Mitochondrial sAC-cAMP-PKA Axis Modulates the ΔΨm-Dependent Control Coefficients of the Respiratory Chain Complexes: Evidence of Respirasome Plasticity
Abstract
:1. Introduction
2. Results
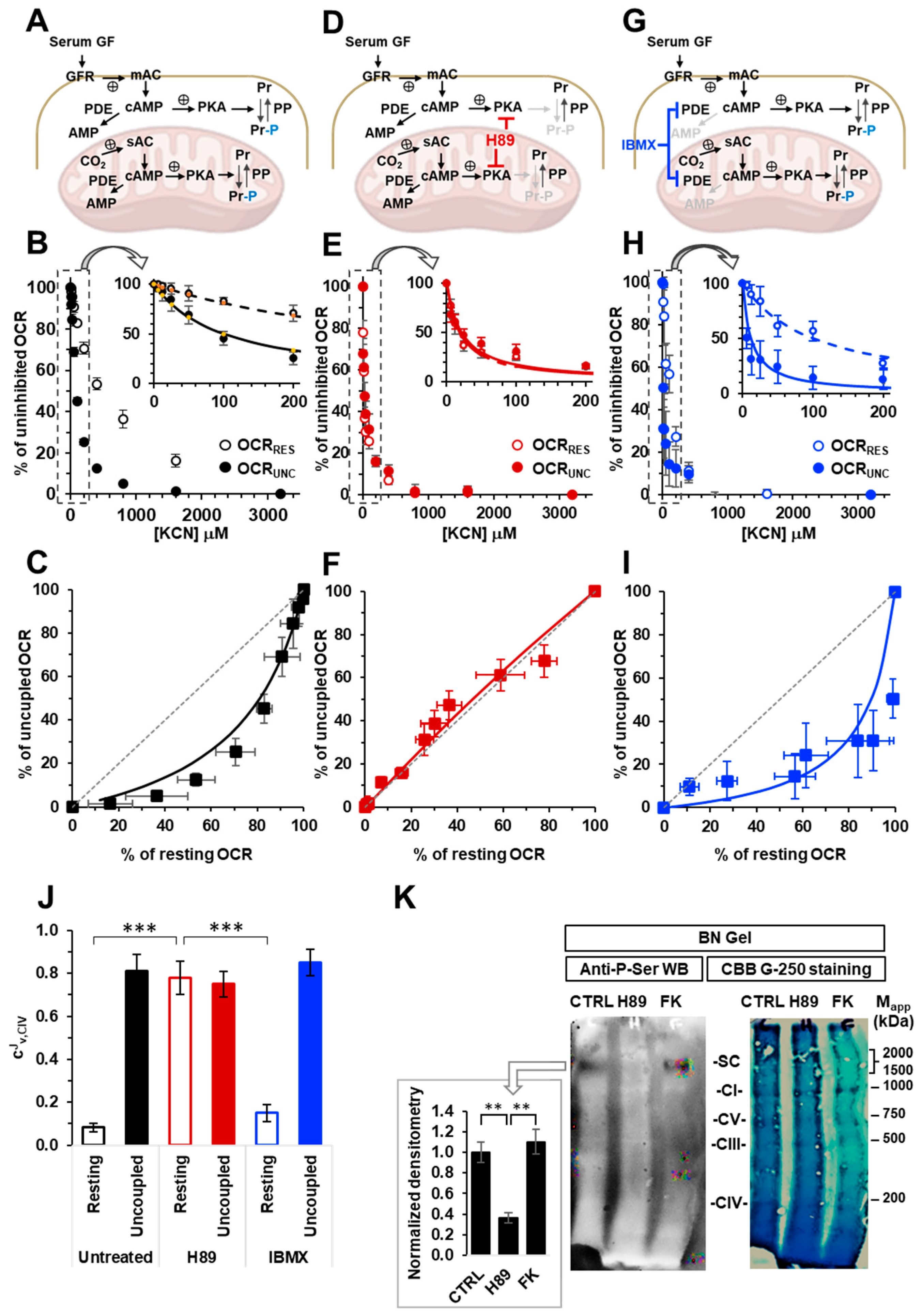
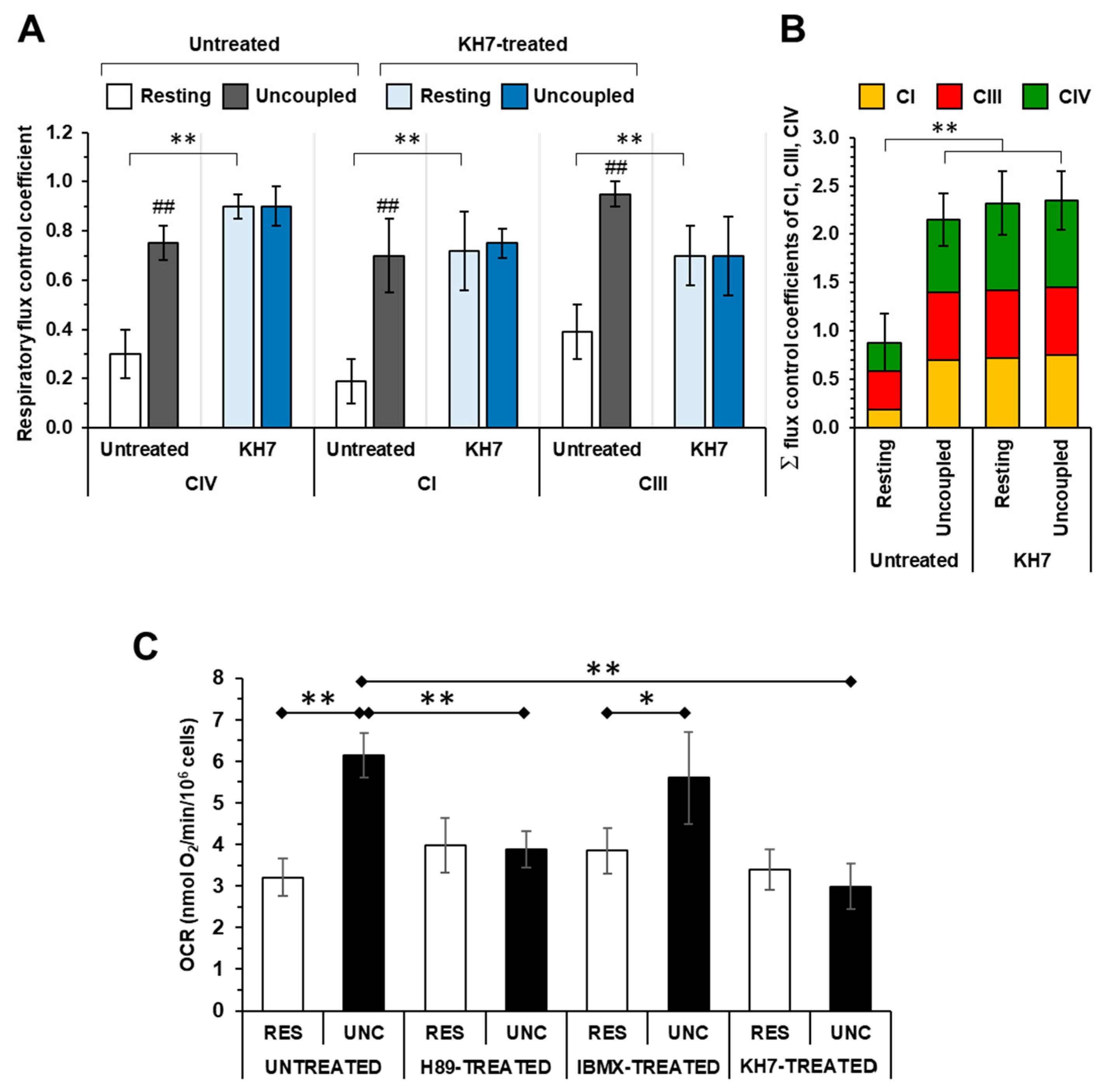
2.1. The Respiratory Flux Control Coefficient of CIV Is Influenced by the Mitochondrial Respiratory State and the Activity of PKA
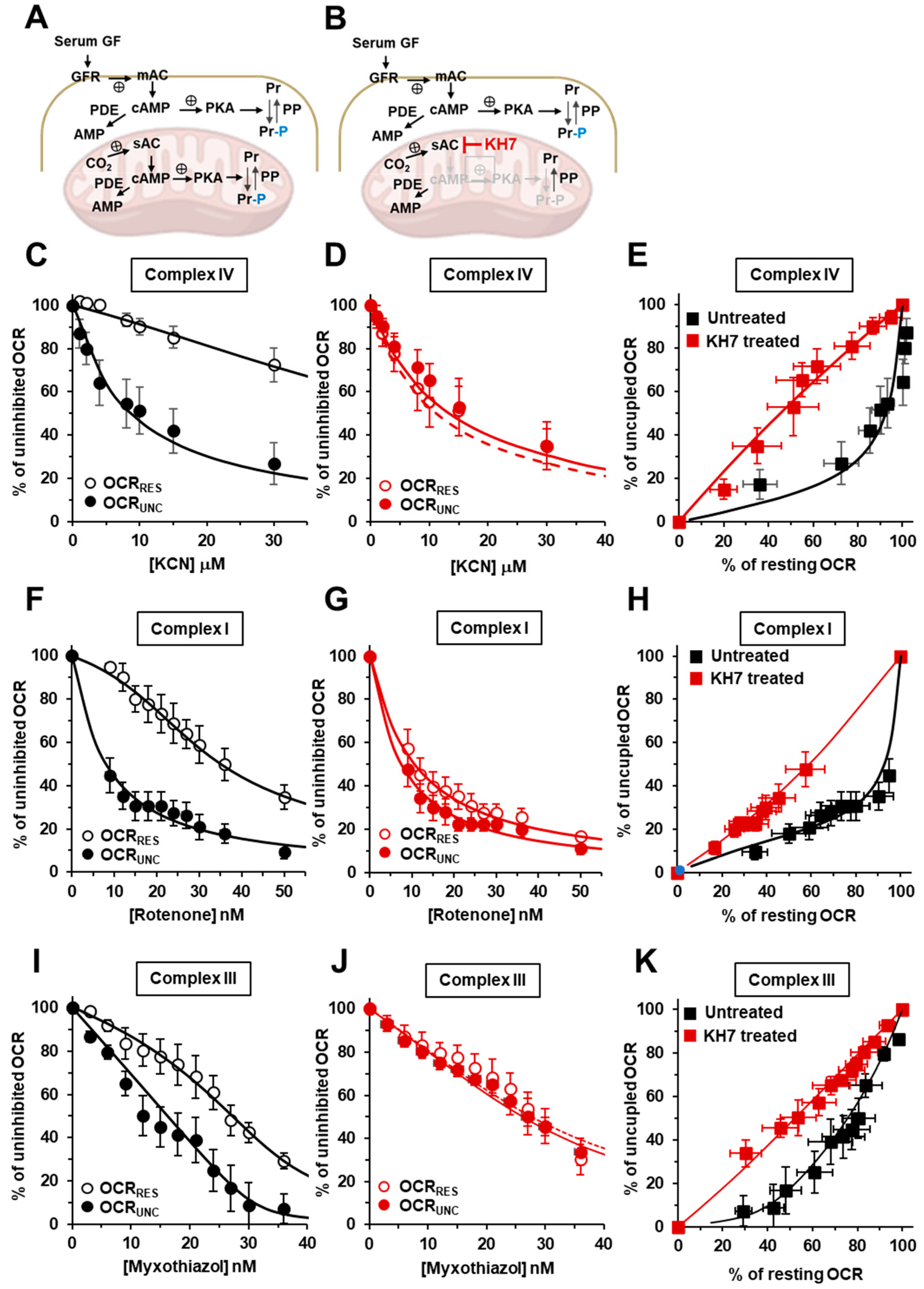
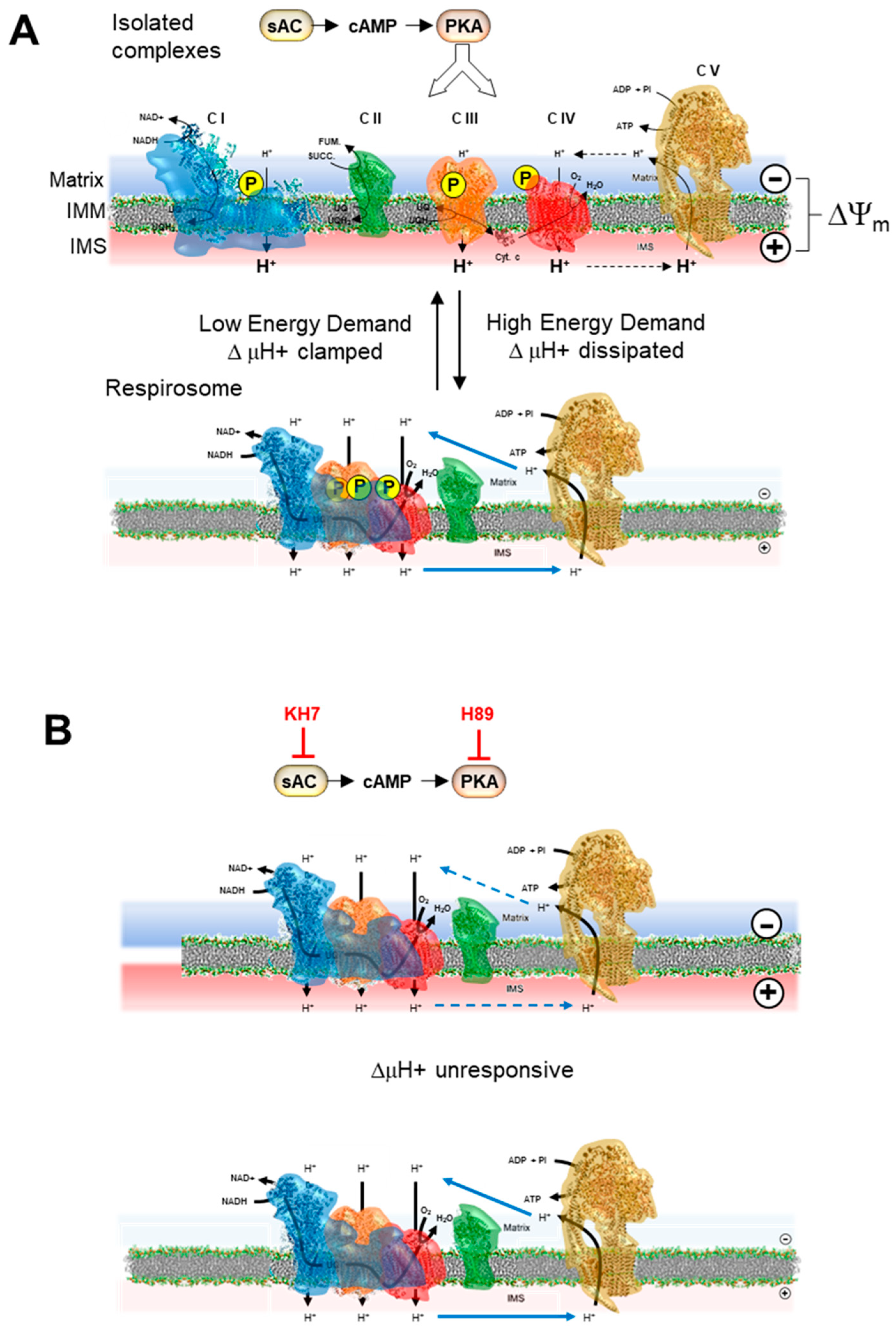
2.2. Inhibition of the Soluble Adenylate Cyclase Affects the Respiratory Flux Control Coefficients of CI, CIII and CIV under the Resting Respiratory State
2.3. The Sum of the Respiratory Flux Control Coefficients of CI, CIII and CIV Indicates the Occurrence of Supercomplexes in Uncoupled Respiration and in the Presence of the Inhibitors of the cAMP/PKA Signaling Pathway
2.4. Inhibition of the cAMP/PKA Axis Does Not Affect the Respiration-Driven Mitochondrial Membrane Potential Generation
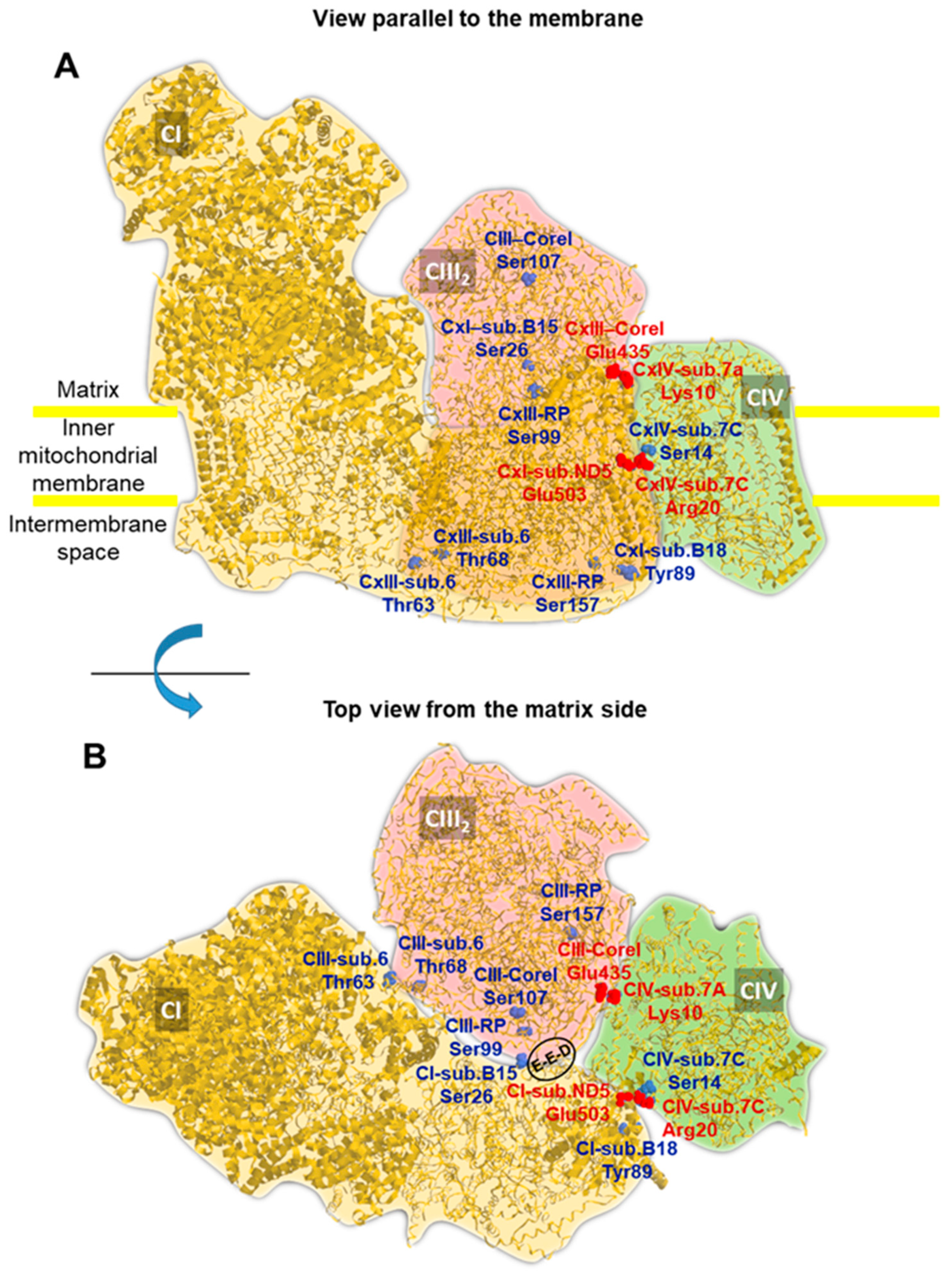
2.5. A Number of PKA-Targeted Serine Residues Are Present at the Contact Sites of CI, CIII and CIV in the Supercomplex
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Respirometry
4.3. Metabolic Flux Control Analysis
a = [I] + KD − E0
4.4. Fluorimetric Measurement of ΔΨm
4.5. Blue Native-PAGE and Western Blotting
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Efremov, R.G.; Baradaran, R.; Sazanov, L.A. The architecture of respiratory complex I. Nature 2010, 465, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Ferguson, S.J. Bioenergetics, 3rd ed.; Academic Press: London, UK, 2002. [Google Scholar]
