, J.M.; Stout, S.A.; Gunster, D.G. Ecological risk assessment of polycyclic aromatic hydrocarbons in sediments: Identifying sources and ecological hazard. Integr. Environ. Assess. Manag. 2005, 1, 22–33. [Google Scholar] [CrossRef]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef]
- Vondráček, J.; Machala, M. The Role of Metabolism in Toxicity of Polycyclic Aromatic Hydrocarbons and their Non-genotoxic Modes of Action. Curr. Drug Metab. 2021, 22, 584–595. [Google Scholar] [CrossRef]
- Yu, H.; Xia, Q.; Yan, J.; Herreno-Saenz, D.; Wu, Y.S.; Tang, I.W.; Fu, P.P. Photoirradiation of polycyclic aromatic hydrocarbons with UVA light—A pathway leading to the generation of reactive oxygen species, lipid peroxidation, and DNA damage. Int. J. Environ. Res. Public Health 2006, 3, 348–354. [Google Scholar] [CrossRef]
- Castaño-Vinyals, G.; D’Errico, A.; Malats, N.; Kogevinas, M. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup. Environ. Med. 2004, 61, e12. [Google Scholar] [CrossRef] [PubMed]
- Ewa, B.; Danuta, M.Š. Polycyclic aromatic hydrocarbons and PAH-related DNA adducts. J. Appl. Genet. 2017, 58, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Thakur, A.K. The Toxicological Mechanisms of Environmental Soot (Black Carbon) and Carbon Black: Focus on Oxidative Stress and Inflammatory Pathways. Front. Immunol. 2017, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Li, X.; Sun, N.; He, F.; Cui, Z.; Li, Y.; Liu, R. The combination of ultrafine carbon black and lead provokes cytotoxicity and apoptosis in mice lung fibroblasts through oxidative stress-activated mitochondrial pathways. Sci. Total Environ. 2021, 799, 149420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wen, H.; Zhou, M.; Lei, T.; Shen, J.; Zhang, D.; Wang, R.; Wu, H.; Jiang, S.; Li, W. Low-dose combined exposure of carboxylated black carbon and heavy metal lead induced potentiation of oxidative stress, DNA damage, inflammation, and apoptosis in BEAS-2B cells. Ecotoxicol. Environ. Saf. 2020, 206, 111388. [Google Scholar] [CrossRef]
- Guan, S.; Tao, S.; Huang, Y.; Jin, Y.; Hu, Y.; Lu, J. Combined toxic effects of CBNPs and Pb on rat alveolar macrophage apoptosis and autophagy flux. Ecotoxicol. Environ. Saf. 2020, 205, 111062. [Google Scholar] [CrossRef]
- Wang, L.; Bao, S.; Liu, X.; Wang, F.; Zhang, J.; Dang, P.; Wang, F.; Li, B.; Lin, Y. Low-dose exposure to black carbon significantly increase lung injury of cadmium by promoting cellular apoptosis. Ecotoxicol. Environ. Saf. 2021, 224, 112703. [Google Scholar] [CrossRef]
- Li, X.; Chu, S.; Song, Z.; He, F.; Cui, Z.; Liu, R. Discrepancy of apoptotic events in mouse hepatocytes and catalase performance: Size-dependent cellular and molecular toxicity of ultrafine carbon black. J. Hazard. Mater. 2022, 421, 126781. [Google Scholar] [CrossRef]
- Colasanti, T.; Fiorito, S.; Alessandri, C.; Serafino, A.; Andreola, F.; Barbati, C.; Morello, F.; Alfè, M.; Di Blasio, G.; Gargiulo, V.; et al. Diesel exhaust particles induce autophagy and citrullination in Normal Human Bronchial Epithelial cells. Cell Death Dis. 2018, 9, 1073. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, R.; Gallo, G.; Vallotto, D.; Marcomini, A.; Pojana, G. Biomarkers in Mytilus galloprovincialis exposed to suspensions of selected nanoparticles (Nano carbon black, C60 fullerene, Nano-TiO2, Nano-SiO2). Aquat. Toxicol. 2010, 100, 168–177. [Google Scholar] [CrossRef]
- Kim, K.; Wang, C.H.; Ok, Y.S.; Lee, S.E. Heart developmental toxicity by carbon black waste generated from oil refinery on zebrafish embryos (Danio rerio): Combined toxicity on heart function by nickel and vanadium. J. Hazard. Mater. 2019, 363, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Saini, S.; Singh, S.P.; Gautam, N.K.; Singh, S. Candle soot derived carbon nanoparticles: An assessment of cellular and progressive toxicity using Drosophila melanogaster model. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 228, 108646. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Naya, M.; Horimoto, M.; Kato, H. Developmental toxicity of diesel exhaust: A review of studies in experimental animals. Reprod. Toxicol. 2013, 42, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Grahame, T.J.; Klemm, R.; Schlesinger, R.B. Public health and components of particulate matter: The changing assessment of black carbon. J. Air Waste Manag. Assoc. 2014, 64, 620–660. [Google Scholar] [CrossRef]
