Immunolocalization of Jasmonates and Auxins in Pea Roots in Connection with Inhibition of Root Growth under Salinity Conditions
Abstract
:1. Introduction
2. Results
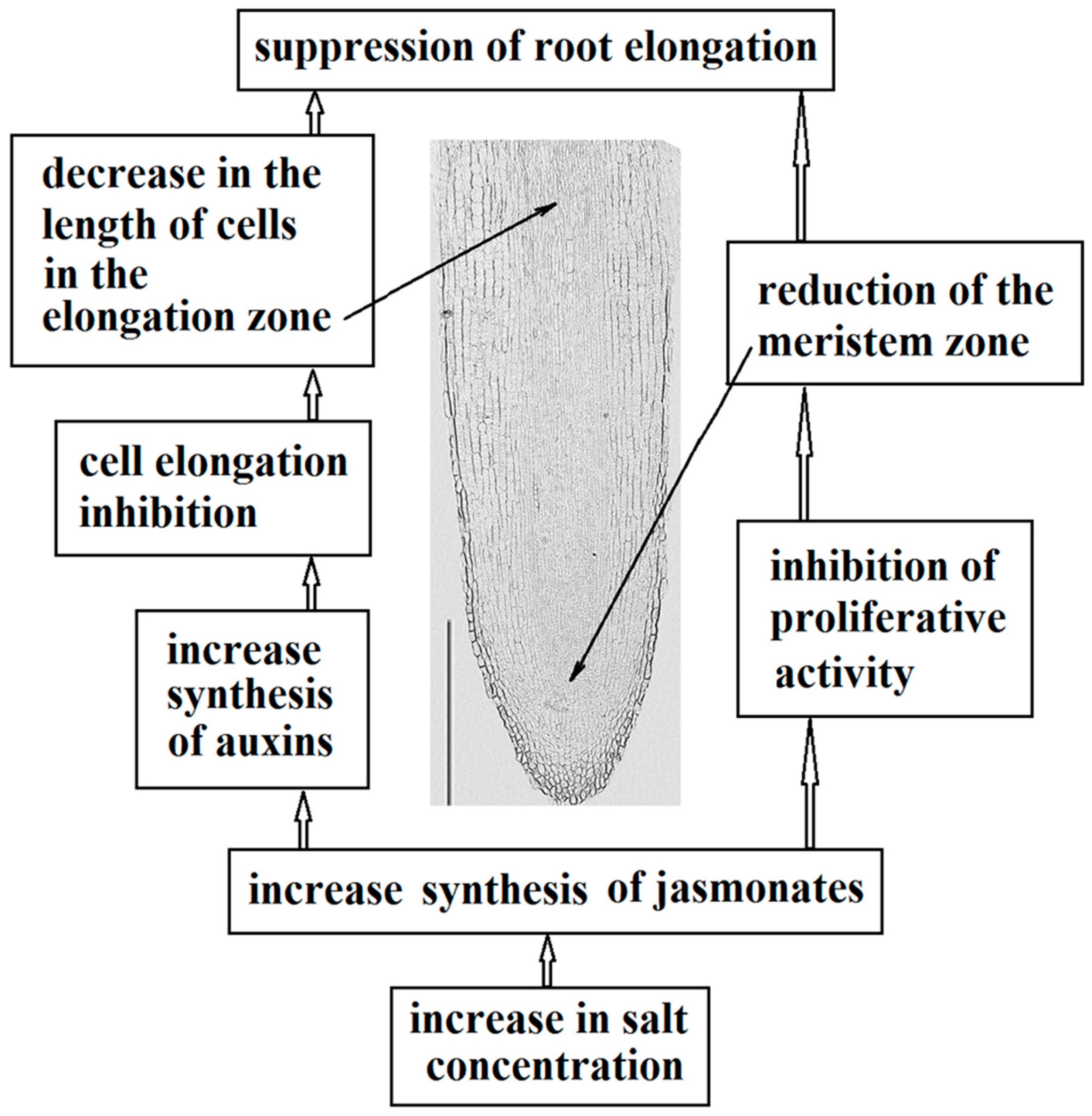
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Collecting Plant Material
4.2. Immunolocalization of Jasmonic Acid and Auxins
4.3. Measurement of the Meristem Zone and Cell Length
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabagh, E.A.; Islam, M.S.; Skalicky, M.; Raza, A.M.; Singh, K.; Hossain, A.M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinha, A.L.; Caperta, A.D. Sustainable agricultural management of saline soils in arid and semi-arid Mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Testerink, C. Root dynamic growth strategies in response to salinity. Plant Cell Environ. 2022, 45, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Ampudia, C.S.; Testerink, C. Salt signals shape the plant root. Curr. Opin. Plant Biol. 2011, 14, 296–302. [Google Scholar] [CrossRef]
- Shao, Y.; Cheng, Y.; Pang, H.; Chang, M.; He, F.; Wang, M.; Davis, D.J.; Zhang, S.; Betz, O.; Fleck, C.; et al. Investigation of salt tolerance mechanisms across a root developmental gradient in almond rootstocks. Front. Plant Sci. 2020, 11, 595055. [Google Scholar] [CrossRef]
