The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress
Abstract
1. Introduction
2. Crosstalk between MSCs and Hepatocytes in Close-to-Homeostasis Conditions
2.1. Chaperon-Like Functions of MSCs in Mixed 3D Cultures
2.2. Crosstalk of Hepatocytes and MSC in 2D Co-Culture
3. MSCs Communication with Hepatocytes in Stressful Environment
3.1. Anti-Apoptotic Effects of MSCs
3.1.1. Involvement of Growth Factors and Bioactive Molecules in the Anti-Apoptotic Effects of MSCs
3.1.2. Involvement of miRNAs in the Anti-Apoptotic Effects of MSCs
3.2. Regenerative Effects of MSCs
3.3. Anti-Ferroptosis and Anti-Pyroptosis Effects of MSC
3.4. MSCs Protection from Lypotoxicity and ER Stress
3.5. Mitochondrial Transfer and Antioxidant Effects of MSCs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N.; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Blachier, M.; Leleu, H.; Peck-Radosavljevic, M.; Valla, D.C.; Roudot-Thoraval, F. The burden of liver disease in Europe: A review of available epidemiological data. J. Hepatol. 2013, 58, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.; Bugianesi, E.; Pryke, R.; Hutchinson, S.J.; Sangro, B.; Martin, N.K.; et al. The EASL-Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022, 399, 61–116. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Fedeli, U.; Barbiellini Amidei, C.; Casotto, V.; Grande, E.; Saia, M.; Zanetto, A.; Russo, F.P. Mortality from chronic liver disease: Recent trends and impact of the COVID-19 pandemic. World J. Gastroenterol. 2023, 29, 4166–4173. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, I.V.; Yarygin, K.N. Cellular Mechanisms of Liver Regeneration and Cell-Based Therapies of Liver Diseases. Biomed. Res. Int. 2017, 2017, 8910821. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, I.V.; Kholodenko, R.V.; Lupatov, A.Y.; Yarygin, K.N. Cell Therapy as a Tool for Induction of Immunological Tolerance after Liver Transplantation. Bull. Exp. Biol. Med. 2018, 165, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Yi, H.; Zheng, J.; Cai, J.; Chen, W.; Lu, T.; Chen, L.; Du, C.; Liu, J.; et al. A novel MSC-based immune induction strategy for ABO-incompatible liver transplantation: A phase I/II randomized, open-label, controlled trial. Stem Cell Res. Ther. 2021, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Huang, Z.; Wei, W.; Li, Z. Mesenchymal Stem Cell Transplantation for Hepatitis B Virus-Related Acute-on-Chronic Liver Failure: A Systematic Review and Meta-Analysis. Curr. Stem Cell Res. Ther. 2023, 18, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Li, Y.Y.; Xu, R.N.; Meng, F.P.; Yu, S.J.; Fu, J.L.; Hu, J.H.; Li, J.X.; Wang, L.F.; Jin, L.; et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: A long-term follow-up analysis of the randomized controlled clinical trial. Hepatol. Int. 2021, 15, 1431–1441. [Google Scholar] [CrossRef]
- Schacher, F.C.; Martins Pezzi da Silva, A.; Silla, L.M.D.R.; Álvares-da-Silva, M.R. Bone Marrow Mesenchymal Stem Cells in Acute-on-Chronic Liver Failure Grades 2 and 3: A Phase I-II Randomized Clinical Trial. Can. J. Gastroenterol. Hepatol. 2021, 2021, 3662776. [Google Scholar] [CrossRef]
- Herrera, M.B.; Bruno, S.; Buttiglieri, S.; Tetta, C.; Gatti, S.; Deregibus, M.C.; Bussolati, B.; Camussi, G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells 2006, 24, 2840–2850. [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, I.V.; Kholodenko, R.V.; Manukyan, G.V.; Yarygin, K.N. Gepatogennaia differentsirovka stromal’nykh kletok vzrosloĭ i fetal’noĭ pecheni in vitro [The hepatic differentiation of adult and fetal liver stromal cells in vitro]. Biomed. Khim. 2016, 62, 674–682. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Kholodenko, I.V.; Gisina, A.M.; Manukyan, G.V.; Majouga, A.G.; Svirshchevskaya, E.V.; Kholodenko, R.V.; Yarygin, K.N. Resistance of Human Liver Mesenchymal Stem Cells to FAS-Induced Cell Death. Curr. Issues Mol. Biol. 2022, 44, 3428–3443. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kurbatov, L.K.; Kholodenko, R.V.; Manukyan, G.V.; Yarygin, K.N. Mesenchymal Stem Cells in the Adult Human Liver: Hype or Hope? Cells 2019, 8, 1127. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.; Kang, S.; Kim, J.; Lee, R.; So, S.; Yoon, Y.I.; Kirchner, V.A.; Song, G.W.; Hwang, S.; et al. Hepatogenic Potential and Liver Regeneration Effect of Human Liver-derived Mesenchymal-Like Stem Cells. Cells 2020, 9, 1521. [Google Scholar] [CrossRef]
- Yigitbilek, F.; Conley, S.M.; Tang, H.; Saadiq, I.M.; Jordan, K.L.; Lerman, L.O.; Taner, T. Comparable in vitro Function of Human Liver-Derived and Adipose Tissue-Derived Mesenchymal Stromal Cells: Implications for Cell-Based Therapy. Front. Cell Dev. Biol. 2021, 9, 641792. [Google Scholar] [CrossRef] [PubMed]
- Raicevic, G.; Najar, M.; Najimi, M.; El Taghdouini, A.; van Grunsven, L.A.; Sokal, E.; Toungouz, M. Influence of inflammation on the immunological profile of adult-derived human liver mesenchymal stromal cells and stellate cells. Cytotherapy 2015, 17, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Girousse, A.; Mathieu, M.; Sastourné-Arrey, Q.; Monferran, S.; Casteilla, L.; Sengenès, C. Endogenous Mobilization of Mesenchymal Stromal Cells: A Pathway for Interorgan Communication? Front. Cell Dev. Biol. 2021, 8, 598520. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Jing, Y.; Zhang, S.; Zong, C.; Jiang, J.; Sun, K.; Li, R.; Gao, L.; Zhao, X.; et al. Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis. Sci. Rep. 2015, 5, 17762. [Google Scholar] [CrossRef]
- Li, C.; Kong, Y.; Wang, H.; Wang, S.; Yu, H.; Liu, X.; Yang, L.; Jiang, X.; Li, L.; Li, L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 2009, 50, 1174–1183. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, L.X.; Shao, J.Z.; Pan, R.L.; Wang, Y.X.; Dong, X.J.; Zhang, G.R. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J. Cell. Mol. Med. 2010, 14, 1494–1508. [Google Scholar] [CrossRef] [PubMed]
- Skurikhin, E.G.; Zhukova, M.A.; Pan, E.S.; Ermakova, N.N.; Pershina, O.V.; Pakhomova, A.V.; Putrova, O.D.; Sandrikina, L.A.; Krupin, V.A.; Kogai, L.V.; et al. Age-Related Features of the Response of the Liver and Stem Cells during Modeling of Liver Cirrhosis. Bull. Exp. Biol. Med. 2021, 171, 127–133. [Google Scholar] [CrossRef]
- Fujii, H.; Hirose, T.; Oe, S.; Yasuchika, K.; Azuma, H.; Fujikawa, T.; Nagao, M.; Yamaoka, Y. Contribution of bone marrow cells to liver regeneration after partial hepatectomy in mice. J. Hepatol. 2002, 36, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Wang, Y.; Qi, L.; Williams, G.M.; Gao, B.; Song, G.; Burdick, J.F.; Sun, Z. Pharmacological Mobilization of Endogenous Bone Marrow Stem Cells Promotes Liver Regeneration after Extensive Liver Resection in Rats. Sci. Rep. 2018, 8, 3587. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Jiang, K.; Li, R.; Dong, C.; Wang, L. Multipotent mesenchymal stromal cells play critical roles in hepatocellular carcinoma initiation, progression and therapy. Mol. Cancer 2018, 17, 178. [Google Scholar] [CrossRef]