- Sheesley, R.J.; Schauer, J.J.; Hemming, J.D.; Barman, M.A.; Geis, S.W.; Tortorelli, J.J. Toxicity of ambient atmospheric particulate matter from the Lake Michigan (USA) airshed to aquatic organisms. Environ. Toxicol. Chem. 2004, 23, 133–140. [Google Scholar] [CrossRef]
- Garner, L.V.; Brown, D.R.; Di Giulio, R.T. Knockdown of AHR1A but not AHR1B exacerbates PAH and PCB-126 toxicity in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2013, 142–143, 336–346. [Google Scholar] [CrossRef]
- Billiard, S.M.; Timme-Laragy, A.R.; Wassenberg, D.M.; Cockman, C.; Di Giulio, R.T. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006, 92, 526–536. [Google Scholar] [CrossRef]
- Cherr, G.N.; Fairbairn, E.; Whitehead, A. Impacts of Petroleum-Derived Pollutants on Fish Development. Annu. Rev. Anim. Biosci. 2017, 5, 185–203. [Google Scholar] [CrossRef]
- Chovanec, A.; Hofer, R.; Schiemer, F. Fish as bioindicators. In Bioindicators and Biomonitors: Principles, Concepts and Applications; Markert, B.A., Breure, A.M., Zechmeister, H.G., Eds.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003; pp. 639–676. [Google Scholar]
- Dragun, Z.; Raspor, B.; Podrug, M. The influence of the season and the biotic factors on the cytosolic metal concentrations in the gills of the European chub (Leuciscus cephalus L.). Chemosphere 2007, 69, 911–919. [Google Scholar] [CrossRef]
- Rašković, B.; Poleksić, V.; Višnjić-Jeftić, Ž.; Skorić, S.; Gačić, Z.; Djikanović, V.; Jarić, I.; Lenhardt, M. Use of histopathology and elemental accumulation in different organs of two benthophagous fish species as indicators of river pollution. Environ. Toxicol. 2015, 30, 1153–1161. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Geffard, O.; Geffard, A.; His, E.; Budzinski, H. Assessment of the bioavailability and toxicity of sediment-associated polycyclic aromatic hydrocarbons and heavy metals applied to Crassostrea gigas embryos and larvae. Mar. Pollut. Bull. 2003, 46, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Handy, R.D.; Eddy, F.B. The Interactions between the Surface of Rainbow Trout, Oncorhynchus Mykiss, and Waterborne Metal Toxicants. Funct. Ecol. 1990, 4, 385–392. [Google Scholar] [CrossRef]
- Leguen, I.; Cravedi, J.P.; Pisam, M.; Prunet, P. Biological functions of trout pavement-like gill cells in primary culture on solid support: pH(i) regulation, cell volume regulation and xenobiotic biotransformation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 207–222. [Google Scholar] [CrossRef]
- Jonz, M.G.; Zachar, P.C.; Da Fonte, D.F.; Mierzwa, A.S. Peripheral chemoreceptors in fish: A brief history and a look ahead. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 186, 27–38. [Google Scholar] [CrossRef]
- Milsom, W.K. New insights into gill chemoreception: Receptor distribution and roles in water and air breathing fish. Respir. Physiol. Neurobiol. 2012, 184, 326–339. [Google Scholar] [CrossRef]
- Sudakov, N.P.; Klimenkov, I.V.; Bedoshvili, Y.D.; Arsent’ev, K.Y.; Gorshkov, A.G.; Izosimova, O.N.; Yakhnenko, V.M.; Kupchinskii, A.B.; Didorenko, S.I.; Likhoshway, Y.V. Early structural and functional changes in Baikal Sculpin gills exposed to suspended soot microparticles in experiment. Chemosphere 2022, 20, 133241. [Google Scholar] [CrossRef]
- Ibrahim, A.; Kabbashi, N.A. Effects of chronic cadmium exposure on the sexual maturity, production and bioaccumulation of pearl gourani Trichogaster leeri. Asian J. Microbiol. Biotechnol. Environ. Sci. 2003, 5, 7–10. [Google Scholar]
- Sumon, K.A.; Yesmin, M.F.; Van den Brink, P.J.; Bosma, R.H.; Peeters, E.T.H.M.; Rashid, H. Effects of long-term chlorpyrifos exposure on mortality and reproductive tissues of Banded Gourami (Trichogaster fasciata). J. Environ. Sci. Health B 2019, 54, 549–559. [Google Scholar] [CrossRef]