- Martynenko, E.; Arkhipova, T.; Safronova, V.; Seldimirova, O.; Galin, I.; Akhtyamova, Z.; Veselov, D.; Ivanov, R.; Kudoyarova, G. Effects of phytohormone-producing rhizobacteria on casparian band formation, ion homeostasis and salt tolerance of durum wheat. Biomolecules 2022, 12, 230. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Melotto, M.; Jian, Y.; He, S.Y. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 2017, 68, 1371–1385. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef]
- Delgado, C.; Mora-Poblete, F.; Ahmar, S.; Chen, J.T.; Figueroa, C.R. Jasmonates and plant salt stress: Molecular players, physiological effects, and improving tolerance by using genome-associated tools. Int. J. Mol. Sci. 2021, 22, 3082. [Google Scholar] [CrossRef]
- Kang, D.-J.; Seo, Y.-J.; Lee, J.D.; Ishii, R.; Kim, K.; Shin, D.; Park, S.; Jang, S.; Lee, I.-J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- Han, X.; Kui, M.; He, K.; Yang, M.; Du, J.; Jiang, Y.; Hu, Y. Jasmonate-regulated root growth inhibition and root hair elongation. J. Exp. Bot. 2023, 74, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhao, P.-X.; Cai, X.-T.; Mao, J.-M.; Miao, Z.-Q.; Xiang, C.