Immunohistochemical Analysis of Knee Chondral Defect Repair after Autologous Particulated Cartilage and Platelet-Rich Plasma Treatment in Sheep
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Study Design and Surgical Procedure
4.3. Preparation and Use of Autologous PRP
4.4. Immunohistochemical Evaluation
4.5. Development of a Semiquantitative Scoring System
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECM | Extracellular matrix |
| HA | Hyaluronic acid |
| MCI | Minced cartilage implantation |
| PACI | Particulated autograft cartilage implantation |
| PACI + PRP | Therapeutic technique based on an autologous matrix composed of healthy hyaline cartilage chips included and mixed in a PRP clot and intraarticular infiltration of PRP |
| PBS | Phosphate-buffered saline |
| PRP | Platelet-rich plasma |
| RLS | Ringer’s lactate solution |
| RT | Room temperature |
References
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J. Orthop. Sports Phy. Ther. 1998, 28, 203–2015. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2012, 34, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Cugat, R.; Alentorn-Geli, E.; Navarro, J.; Cuscó, X.; Steinbacher, G.; Seijas, R.; Álvarez-Díaz, P.; Barastegui, D.; Laiz, P.; Samitier, G.; et al. A novel autologous-made matrix using hyaline cartilage chips and platelet-rich growth factors for the treatment of full-thickness cartilage or osteochondral defects: Preliminary results. J. Orthop. Surg. 2019, 28, 2309499019887547. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Simon, T.M.; Jackson, D.W. Articular cartilage: Injury pathways and treatment options. Sports Med. Arthrosc. Rev. 2006, 14, 146–154. [Google Scholar] [CrossRef]
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and minor types of collagens in the articular cartilage: The role of collagens in repair tissue evaluation in chondral defects. Int. J. Mol. Sci. 2021, 22, 13129. [Google Scholar] [CrossRef]
- Albrecht, F.; Roessner, A.; Zimmermann, E. Closure of osteochondral lesions using chondral fragments and fibrin adhesive. Arch. Orthop. Trauma. Surg. 1983, 101, 213–217. [Google Scholar] [CrossRef]
- Farr, J.; Cole, B.J.; Sherman, S.; Karas, V. Particulated articular cartilage: CAIS and DeNovo NT. J. Knee Surg. 2012, 25, 23–29. [Google Scholar] [CrossRef]
- Mariani, E.; Pulsatelli, L. Platelet concentrates in musculoskeletal medicine. Int. J. Mol. Sci. 2020, 21, 1328. [Google Scholar] [CrossRef]