- Low, K.W.; Sin, Y.M. Effects of mercuric chloride and sodium selenite on some immune responses of blue gourami, Trichogaster trichopterus (Pallus). Sci. Total Environ. 1998, 214, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, C.P.; Lin, H.C. Morphological and biochemical variations in the gills of 12 aquatic air-breathing anabantoid fish. Physiol. Biochem. Zool. 2011, 84, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.; McGoran, R.E.; White, C.R.; Portugal, S.J. Life in a bubble: The role of the labyrinth organ in determining territory, mating and aggressive behaviours in anabantoids. J. Fish Biol. 2017, 91, 723–749. [Google Scholar] [CrossRef] [PubMed]
- Sanchís, J.; Llorca, M.; Olmos, M.; Schirinzi, G.F.; Bosch-Orea, C.; Abad, E.; Barceló, D.; Farré, M. Metabolic Responses of Mytilus galloprovincialis to Fullerenes in Mesocosm Exposure Experiments. Environ. Sci. Technol. 2018, 52, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Sandbichler, A.M.; Farkas, J.; Salvenmoser, W.; Pelster, B. Cortisol affects tight junction morphology between pavement cells of rainbow trout gills in single-seeded insert culture. J. Comp. Physiol. B 2011, 181, 1023–1034. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef]
- Steinke, A.; Meier-Stiegen, S.; Drenckhahn, D.; Asan, E. Molecular composition of tight and adherens junctions in the rat olfactory epithelium and fila. Histochem. Cell Biol. 2008, 130, 339–361. [Google Scholar] [CrossRef]
- Kovacs, E.M.; Goodwin, M.; Ali, R.G.; Paterson, A.D.; Yap, A.S. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 2002, 12, 379–382. [Google Scholar] [CrossRef]
- Smith, M.G.; Swamy, S.R.; Pon, L.A. The life cycle of actin patches in mating yeast. J. Cell Sci. 2001, 114, 1505–1513. [Google Scholar] [CrossRef]
- Planade, J.; Belbahri, R.; Boiero Sanders, M.; Guillotin, A.; du Roure, O.; Michelot, A.; Heuvingh, J. Mechanical stiffness of reconstituted actin patches correlates tightly with endocytosis efficiency. PLoS Biol. 2019, 17, e3000500. [Google Scholar] [CrossRef] [PubMed]
- Alessa, L.; Kropf, D.L. F-actin marks the rhizoid pole in living Pelvetia compressa zygotes. Development 1999, 126, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Panteris, E.; Apostolakos, P.; Galatis, B. Cytoskeletal asymmetry in Zea mays subsidiary cell mother cells: A monopolar prophase microtubule half-spindle anchors the nucleus to its polar position. Cell Motil. Cytoskelet. 2006, 63, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.; Reis-Rodrigues, P.; de Vries, I.; Hons, M.; Aguilera, J.; Riedl, M.; Leithner, A.; Tasciyan, S.; Kopf, A.; Merrin, J.; et al. WASp triggers mechanosensitive actin patches to facilitate immune cell migration in dense tissues. Dev. Cell 2022, 57, 47–62.e9. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.B.; Gallo, G. Structure meets function: Actin filaments and myosin motors in the axon. J. Neurochem. 2014, 129, 213–220. [Google Scholar] [CrossRef]
- Schott, D.; Huffaker, T.; Bretscher, A. Microfilaments and microtubules: The news from yeast. Curr. Opin. Microbiol. 2002, 5, 564–574. [Google Scholar] [CrossRef]
- Balasanyan, V.; Watanabe, K.; Dempsey, W.P.; Lewis, T.L., Jr.; Trinh, L.A.; Arnold, D.B. Structure and Function of an Actin-Based Filter in the Proximal Axon. Cell Rep. 2017, 21, 2696–2705. [Google Scholar] [CrossRef]
- van Bommel, B.; Konietzny, A.; Kobler, O.; Bär, J.; Mikhaylova, M. F-actin patches associated with glutamatergic synapses control positioning of dendritic lysosomes. EMBO J. 2019, 38, e101183. [Google Scholar] [CrossRef]
- Baldauf, L.; Frey, F.; Arribas Perez, M.; Idema, T.; Koenderink, G.H. Branched actin cortices reconstituted in vesicles sense membrane curvature. Biophys. J. 2023, 122, 2311–2324. [Google Scholar] [CrossRef]
- Gipson, I.K.; Tisdale, A.S. Visualization of conjunctival goblet cell actin cytoskeleton and mucin content in tissue whole mounts. Exp. Eye Res. 1997, 65, 407–415. [Google Scholar] [CrossRef]
