Luminescence of In(III)Cl-etioporphyrin-I
Abstract
:1. Introduction
2. Results and Discussion
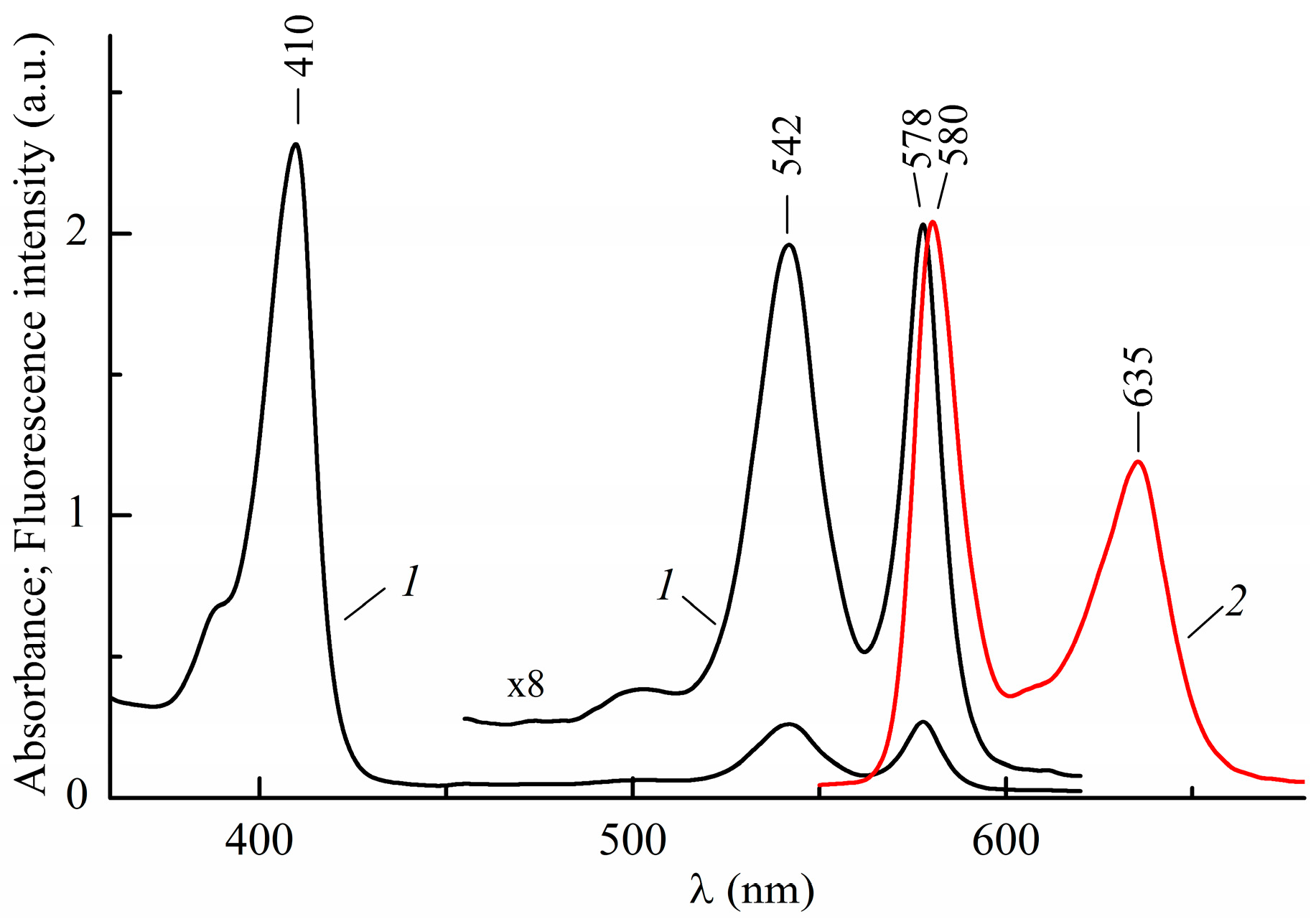
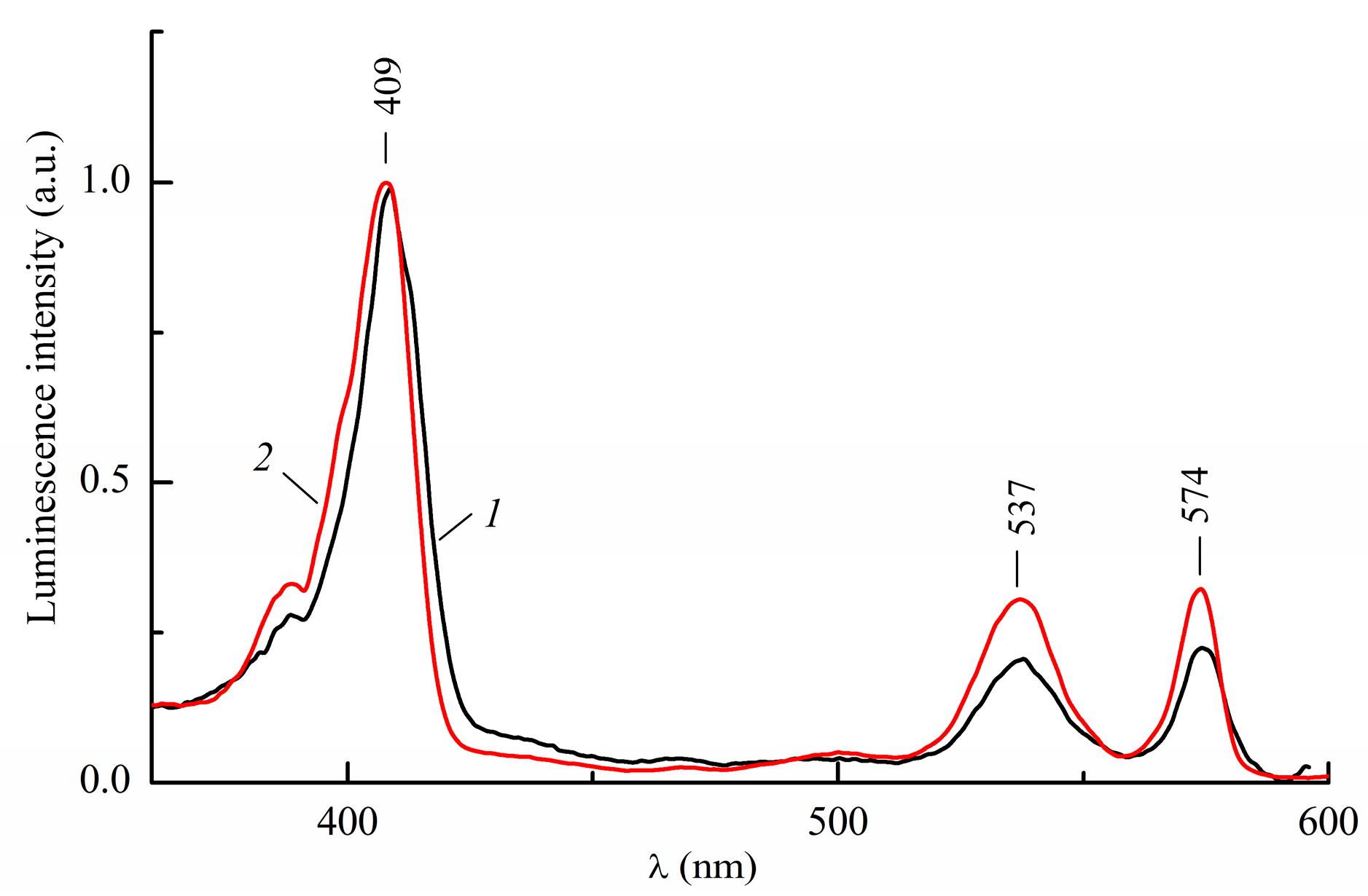
2.1. Absorption and Luminescence Spectra of InCl-EtioP-I
2.2. DFT Calculations
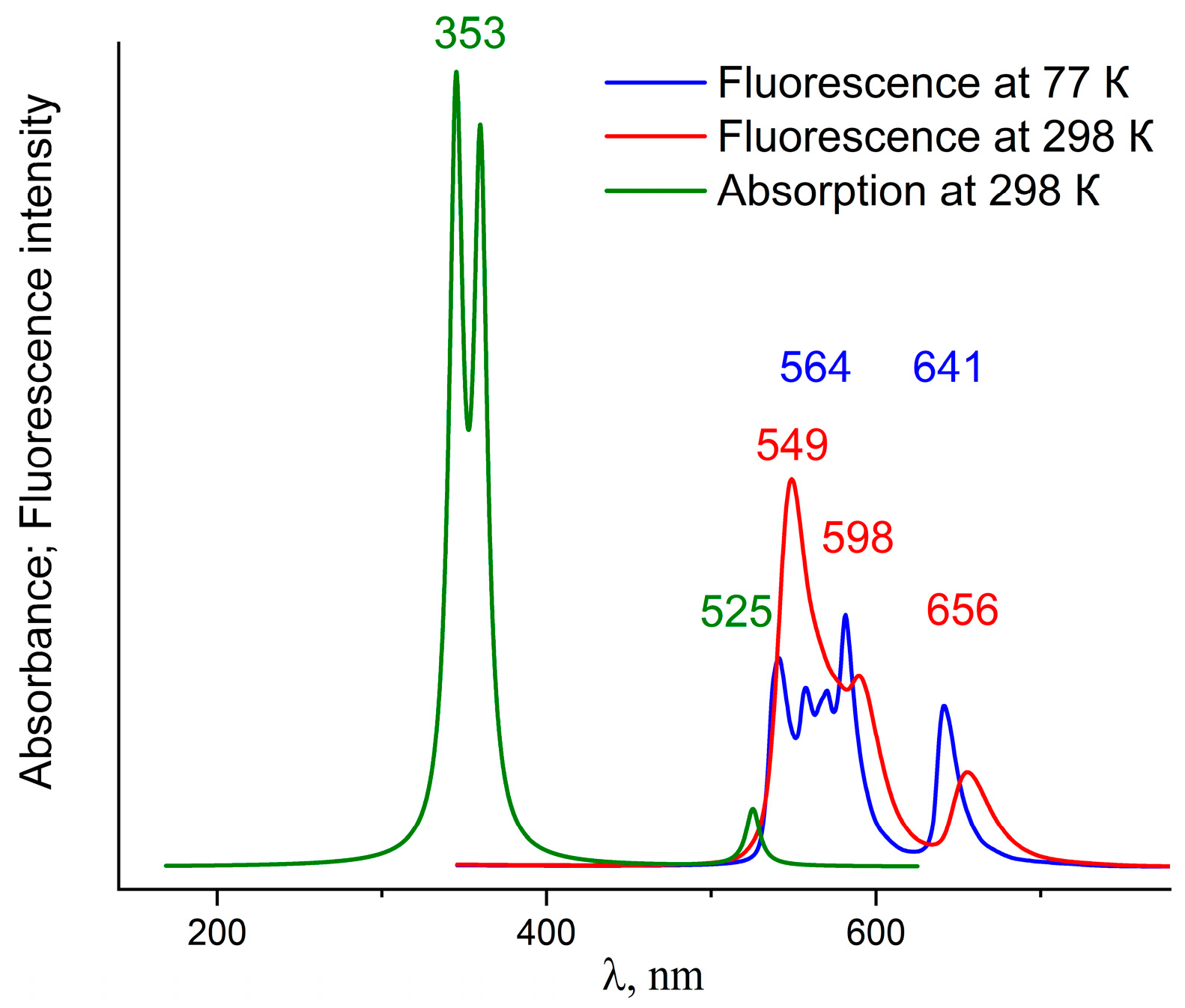
2.3. Photophysical Properties
3. Materials and Methods
3.1. Spectral Measurements
3.2. Photophysical Measurements
3.3. Quantum Chemistry
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dechan, P.; Bajju, G.D. Synthesis and spectroscopic properties of axial phenoxide and para amino phenoxide incorporated indium (III) porphyrins. J. Mol. Struct. 2019, 1195, 140–152. [Google Scholar] [CrossRef]
- Harriman, A. Luminescence of porphyrins and metalloporphyrins. Part 3.—Heavy-atom effects. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1981, 77, 1281–1291. [Google Scholar] [CrossRef]
- Tsvirko, M.P.; Solov’ev, K.N.; Gradyushko, A.T.; Dvornikov, S.S. The phosphorescence of etioporphyrin I and its complexes with light metals. J. Appl. Spectrosc. 1974, 20, 403–405. [Google Scholar] [CrossRef]
- Tsvirko, M.P.; Solov’ev, K.N. Photophysical processes in dimers of etioporphyrin and its metal complexes. J. Appl. Spectrosc. 1974, 20, 90–95. [Google Scholar] [CrossRef]
- Gradyushko, A.T.; Solov’ev, K.N.; Khokhlova, S.G. Quasiline luminescence spectra of etioporphyrin i metal complexes. J. Appl. Spectrosc. 1974, 21, 1034–1038. [Google Scholar] [CrossRef]
- Whitten, D.G.; Lopp, I.G.; Wildes, P.D. Fluorescence of zinc and magnesium etioporphyrin. I. Quenching and wavelength shifts due to complex formation. J. Am. Chem. Soc. 1968, 90, 7196–7200. [Google Scholar] [CrossRef]