- Abrams, G.D.; Frank, R.M.; Fortier, L.A.; Cole, B.J. Platelet-rich plasma for articular cartilage repair. Sports Med. Arthrosc. Rev. 2013, 21, 213–219. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, H.; Liang, G.; Zeng, L.F.; Yang, W.; Liu, J. Effects and safety of the combination of platelet-rich plasma (PRP) and hyaluronic acid (HA) in the treatment of knee osteoarthritis: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 224. [Google Scholar] [CrossRef] [PubMed]
- Cugat, R.; Alentorn-Geli, E.; Steinbacher, G.; Álvarez-Díaz, P.; Cuscó, X.; Seijas, R.; Barastegui, D.; Navarro, J.; Laiz, P.; García-Balletbó, M. Treatment of knee osteochondral lesions using a novel clot of autologous plasma rich in growth factors mixed with healthy hyaline cartilage chips and intra-articular injection of PRGF. Case Rep. Orthop. 2017, 2017, 8284548. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Pérez, J.M.; Fernández-Sarmiento, J.A.; Aguilar-García, D.; Granados-Machuca, M.D.M.; Morgaz-Rodríguez, J.; Navarrete-Calvo, R.; Pérez-Arévalo, J.; Carrillo-Poveda, J.M.; Alentorn-Geli, E.; Laiz-Boada, P.; et al. Cartilage regeneration using a novel autologous growth factors-based matrix for full-thickness defects in sheep. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.; Kandel, R.; Roberts, S.; Saris, D.B.F.; Creemers, L.; Mainil-Varlet, P.; Méthot, S.; Hollander, A.P.; Buschmann, M.D. International cartilage repair society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage 2011, 2, 153–172. [Google Scholar] [CrossRef]
- Alcaide-Ruggiero, L.; Cugat, R.; Domínguez, J.M. Proteoglycans in articular cartilage and their contribution to chondral injury and repair mechanisms. Int. J. Mol. Sci. 2023, 24, 10824. [Google Scholar] [CrossRef]
- Sophia-Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Decker, R.S. Articular cartilage and joint development from embryogenesis to adulthood. Semin. Cell Dev. Biol. 2017, 62, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Musumeci, G.; Castrogiovanni, P.; Leonardi, R.; Trovato, F.M.; Szychlinska, M.A.; Di Giunta, A.; Loreto, C.; Castorina, R. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J. Orthop. 2014, 5, 80–88. [Google Scholar] [CrossRef]
- Cugat, R.; Samitier, G.; Vinagre, G.; Sava, M.; Alentorn-Geli, E.; García-Balletbó, M.; Cuscó, X.; Seijas, R.; Barastegui, D.; Navarro, J.; et al. Particulated autologous chondral−platelet-rich plasma matrix implantation (PACI) for treatment of full-thickness cartilage osteochondral defects. Arthrosc. Tech. 2021, 10, 239–544. [Google Scholar] [CrossRef]