- Hackenbrock, C.R.; Chazotte, B.; Gupte, S.S. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J. Bioenerg. Biomembr. 1986, 18, 331–368. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; Pfeiffer, K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000, 19, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G.; Genova, M.L. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: Random collisions vs. solid state electron channeling. Am. J. Physiol. Cell Physiol. 2007, 292, C1221–C1239. [Google Scholar] [CrossRef] [PubMed]
- Genova, M.L.; Lenaz, G. Functional role of mitochondrial respiratory supercomplexes. Biochim. Biophys Acta 2014, 1837, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Acin-Perez, R.; Enriquez, J.A. The function of the respiratory supercomplexes: The plasticity model. Biochim. Biophys Acta 2014, 1837, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Porras, C.A.; Bai, Y. Respiratory supercomplexes: Plasticity and implications. Front. Biosci. 2015, 20, 621–634. [Google Scholar]
- Cogliati, S.; Lorenzi, I.; Rigoni, G.; Caicci, F.; Soriano, M.E. Regulation of Mitochondrial Electron Transport Chain Assembly. J. Mol. Biol. 2018, 430, 4849–4873. [Google Scholar] [CrossRef]
- Fernández-Vizarra, E.; López-Calcerrada, S.; Sierra-Magro, A.; Pérez-Pérez, R.; Formosa, L.E.; Hock, D.H.; Illescas, M.; Peñas, A.; Brischigliaro, M.; Ding, S.; et al. Two independent respiratory chains adapt OXPHOS performance to glycolytic switch. Cell Metab. 2022, 34, 1792–1808. [Google Scholar] [CrossRef]