- Katagiri, H.; Kushida, Y.; Nojima, M.; Kuroda, Y.; Wakao, S.; Ishida, K.; Endo, F.; Kume, K.; Takahara, T.; Nitta, H.; et al. A Distinct Subpopulation of Bone Marrow Mesenchymal Stem Cells, Muse Cells, Directly Commit to the Replacement of Liver Components. Am. J. Transplant. 2016, 16, 468–483. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Battle, M.A.; Konopka, G.; Parviz, F.; Gaggl, A.L.; Yang, C.; Sladek, F.M.; Duncan, S.A. Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc. Natl. Acad. Sci. USA 2006, 103, 8419–8424. [Google Scholar] [CrossRef]
- Ober, E.A.; Lemaigre, F.P. Development of the liver: Insights into organ and tissue morphogenesis. J. Hepatol. 2018, 68, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Yoshitomi, H.; Rossant, J.; Zaret, K.S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001, 294, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, A.; D’Ambrosio, V.; Corno, S.; Pajno, C.; Carpino, G.; Amato, G.; Vena, F.; Mondo, A.; Spiniello, L.; Monti, M.; et al. Current protocols and clinical efficacy of human fetal liver cell therapy in patients with liver disease: A literature review. Cytotherapy 2022, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Yanagida, A.; Okada, K.; Yamazaki, Y.; Nakauchi, H.; Kamiya, A. Mesenchymal progenitor cells in mouse foetal liver regulate differentiation and proliferation of hepatoblasts. Liver Int. 2014, 34, 1378–1390. [Google Scholar] [CrossRef]
- Kamo, N.; Yasuchika, K.; Fujii, H.; Hoppo, T.; Machimoto, T.; Ishii, T.; Fujita, N.; Tsuruo, T.; Yamashita, J.K.; Kubo, H.; et al. Two populations of Thy1-positive mesenchymal cells regulate in vitro maturation of hepatic progenitor cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G526–G534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takagi, C.; Yagi, H.; Hieda, M.; Tajima, K.; Hibi, T.; Abe, Y.; Kitago, M.; Shinoda, M.; Itano, O.; Kitagawa, Y. Mesenchymal Stem Cells Contribute to Hepatic Maturation of Human Induced Pluripotent Stem Cells. Eur. Surg. Res. 2017, 58, 27–39. [Google Scholar] [CrossRef]
- He, Y.T.; Zhu, X.L.; Li, S.F.; Zhang, B.Q.; Li, Y.; Wu, Q.; Zhang, Y.L.; Zhou, Y.Y.; Li, L.; Qi, Y.N.; et al. Creating rat hepatocyte organoid as an in vitro model for drug testing. World. J. Stem Cells 2020, 12, 1184–1195. [Google Scholar] [CrossRef]
- Li, J.; Xing, F.; Chen, F.; He, L.; So, K.F.; Liu, Y.; Xiao, J. Functional 3D Human Liver Bud Assembled from MSC-Derived Multiple Liver Cell Lineages. Cell Transplant. 2019, 28, 510–521, Erratum in Cell Transplant. 2022, 31, 9636897221126859. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.T.U.H.; Dan, Y.Y.; Chan, Y.S.; Ng, H.H. Emerging liver organoid platforms and technologies. Cell Regen. 2021, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Prior, N.; Hindley, C.J.; Rost, F.; Meléndez, E.; Lau, W.W.Y.; Göttgens, B.; Rulands, S.; Simons, B.D.; Huch, M. Lgr5+ stem and progenitor cells reside at the apex of a heterogeneous embryonic hepatoblast pool. Development 2019, 146, dev174557. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19. [Google Scholar] [CrossRef]
- Peng, W.C.; Logan, C.Y.; Fish, M.; Anbarchian, T.; Aguisanda, F.; Álvarez-Varela, A.; Wu, P.; Jin, Y.; Zhu, J.; Li, B.; et al. Inflammatory Cytokine TNFα Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell 2018, 175, 1607–1619.e15. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Sullivan, K.M.; Baik, S.; Ko, E.; Kim, M.J.; Kim, Y.J.; Kong, H. Decellularized Matrix Produced by Mesenchymal Stem Cells Modulates Growth and Metabolic Activity of Hepatic Cell Cluster. ACS. Biomater. Sci. Eng. 2018, 4, 456–462. [Google Scholar] [CrossRef]
- Rebelo, S.P.; Costa, R.; Silva, M.M.; Marcelino, P.; Brito, C.; Alves, P.M. Three-dimensional co-culture of human hepatocytes and mesenchymal stem cells: Improved functionality in long-term bioreactor cultures. J. Tissue Eng. Regen. Med. 2017, 11, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Enomura, M.; Yoshizawa, E.; Kimura, M.; Koike, H.; Ueno, Y.; Matsuzaki, T.; Yamazaki, T.; Toyohara, T.; Osafune, K.; et al. Vascularized and Complex Organ Buds from Diverse Tissues via Mesenchymal Cell-Driven Condensation. Cell Stem Cell 2015, 16, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Bertet, C.; Sulak, L.; Lecuit, T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 2004, 429, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Shindo, A.; Wallingford, J.B. PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science 2014, 343, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Haga, H.; Koyama, Y.; Takahashi, M.; Kawabata, K. Diphosphorylation of the myosin regulatory light chain enhances the tension acting on stress fibers in fibroblasts. J. Cell. Physiol. 2006, 209, 726–731. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Kimura, M.; Yoshizawa, E.; Ayano, S.; Koido, M.; Funayama, S.; Nakanishi, N.; Hisai, T.; Kobayashi, T.; et al. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Rep. 2017, 21, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Asahina, K.; Tsai, S.Y.; Li, P.; Ishii, M.; Maxson, R.E., Jr.; Sucov, H.M.; Tsukamoto, H. Mesenchymal origin of hepatic stellate cells, submesothelial cells, and perivascular mesenchymal cells during mouse liver development. Hepatology 2009, 49, 998–1011. [Google Scholar] [CrossRef]
- Asahina, K.; Zhou, B.; Pu, W.T.; Tsukamoto, H. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 2011, 53, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Yagi, H.; Inomata, K.; Matsubara, K.; Hibi, T.; Abe, Y.; Kitago, M.; Shinoda, M.; Obara, H.; Itano, O.; et al. Mesenchymal stem cells support hepatocyte function in engineered liver grafts. Organogenesis 2014, 10, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Novoseletskaya, E.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Eremichev, R.; Milovskaya, I.; Kulebyakin, K.; Kulebyakina, M.; Rodionov, S.; Omelyanenko, N.; et al. Mesenchymal Stromal Cell-Produced Components of Extracellular Matrix Potentiate Multipotent Stem Cell Response to Differentiation Stimuli. Front. Cell Dev. Biol. 2020, 8, 555378. [Google Scholar] [CrossRef]
- Burk, J.; Sassmann, A.; Kasper, C.; Nimptsch, A.; Schubert, S. Extracellular Matrix Synthesis and Remodeling by Mesenchymal Stromal Cells Is Context-Sensitive. Int. J. Mol. Sci. 2022, 23, 1758. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Aihara, E.; Watson, C.; Mourya, R.; Mizuochi, T.; Shivakumar, P.; Phelan, K.; Mayhew, C.; Helmrath, M.; Takebe, T.; et al. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development 2017, 144, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Lo Nigro, A.; Gallo, A.; Bulati, M.; Vitale, G.; Paini, D.S.; Pampalone, M.; Galvagno, D.; Conaldi, P.G.; Miceli, V. Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration. Front. Med. 2021, 8, 746298. [Google Scholar] [CrossRef] [PubMed]
- Montanari, E.; Pimenta, J.; Szabó, L.; Noverraz, F.; Passemard, S.; Meier, R.P.H.; Meyer, J.; Sidibe, J.; Thomas, A.; Schuurman, H.J.; et al. Beneficial Effects of Human Mesenchymal Stromal Cells on Porcine Hepatocyte Viability and Albumin Secretion. J. Immunol. Res. 2018, 2018, 1078547. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aristizábal, A.; Keating, A.; Davies, J.E. Mesenchymal stromal cells as supportive cells for hepatocytes. Mol. Ther. 2009, 17, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.H.; Filippi, C.; Sun, S.; Lehec, S.; Dhawan, A.; Hughes, R.D. Hypoxic preconditioning potentiates the trophic effects of mesenchymal stem cells on co-cultured human primary hepatocytes. Stem Cell Res. Ther. 2015, 6, 237. [Google Scholar] [CrossRef]