- Pati, R.; Das, I.; Mehta, R.K.; Sahu, R.; Sonawane, A. Zinc-Oxide Nanoparticles Exhibit Genotoxic, Clastogenic, Cytotoxic and Actin Depolymerization Effects by Inducing Oxidative Stress Responses in Macrophages and Adult Mice. Toxicol. Sci. 2016, 150, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Davis, H.W. Hydrogen peroxide-induced cytoskeletal rearrangement in cultured pulmonary endothelial cells. J. Cell. Physiol. 1998, 174, 370–379. [Google Scholar] [CrossRef]
- Huot, J.; Houle, F.; Spitz, D.R.; Landry, J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996, 56, 273–279. [Google Scholar]
- Bellomo, G.; Mirabelli, F.; Vairetti, M.; Iosi, F.; Malorni, W. Cytoskeleton as a target in menadione-induced oxidative stress in cultured mammalian cells. I. Biochemical and immunocytochemical features. J. Cell. Physiol. 1990, 143, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lai, C.H.; Lung, S.C.; Wang, W.C.; Huang, C.C.; Chen, G.W.; Suo, G.; Choug, C.T.; Lin, C.H. Carbon black aggregates cause endothelial dysfunction by activating ROCK. J. Hazard. Mater. 2017, 338, 66–75. [Google Scholar] [CrossRef]
- Walker, V.G.; Li, Z.; Hulderman, T.; Schwegler-Berry, D.; Kashon, M.L.; Simeonova, P.P. Potential in vitro effects of carbon nanotubes on human aortic endothelial cells. Toxicol. Appl. Pharmacol. 2009, 236, 319–328. [Google Scholar] [CrossRef]
- Gómez-Mendikute, A.; Etxeberria, A.; Olabarrieta, I.; Cajaraville, M.P. Oxygen radicals production and actin filament disruption in bivalve haemocytes treated with benzo(a)pyrene. Mar. Environ. Res. 2002, 54, 431–436. [Google Scholar] [CrossRef]
- Hooven, L.A.; Baird, W.M. Proteomic analysis of MCF-7 cells treated with benzo[a]pyrene, dibenzo[a,l]pyrene, coal tar extract, and diesel exhaust extract. Toxicology 2008, 249, 1–10. [Google Scholar] [CrossRef]
- Huot, J.; Houle, F.; Rousseau, S.; Deschesnes, R.G.; Shah, G.M.; Landry, J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J. Cell Biol. 1998, 143, 1361–1373. [Google Scholar] [CrossRef]
- Huot, J.; Houle, F.; Marceau, F.; Landry, J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ. Res. 1997, 80, 383–392. [Google Scholar] [CrossRef]
- Ju, S.; Lim, L.; Ki, Y.J.; Choi, D.H.; Song, H. Oxidative stress generated by polycyclic aromatic hydrocarbons from ambient particulate matter enhance vascular smooth muscle cell migration through MMP upregulation and actin reorganization. Part. Fibre Toxicol. 2022, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Tomkiewicz, C.; Herry, L.; Bui, L.C.; Métayer, C.; Bourdeloux, M.; Barouki, R.; Coumoul, X. The aryl hydrocarbon receptor regulates focal adhesion sites through a non-genomic FAK/Src pathway. Oncogene 2013, 32, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, B.; Tyther, R.; Sheehan, D. Carbonylation and glutathionylation of proteins in the blue mussel Mytilus edulis detected by proteomic analysis and Western blotting: Actin as a target for oxidative stress. Aquat. Toxicol. 2005, 73, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Di Rosa, I.; Simoncelli, F.; Pipe, R.K.; Panara, F.; Pascolini, R. The effects of copper on actin and fibronectin organization in Mytilus galloprovincialis haemocytes. Dev. Comp. Immunol. 1996, 20, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mendikute, A.; Cajaraville, M.P. Comparative effects of cadmium, copper, paraquat and benzo[a]pyrene on the actin cytoskeleton and production of reactive oxygen species (ROS) in mussel haemocytes. Toxicol. Vitro 2003, 17, 539–546. [Google Scholar] [CrossRef]
- Jiang, H.; Sha, S.H.; Schacht, J. Rac/Rho pathway regulates actin depolymerization induced by aminoglycoside antibiotics. J. Neurosci. Res. 2006, 83, 1544–1551. [Google Scholar] [CrossRef]
- Torres-Cruz, F.M.; Rodríguez-Cruz, F.; Escobar-Herrera, J.; Barragán-Andrade, N.; Basurto-Islas, G.; Ripova, D.; Ávila, J.; Garcia-Sierra, F. Expression of Tau Produces Aberrant Plasma Membrane Blebbing in Glial Cells Through RhoA-ROCK-Dependent F-Actin Remodeling. J. Alzheimers Dis. 2016, 52, 463–482. [Google Scholar] [CrossRef]