- Kacser, H.; Burns, J.A. The control of flux. Symp. Soc. Exp. Biol. 1973, 27, 65–104. [Google Scholar] [CrossRef]
- Heinrich, R.; Rapoport, T.A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur. J. Biochem. 1974, 42, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Reder, C. Metabolic control theory: A structural approach. J. Theor. Biol. 1988, 135, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Groen, A.K.; Wanders, R.J.; Westerhoff, H.V.; van der Meer, R.; Tager, J.M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J. Biol. Chem. 1982, 257, 2754–2757. [Google Scholar] [CrossRef] [PubMed]
- Tager, J.M.; Wanders, R.J.; Groen, A.K.; Kunz, W.; Bohnensack, R.; Küster, U.; Letko, G.; Böhme, G.; Duszynski, J.; Wojtczak, L. Control of mitochondrial respiration. FEBS Lett. 1983, 151, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem. J. 1992, 284 Pt 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, C.; Scrima, R.; Boffoli, D.; Capitanio, N. Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem. J. 2006, 396, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Quarato, G.; Piccoli, C.; Scrima, R.; Capitanio, N. Variation of flux control coefficient of cytochrome c oxidase and of the other respiratory chain complexes at different values of protonmotive force occurs by a threshold mechanism. Biochim. Biophys Acta 2011, 1807, 1114–1124. [Google Scholar] [CrossRef]
- Stram, A.R.; Payne, R.M. Post-translational modifications in mitochondria: Protein signaling in the powerhouse. Cell Mol. Life Sci. 2016, 73, 4063–4073. [Google Scholar] [CrossRef]