- Tiegs, G.; Horst, A.K. TNF in the liver: Targeting a central player in inflammation. Semin. Immunopathol. 2022, 44, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Gu, J.Y.; Zhang, Y.; Han, B.; Xiao, J.Q.; Yuan, X.W.; Zhang, N.; Ding, Y.T. Protective effects of ACLF sera on metabolic functions and proliferation of hepatocytes co-cultured with bone marrow MSCs in vitro. World J. Gastroenterol. 2011, 17, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.P.; Bozyk, P.D.; Goldsmith, A.M.; Linn, M.J.; Lei, J.; Bentley, J.K.; Hershenson, M.B. Autocrine production of TGF-beta1 promotes myofibroblastic differentiation of neonatal lung mesenchymal stem cells. Am. J. Physiol. Lung. Cell Mol. Physiol. 2010, 298, L735–L743. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.K.; Albrecht, J.H. Regulation of the hepatocyte cell cycle by type I collagen matrix: Role of cyclin D1. J. Cell Sci. 1999, 112 Pt 17, 2971–2981. [Google Scholar] [CrossRef]
- Moghe, P.V.; Coger, R.N.; Toner, M.; Yarmush, M.L. Cell–cell interactions are essential for maintenance of hepatocyte function in collagen gel but not on matrigel. Biotechnol. Bioeng. 1997, 56, 706–711. [Google Scholar] [CrossRef]
- Gu, J.; Shi, X.; Zhang, Y.; Ding, Y. Heterotypic interactions in the preservation of morphology and functionality of porcine hepatocytes by bone marrow mesenchymal stem cells in vitro. J. Cell. Physiol. 2009, 219, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aristizábal, A.; Ng, C.; Ng, J.; Davies, J.E. Effects of two mesenchymal cell populations on hepatocytes and lymphocytes. Liver Transpl. 2012, 18, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Parekkadan, B.; Suganuma, K.; Soto-Gutierrez, A.; Tompkins, R.G.; Tilles, A.W.; Yarmush, M.L. Long-term superior performance of a stem cell/hepatocyte device for the treatment of acute liver failure. Tissue Eng. Part A 2009, 15, 3377–3388. [Google Scholar] [CrossRef] [PubMed]
- Azhdari Tafti, Z.; Mahmoodi, M.; Hajizadeh, M.R.; Ezzatizadeh, V.; Baharvand, H.; Vosough, M.; Piryaei, A. Conditioned Media Derived from Human Adipose Tissue Mesenchymal Stromal Cells Improves Primary Hepatocyte Maintenance. Cell J. 2018, 20, 377–387, Erratum in Cell J. 2021, 23, 143–144. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Wu, Y.; Dhadda, P.; Hughes, R.D.; Mitry, R.R.; Qin, H.; Lehec, S.C.; Heaton, N.D.; Dhawan, A. Coculture with mesenchymal stem cells results in improved viability and function of human hepatocytes. Cell Transplant. 2015, 24, 73–83. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kholodenko, R.V.; Majouga, A.G.; Yarygin, K.N. Apoptotic MSCs and MSC-Derived Apoptotic Bodies as New Therapeutic Tools. Curr. Issues Mol. Biol. 2022, 44, 5153–5172. [Google Scholar] [CrossRef]
- Fouraschen, S.M.; Pan, Q.; de Ruiter, P.E.; Farid, W.R.; Kazemier, G.; Kwekkeboom, J.; Ijzermans, J.N.; Metselaar, H.J.; Tilanus, H.W.; de Jonge, J.; et al. Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev. 2012, 21, 2410–2419. [Google Scholar] [CrossRef]
- Pinheiro, D.; Dias, I.; Freire, T.; Thole, A.A.; Stumbo, A.C.; Cortez, E.A.C.; de Carvalho, L.; de Carvalho, S.N. Effects of mesenchymal stem cells conditioned medium treatment in mice with cholestatic liver fibrosis. Life Sci. 2021, 281, 119768. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Yang, L.; Zhang, G.; Wang, Y.; Zhang, S. The Effects of Conditioned Medium Derived from Mesenchymal Stem Cells Cocultured with Hepatocytes on Damaged Hepatocytes and Acute Liver Failure in Rats. Stem Cells Int. 2018, 2018, 9156560. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Fan, X.; Wang, Y.; Shen, M.; Zheng, Y.; Zhao, S.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver Immunity and Therapy. Front. Immunol. 2022, 13, 833878. [Google Scholar] [CrossRef]
- Zhang, S.; Hou, Y.; Yang, J.; Xie, D.; Jiang, L.; Hu, H.; Hu, J.; Luo, C.; Zhang, Q. Application of mesenchymal stem cell exosomes and their drug-loading systems in acute liver failure. J. Cell. Mol. Med. 2020, 24, 7082–7093. [Google Scholar] [CrossRef]
- Hsu, M.J.; Karkossa, I.; Schäfer, I.; Christ, M.; Kühne, H.; Schubert, K.; Rolle-Kampczyk, U.E.; Kalkhof, S.; Nickel, S.; Seibel, P.; et al. Mitochondrial Transfer by Human Mesenchymal Stromal Cells Ameliorates Hepatocyte Lipid Load in a Mouse Model of NASH. Biomedicines 2020, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Lee, O.K. Exosomes from mesenchymal stem cells induce the conversion of hepatocytes into progenitor oval cells. Stem Cell Res. Ther. 2017, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Najimi, M.; Smets, F.; Sokal, E. Hepatocyte apoptosis. Methods Mol. Biol. 2009, 481, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.S.; Cortez-Pinto, H.; Sola, S.; Castro, R.E.; Ramalho, R.M.; Baptista, A.; Moura, M.C.; Camilo, M.E.; Rodrigues, C.M. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am. J. Gastroenterol. 2004, 99, 1708–1717. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Canbay, A.; Angulo, P.; Taniai, M.; Burgart, L.J.; Lindor, K.D.; Gores, G.J. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003, 125, 437–443. [Google Scholar] [CrossRef]
- Yoneyama, K.; Goto, T.; Miura, K.; Mikami, K.; Ohshima, S.; Nakane, K.; Lin, J.G.; Sugawara, M.; Nakamura, N.; Shirakawa, K.; et al. The expression of Fas and Fas ligand, and the effects of interferon in chronic liver diseases with hepatitis C virus. Hepatol. Res. 2002, 24, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.T.; Liu, W.; Wu, S.X.; He, Y.; Lin, Y.T.; Chen, W.N.; Lin, X.J.; Lin, X. Hepatitis B Virus Surface Antigen Enhances the Sensitivity of Hepatocytes to Fas-Mediated Apoptosis via Suppression of AKT Phosphorylation. J. Immunol. 2018, 201, 2303–2314, Erratum in J. Immunol. 2020, 205, 300–301. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Gores, G.J. Apoptosis: A mechanism of acute and chronic liver injury. Gut 2005, 54, 1024–1033. [Google Scholar] [CrossRef]
- Su, F.; Schneider, R.J. Hepatitis B virus HBX protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 1997, 94, 8744–8749. [Google Scholar] [CrossRef]
- Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014, 5, e996. [Google Scholar] [CrossRef] [PubMed]
- Woo, G.A.; O’Brien, C. Long-term management of alcoholic liver disease. Clin. Liver Dis. 2012, 16, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.K.; Yates, E.; Lilly, K.; Dhanda, A.D. Oxidative stress in alcohol-related liver disease. World J. Hepatol. 2020, 12, 332–349. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Y.J. Interference of Apoptosis by Hepatitis B Virus. Viruses 2017, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, J.; Zheng, S.; Zhang, K.; Ouyang, Y.; Zhang, Y.; Li, C.; Wu, D. Human umbilical cord mesenchymal stem cells ameliorate acute liver failure by inhibiting apoptosis, inflammation and pyroptosis. Ann. Transl. Med. 2021, 9, 1615. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Lu, T.; Zhang, Y.; Sui, X.; Li, Y.; Huang, X.; He, L.; Cai, J.; Zhou, C.; et al. MSCs ameliorate hepatocellular apoptosis mediated by PINK1-dependent mitophagy in liver ischemia/reperfusion injury through AMPKα activation. Cell Death Dis. 2020, 11, 256. [Google Scholar] [CrossRef]