- Farah, M.E.; Sirotkin, V.; Haarer, B.; Kakhniashvili, D.; Amberg, D.C. Diverse protective roles of the actin cytoskeleton during oxidative stress. Cytoskeleton 2011, 68, 340–354. [Google Scholar] [CrossRef]
- DeMeester, S.L.; Cobb, J.P.; Hotchkiss, R.S.; Osborne, D.F.; Karl, I.E.; Tinsley, K.W.; Buchman, T.G. Stress-induced fractal rearrangement of the endothelial cell cytoskeleton causes apoptosis. Surgery 1998, 124, 362–371. [Google Scholar] [CrossRef]
- Nezis, I.P.; Stravopodis, D.J.; Papassideri, I.; Margaritis, L.H. Actin cytoskeleton reorganization of the apoptotic nurse cells during the late developmental stages of oogenesis in Dacus oleae. Cell Motil. Cytoskelet. 2001, 48, 224–233. [Google Scholar] [CrossRef]
- Posey, S.C.; Bierer, B.E. Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J. Biol. Chem. 1999, 274, 4259–4265. [Google Scholar] [CrossRef]
- Louzao, M.C.; Ares, I.R.; Cagide, E.; Espiña, B.; Vilariño, N.; Alfonso, A.; Vieytes, M.R.; Botana, L.M. Palytoxins and cytoskeleton: An overview. Toxicon 2011, 57, 460–469. [Google Scholar] [CrossRef]
- Gilbert, P.M.; Weaver, V.M. Cellular adaptation to biomechanical stress across length scales in tissue homeostasis and disease. Semin. Cell Dev. Biol. 2017, 67, 141–152. [Google Scholar] [CrossRef]
- Klimenkov, I.V.; Sudakov, N.P.; Pastukhov, M.V.; Svinov, M.M.; Kositsyn, N.S. Rearrangement of Actin Microfilaments in the Development of Olfactory Receptor Cells in Fish. Sci. Rep. 2018, 8, 3692. [Google Scholar] [CrossRef] [PubMed]
- Duszyc, K.; Gomez, G.A.; Schroder, K.; Sweet, M.J.; Yap, A.S. In life there is death: How epithelial tissue barriers are preserved despite the challenge of apoptosis. Tissue Barriers 2017, 5, e1345353. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wilson, G. Apoptosis in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1017–1025. [Google Scholar]
- Barker, N.; van de Wetering, M.; Clevers, H. The intestinal stem cell. Genes Dev. 2008, 22, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tian, D.; He, J.; Yan, X.; Zhao, J.; Yuan, X.; Peng, S. Prolonged exposure to carbon nanoparticles induced methylome remodeling and gene expression in zebrafish heart. J. Appl. Toxicol. 2019, 39, 322–332. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Gao, S.; Liu, R. Assessing the in vitro and in vivo toxicity of ultrafine carbon black to mouse liver. Sci. Total Environ. 2019, 655, 1334–1341. [Google Scholar] [CrossRef]
- Chu, C.; Zhou, L.; Xie, H.; Pei, Z.; Zhang, M.; Wu, M.; Zhang, S.; Wang, L.; Zhao, C.; Shi, L.; et al. Pulmonary toxicities from a 90-day chronic inhalation study with carbon black nanoparticles in rats related to the systemical immune effects. Int. J. Nanomed. 2019, 14, 2995–3013. [Google Scholar] [CrossRef]
- Onyeso, G.; Bohr, E.L.; Nkpaa, K.W.; Amadi, P.N.; Ugwu, A.C. Black soot exposure induced hypothalamic and testicular oxidative stress and apoptosis in male rats. Andrologia 2020, 52, e13866. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Huang, Y.; Shang, J.; Liu, Y.; Chen, C. Black Carbon Induces Cytotoxicity and NLRP3 Inflammasome Activation in Human Corneal Epithelial Cells. Curr. Eye Res. 2020, 45, 680–685. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhou, Q.; Qian, G.; Wang, T.; Wu, M.; Zhu, T.; Qiu, X.; Shang, Y.; Shang, J. Comparison of gene expression profiles induced by fresh or ozone-oxidized black carbon particles in A549 cells. Chemosphere 2017, 180, 212–220. [Google Scholar] [CrossRef]
- Zhen, X.; Ng, W.C.; Fendy; Tong, Y.W.; Dai, Y.; Neoh, K.G.; Wang, C.H. Toxicity assessment of carbon black waste: A by-product from oil refineries. J. Hazard. Mater. 2017, 321, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Meng, T.; Niu, Y.; Dai, Y.; Zhang, L.; Zheng, X.; Jalava, P.; Dong, G.; Gao, W.; et al. Oxidative stress induced by ultrafine carbon black particles can elicit apoptosis in vivo and in vitro. Sci. Total Environ. 2020, 709, 135802. [Google Scholar] [CrossRef]