- Zhao, X.; León, I.R.; Bak, S.; Mogensen, M.; Wrzesinski, K.; Højlund, K.; Jensen, O.N. Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol. Cell Proteom. 2011, 10, M110.000299. [Google Scholar] [CrossRef]
- Castellanos, E.; Lanning, N.J. Phosphorylation of OXPHOS Machinery Subunits: Functional Implications in Cell Biology and Disease. Yale J. Biol. Med. 2019, 92, 523–531. [Google Scholar]
- Niemi, N.M.; Pagliarini, D.J. The extensive and functionally uncharacterized mitochondrial phosphoproteome. J. Biol. Chem. 2021, 297, 100880. [Google Scholar] [CrossRef] [PubMed]
- Thomson, M. Evidence of undiscovered cell regulatory mechanisms: Phosphoproteins and protein kinases in mitochondria. Cell Mol. Life. Sci. 2002, 59, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Horbinski, C.; Chu, C.T. Kinase signaling cascades in the mitochondrion: A matter of life or death. Free Radic. Biol. Med. 2005, 38, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Gellerich, F.N.; Kunz, W.S.; Bohnensack, R. Estimation of flux control coefficients from inhibitor titrations by non-linear regression. FEBS Lett. 1990, 274, 167–170. [Google Scholar] [PubMed]
- Small, J.R. Flux control coefficients determined by inhibitor titration: The design and analysis of experiments to minimize errors. Biochem. J. 1993, 296, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Schägger, H.; Pfeiffer, K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J. Biol. Chem. 2001, 276, 37861–37867. [Google Scholar] [CrossRef] [PubMed]