- Quintanilha, L.F.; Takami, T.; Hirose, Y.; Fujisawa, K.; Murata, Y.; Yamamoto, N.; Goldenberg, R.C.; Terai, S.; Sakaida, I. Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol. Res. 2014, 44, E206–E217. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, H.; Wang, J.; Yang, F.; Li, J.; Zhou, Y.; Yuan, X.; Zhu, W.; Shi, X. Prostaglandin E(2) secreted by mesenchymal stem cells protects against acute liver failure via enhancing hepatocyte proliferation. FASEB J. 2019, 33, 2514–2525. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Y.; Pu, Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des. Devel. Ther. 2019, 13, 2887–2897. [Google Scholar] [CrossRef]
- Tan, C.Y.; Lai, R.C.; Wong, W.; Dan, Y.Y.; Lim, S.K.; Ho, H.K. Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res. Ther. 2014, 5, 76. [Google Scholar] [CrossRef]
- Ishida, K.; Seki, A.; Kawaguchi, K.; Nasti, A.; Yamato, M.; Inui, H.; Komura, T.; Yamashita, T.; Arai, K.; Yamashita, T.; et al. Restorative effect of adipose tissue-derived stem cells on impaired hepatocytes through Notch signaling in non-alcoholic steatohepatitis mice. Stem Cell Res. 2021, 54, 102425. [Google Scholar] [CrossRef]
- Feng, G.; Kaplowitz, N. Mechanism of staurosporine-induced apoptosis in murine hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G825–G834. [Google Scholar] [CrossRef]
- Allan, L.A.; Clarke, P.R. Apoptosis and autophagy: Regulation of caspase-9 by phosphorylation. FEBS J. 2009, 276, 6063–6073. [Google Scholar] [CrossRef]
- Hikita, H.; Takehara, T.; Kodama, T.; Shimizu, S.; Shigekawa, M.; Hosui, A.; Miyagi, T.; Tatsumi, T.; Ishida, H.; Li, W.; et al. Delayed-onset caspase-dependent massive hepatocyte apoptosis upon Fas activation in Bak/Bax-deficient mice. Hepatology 2011, 54, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Y.; He, X.; Kim, T.H.; Kuharsky, D.K.; Rabinowich, H.; Chen, J.; Du, C.; Yin, X.M. Relief of extrinsic pathway inhibition by the Bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. J. Biol. Chem. 2002, 277, 26912–26920. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Kojima, M.; Takeda, M.; Higashiyama, S.; Kawase, M.; Yagi, K. Maintenance of hepatocyte functions by coculture with bone marrow stromal cells. J. Biosci. Bioeng. 2004, 97, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, S.; Liu, T.; Liu, Y.; Wang, Y. Maintenance of rat hepatocytes under inflammation by coculture with human orbital fat-derived stem cells. Cell Mol. Biol. Lett. 2012, 17, 182–195. [Google Scholar] [CrossRef]
- De Bartolo, L.; Salerno, S.; Morelli, S.; Giorno, L.; Rende, M.; Memoli, B.; Procino, A.; Andreucci, V.E.; Bader, A.; Drioli, E. Long-term maintenance of human hepatocytes in oxygen-permeable membrane bioreactor. Biomaterials 2006, 27, 4794–4803. [Google Scholar] [CrossRef]
- Tiberio, L.; Tiberio, G.A.; Bardella, L.; Cervi, E.; Cerea, K.; Dreano, M.; Garotta, G.; Fra, A.; Montani, N.; Ferrari-Bravo, A.; et al. Mechanisms of interleukin-6 protection against ischemia-reperfusion injury in rat liver. Cytokine 2006, 34, 131–142. [Google Scholar] [CrossRef]
- Ren, X.; Hogaboam, C.; Carpenter, A.; Colletti, L. Stem cell factor restores hepatocyte proliferation in IL-6 knockout mice following 70% hepatectomy. J. Clin. Investig. 2003, 112, 1407–1418. [Google Scholar] [CrossRef]
- Sun, R.; Jaruga, B.; Kulkarni, S.; Sun, H.; Gao, B. IL-6 modulates hepatocyte proliferation via induction of HGF/p21cip1: Regulation by SOCS3. Biochem. Biophys. Res. Commun. 2005, 338, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef]
- Kishimoto, T. Interleukin-6: From basic science to medicine--40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef]
- Peters, M.; Blinn, G.; Solem, F.; Fischer, M.; Meyer zum Büschenfelde, K.H.; Rose-John, S. In vivo and in vitro activities of the gp130-stimulating designer cytokine Hyper-IL-6. J. Immunol. 1998, 161, 3575–3581. [Google Scholar] [CrossRef]
- Peters, M.; Schirmacher, P.; Goldschmitt, J.; Odenthal, M.; Peschel, C.; Fattori, E.; Ciliberto, G.; Dienes, H.P.; Meyer zum Büschenfelde, K.H.; Rose-John, S. Extramedullary expansion of hematopoietic progenitor cells in interleukin (IL)-6-sIL-6R double transgenic mice. J. Exp. Med. 1997, 185, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Schirmacher, P.; Peters, M.; Ciliberto, G.; Blessing, M.; Lotz, J.; Meyer zum Büschenfelde, K.H.; Rose-John, S. Hepatocellular hyperplasia, plasmacytoma formation, and extramedullary hematopoiesis in interleukin (IL)-6/soluble IL-6 receptor double-transgenic mice. Am. J. Pathol. 1998, 153, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cui, Y.; Wang, J.; Liu, D.; Tian, Y.; Liu, K.; Wang, X.; Liu, L.; He, Y.; Pei, Y.; et al. Mesenchymal stem cells protect against acetaminophen hepatotoxicity by secreting regenerative cytokine hepatocyte growth factor. Stem Cell Res. Ther. 2022, 13, 94. [Google Scholar] [CrossRef]
- Weiskirchen, R. Letter to the Editor: LO2, a misidentified cell line: Some data should be interpreted with caution. Hepatology 2023, 77, E66. [Google Scholar] [CrossRef]
- Lee, C.W.; Chen, Y.F.; Wu, H.H.; Lee, O.K. Historical perspectives and advances in mesenchymal stem cell research for the treatment of liver diseases. Gastroenterology 2018, 154, 46–56. [Google Scholar] [CrossRef]
- Van Poll, D.; Parekkadan, B.; Cho, C.H.; Berthiaume, F.; Nahmias, Y.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008, 47, 1634–1643. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakamura, T. Hepatocyte growth factor: Renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001, 59, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Molnarfi, N.; Benkhoucha, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte growth factor: A regulator of inflammation and autoimmunity. Autoimmun. Rev. 2015, 14, 293–303. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588–610. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Xu, X.; Liu, A.; He, H.; Xu, J.; Chen, Q.; Liu, S.; Liu, L.; Qiu, H.; et al. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res. Ther. 2016, 7, 66. [Google Scholar] [CrossRef]
- Chen, Q.H.; Wu, F.; Liu, L.; Chen, H.B.; Zheng, R.Q.; Wang, H.L.; Yu, L.N. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res. Ther. 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.F.; Jin, J.; Wang, H.; Wang, L.S.; Wu, C.T. Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings. J. Cell. Mol. Med. 2022, 26, 4745–4755. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, A.H.; Chen, N.; Pu, L.Y.; Fan, Y.; Lv, L.; Sun, B.C.; Li, G.Q.; Wang, X.H. Mesenchymal stem cells over-expressing hepatocyte growth factor improve small-for-size liver grafts regeneration. Mol. Ther. 2007, 15, 1382–1389. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, L.; Qian, X.; Chen, N.; Yao, A.; Pu, L.; Zhang, F.; Li, X.; Kong, L.; Sun, B.; et al. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2010, 19, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Sohn, S.Y.; Bhang, D.H.; Nam, M.J.; Lee, H.W.; Youn, H.Y. Therapeutic effects of hepatocyte growth factor-overexpressing human umbilical cord blood-derived mesenchymal stem cells on liver fibrosis in rats. Cell Biol. Int. 2014, 38, 106–116. [Google Scholar] [CrossRef]