-B. Integration of jasmonic acid and ethylene into auxin signaling in root development. Front. Plant Sci. 2020, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Gyohda, A.; Tominaga, M.; Kawakatsu, M.; Hatakeyama, A.; Ishii, N.; Shimaya, K.; Nishimura, T.; Riemann, M.; Nick, P.; et al. RSOsPR10 expression in response to environmental stresses is regulated antagonistically by jasmonate/ethylene and salicylic acid signaling pathways in rice roots. Plant Cell Physiol. 2011, 52, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Abdel Latef, A.A.H.; Tahjib-Ul-Arif, M.; Rhaman, M.S. Exogenous Auxin-Mediated Salt Stress Alleviation in Faba Bean (Vicia faba L.). Agronomy 2021, 11, 547. [Google Scholar] [CrossRef]
- Verma, S.; Negi, N.P.; Pareek, S.; Mudgal, G.; Kumar, D. Auxin response factors in plant adaptation to drought and salinity stress. Physiol. Plant. 2022, 174, e13714. [Google Scholar] [CrossRef]
- Hentrich, M.; Böttcher, C.; Düchting, P.; Cheng, Y.; Zhao, Y.; Berkowitz, O.; Masle, J.; Medina, J.; Pollmann, S. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J. 2013, 74, 626–637. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.-M.; Sánchez-Parra, B.; Ortiz-García, P.; Santamaría, M.E.; Díaz, I.; Pollmann, S. Jasmonic Acid-Dependent MYC Transcription Factors Bind to a Tandem G-Box Motif in the YUCCA8 and YUCCA9 Promoters to Regulate Biotic Stress Responses. Int. J. Mol. Sci. 2021, 22, 9768. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, J.; Zhai, Q.; Zhou, W.; Qi, L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.; Qi, J.; et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, H.; Shang, C.; Ma, S.; Liu, L.; Cheng, J. The Roles of Auxin Biosynthesis YUCCA Gene Family in Plants. Int. J. Mol. Sci. 2019, 20, 6343. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, M.V.; Lloret, P.G.; Salguero, J. Synergistic action of auxin and ethylene on root elongation inhibition is caused by a reduction of epidermal cell length. Plant Signal Behav. 2014, 9, e28361. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Arkhipova, T.; Veselov, D. Water relations in plants treated with growth promoting rhizosphere bacteria. Plant Soil 2023, in press. [Google Scholar] [CrossRef]
- Valenzuela, C.E.; Acevedo-Acevedo, O.; Miranda, G.S.; Vergara-Barros, P.; Holuigue, L.; Figueroa, C.R.; Figueroa, R.M. Salt stress response triggers activation of the jasmonate signaling pathway leading to inhibition of cell elongation in Arabidopsis primary root. J. Exp. Bot. 2016, 67, 4209–4220. [Google Scholar] [CrossRef]
- Smolko, A.; Bauer, N.; Pavlović, I.; Pěnčík, A.; Novák, O.; Salopek-Sondi, B. Altered root growth, auxin metabolism and distribution in Arabidopsis thaliana exposed to salt and osmotic stress. Int. J. Mol. Sci. 2021, 22, 7993. [Google Scholar] [CrossRef]