- Torres-Quesada, O.; Mayrhofer, J.E.; Stefan, E. The many faces of compartmentalized PKA signalosomes. Cell Signal. 2017, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ould Amer, Y.; Hebert-Chatelain, E. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim. Biophys Acta Bioenerg. 2018, 1859, 868–877. [Google Scholar] [CrossRef]
- Valsecchi, F.; Konrad, C.; Manfredi, G. Role of soluble adenylyl cyclase in mitochondria. Biochim. Biophys Acta 2014, 1842 Pt B, 2555–2560. [Google Scholar] [CrossRef]
- Bitterman, J.L.; Ramos-Espiritu, L.; Diaz, A.; Levin, L.R.; Buck, J. Pharmacological distinction between soluble and transmembrane adenylyl cyclases. J. Pharmacol. Exp. Ther. 2013, 347, 589–598. [Google Scholar] [CrossRef]
- Wu, M.; Gu, J.; Guo, R.; Huang, Y.; Yang, M. Structure of Mammalian Respiratory Supercomplex I1III2IV1. Cell 2016, 167, 1598–1609. [Google Scholar] [CrossRef]
- Milenkovic, D.; Misic, J.; Hevler, J.F.; Molinie’, T.; Chung, I.; Atanassov, I.; Li, X.; Filograna, R.; Mesaros, A.; Mourier, A.; et al. Preserved respiratory chain capacity and physiology in mice with profoundly reduced levels of mitochondrial respirasomes. Cell Metab. 2023, 35, 1799–1813.e7. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zong, S.; Wu, M.; Gu, J.; Yang, M. Architecture of Human Mitochondrial Respiratory Megacomplex I2III2IV2. Cell 2017, 170, 1247–1257.e12. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vizarra, E.; Ugalde, C. Cooperative assembly of the mitochondrial respiratory chain. Trends Biochem. Sci. 2022, 47, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Maranzana, E.; Barbero, G.; Falasca, A.I.; Lenaz, G.; Genova, M.L. Mitochondrial Respiratory Supercomplex Association Limits Production of Reactive Oxygen Species from Complex I. Antioxid. Redox Signal. 