- Delman, C.; Wuellner, J.; Kreulen, C.; Lundeen, G.; Giza, E. Particulated autograft cartilage implantation for the treatment of osteochondral lesions of the talus: A novel technique. Foot Ankle Spec. 2018, 11, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Bonasia, D.E.; Marmotti, A.; Rosso, F.; Collo, G.; Rossi, R. Use of chondral fragments for one stage cartilage repair: A systematic review. World J. Orthop. 2015, 6, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Prado, R.; Guadilla, J.; Alkhraisat, M.H.; Laiz, P.; Padilla, S.; García-Balletbó, M.; Cugat, R. The dual-responsive interaction of particulated hyaline cartilage and plasma rich in growth factors (PRGF) in the repair of cartilage defects: An in vitro study. Int. J. Mol. Sci. 2023, 24, 11581. [Google Scholar] [CrossRef]
- Marmotti, A.; Bruzzone, M.; Bonasia, D.E.; Castoldi, F.; Von-Degerfeld, M.M.; Bignardi, C.; Mattia, S.; Maiello, R.R.; Peretti, G.M. Autologous cartilage fragments in a composite scaffold for one stage osteochondral repair in a goat model. Eur. Cell Mater. 2013, 26, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Morgaz, J.; Fernández-Sarmiento, J.A.; Granados, M.M.; Navarrete-Calvo, R.; Pérez, J.; Quirós-Carmona, S.; Carrillo, J.M.; Cugat, R.; et al. Particulate cartilage and platelet-rich plasma treatment for knee chondral defects in sheep. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2944–2955. [Google Scholar] [CrossRef]
- Levinson, C.; Cavalli, E.; Sindi, D.M.; Kessel, B.; Zenobi-Wong, M.; Preiss, S.; Salzmann, G.; Neidencach, P. Chondrocytes from device-minced articular cartilage show potent outgrowth into fibrin and collagen hydrogels. Orthop. J. Sports Med. 2019, 7, 2325967119867618. [Google Scholar] [CrossRef] [PubMed]
- Bonasia, D.E.; Marmotti, A.; Mattia, S.; Cosentino, A.; Spolaore, S.; Governale, G.; Castoldi, F.; Rossi, R. The degree of chondral fragmentation affects extracellular matrix production in cartilage autograft implantation: An in vitro study. Arthroscopy 2015, 31, 2335–2341. [Google Scholar] [CrossRef]
- Bhagat, V.; Becker, M.L. Degradable adhesives for surgery and tissue engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef]