- Arkhipova, T.; Sharipova, G.; Akhiyarova, G.; Kuzmina, L.; Galin, I.; Martynenko, E.; Seldimirova, O.; Nuzhnaya, T.; Feoktistova, A.; Timergalin, M.; et al. The effects of rhizosphere inoculation with Pseudomonas mandelii on formation of apoplast barriers, HvPIP2 aquaporins and hydraulic conductance of barley. Microorganisms 2022, 10, 935. [Google Scholar] [CrossRef]
- Martynenko, E.; Arkhipova, T.; Akhiyarova, G.; Sharipova, G.; Galin, I.; Seldimirova, O.; Ivanov, R.; Nuzhnaya, T.; Finkina, E.; Ovchinnikova, T.; et al. Effects of a Pseudomonas strain on the lipid transfer proteins, appoplast barriers and activity of aquaporins associated with hydraulic conductance of pea plants. Membranes 2023, 13, 208. [Google Scholar] [CrossRef]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Koevoets, I.T.; Mol, S.; Hoefsloot, H.; Feron, R.; Tester, M.A.; Keurentjes, J.J.B.; Korte, A.; Haring, M.A.; Boer, D.G.-J.; et al. Genetic components of root architecture remodeling in response to salt stress. Plant Cell 2017, 29, 3198–3213. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Li, X. Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J. Plant Physiol. 2009, 166, 1637–1645. [Google Scholar] [CrossRef]
- Liu, W.; Li, R.-J.; Han, T.-T.; Cai, W.; Fu, Z.-W.; Lu, J.-T. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol. 2015, 168, 343–356. [Google Scholar] [CrossRef]