- Moon, S.H.; Lee, C.M.; Park, S.H.; Jin, N.M. Effects of hepatocyte growth factor gene-transfected mesenchymal stem cells on dimethylnitrosamine-induced liver fibrosis in rats. Growth Factors 2019, 37, 105–119. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Zhou, Y.; Feng, F.; Wang, Q.; Zhu, X.; Ai, H.; Huang, X.; Zhang, X. Hepatocyte growth factor gene-modified adipose-derived mesenchymal stem cells ameliorate radiation induced liver damage in a rat model. PLoS ONE 2014, 9, e114670. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.D.; Kim, S.S.; Cha, H.Y.; Jang, S.H.; Chang, D.Y.; Kim, W.; Suh-Kim, H.; Lee, J.H. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp. Mol. Med. 2014, 46, e110. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Jo, J.; Tabata, Y. Liver anti-fibrosis therapy with mesenchymal stem cells secreting hepatocyte growth factor. J. Biomater. Sci. Polym. Ed. 2012, 23, 2259–2272. [Google Scholar] [CrossRef]
- Yin, F.; Wang, W.Y.; Mao, L.C.; Cai, Q.Q.; Jiang, W.H. Effect of human umbilical cord mesenchymal stem cells transfected with HGF on TGF-β1/Smad signaling pathway in carbon tetrachloride-induced liver fibrosis rats. Stem Cells Dev. 2020, 29, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Q.; Meng, F.; Huang, X.; Li, C.; Zhou, X.; Zeng, X.; He, Y.; Liu, J.; Hu, X.; et al. Therapeutic Potential of HGF-Expressing Human Umbilical Cord Mesenchymal Stem Cells in Mice with Acute Liver Failure. Int. J. Hepatol. 2016, 2016, 5452487. [Google Scholar] [CrossRef]
- Russell, J.O.; Camargo, F.D. Hippo signalling in the liver: Role in development, regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 297–312. [Google Scholar] [CrossRef]
- Pibiri, M.; Simbula, G. Role of the Hippo pathway in liver regeneration and repair: Recent advances. Inflamm. Regen. 2022, 42, 59. [Google Scholar] [CrossRef] [PubMed]
- Driskill, J.H.; Pan, D. The Hippo Pathway in Liver Homeostasis and Pathophysiology. Annu. Rev. Pathol. 2021, 16, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Yimlamai, D.; Fowl, B.H.; Camargo, F.D. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J. Hepatol. 2015, 63, 1491–1501. [Google Scholar] [CrossRef]
- Nishina, H. Physiological and pathological roles of the Hippo-YAP/TAZ signaling pathway in liver formation, homeostasis, and tumorigenesis. Cancer Sci. 2022, 113, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Gao, Y.; Qu, A.; Jiang, Y.; Li, H.; Xie, G.; Yao, X.; Yang, X.; Zhu, S.; Yagai, T.; et al. YAP-TEAD mediates PPAR α-induced hepatomegaly and liver regeneration in mice. Hepatology 2022, 75, 74–88. [Google Scholar] [CrossRef]
- Oh, S.H.; Swiderska-Syn, M.; Jewell, M.L.; Premont, R.T.; Diehl, A.M. Liver regeneration requires Yap1-TGFβ-dependent epithelial-mesenchymal transition in hepatocytes. J. Hepatol. 2018, 69, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, Q.; Li, R.; Zhang, J.; Ye, X.; Li, X. YAP1 protects against septic liver injury via ferroptosis resistance. Cell Biosci. 2022, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lu, Y.; Piao, W.; Jin, H. The Translational Regulation in mTOR Pathway. Biomolecules 2022, 12, 802. [Google Scholar] [CrossRef]
- Sun, E.J.; Wankell, M.; Palamuthusingam, P.; McFarlane, C.; Hebbard, L. Targeting the PI3K/Akt/mTOR Pathway in Hepatocellular Carcinoma. Biomedicines 2021, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Marcondes-de-Castro, I.A.; Reis-Barbosa, P.H.; Marinho, T.S.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. AMPK/mTOR pathway significance in healthy liver and non-alcoholic fatty liver disease and its progression. J. Gastroenterol. Hepatol. 2023, 13, 970292. [Google Scholar] [CrossRef] [PubMed]
- Honda, D.; Okumura, M.; Chihara, T. Crosstalk between the mTOR and Hippo pathways. Dev. Growth Differ. 2023, 65, 337–347. [Google Scholar] [CrossRef]
- Mahesh, G.; Anil Kumar, K.; Reddanna, P. Overview on the Discovery and Development of Anti-Inflammatory Drugs: Should the Focus Be on Synthesis or Degradation of PGE2? J. Inflamm. Res. 2021, 14, 253–263. [Google Scholar] [CrossRef]
- Nissim, S.; Sherwood, R.I.; Wucherpfennig, J.; Saunders, D.; Harris, J.M.; Esain, V.; Carroll, K.J.; Frechette, G.M.; Kim, A.J.; Hwang, K.L.; et al. Prostaglandin E2 regulates liver versus pancreas cell-fate decisions and endodermal outgrowth. Dev. Cell 2014, 28, 423–437. [Google Scholar] [CrossRef]
- Masaki, N.; Ohta, Y.; Shirataki, H.; Ogata, I.; Hayashi, S.; Yamada, S.; Hirata, K.; Nagoshi, S.; Mochida, S.; Tomiya, T.; et al. Hepatocyte membrane stabilization by prostaglandins E1 and E2: Favorable effects on rat liver injury. Gastroenterology 1992, 102, 572–576. [Google Scholar] [CrossRef]
- Wanner, G.A.; Müller, P.; Ertel, W.; Busch, C.J.; Menger, M.D.; Messmer, K. Differential effect of cyclooxygenase metabolites on proinflammatory cytokine release by Kupffer cells after liver ischemia and reperfusion. Am. J. Surg. 1998, 175, 146–151. [Google Scholar] [CrossRef]
- Arai, M.; Peng, X.X.; Currin, R.T.; Thurman, R.G.; Lemasters, J.J. Protection of sinusoidal endothelial cells against storage/reperfusion injury by prostaglandin E2 derived from Kupffer cells. Transplantation 1999, 68, 440–445. [Google Scholar] [CrossRef]
- Okumura, T.; Kanemaki, T.; Kitade, H. Stimulation of glucose incorporation into glycogen by E-series prostaglandins in cultured rat hepatocytes. Biochim. Biophys. Acta 1993, 1176, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kanemaki, T.; Kitade, H.; Hiramatsu, Y.; Kamiyama, Y.; Okumura, T. Stimulation of glycogen degradation by prostaglandin E2 in primary cultured rat hepatocytes. Prostaglandins 1993, 45, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Püschel, G.P.; Kirchner, C.; Schröder, A.; Jungermann, K. Glycogenolytic and antiglycogenolytic prostaglandin E2 actions in rat hepatocytes are mediated via different signalling pathways. Eur. J. Biochem. 1993, 218, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Püschel, G.P.; Jungermann, K. Integration of function in the hepatic acinus: Intercellular communication in neural and humoral control of liver metabolism. Prog. Liver Dis. 1994, 12, 19–46. [Google Scholar] [PubMed]
- Henkel, J.; Neuschäfer-Rube, F.; Pathe-Neuschäfer-Rube, A.; Püschel, G.P. Aggravation by prostaglandin E2 of interleukin-6-dependent insulin resistance in hepatocytes. Hepatology 2009, 50, 781–790. [Google Scholar] [CrossRef]
- Hespeling, U.; Jungermann, K.; Püschel, G.P. Feedback-inhibition of glucagon-stimulated glycogenolysis in hepatocyte/Kupffer cell cocultures by glucagon-elicited prostaglandin production in Kupffer cells. Hepatology 1995, 22, 1577–1583. [Google Scholar]
- Fennekohl, A.; Lucas, M.; Puschel, G.P. Induction by interleukin 6 of G(s)-coupled prostaglandin E(2) receptors in rat hepatocytes mediating a prostaglandin E(2)-dependent inhibition of the hepatocyte’s acute phase response. Hepatology 2000, 31, 1128–1134. [Google Scholar] [CrossRef]
- Pérez, S.; Aspichueta, P.; Ochoa, B.; Chico, Y. The 2-series prostaglandins suppress VLDL secretion in an inflammatory condition-dependent manner in primary rat hepatocytes. Biochim. Biophys. Acta 2006, 1761, 160–171. [Google Scholar] [CrossRef]