- Hussain, S.; Thomassen, L.C.; Ferecatu, I.; Borot, M.C.; Andreau, K.; Martens, J.A.; Fleury, J.; Baeza-Squiban, A.; Marano, F.; Boland, S. Carbon black and titanium dioxide nanoparticles elicit distinct apoptotic pathways in bronchial epithelial cells. Part. Fibre Toxicol. 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Deweirdt, J.; Quignard, J.F.; Lacomme, S.; Gontier, E.; Mornet, S.; Savineau, J.P.; Marthan, R.; Guibert, C.; Baudrimont, I. In vitro study of carbon black nanoparticles on human pulmonary artery endothelial cells: Effects on calcium signaling and mitochondrial alterations. Arch. Toxicol. 2020, 94, 2331–2348. [Google Scholar] [CrossRef]
- Hou, L.; Guan, S.; Jin, Y.; Sun, W.; Wang, Q.; Du, Y.; Zhang, R. Cell metabolomics to study the cytotoxicity of carbon black nanoparticles on A549 cells using UHPLC-Q/TOF-MS and multivariate data analysis. Sci. Total Environ. 2020, 698, 134122. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Y.X.; Sun, L.; Chen, Y.J.; Liu, C.; Huang, L.L.; Lu, W.Q.; Zeng, Q. Urinary metabolites of polycyclic aromatic hydrocarbons, sperm DNA damage and spermatozoa apoptosis. J. Hazard. Mater. 2017, 329, 241–248. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, A.; Wang, L.; Jin, L.; Lin, S.; Li, Z.; McDonald, J.A. Casp8 hypomethylation and neural tube defects in association with polycyclic aromatic hydrocarbon exposure. Clin. Epigenet. 2019, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Tithof, P.K.; Elgayyar, M.; Cho, Y.; Guan, W.; Fisher, A.B.; Peters-Golden, M. Polycyclic aromatic hydrocarbons present in cigarette smoke cause endothelial cell apoptosis by a phospholipase A2-dependent mechanism. FASEB J. 2002, 16, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.K.; Matulka, R.A.; Hahn, M.E.; Trombino, A.F.; Lawrence, B.P.; Kerkvliet, N.I.; Sherr, D.H. The role of polycyclic aromatic hydrocarbon metabolism in dimethylbenz[a]anthracene-induced pre-B lymphocyte apoptosis. Toxicol. Appl. Pharmacol. 1999, 161, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Pan, L.; Miao, J.; Lei, F.; Xu, R.; Zhang, X. The mechanism of apoptosis of Chlamys farreri hemocytes under benzopyrene stress in vitro. Sci. Total Environ. 2021, 794, 148731. [Google Scholar] [CrossRef]
- Andrysík, Z.; Machala, M.; Chramostová, K.; Hofmanová, J.; Kozubík, A.; Vondrácek, J. Activation of ERK1/2 and p38 kinases by polycyclic aromatic hydrocarbons in rat liver epithelial cells is associated with induction of apoptosis. Toxicol. Appl. Pharmacol. 2006, 211, 198–208. [Google Scholar] [CrossRef]
- Allan, L.L.; Mann, K.K.; Matulka, R.A.; Ryu, H.Y.; Schlezinger, J.J.; Sherr, D.H. Bone marrow stromal-B cell interactions in polycyclic aromatic hydrocarbon-induced pro/pre-B cell apoptosis. Toxicol. Sci. 2003, 76, 357–365. [Google Scholar] [CrossRef]
- Bahri, R.; Saidane-Mosbahi, D.; Rouabhia, M. Cytokine release and cytotoxicity in human keratinocytes induced by polycyclic aromatic hydrocarbons (1-methylpyrene and perylene). J. Toxicol. Environ. Health A 2010, 73, 552–564. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Near, R.; Shneider, A.; Cui, H.; Ju, S.T.; Sherr, D.H. Fluoranthene-induced apoptosis in murine T cell hybridomas is independent of the aromatic hydrocarbon receptor. Toxicol. Appl. Pharmacol. 1996, 139, 144–152. [Google Scholar] [CrossRef]
- Asare, N.; Lagadic-Gossmann, D.; Holme, J.A. 3-nitrofluoranthene (3-NF)-induced apoptosis and programmed necrosis. Autophagy 2009, 5, 751–752. [Google Scholar] [CrossRef]
- Nogueira, D.J.; Arl, M.; Köerich, J.S.; Simioni, C.; Ouriques, L.C.; Vicentini, D.S.; Matias, W.G. Comparison of cytotoxicity of α-Al2O3 and η-Al2O3 nanoparticles toward neuronal and bronchial cells. Toxicol. Vitro 2019, 61, 104596. [Google Scholar] [CrossRef]