- Eastwood, D.; Gouterman, M. Porphyrins. J. Mol. Spectrosc. 1970, 35, 359–375. [Google Scholar] [CrossRef]
- Wamser, C.C.; Ghosh, A. The Hyperporphyrin Concept: A Contemporary Perspective. JACS Au 2022, 2, 1543–1560. [Google Scholar] [CrossRef]
- Solov’ev, K.N.; Borisevich, E.A. Intramolecular heavy-atom effect in the photophysics of organic molecules. Phys.-Uspekhi 2005, 48, 231–253. [Google Scholar] [CrossRef]
- Koifman, O.; Rychikhina, E.; Yunin, P.; Koptyaev, A.; Sachkov, Y.; Pakhomov, G. Vacuum-deposited petroporphyrins: Effect of regioisomerism on film morphology. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129284. [Google Scholar] [CrossRef]
- Koifman, O.; Koptyaev, A.; Travkin, V.; Yunin, P.; Somov, N.; Masterov, D.; Pakhomov, G. Aggregation and Conductivity in Hot-Grown Petroporphyrin Films. Colloids Interfaces 2022, 6, 77. [Google Scholar] [CrossRef]
- Koifman, O.I.; Rychikhina, E.D.; Travkin, V.V.; Sachkov, Y.I.; Stuzhin, P.A.; Somov, N.V.; Yunin, P.A.; Zhabanov, Y.A.; Pakhomov, G.L. An Indium Synthetic Etioporphyrin for Organic Electronics: Aggregation and Photoconductivity in Thin Films. ChemPlusChem 2023, 88, e202300141. [Google Scholar] [CrossRef] [PubMed]
- Travkin, V.; Sachkov, Y.; Koptyaev, A.; Pakhomov, G. Isomer-dependent performance of thin-film solar cells based on petroporphyrins. Chem. Phys. 2023, 573, 112014. [Google Scholar] [CrossRef]