- Sack, L.; John, G.P.; Buckley, T.N. ABA Accumulation in dehydrating leaves is associated with decline in cell volume, not turgor pressure. Plant Physiol. 2018, 176, 489–495. [Google Scholar] [CrossRef]
- Noir, S.; Bömer, M.; Takahashi, N.; Ishida, T.; Tsui, T.-L.; Balbi, V.; Shanahan, H.; Sugimoto, K.; Devoto, A. Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol. 2013, 161, 1930–1951. [Google Scholar] [CrossRef]
- Uyehara, A.N.; Del Valle-Echevarria, A.R.; Hunter, C.T.; Nelissen, H.; Demuynck, K.; Cahill, J.F.; Gorman, Z.; Jander, G.; Muszynski, M.G. Cytokinin Promotes Jasmonic Acid Accumulation in the Control of Maize Leaf Growth. Plants 2023, 12, 3014. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Moe-Lange, J.; Hennet, L.; Feldman, L.J. Salt Stress Affects the Redox Status of Arabidopsis Root Meristems. Front. Plant Sci. 2016, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Ferrandi, P.; Pencík, A.; Gehring, C.; Novák, O.; Ingle, R.A.; Donaldson, L. Salt-specific gene expression reveals elevated auxin levels in Arabidopsis thaliana plants grown under saline conditions. Front. Plant Sci. 2022, 13, 804716. [Google Scholar] [CrossRef]
- Chen, Q.; Dai, X.; De-Paoli, H.; Cheng, Y.; Takebayashi, Y.; Kasahara, H.; Kamiya, Y.; Zhao, Y. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol. 2014, 55, 1072–1079. [Google Scholar] [CrossRef]