2013, 19, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.; Meyrat, A.; Stoldt, S.; Santiago, R.; Wenzel, D.; Jakobs, S.; von Ballmoos, C.; Ott, M. Kinetic coupling of the respiratory chain with ATP synthase, but not proton gradients, drives ATP production in cristae membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Jarne, T.; Ugalde, C. Respiratory chain supercomplexes: Structures, function and biogenesis. Semin. Cell Dev. Biol. 2018, 76, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, L.; Wang, B.; Chen, D.; Ye, X.; Han, X.; Fang, Q.; Yu, C.; Wu, J.; Guo, S.; et al. Mitochondrial supercomplex assembly regulates metabolic features and glutamine dependency in mammalian cells. Theranostics 2023, 13, 3165–3187. [Google Scholar] [CrossRef] [PubMed]
- Villani, G.; Attardi, G. In vivo measurements of respiration control by cytochrome c oxidase and in situ analysis of oxidative phosphorylation. Methods Cell Biol. 2001, 65, 119–131. [Google Scholar]
- Scacco, S.; Vergari, R.; Scarpulla, R.C.; Technikova-Dobrova, Z.; Sardanelli, A.; Lambo, R.; Lorusso, V.; Papa, S. cAMP-dependent phosphorylation of the nuclear encoded 18-kDa (IP) subunit of respiratory complex I and activation of the complex in serum-starved mouse fibroblast cultures. J. Biol. Chem. 2000, 275, 17578–17582. [Google Scholar] [CrossRef]