- Du, Z.; Zhao, D.; Jing, L.; Cui, G.; Jin, M.; Li, Y.; Liu, X.; Liu, Y.; Du, H.; Guo, C.; et al. Cardiovascular toxicity of different sizes amorphous silica nanoparticles in rats after intratracheal instillation. Cardiovasc. Toxicol. 2013, 13, 194–207. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Pan, J.; Han, D.; Lin, J.; Niu, Q.; Liu, R. Toxic effect and mechanism of ultrafine carbon black on mouse primary splenocytes and two digestive enzymes. Ecotoxicol. Environ. Saf. 2021, 212, 111980. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Zeng, S.; Ouyang, Y. RIPK1 is a key factor in black carbon-induced cell death. Biomed. Res. 2022, 43, 23–30. [Google Scholar] [CrossRef]
- Gao, X.; Xu, H.; Shang, J.; Yuan, L.; Zhang, Y.; Wang, L.; Zhang, W.; Luan, X.; Hu, G.; Chu, H.; et al. Ozonized carbon black induces mitochondrial dysfunction and DNA damage. Environ. Toxicol. 2017, 32, 944–955. [Google Scholar] [CrossRef]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef]
- Lee, W.; Huang, C.Y.; Lin, H.C. The source of lamellar mitochondria-rich cells in the air-breathing fish, Trichogaster leeri. J. Exp. Zool. A Ecol. Genet. Physiol. 2008, 309, 198–205. [Google Scholar] [CrossRef]
- Mierzwa, A.S.; Nguyen, F.; Xue, M.; Jonz, M.G. Regeneration of the gill filaments and replacement of serotonergic neuroepithelial cells in adult zebrafish (Danio rerio). Respir. Physiol. Neurobiol. 2020, 274, 103366. [Google Scholar] [CrossRef]
- Nguyen, F.; Jonz, M.G. Replacement of mitochondrion-rich cells during regeneration of the gills and opercular epithelium in zebrafish (Danio rerio). Acta Histochem. 2021, 123, 151738. [Google Scholar] [CrossRef]
- Cadiz, L.; Jonz, M.G. A comparative perspective on lung and gill regeneration. J. Exp. Biol. 2020, 223, jeb226076. [Google Scholar] [CrossRef]
- Vuong, N.Q.; Goegan, P.; Mohottalage, S.; Breznan, D.; Ariganello, M.; Williams, A.; Elisma, F.; Karthikeyan, S.; Vincent, R.; Kumarathasan, P. Proteomic changes in human lung epithelial cells (A549) in response to carbon black and titanium dioxide exposures. J. Proteom. 2016, 149, 53–63. [Google Scholar] [CrossRef]
- An, J.; He, H.; Wang, L.; Jin, Y.; Kong, J.; Zhong, Y.; Liu, M.; Shang, Y. Fresh and ozonized black carbon promoted DNA damage and repair responses in A549 cells. Toxicol. Res. 2018, 8, 180–187. [Google Scholar] [CrossRef]
- Niranjan, R.; Mishra, K.P.; Tripathi, S.N.; Thakur, A.K. Proliferation of Lung Epithelial Cells Is Regulated by the Mechanisms of Autophagy Upon Exposure of Soots. Front. Cell Dev. Biol. 2021, 9, 662597. [Google Scholar] [CrossRef]
- Pink, M.; Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Integrated proteomic and metabolomic analysis to assess the effects of pure and benzo[a]pyrene-loaded carbon black particles on energy metabolism and motility in the human endothelial cell line EA.hy926. Arch. Toxicol. 2014, 88, 913–934. [Google Scholar] [CrossRef]
- Pinkerton, K.E.; Zhou, Y.M.; Teague, S.V.; Peake, J.L.; Walther, R.C.; Kennedy, I.M.; Leppert, V.J.; Aust, A.E. Reduced lung cell proliferation following short-term exposure to ultrafine soot and iron particles in neonatal rats: Key to impaired lung growth? Inhal. Toxicol. 2004, 16, 73–81. [Google Scholar] [CrossRef]
- Chramostová, K.; Vondrácek, J.; Sindlerová, L.; Vojtesek, B.; Kozubík, A.; Machala, M. Polycyclic aromatic hydrocarbons modulate cell proliferation in rat hepatic epithelial stem-like WB-F344 cells. Toxicol. Appl. Pharmacol. 2004, 196, 136–148. [Google Scholar] [CrossRef]
- Barouki, R.; Aggerbeck, M.; Aggerbeck, L.; Coumoul, X. The aryl hydrocarbon receptor system. Drug Metabol. Drug Interact. 2012, 27, 3–8. [Google Scholar] [CrossRef]
- Volkov, M.S.; Bolotina, N.A.; Evteev, V.A.; Koblyakov, V.A. Ah-receptor-independent stimulation of hepatoma 27 culture cell proliferation by polycyclic aromatic hydrocarbons. Biochemistry 2012, 77, 201–207. [Google Scholar] [CrossRef]
- Fan, Y.; Bergmann, A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends. Cell Biol. 2008, 18, 467–473. [Google Scholar] [CrossRef]
- Malavia, N.K.; Raub, C.B.; Mahon, S.B.; Brenner, M.; Panettieri, R.A., Jr.; George, S.C. Airway epithelium stimulates smooth muscle proliferation. Am. J. Respir. Cell Mol. Biol. 2009, 41, 297–304. [Google Scholar] [CrossRef]
- Adamson, I.Y. Drug-induced pulmonary fibrosis. Environ. Health Perspect. 1984, 55, 25–36. [Google Scholar] [CrossRef]