- Chapman, E.J.; Estelle, M. Cytokinin and auxin intersection in root meristems. Genome Biol. 2009, 10, 210. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Zhou, J.; Qiao, J.; Li, Y.; Quan, R.; Huang, R. Abscisic acid promotes auxin biosynthesis to inhibit primary root elongation in rice. Plant Physiol. 2023, 191, 1953–1967. [Google Scholar] [CrossRef]
- Mielke, K.; Forner, S.; Kramell, R.; Conrad, U.; Hause, B. Cell-specific visualization of jasmonates in wounded tomato and Arabidopsis leaves using jasmonate-specific antibodies. New Phytol. 2011, 190, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.; Akhiyarova, G.; Feoktistova, A.; Akhtyamova, Z.; Korobova, A.; Ivanov, I.; Kudoyarova, G.; Dodd, I.; Kuluev, B. Effects of phosphate shortage on root growth and hormone content of barley depend on capacity of the roots to accumulate ABA. Plants 2020, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
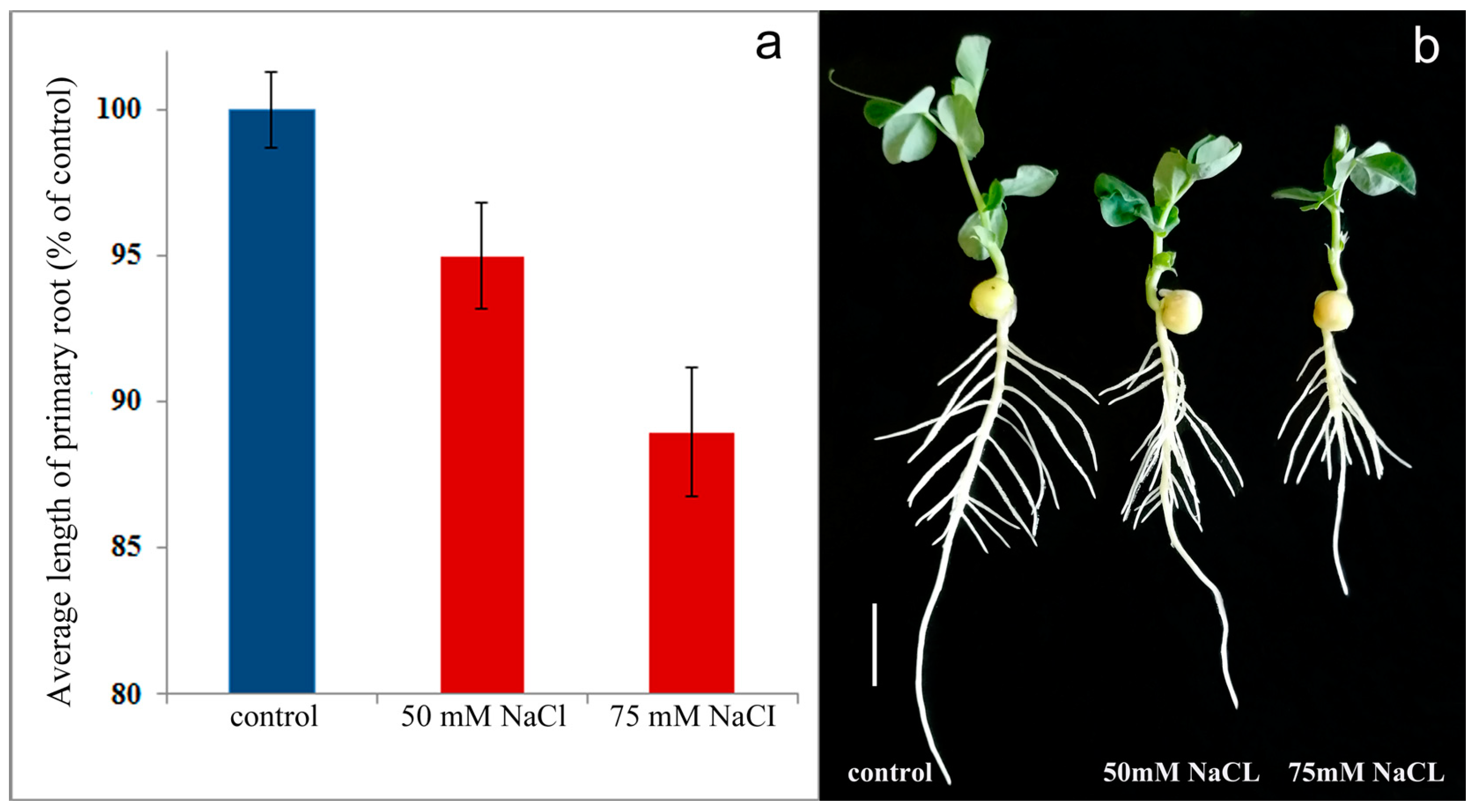
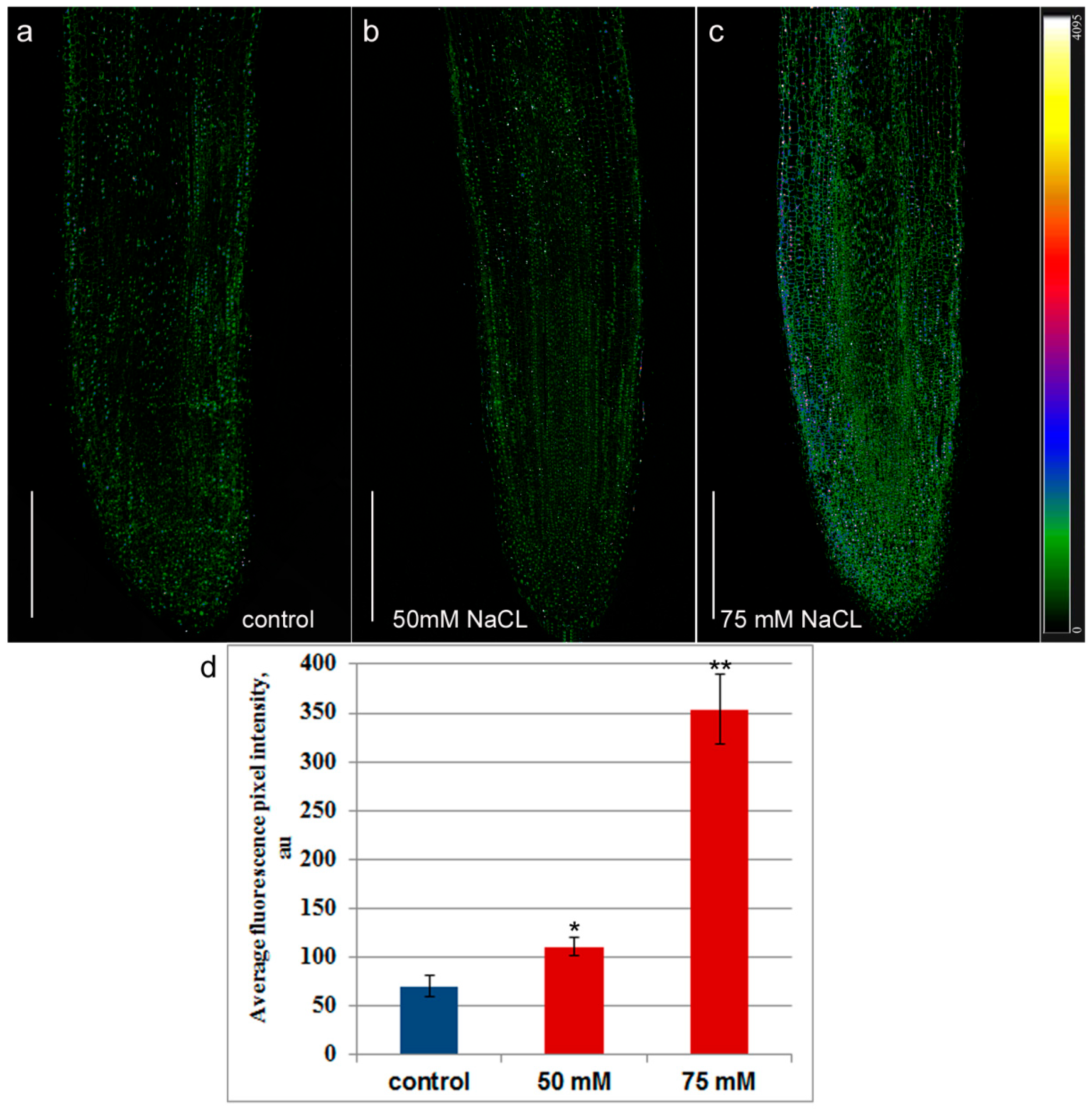

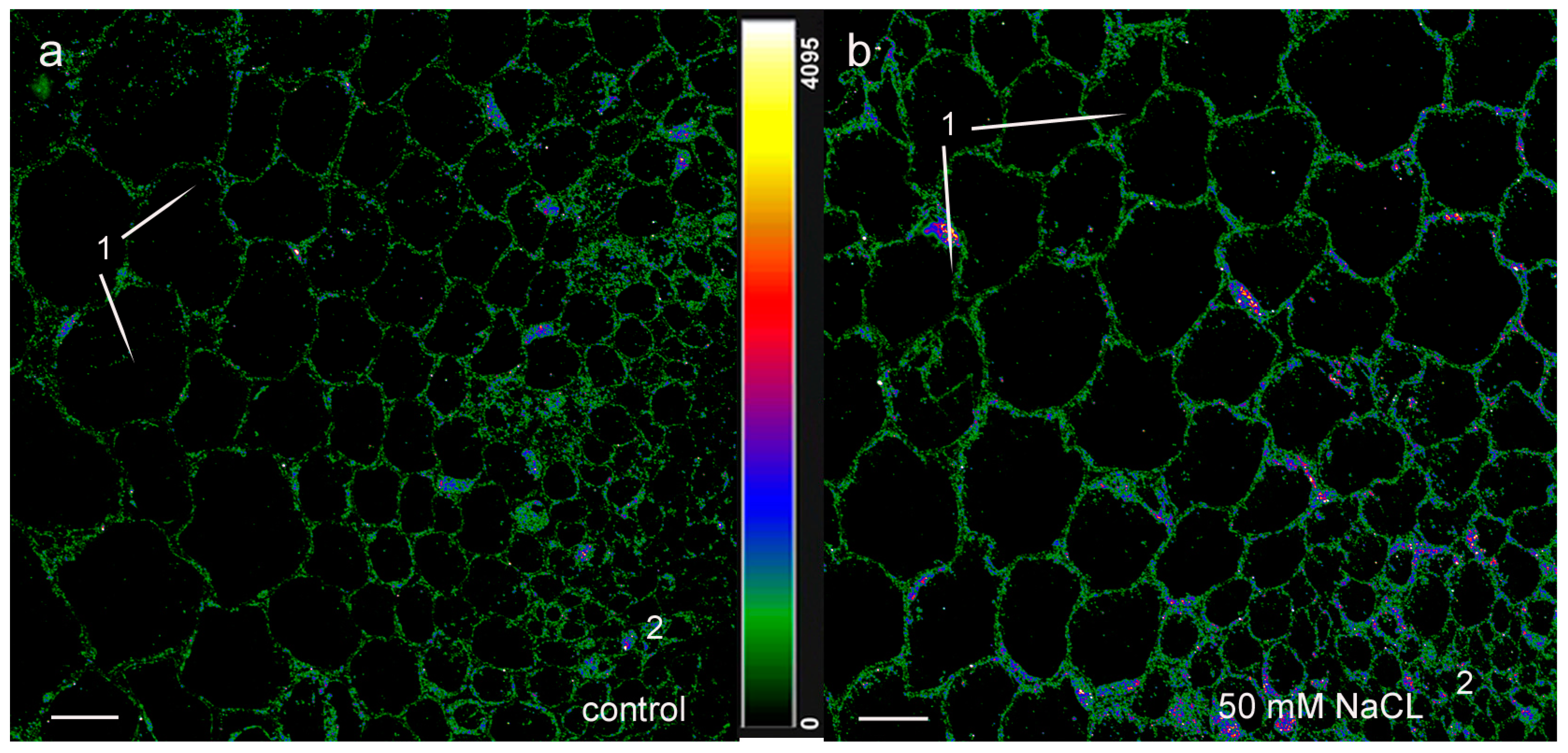
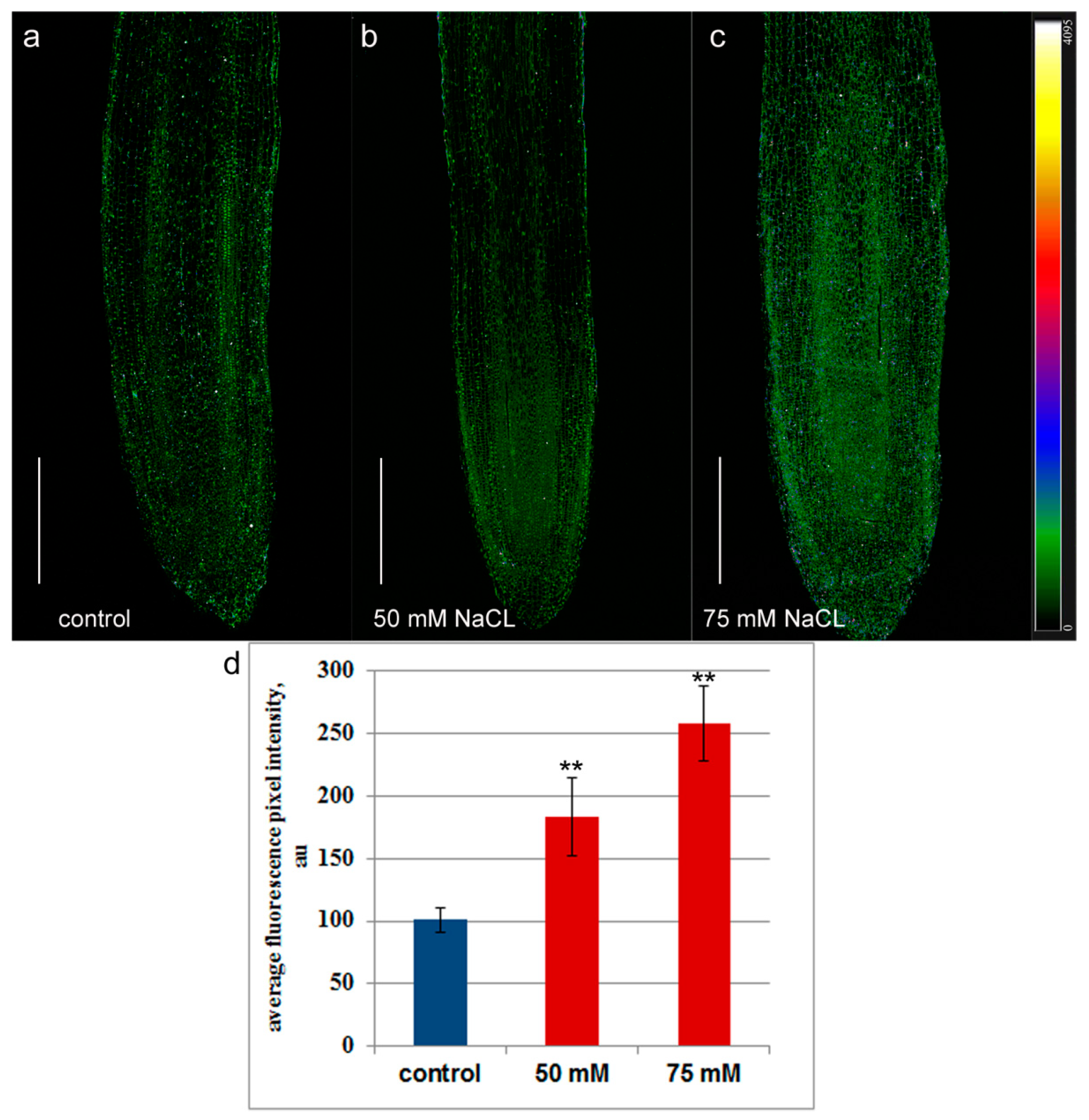
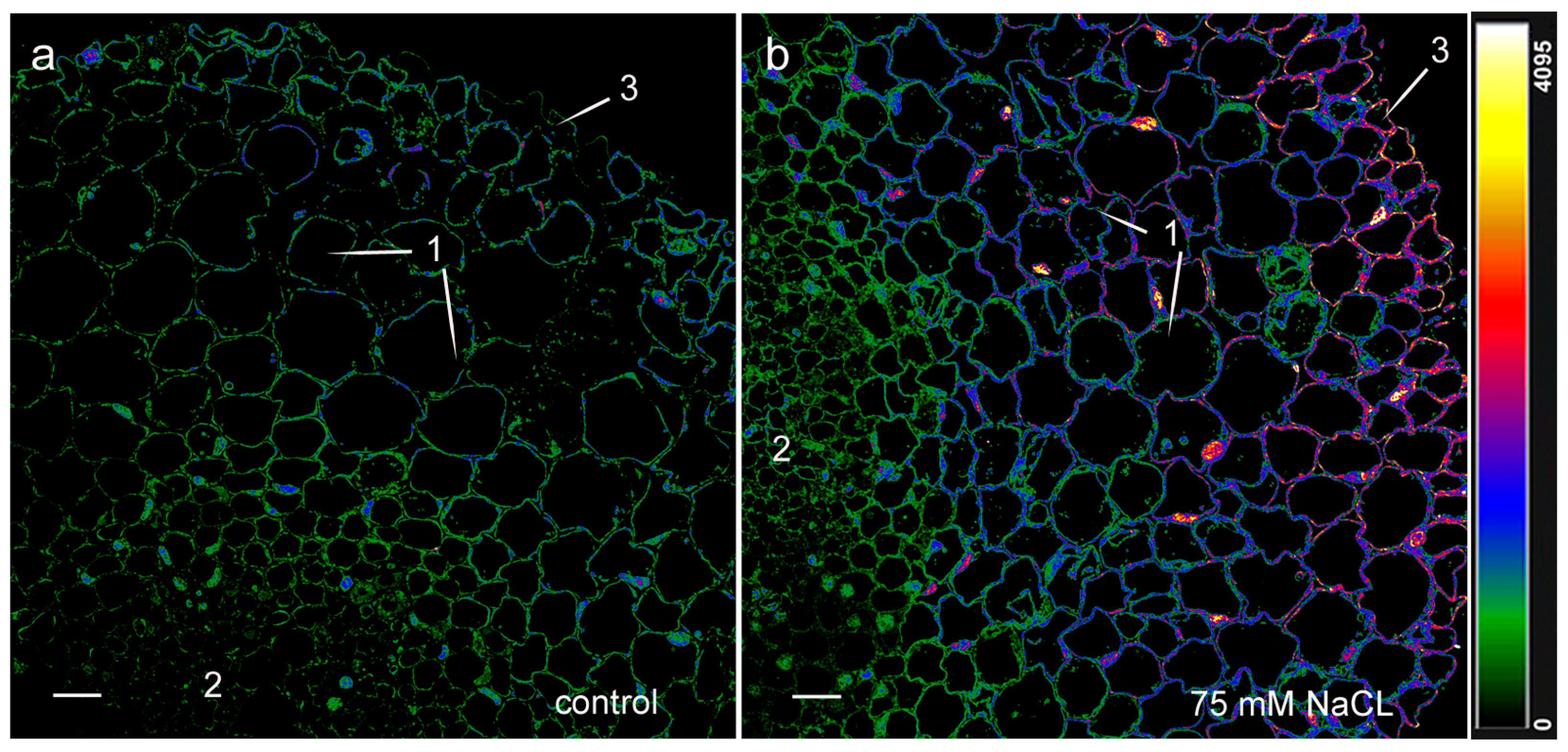
| NaCl Stress | Meristem Size, µm | Mean Length of Cells in Elongating Root Zone, µm |
|---|---|---|