- Piccoli, C.; Scacco, S.; Bellomo, F.; Signorile, A.; Iuso, A.; Boffoli, D.; Scrima, R.; Capitanio, N.; Papa, S. cAMP controls oxygen metabolism in mammalian cells. FEBS Lett. 2006, 580, 4539–4543. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, E.; Andersen, J.V.; Christensen, S.K.; Siamka, O.; Larsen, M.R.; Waagepetersen, H.S.; Aldana, B.I.; Bak, L.K. Pharmacological inhibition of mitochondrial soluble adenylyl cyclase in astrocytes causes activation of AMP-activated protein kinase and induces breakdown of glycogen. Glia 2021, 69, 2828–2844. [Google Scholar] [CrossRef] [PubMed]
- Signorile, A.; Pacelli, C.; Palese, L.L.; Santeramo, A.; Roca, E.; Cocco, T.; De Rasmo, D. cAMP/PKA Signaling Modulates Mitochondrial Supercomplex Organization. Int. J. Mol. Sci. 2022, 23, 9655. [Google Scholar] [CrossRef] [PubMed]
- Kasimova, M.A.; Lindahl, E.; Delemotte, L. Determining the molecular basis of voltage sensitivity in membrane proteins. J. Gen. Physiol. 2018, 150, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Conti, F.; Franciolini, F. Fifty years of gating currents and channel gating. J. Gen. Physiol. 2023, 155, e202313380. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell. Biol. 2008, 9, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Li, H. Interplay between the electrostatic membrane potential and conformational changes in membrane proteins. Protein Sci. 2019, 28, 502–512. [Google Scholar] [CrossRef]
- Beavo, J.A.; Brunton, L.L. Cyclic nucleotide research—Still expanding after half a century. Nat. Rev. Mol. Cell Biol. 2002, 3, 710–718. [Google Scholar] [CrossRef]