- Enomoto, N.; Ikejima, K.; Yamashina, S.; Enomoto, A.; Nishiura, T.; Nishimura, T.; Brenner, D.A.; Schemmer, P.; Bradford, B.U.; Rivera, C.A.; et al. Kupffer cell-derived prostaglandin E(2) is involved in alcohol-induced fat accumulation in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G100–G106. [Google Scholar] [CrossRef]
- Mater, M.K.; Thelen, A.P.; Jump, D.B. Arachidonic acid and PGE2 regulation of hepatic lipogenic gene expression. J. Lipid Res. 1999, 40, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, O.G.; Sparks, J.D.; Sparks, C.E.; Gibbons, G.F. Prostaglandins suppress VLDL secretion in primary rat hepatocyte cultures: Relationships to hepatic calcium metabolism. J. Lipid Res. 1992, 33, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.; Frede, K.; Schanze, N.; Vogel, H.; Schürmann, A.; Spruss, A.; Bergheim, I.; Püschel, G.P. Stimulation of fat accumulation in hepatocytes by PGE₂-dependent repression of hepatic lipolysis, β-oxidation and VLDL-synthesis. Lab. Investig. 2012, 92, 1597–1606. [Google Scholar] [CrossRef]
- Tsujii, H.; Okamoto, Y.; Kikuchi, E.; Matsumoto, M.; Nakano, H. Prostaglandin E2 and rat liver regeneration. Gastroenterology 1993, 105, 495–499. [Google Scholar] [CrossRef]
- Rudnick, D.A.; Perlmutter, D.H.; Muglia, L.J. Prostaglandins are required for CREB activation and cellular proliferation during liver regeneration. Proc. Natl. Acad. Sci. USA 2001, 98, 8885–8890. [Google Scholar] [CrossRef]
- Motiño, O.; Francés, D.E.; Casanova, N.; Fuertes-Agudo, M.; Cucarella, C.; Flores, J.M.; Vallejo-Cremades, M.T.; Olmedilla, L.; Pérez Peña, J.; Bañares, R.; et al. Protective Role of Hepatocyte Cyclooxygenase-2 Expression Against Liver Ischemia-Reperfusion Injury in Mice. Hepatology 2019, 70, 650–665. [Google Scholar] [CrossRef]
- Hashimoto, N.; Watanabe, T.; Ikeda, Y.; Yamada, H.; Taniguchi, S.; Mitsui, H.; Kurokawa, K. Prostaglandins induce proliferation of rat hepatocytes through a prostaglandin E2 receptor EP3 subtype. Am. J. Physiol. 1997, 272 Pt 1, G597–G604. [Google Scholar] [CrossRef]
- Refsnes, M.; Dajani, O.F.; Sandnes, D.; Thoresen, G.H.; Røttingen, J.A.; Iversen, J.G.; Christoffersen, T. On the mechanisms of the growth-promoting effect of prostaglandins in hepatocytes: The relationship between stimulation of DNA synthesis and signaling mediated by adenylyl cyclase and phosphoinositide-specific phospholipase C. J. Cell. Physiol. 1995, 164, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Refsnes, M.; Thoresen, G.H.; Dajani, O.F.; Christoffersen, T. Stimulation of hepatocyte DNA synthesis by prostaglandin E2 and prostaglandin F2 alpha: Additivity with the effect of norepinephrine, and synergism with epidermal growth factor. J. Cell. Physiol. 1994, 159, 35–40. [Google Scholar] [CrossRef]
- Dajani, O.F.; Meisdalen, K.; Guren, T.K.; Aasrum, M.; Tveteraas, I.H.; Lilleby, P.; Thoresen, G.H.; Sandnes, D.; Christoffersen, T. Prostaglandin E2 upregulates EGF-stimulated signaling in mitogenic pathways involving Akt and ERK in hepatocytes. J. Cell. Physiol. 2008, 214, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Meisdalen, K.; Dajani, O.F.; Christoffersen, T.; Sandnes, D. Prostaglandins enhance epidermal growth factor-induced DNA synthesis in hepatocytes by stimulation of E prostanoid 3 and F prostanoid receptors. J. Pharmacol. Exp. Ther. 2007, 322, 1044–1050. [Google Scholar] [CrossRef]
- Nishizawa, N.; Ito, Y.; Eshima, K.; Ohkubo, H.; Kojo, K.; Inoue, T.; Raouf, J.; Jakobsson, P.J.; Uematsu, S.; Akira, S.; et al. Inhibition of microsomal prostaglandin E synthase-1 facilitates liver repair after hepatic injury in mice. J. Hepatol. 2018, 69, 110–120. [Google Scholar] [CrossRef]
- Chan, P.C.; Hsiao, F.C.; Chang, H.M.; Wabitsch, M.; Hsieh, P.S. Importance of adipocyte cyclooxygenase-2 and prostaglandin E2-prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance. FASEB J. 2016, 30, 2282–2297. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.S.; Jin, J.S.; Chiang, C.F.; Chan, P.C.; Chen, C.H.; Shih, K.C. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity 2009, 17, 1150–1157. [Google Scholar] [CrossRef]
- Lan, Y.; Qian, B.; Huang, H.Y.; Wang, P.; Li, T.; Yuan, Q.; Zhang, H.Y.; Lin, Y.C.; Lin, Z.N. Hepatocyte-Derived Prostaglandin E2-Modulated Macrophage M1-Type Polarization via mTOR-NPC1 Axis-Regulated Cholesterol Transport from Lysosomes to the Endoplasmic Reticulum in Hepatitis B Virus x Protein-Related Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2022, 23, 11660. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Ding, H.; Shi, X.; Ren, H. Mesenchymal stem cell-secreted prostaglandin E2 ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res. Ther. 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Huang, H.; Guo, Z.; Chang, Y.; Li, Z. Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 2021, 11, 8836–8854. [Google Scholar] [CrossRef] [PubMed]
- Kweon, M.; Kim, J.Y.; Jun, J.H.; Kim, G.J. Research Trends in the Efficacy of Stem Cell Therapy for Hepatic Diseases Based on MicroRNA Profiling. Int. J. Mol. Sci. 2020, 22, 239. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, D.; Li, X.; Shi, Q.; Ju, X. Mesenchymal stem cell conditioned medium alleviates oxidative stress injury induced by hydrogen peroxide via regulating miR143 and its target protein in hepatocytes. BMC Immunol. 2017, 18, 51. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, X.; Wang, Y.; Tang, H.; Tong, D.; Ji, F. microRNA-143, down-regulated in osteosarcoma, promotes apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol. Rep. 2010, 24, 1363–1369. [Google Scholar] [CrossRef][Green Version]
- Zhou, P.; Chen, W.G.; Li, X.W. MicroRNA-143 acts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am. J. Cancer Res. 2015, 5, 2056–2063. [Google Scholar]
- DeWaal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat. Commun. 2018, 9, 446, Erratum in Nat. Commun. 2018, 9, 2539. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transpl. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, S.; Lin, C.; Cheng, X.; Meng, Z. Mesenchymal stem cell conditioned medium attenuates oxidative stress injury in hepatocytes partly by regulating the miR-486-5p/PIM1 axis and the TGF-β/Smad pathway. Bioengineered 2021, 12, 6434–6447. [Google Scholar] [CrossRef]
- Zhou, Q.; Rong, C.; Gu, T.; Li, H.; Wu, L.; Zhuansun, X.; Zhao, X.; Xiao, Z.; Kuang, Y.; Xu, S.; et al. Mesenchymal stem cells improve liver fibrosis and protect hepatocytes by promoting microRNA-148a-5p-mediated inhibition of Notch signaling pathway. Stem Cell Res. Ther. 2022, 13, 354. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, Z.; Pan, Y.; Zheng, W.; Li, W.; Zhang, Z.; Xiong, P.; Xu, D.; Du, M.; Wang, B.; et al. H19/miR-148a/USP4 axis facilitates liver fibrosis by enhancing TGF-β signaling in both hepatic stellate cells and hepatocytes. J. Cell. Physiol. 2019, 234, 9698–9710. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Li, X.; Lin, D.; Li, Z.; Wang, J.; Chen, J.; Gao, Z.; Lin, B. Bone marrow mesenchymal stem cell-derived small extracellular vesicles promote liver regeneration via miR-20a-5p/PTEN. Front. Pharmacol. 2023, 14, 1168545. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Wang, S.; Kim, J.; Kim, G.J.; Jung, Y. MicroRNA125b-mediated Hedgehog signaling influences liver regeneration by chorionic plate-derived mesenchymal stem cells. Sci. Rep. 2015, 5, 14135. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, Q.; Cai, X.; Li, F.; Ma, Z.; Xu, M.; Lu, L. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 2017, 21, 2491–2502. [Google Scholar] [CrossRef]