- Siebentritt, S.; Weiss, T.P.; Sood, M.; Wolter, M.H.; Lomuscio, A.; Ramirez, O. How photoluminescence can predict the efficiency of solar cells. J. Phys. Mater. 2021, 4, 042010. [Google Scholar] [CrossRef]
- Gouterman, M.; Khalil, G.E. Porphyrin free base phosphorescence. J. Mol. Spectrosc. 1974, 53, 88–100. [Google Scholar] [CrossRef]
- Gouterman, M.; Wagnière, G.H.; Snyder, L.C. Spectra of porphyrins. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Sundholm, D. Density functional theory study of the electronic absorption spectrum of Mg-porphyrin and Mg-etioporphyrin-I. Chem. Phys. Lett. 2000, 317, 392–399. [Google Scholar] [CrossRef]
- Arabei, S.M. Influence of temperature on the electronic spectra of tetrabenzoporphin in n-octane. J. Appl. Spectrosc. 1992, 57, 572–576. [Google Scholar] [CrossRef]
- Arabei, S.; Galaup, J.P.; Solovyov, K.; Donyagina, V. Fine-structure vibronic spectra and NH-phototautomerism in free-base unsubstituted 2,3-naphthalocyanine in naphthalene at 6 K. Chem. Phys. 2005, 311, 307–319. [Google Scholar] [CrossRef]
- Dvornikov, S.S.; Solov’ev, K.N.; Tsvirko, M.P.; Gradyushko, A.T. External effects of the heavy atom in the case of porphyrins and metalloporphyrins. J. Appl. Spectrosc. 1976, 25, 1522–1526. [Google Scholar] [CrossRef]
- Eroshin, A.V.; Otlyotov, A.A.; Kuzmin, I.A.; Stuzhin, P.A.; Zhabanov, Y.A. DFT Study of the Molecular and Electronic Structure of Metal-Free Tetrabenzoporphyrin and Its Metal Complexes with Zn, Cd, Al, Ga, In. Int. J. Mol. Sci. 2022, 23, 939. [Google Scholar] [CrossRef]