- Kreuz, P.C.; Krüger, J.P.; Metzlaff, S.; Freymann, U.; Endres, M.; Pruss, A.; Petersen, W.; Kaps, C. Platelet-rich plasma preparation types show impact on chondrogenic differentiation, migration, and proliferation of human subchondral mesenchymal progenitor cells. Arthroscopy 2015, 31, 1951–1961. [Google Scholar] [CrossRef]
- Anitua, E.; Sanchez, M.; Nurden, A.T.; Zalduendo, M.; De La Fuente, M.; Orive, G.; Azofra, J.; Andia, I. Autologous fibrin matrices: A potential source of biological mediators that modulate tendon cell activities. J. Biomed. Mater. Res. A. 2006, 77, 285–293. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, M.; Meng, H.Y.; Wang, A.Y.; Guo, Q.Y.; Wang, Y.; Peng, J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthr. Cartil. 2013, 21, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Peck, Y.; He, P.; Chilla, G.S.V.N.; Poh, C.L.; Wang, D.A. A preclinical evaluation of an autologous living hyaline-like cartilaginous graft for articular cartilage repair: A pilot study. Sci. Rep. 2015, 5, 16225. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, X.; Ye, Y.; Song, K.; Cheng, Y.; Di, J.; Hu, Q.; Li, J.; Ju, H.; Jiang, Q.; et al. Photo-cross-linked scaffold with kartogenin-encapsulated nanoparticles for cartilage regeneration. ACS Nano. 2016, 10, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xu, X.; Xu, Q.; Sun, Z.; Jiang, Q.; Shi, D. Platelet-rich plasma combined with injectable hyaluronic acid hydrogel for porcine cartilage regeneration: A 6-month follow-up. Regen. Biomater. 2020, 7, 77–90. [Google Scholar] [CrossRef]
- Bourgot, I.; Primac, I.; Louis, T.; Noël, A.; Maquoi, E. Reciprocal interplay between fibrillar collagens and collagen-binding integrins: Implications in cancer progression and metastasis. Front. Oncol. 2020, 10, 1488. [Google Scholar] [CrossRef]
- Parsons, P.; Gilbert, S.J.; Vaughan-Thomas, A.; Sorrell, D.A.; Notman, R.; Bishop, M.; Haynes, A.J.; Mason, D.J.; Duance, V.C. Type IX collagen interacts with fibronectin providing an important molecular bridge in articular cartilage. J. Biol. Chem. 2011, 286, 34986–34997. [Google Scholar] [CrossRef]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef]
- He, Y.; Siebuhr, A.S.; Brandt-Hansen, N.U.; Wang, J.; Su, D.; Zheng, Q.; Simonsen, O.; Petersen, K.K.; Arendt-Nielsen, L.; Eskehave, T.; et al. Type X collagen levels are elevated in serum from human osteoarthritis patients and associated with biomarkers of cartilage degradation and inflammation. BMC Musculoskelet. Disord. 2014, 15, 309. [Google Scholar] [CrossRef]