- Song, X.J.; Zhang, L.; Li, Q.; Li, Y.; Ding, F.H.; Li, X. hUCB-MSC derived exosomal miR-124 promotes rat liver regeneration after partial hepatectomy via downregulating Foxg1. Life Sci. 2021, 265, 118821. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, I.G.; Katselis, C.; Apostolou, K.; Feretis, T.; Lymperi, M.; Konstadoulakis, M.M.; Papalois, A.E.; Zografos, G.C. Mesenchymal Stem Cells Transplantation following Partial Hepatectomy: A New Concept to Promote Liver Regeneration-Systematic Review of the Literature Focused on Experimental Studies in Rodent Models. Stem Cells Int. 2017, 2017, 7567958. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, S.; Benzing, C.; Krenzien, F.; Splith, K.; Haber, P.K.; Arnold, A.; Nösser, M.; Kamali, C.; Hermann, F.; Günther, C.; et al. Human Stem Cells Promote Liver Regeneration After Partial Hepatectomy in BALB/C Nude Mice. J. Surg. Res. 2019, 239, 191–200. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wen, D.G.; Gong, J.P.; Liu, Z.J. Research Status of Mesenchymal Stem Cells in Liver Transplantation. Cell Transpl. 2019, 28, 1490–1506. [Google Scholar] [CrossRef]
- Owen, A.; Newsome, P.N. Mesenchymal Stromal Cells, a New Player in Reducing Complications From Liver Transplantation? Front. Immunol. 2020, 11, 1306. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, X.; Li, H.; Zheng, J.; Chen, X.; Liu, W.; Tai, Y.; Zhang, Y.; Wang, G.; Yang, Y. Mesenchymal Stem Cells Ameliorate Hepatic Ischemia/Reperfusion Injury via Inhibition of Neutrophil Recruitment. J. Immunol. Res. 2018, 2018, 7283703. [Google Scholar] [CrossRef] [PubMed]
- Saat, T.C.; van den Engel, S.; Bijman-Lachger, W.; Korevaar, S.S.; Hoogduijn, M.J.; IJzermans, J.N.; de Bruin, R.W. Fate and Effect of Intravenously Infused Mesenchymal Stem Cells in a Mouse Model of Hepatic Ischemia Reperfusion Injury and Resection. Stem Cells Int. 2016, 2016, 5761487. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.P.; Mahou, R.; Morel, P.; Meyer, J.; Montanari, E.; Muller, Y.D.; Christofilopoulos, P.; Wandrey, C.; Gonelle-Gispert, C.; Bühler, L.H. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol. 2015, 62, 634–641. [Google Scholar] [CrossRef]
- Xu, J.; Chen, P.; Yu, C.; Shi, Q.; Wei, S.; Li, Y.; Qi, H.; Cao, Q.; Guo, C.; Wu, X.; et al. Hypoxic bone marrow mesenchymal stromal cells-derived exosomal miR-182-5p promotes liver regeneration via FOXO1-mediated macrophage polarization. FASEB J. 2022, 36, e22553. [Google Scholar] [CrossRef] [PubMed]
- Vištejnová, L.; Liška, V.; Kumar, A.; Křečková, J.; Vyčítal, O.; Brůha, J.; Beneš, J.; Kolinko, Y.; Blassová, T.; Tonar, Z.; et al. Mesenchymal Stromal Cell Therapy in Novel Porcine Model of Diffuse Liver Damage Induced by Repeated Biliary Obstruction. Int. J. Mol. Sci. 2021, 22, 4304. [Google Scholar] [CrossRef]
- Van der Helm, D.; Barnhoorn, M.C.; de Jonge-Muller, E.S.M.; Molendijk, I.; Hawinkels, L.J.A.C.; Coenraad, M.J.; van Hoek, B.; Verspaget, H.W. Local but not systemic administration of mesenchymal stromal cells ameliorates fibrogenesis in regenerating livers. J. Cell. Mol. Med. 2019, 23, 6238–6250. [Google Scholar] [CrossRef]
- Ezquer, F.; Bahamonde, J.; Huang, Y.L.; Ezquer, M. Administration of multipotent mesenchymal stromal cells restores liver regeneration and improves liver function in obese mice with hepatic steatosis after partial hepatectomy. Stem Cell Res. Ther. 2017, 8, 20. [Google Scholar] [CrossRef]
- Kim, O.H.; Hong, H.E.; Seo, H.; Kwak, B.J.; Choi, H.J.; Kim, K.H.; Ahn, J.; Lee, S.C.; Kim, S.J. Generation of induced secretome from adipose-derived stem cells specialized for disease-specific treatment: An experimental mouse model. World J. Stem Cells 2020, 12, 70–86. [Google Scholar] [CrossRef]
- Qi, X.; Ng, K.T.; Lian, Q.; Li, C.X.; Geng, W.; Ling, C.C.; Yeung, W.H.; Ma, Y.Y.; Liu, X.B.; Liu, H.; et al. Glutathione Peroxidase 3 Delivered by hiPSC-MSCs Ameliorated Hepatic IR Injury via Inhibition of Hepatic Senescence. Theranostics 2018, 8, 212–222. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, S.; Kim, G.D.; Kim, J.Y.; Jun, J.H.; Bae, S.H.; Baik, S.K.; Hwang, S.G.; Kim, G.J. Increased Phosphatase of Regenerating Liver-1 by Placental Stem Cells Promotes Hepatic Regeneration in a Bile-Duct-Ligated Rat Model. Cells 2021, 10, 2530. [Google Scholar] [CrossRef]
- Zeng, Q.; Hong, W.; Tan, Y. Mouse PRL-2 and PRL-3, two potentially prenylated protein tyrosine phosphatases homologous to PRL-1. Biochem. Biophys. Res. Commun. 1998, 244, 421–427. [Google Scholar] [CrossRef]
- Jiao, Y.; Ye, D.Z.; Li, Z.; Teta-Bissett, M.; Peng, Y.; Taub, R.; Greenbaum, L.E.; Kaestner, K.H. Protein tyrosine phosphatase of liver regeneration-1 is required for normal timing of cell cycle progression during liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G85–G91. [Google Scholar] [CrossRef]
- Taub, R.; Greenbaum, L.E.; Peng, Y. Transcriptional regulatory signals define cytokine-dependent and -independent pathways in liver regeneration. Semin. Liver Dis. 1999, 19, 117–127. [Google Scholar] [CrossRef]
- Gungabeesoon, J.; Tremblay, M.L.; Uetani, N. Localizing PRL-2 expression and determining the effects of dietary Mg2+ on expression levels. Histochem. Cell Biol. 2016, 146, 99–111. [Google Scholar] [CrossRef]
- Uetani, N.; Hardy, S.; Gravel, S.P.; Kiessling, S.; Pietrobon, A.; Wong, N.N.; Chénard, V.; Cermakian, N.; St-Pierre, J.; Tremblay, M.L. PRL2 links magnesium flux and sex-dependent circadian metabolic rhythms. JCI Insight 2017, 2, e91722. [Google Scholar] [CrossRef]
- Hardy, S.; Kostantin, E.; Wang, S.J.; Hristova, T.; Galicia-Vázquez, G.; Baranov, P.V.; Pelletier, J.; Tremblay, M.L. Magnesium-sensitive upstream ORF controls PRL phosphatase expression to mediate energy metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 2925–2934. [Google Scholar] [CrossRef]
- Dumaual, C.M.; Sandusky, G.E.; Soo, H.W.; Werner, S.R.; Crowell, P.L.; Randall, S. Tissue-specific alterations of PRL-1 and PRL-2 expression in cancer. Am. J. Transl. Res. 2012, 4, 83–101. [Google Scholar]
- Liu, L.Z.; He, Y.Z.; Dong, P.P.; Ma, L.J.; Wang, Z.C.; Liu, X.Y.; Duan, M.; Yang, L.X.; Shi, J.Y.; Zhou, J.; et al. Protein tyrosine phosphatase PTP4A1 promotes proliferation and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma via the PI3K/AKT pathway. Oncotarget 2016, 7, 75210–75220. [Google Scholar] [CrossRef]
- Shinmei, S.; Sentani, K.; Hayashi, T.; Sakamoto, N.; Goto, K.; Oo, H.Z.; Naito, Y.; Teishima, J.; Matsubara, A.; Oue, N.; et al. Identification of PRL1 as a novel diagnostic and therapeutic target for castration-resistant prostate cancer by the Escherichia coli ampicillin secretion trap (CAST) method. Urol. Oncol. 2014, 32, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zeng, H.; Zhang, X.; Zhao, Y.; Sha, H.; Ge, X.; Zhang, M.; Gao, X.; Xu, Q. Phosphatase of regenerating liver-3 promotes motility and metastasis of mouse melanoma cells. Am. J. Pathol. 2004, 164, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.S.; Argani, P.; Cook, B.P.; Liangfeng, H.; Chartrand, S.D.; Zhang, M.; Saha, S.; Bardelli, A.; Jiang, Y.; St Martin, T.B.; et al. Alterations in vascular gene expression in invasive breast carcinoma. Cancer Res. 2004, 64, 7857–7866, Erratum in Cancer Res. 2004, 64, 8794. [Google Scholar] [CrossRef]