| 0 mM NaCl | 1066 ± 86 a | 60 ± 2 a |
| 50 mM NaCl | 899 ± 29 b | 54 ± 1 b |
| 75 mM NaCl | 822 ± 45 b | 50 ± 3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhiyarova, G.; Vafina, G.; Veselov, D.; Kudoyarova, G. Immunolocalization of Jasmonates and Auxins in Pea Roots in Connection with Inhibition of Root Growth under Salinity Conditions. Int. J. Mol. Sci. 2023, 24, 15148. https://doi.org/10.3390/ijms242015148
Akhiyarova G, Vafina G, Veselov D, Kudoyarova G. Immunolocalization of Jasmonates and Auxins in Pea Roots in Connection with Inhibition of Root Growth under Salinity Conditions. International Journal of Molecular Sciences. 2023; 24(20):15148. https://doi.org/10.3390/ijms242015148
Chicago/Turabian StyleAkhiyarova, Guzel, Gyulnar Vafina, Dmitriy Veselov, and Guzel Kudoyarova. 2023. "Immunolocalization of Jasmonates and Auxins in Pea Roots in Connection with Inhibition of Root Growth under Salinity Conditions" International Journal of Molecular Sciences 24, no. 20: 15148. https://doi.org/10.3390/ijms242015148
APA StyleAkhiyarova, G., Vafina, G., Veselov, D., & Kudoyarova, G. (2023). Immunolocalization of Jasmonates and Auxins in Pea Roots in Connection with Inhibition of Root Growth under Salinity Conditions. International Journal of Molecular Sciences, 24(20), 15148. https://doi.org/10.3390/ijms242015148