- Takroni, T.; Laouar, L.; Adesida, A.; Elliott, J.A.W.; Jomha, N.M. Anatomical study: Comparing the human, sheep and pig knee meniscus. J. Exp. Orthop. 2016, 3, 35. [Google Scholar] [CrossRef]
- Kaul, G.; Cucchiarini, M.; Remberger, K.; Kohn, D.; Madry, H. Failed cartilage repair for early osteoarthritis defects: A biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow-stimulation techniques. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Landmann, M.; Scheibner, D.; Graaf, A.; Gischke, M.; Koethe, S.; Fatola, O.I.; Raddatz, B.; Mettenleiter, T.C.; Beer, M.; Grund, C.; et al. A semiquantitative scoring system for histopathological and immunohistochemical assessment of lesions and tissue tropism in avian influenza. Viruses 2021, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.A. Quantification of immunohistochemistry—Issues concerning methods, utility and semiquantitative assessment I. Histopathology 2006, 49, 406–410. [Google Scholar] [CrossRef] [PubMed]
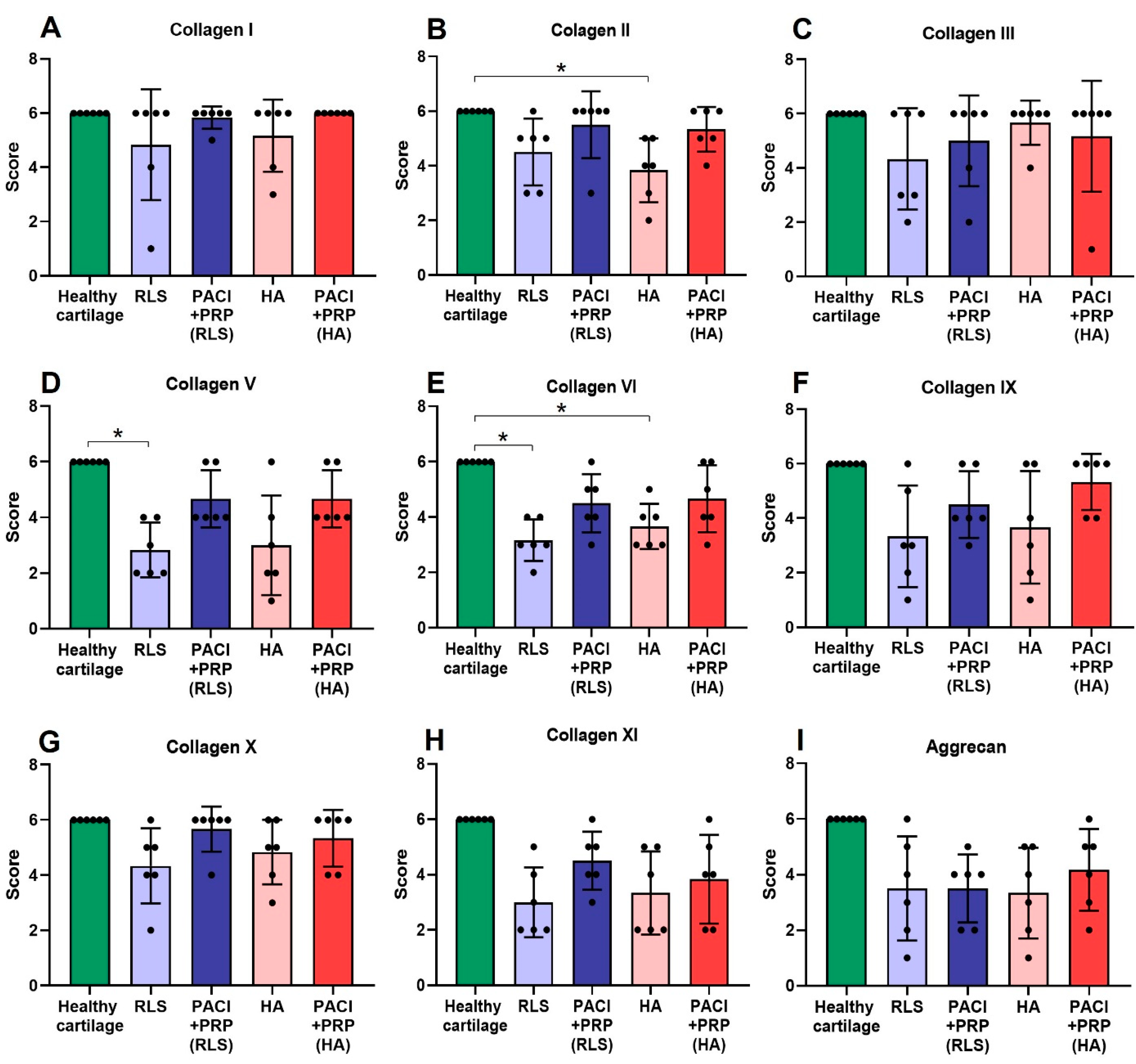

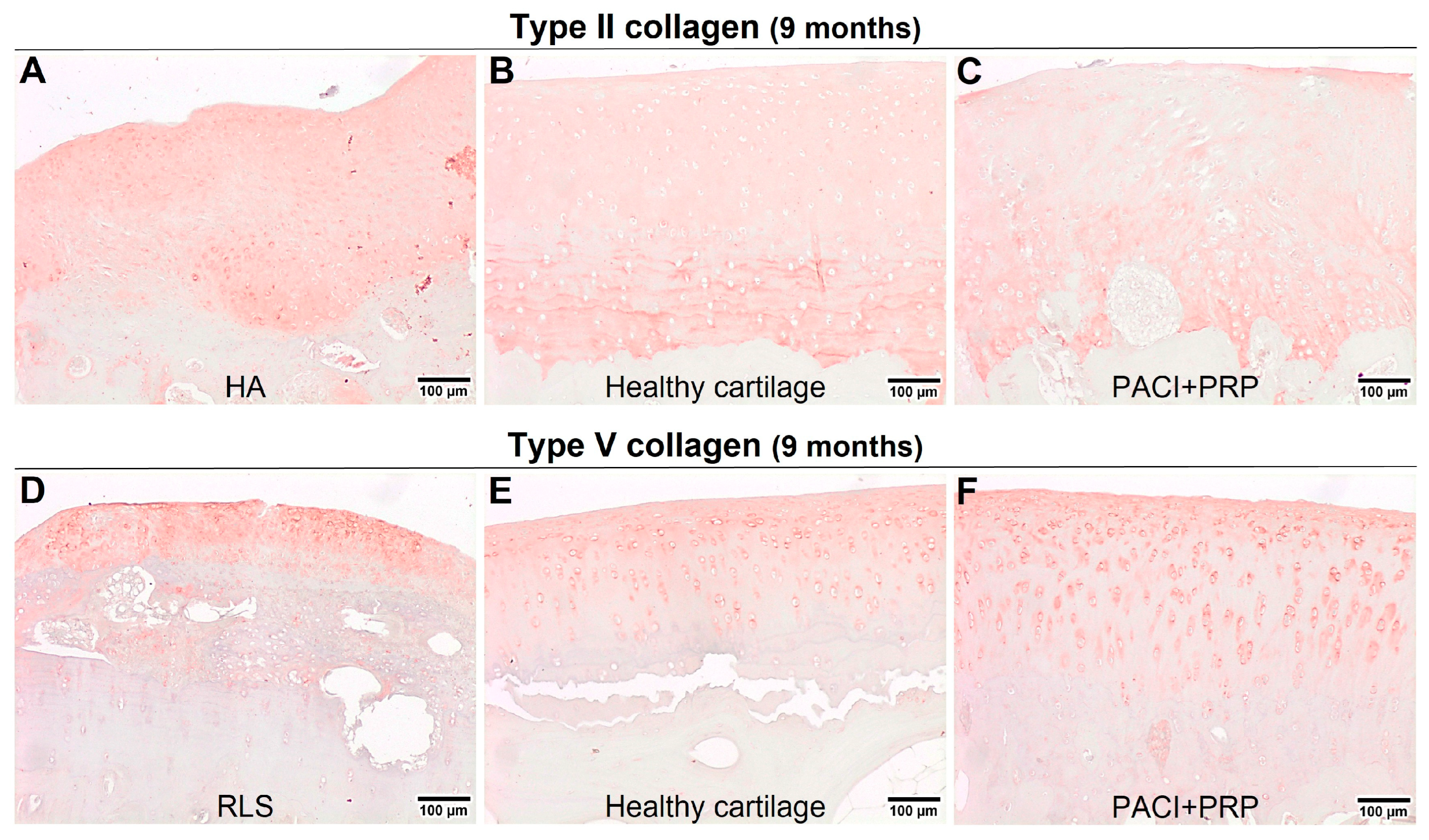


| Month | Variable | Group | ||||
|---|---|---|---|---|---|---|
| HC | RLS | PACI + PRP (RLS) | HA | PACI + PRP (HA) | ||
| 9 | Col I | 6 ± 0 | 6 ± 2.04 | 6 ± 0.41 | 6 ± 1.33 | 6 ± 0 |
| Col II | 6 ± 0 b | 5 ± 1.22 | 6 ± 1.22 | 4 ± 1.17 b | 5.50 ± 0.82 | |
| Col III | 6 ± 0 | 4.50 ± 1.86 | 6 ± 1.67 | 6 ± 0.82 | 6 ± 2.04 | |
| Col V | 6 ± 0 a | 2.50 ± 0.98 a | 4 ± 1.03 | 2.50 ± 1.79 | 4 ± 1.03 | |
| Col VI | 6 ± 0 a,b | 3 ± 0.75 a | 4.50 ± 1.05 | 3.50 ± 0.82 b | 4.50 ± 1.21 | |
| Col IX | 6 ± 0 | 3 ± 1.86 | 4 ± 1.22 | 3.50 ± 2.06 | 6 ± 1.03 | |
| Col X | 6 ± 0 | 4.50 ± 1.37 | 6 ± 0.82 | 5 ± 1.17 | 6 ± 1.03 | |
| Col XI | 6 ± 0 | 4 ± 1.17 | 4.50 ± 1.05 | 4.50 ± 1.67 | 4.50 ± 1.83 | |
| Aggrecan | 6 ± 0 | 3.50 ± 1.87 | 3 ± 1.76 | 3.50 ± 1.26 | 4.50 ± 1.21 | |
| 18 | Col I | 6 ± 0 | 6 ± 2.04 | 6 ± 0 | 6 ± 2.27 | 6 ± 0 |
| Col II | 6 ± 0 | 5 ± 1.03 | 6 ± 0.41 | 6 ± 1.25 | 6 ± 0.38 | |
| Col III | 6 ± 0 | 3.50 ± 1.67 | 5.50 ± 1.27 | 3 ± 2.34 | 6 ± 1.53 | |
| Col V | 6 ± 0 | 4.50 ± 0.82 | 6 ± 0.52 | 4 ± 1.90 | 6 ± 0.97 | |
| Col VI | 6 ± 0 a,b | 4 ± 0.41 a | 6 ± 1.33 | 3 ± 1.21 b | 5 ± 0.75 | |
| Col IX | 6 ± 0 b | 2 ± 2.34 | 4 ± 1.60 | 4 ± 1.99 b | 6 ± 0.75 | |
| Col X | 6 ± 0 a | 3 ± 1.03 a | 4.5 ± 1.21 | 5 ± 1.60 | 6 ± 0.53 | |
| Col XI | 6 ± 0 | 4.5 ± 1.05 | 5± 0.89 | 5 ± 0.90 | 5 ± 0.69 | |