- Eroshin, A.V.; Koptyaev, A.I.; Otlyotov, A.A.; Minenkov, Y.; Zhabanov, Y.A. Iron(II) Complexes with Porphyrin and Tetrabenzoporphyrin: CASSCF/MCQDPT2 Study of the Electronic Structures and UV–Vis Spectra by sTD-DFT. Int. J. Mol. Sci. 2023, 24, 7070. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Classen, A.; Chochos, C.L.; Lüer, L.; Gregoriou, V.G.; Wortmann, J.; Osvet, A.; Forberich, K.; McCulloch, I.; Heumüller, T.; Brabec, C.J. The role of exciton lifetime for charge generation in organic solar cells at negligible energy-level offsets. Nat. Energy 2020, 5, 711–719. [Google Scholar] [CrossRef]
- Baldo, M.; Segal, M. Phosphorescence as a probe of exciton formation and energy transfer in organic light emitting diodes. Phys. Status Solidi (A) 2004, 201, 1205–1214. [Google Scholar] [CrossRef]
- Gradyushko, A.; Tsvirko, M. Probabilities of intercombination transitions in porphyrin and metalloporphyrin molecules. Opt. Spectrosc. (USSR) 1971, 31, 291–295. [Google Scholar]
- Pershukevich, P.P.; Volkovich, D.I.; Makarova, E.A.; Lukyanets, E.A.; Solovyov, K.N. The Luminescence of Pd and Pt Benzohydroporphyrazines in the Near-IR Range. Opt. Spectrosc. 2020, 128, 1789–1799. [Google Scholar] [CrossRef]
- Shushkevich, I.K.; Pershukevich, P.P.; Stupak, A.P.; Solov’ev, K.N. Influence of a Solvent on the Quantum Yield and Duration of the Fluorescence of Tetraazoporphin. J. Appl. Spectrosc. 2005, 72, 767–770. [Google Scholar] [CrossRef]
- Pershukevich, P.P.; Galievsky, V.A.; Stasheuski, A.S.; Makarova, E.A.; Luk’yanets, E.A.; Solovyov, K.N. Phosphorescence of palladium and platinum complexes of benzo-fused hydroporphyrazines. J. Appl. Spectrosc. 2011, 77, 790–801. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Metz, B.; Stoll, H.; Dolg, M. Small-core multiconfiguration-Dirac–Hartree–Fock-adjusted pseudopotentials for post-d main group elements: Application to PbH and PbO. J. Chem. Phys. 2000, 113, 2563–2569. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]



| Solvent | T, K. | τF, ns * | φF, % | IP/IF | φP, % | φΔ, % | τp, ms | |
|---|---|---|---|---|---|---|---|---|
| τ1, ns/B1, % | τ2, ns/B2, % | |||||||
| Toluene | 298 | 0.19/96.5 | 3.31/3.5 | 0.33 | - | - | 81 | - |
| toluene + diethyl ether (1:2) | 298 | 0.21/51.8 | 1.63/48.2 | 0.30 | - | - | 58 | - |
| 77 | 0.42/34.2 | 2.19/65.8 | 0.39 | 26.1 | 10.2 | - | 17.0 | |
| toluene + diethyl ether + CH3I (1:2:1) | 298 | 0.13/74.2 | 3.77/25.8 | 0.19 | - | - | 62 | - |
| 77 | 0.25/74.4 | 4.23/25.6 | 0.37 | 19.2 | 7.1 | - | 6.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koptyaev, A.I.; Zhabanov, Y.A.; Pakhomov, G.L.; Pershukevich, P.P.; Arabei, S.M.; Stuzhin, P.A. Luminescence of In(III)Cl-etioporphyrin-I. Int. J. Mol. Sci. 2023, 24, 15168. https://doi.org/10.3390/ijms242015168
Koptyaev AI, Zhabanov YA, Pakhomov GL, Pershukevich PP, Arabei SM, Stuzhin PA. Luminescence of In(III)Cl-etioporphyrin-I. International Journal of Molecular Sciences. 2023; 24(20):15168. https://doi.org/10.3390/ijms242015168
Chicago/Turabian StyleKoptyaev, Andrey I., Yuriy A. Zhabanov, Georgy L. Pakhomov, Piotr P. Pershukevich, Serguei M. Arabei, and Pavel A. Stuzhin. 2023. "Luminescence of In(III)Cl-etioporphyrin-I" International Journal of Molecular Sciences 24, no. 20: 15168. https://doi.org/10.3390/ijms242015168