- Akerman, K.E.; Wikström, M.K. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976, 68, 191–197. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scrima, R.; Cela, O.; Rosiello, M.; Nabi, A.Q.; Piccoli, C.; Capitanio, G.; Tucci, F.A.; Leone, A.; Quarato, G.; Capitanio, N. Mitochondrial sAC-cAMP-PKA Axis Modulates the ΔΨm-Dependent Control Coefficients of the Respiratory Chain Complexes: Evidence of Respirasome Plasticity. Int. J. Mol. Sci. 2023, 24, 15144. https://doi.org/10.3390/ijms242015144
Scrima R, Cela O, Rosiello M, Nabi AQ, Piccoli C, Capitanio G, Tucci FA, Leone A, Quarato G, Capitanio N. Mitochondrial sAC-cAMP-PKA Axis Modulates the ΔΨm-Dependent Control Coefficients of the Respiratory Chain Complexes: Evidence of Respirasome Plasticity. International Journal of Molecular Sciences. 2023; 24(20):15144. https://doi.org/10.3390/ijms242015144
Chicago/Turabian StyleScrima, Rosella, Olga Cela, Michela Rosiello, Ari Qadir Nabi, Claudia Piccoli, Giuseppe Capitanio, Francesco Antonio Tucci, Aldo Leone, Giovanni Quarato, and Nazzareno Capitanio. 2023. "Mitochondrial sAC-cAMP-PKA Axis Modulates the ΔΨm-Dependent Control Coefficients of the Respiratory Chain Complexes: Evidence of Respirasome Plasticity" International Journal of Molecular Sciences 24, no. 20: 15144. https://doi.org/10.3390/ijms242015144
APA StyleScrima, R., Cela, O., Rosiello, M., Nabi, A. Q., Piccoli, C., Capitanio, G., Tucci, F. A., Leone, A., Quarato, G., & Capitanio, N. (2023). Mitochondrial sAC-cAMP-PKA Axis Modulates the ΔΨm-Dependent Control Coefficients of the Respiratory Chain Complexes: Evidence of Respirasome Plasticity. International Journal of Molecular Sciences, 24(20), 15144. https://doi.org/10.3390/ijms242015144