| Aggrecan | 6 ± 0 | 4.5 ± 1.47 | 5 ± 0.75 | 5 ± 1.38 | 5 ± 0.82 | |
| Target | Source | Reference | Host/Clonality | Dilution | Host Species |
|---|---|---|---|---|---|
| Collagen I | Abcam φ | ab34710 | Rabbit polyclonal | 1/200 | Rabbit |
| Collagen II | Abcam φ | ab34712 | Rabbit polyclonal | 1/100 | Rabbit |
| Collagen III | Abcam φ | ab7778 | Rabbit polyclonal | 1/200 | Rabbit |
| Collagen V | Abcam φ | ab7046 | Rabbit polyclonal | 1/200 | Rabbit |
| Collagen VI | Abcam φ | ab6588 | Rabbit polyclonal | 1/1000 | Rabbit |
| Collagen IX | Abcam φ | ab134568 | Rabbit polyclonal | 1/200 | Rabbit |
| Collagen X | Abcam φ | ab49945 | Mouse monoclonal | 1/500 | Mouse |
| Collagen XI alpha 2 | Abcam φ | ab196613 | Rabbit polyclonal | 1/50 | Rabbit |
| Aggrecan | Abcam φ | ab3778 | Mouse monoclonal | 1/100 | Mouse |
| Parameters | Points | Final Score | |
|---|---|---|---|
| Immunostaining Pattern | Severely abnormal | 0 | 6 |
| Abnormal | 1 | ||
| Nearly normal | 2 | ||
| Normal | 3 | ||
| Immunostaining Intensity | Severely abnormal | 0 | |
| Abnormal | 1 | ||
| Nearly normal | 2 | ||
| Normal | 3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaide-Ruggiero, L.; Molina-Hernández, V.; Morgaz, J.; Fernández-Sarmiento, J.A.; Granados, M.M.; Navarrete-Calvo, R.; Pérez, J.; Quirós-Carmona, S.; Carrillo, J.M.; Cugat, R.; et al. Immunohistochemical Analysis of Knee Chondral Defect Repair after Autologous Particulated Cartilage and Platelet-Rich Plasma Treatment in Sheep. Int. J. Mol. Sci. 2023, 24, 15157. https://doi.org/10.3390/ijms242015157
Alcaide-Ruggiero L, Molina-Hernández V, Morgaz J, Fernández-Sarmiento JA, Granados MM, Navarrete-Calvo R, Pérez J, Quirós-Carmona S, Carrillo JM, Cugat R, et al. Immunohistochemical Analysis of Knee Chondral Defect Repair after Autologous Particulated Cartilage and Platelet-Rich Plasma Treatment in Sheep. International Journal of Molecular Sciences. 2023; 24(20):15157. https://doi.org/10.3390/ijms242015157
Chicago/Turabian StyleAlcaide-Ruggiero, Lourdes, Verónica Molina-Hernández, Juan Morgaz, J. Andrés Fernández-Sarmiento, María M. Granados, Rocío Navarrete-Calvo, José Pérez, Setefilla Quirós-Carmona, José M. Carrillo, Ramón Cugat, and et al. 2023. "Immunohistochemical Analysis of Knee Chondral Defect Repair after Autologous Particulated Cartilage and Platelet-Rich Plasma Treatment in Sheep" International Journal of Molecular Sciences 24, no. 20: 15157. https://doi.org/10.3390/ijms242015157