- Puchelle, E. Airway epithelium wound repair and regeneration after injury. Acta Otorhinolaryngol. Belg. 2000, 54, 263–270. [Google Scholar]
- Young, H.E.; Duplaa, C.; Romero-Ramos, M.; Chesselet, M.F.; Vourc’h, P.; Yost, M.J.; Ericson, K.; Terracio, L.; Asahara, T.; Masuda, H.; et al. Adult reserve stem cells and their potential for tissue engineering. Cell Biochem. Biophys. 2004, 40, 1–80. [Google Scholar] [CrossRef]
- Stolper, J.; Ambrosio, E.M.; Danciu, D.P.; Buono, L.; Elliott, D.A.; Naruse, K.; Martínez-Morales, J.R.; Marciniak-Czochra, A.; Centanin, L. Stem cell topography splits growth and homeostatic functions in the fish gill. eLife 2019, 8, e43747. [Google Scholar] [CrossRef]
- Kuramitsu, K.; Sverdlov, D.Y.; Liu, S.B.; Csizmadia, E.; Burkly, L.; Schuppan, D.; Hanto, D.W.; Otterbein, L.E.; Popov, Y. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am. J. Pathol. 2013, 183, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.C.; Teisanu, R.M.; Stripp, B.R. Endogenous lung stem cells and contribution to disease. J. Pathol. 2009, 217, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Goldberg, V.M.; Caplan, A.I. Cartilage regeneration using principles of tissue engineering. Clin. Orthop. Relat. Res. 2001, 391, S161–S170. [Google Scholar] [CrossRef]
- Li, M.; Bao, F.; Zhang, Y.; Sheng, H.; Chen, C.; Zhao, J. Photochemical Aging of Soot in the Aqueous Phase: Release of Dissolved Black Carbon and the Formation of 1O2. Environ. Sci. Technol. 2019, 53, 12311–12319. [Google Scholar] [CrossRef]
- Dittmar, T. The molecular level determination of black carbon in marine dissolved organic matter. Org. Geochem. 2008, 39, 396–407. [Google Scholar] [CrossRef]
- Macirella, R.; Madeo, G.; Sesti, S.; Tripepi, M.; Bernabò, I.; Godbert, N.; La Russa, D.; Brunelli, E. Exposure and post-exposure effects of chlorpyrifos on Carassius auratus gills: An ultrastructural and morphofunctional investigation. Chemosphere 2020, 251, 126434. [Google Scholar] [CrossRef]
- Klimenkov, I.V.; Sudakov, N.P.; Pastukhov, M.V.; Kositsyn, N.S. The Phenomenon of Compensatory Cell Proliferation in Olfactory Epithelium in Fish Caused by Prolonged Exposure to Natural Odorants. Sci. Rep. 2020, 10, 8908. [Google Scholar] [CrossRef]
- Gratzner, H.G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science 1982, 218, 474–475. [Google Scholar] [CrossRef]
- Kinkel, M.D.; Eames, S.C.; Philipson, L.H.; Prince, V.E. Intraperitoneal injection into adult zebrafish. J. Vis. Exp. 2010, 42, 2126. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudakov, N.P.; Chang, H.-M.; Renn, T.-Y.; Klimenkov, I.V. Degenerative and Regenerative Actin Cytoskeleton Rearrangements, Cell Death, and Paradoxical Proliferation in the Gills of Pearl Gourami (Trichogaster leerii) Exposed to Suspended Soot Microparticles. Int. J. Mol. Sci. 2023, 24, 15146. https://doi.org/10.3390/ijms242015146
Sudakov NP, Chang H-M, Renn T-Y, Klimenkov IV. Degenerative and Regenerative Actin Cytoskeleton Rearrangements, Cell Death, and Paradoxical Proliferation in the Gills of Pearl Gourami (Trichogaster leerii) Exposed to Suspended Soot Microparticles. International Journal of Molecular Sciences. 2023; 24(20):15146. https://doi.org/10.3390/ijms242015146
Chicago/Turabian StyleSudakov, Nikolay P., Hung-Ming Chang, Ting-Yi Renn, and Igor V. Klimenkov. 2023. "Degenerative and Regenerative Actin Cytoskeleton Rearrangements, Cell Death, and Paradoxical Proliferation in the Gills of Pearl Gourami (Trichogaster leerii) Exposed to Suspended Soot Microparticles" International Journal of Molecular Sciences 24, no. 20: 15146. https://doi.org/10.3390/ijms242015146
APA StyleSudakov, N. P., Chang, H.-M., Renn, T.-Y., & Klimenkov, I. V. (2023). Degenerative and Regenerative Actin Cytoskeleton Rearrangements, Cell Death, and Paradoxical Proliferation in the Gills of Pearl Gourami (Trichogaster leerii) Exposed to Suspended Soot Microparticles. International Journal of Molecular Sciences, 24(20), 15146. https://doi.org/10.3390/ijms242015146