- Vandsemb, E.N.; Rye, M.B.; Steiro, I.J.; Elsaadi, S.; Rø, T.B.; Slørdahl, T.S.; Sponaas, A.M.; Børset, M.; Abdollahi, P. PRL-3 induces a positive signaling circuit between glycolysis and activation of STAT1/2. FEBS J. 2021, 288, 6700–6715. [Google Scholar] [CrossRef]
- Smith, C.N.; Blackburn, J.S. PRL-3 promotes a positive feedback loop between STAT1/2-induced gene expression and glycolysis in multiple myeloma. FEBS J. 2021, 288, 6674–6676. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, Y.; Liu, L.; Gao, Q.; Jin, S.; Lan, Q.; Lai, W.; Luo, X.; Wu, H.; Huang, Y.; et al. PRL-3 improves colorectal cancer cell proliferation and invasion through IL-8 mediated glycolysis metabolism. Int. J. Oncol. 2017, 51, 1271–1279. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, F.; Zhang, Z.-Y. PRL1 promotes cell migration and invasion by increasing MMP2 and MMP9 expression through Src and ERK1/2 pathways. Biochemistry 2009, 48, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jun, J.H.; Park, S.Y.; Yang, S.W.; Bae, S.H.; Kim, G.J. Dynamic regulation of miRNA expression by functionally enhanced placental mesenchymal stem cells promotes hepatic regeneration in a rat model with bile duct ligation. Int. J. Mol. Sci. 2019, 20, 5299. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, S.H.; Seok, J.; Bae, S.H.; Hwang, S.G.; Kim, G.J. Increased PRL-1 in BM-derived MSCs triggers anaerobic metabolism via mitochondria in a cholestatic rat model. Mol. Ther. Nucleic Acids 2023, 31, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, W.; Abdul Razak, S.R.; Han, T.; Ahmad, N.H.; Li, X. Ferroptosis as a potential target for cancer therapy. Cell Death Dis. 2023, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rodríguez, R.U.; Inzaugarat, M.E.; Ruiz-Margáin, A.; Nelson, L.J.; Trautwein, C.; Cubero, F.J. Reclassifying Hepatic Cell Death during Liver Damage: Ferroptosis-A Novel Form of Non-Apoptotic Cell Death? Int. J. Mol. Sci. 2020, 21, 1651. [Google Scholar] [CrossRef]
- Yamada, N.; Karasawa, T.; Kimura, H.; Watanabe, S.; Komada, T.; Kamata, R.; Sampilvanjil, A.; Ito, J.; Nakagawa, K.; Kuwata, H.; et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, H.; Wang, J.; Guo, X.; Lin, H.; Chen, H.; Jiang, C.; Chen, L.; Yao, P.; Tang, Y. Oxidative stress-dependent frataxin inhibition mediated alcoholic hepatocytotoxicity through ferroptosis. Toxicology 2020, 445, 152584. [Google Scholar] [CrossRef]
- Cheng, Z.; Chu, H.; Zhu, Q.; Yang, L. Ferroptosis in non-alcoholic liver disease: Molecular mechanisms and therapeutic implications. Front. Nutr. 2023, 10, 1090338. [Google Scholar] [CrossRef]
- Tsurusaki, S.; Tsuchiya, Y.; Koumura, T.; Nakasone, M.; Sakamoto, T.; Matsuoka, M.; Imai, H.; Yuet-Yin Kok, C.; Okochi, H.; Nakano, H.; et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019, 10, 449. [Google Scholar] [CrossRef]
- Wang, H.; An, P.; Xie, E.; Wu, Q.; Fang, X.; Gao, H.; Zhang, Z.; Li, Y.; Wang, X.; Zhang, J.; et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 2017, 66, 449–465. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chen, W.; Zhou, J.; Zhu, J.; Yao, Q.; Feng, B.; Feng, X.; Shi, X.; Pan, Q.; Yu, J.; et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. 2022, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jiang, L.; Tavana, O.; Gu, W. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 2019, 79, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wu, L.; Li, X.; Zheng, W.; Zuo, H.; Song, H. Exosomes derived from bone marrow mesenchymal stem cells alleviate biliary ischemia reperfusion injury in fatty liver transplantation by inhibiting ferroptosis. Mol. Cell Biochem. 2023. [Google Scholar] [CrossRef]
- Song, Y.; Wang, B.; Zhu, X.; Hu, J.; Sun, J.; Xuan, J.; Ge, Z. Human Umbilical Cord Blood-Derived MSCs Exosome Attenuate Myocardial Injury by Inhibiting Ferroptosis in Acute Myocardial Infarction Mice. Cell Biol. Toxicol. 2021, 37, 51–64. [Google Scholar] [CrossRef]
- Zhou, Z.; You, B.; Ji, C.; Zhang, L.; Wu, F.; Qian, H. Implications of Crosstalk between Exosome-Mediated Ferroptosis and Diseases for Pathogenesis and Treatment. Cells 2023, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, S.; Wan, B.; Velani, B.; Zhu, Y. Pyroptosis in Liver Disease: New Insights into Disease Mechanisms. Aging Dis. 2019, 10, 1094–1108. [Google Scholar] [CrossRef]
- Gan, C.; Cai, Q.; Tang, C.; Gao, J. Inflammasomes and Pyroptosis of Liver Cells in Liver Fibrosis. Front. Immunol. 2022, 13, 896473. [Google Scholar] [CrossRef]
- Gaul, S.; Leszczynska, A.; Alegre, F.; Kaufmann, B.; Johnson, C.D.; Adams, L.A.; Wree, A.; Damm, G.; Seehofer, D.; Calvente, C.J.; et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 2021, 74, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Z.; Xie, J.; Ji, W.; Cui, Y.; Ai, Z.; Liang, G. Inflammasome and pyroptosis in autoimmune liver diseases. Front. Immunol. 2023, 14, 1150879. [Google Scholar] [CrossRef]
- Yang, H.; Wang, J.; Liu, Z.G. Multi-faceted role of pyroptosis mediated by inflammasome in liver fibrosis. J. Cell. Mol. Med. 2022, 26, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xue, W.; Wei, H.; Fan, Q.; Li, X.; Qiu, Y.; Cui, D. Research Progress of Pyroptosis in Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 13065. [Google Scholar] [CrossRef] [PubMed]
- Beier, J.I.; Banales, J.M. Pyroptosis: An inflammatory link between NAFLD and NASH with potential therapeutic implications. J. Hepatol. 2018, 68, 643–645. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, K.; Zhang, Y.; He, J.; Ouyang, Y.; Lang, R.; Ao, C.; Jiang, Y.; Xiao, H.; Li, Y.; et al. Human Umbilical Cord Mesenchymal Stem Cells Inhibit Pyroptosis of Renal Tubular Epithelial Cells through miR-342-3p/Caspase1 Signaling Pathway in Diabetic Nephropathy. Stem Cells Int. 2023, 2023, 5584894. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y.; Du, Z.; Wu, T.; Yang, C. Hair follicle mesenchymal stem cell exosomal lncRNA H19 inhibited NLRP3 pyroptosis to promote diabetic mouse skin wound healing. Aging 2023, 15, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, H.; Yuan, X.; Ma, H.; Shi, X.; Ding, Y. Interleukin-10 secreted by mesenchymal stem cells attenuates acute liver failure through inhibiting pyroptosis. Hepatol. Res. 2018, 48, E194–E202. [Google Scholar] [CrossRef] [PubMed]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, Y.K.; Han, C.S.; Chen, L.J.; Wang, Y.M.; Zhuang, Z.M.; Lin, S.; Zhou, Y.H.; Jiang, J.H.; Yang, R.L. Stem Cells From Human Exfoliated Deciduous Teeth Alleviate Liver Cirrhosis via Inhibition of Gasdermin D-Executed Hepatocyte Pyroptosis. Front. Immunol. 2022, 13, 860225. [Google Scholar] [CrossRef]
- Li, L.; Zeng, X.; Liu, Z.; Chen, X.; Li, L.; Luo, R.; Liu, X.; Zhang, J.; Liu, J.; Lu, Y.; et al. Mesenchymal stromal cells protect hepatocytes from lipotoxicity through alleviation of endoplasmic reticulum stress by restoring SERCA activity. J. Cell. Mol. Med. 2021, 25, 2976–2993. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Hua, S.; Li, W.; Han, J.; Li, F.; Chen, H.; Zhang, Z.; Xie, Y.; Ouyang, Q.; Zou, X.; et al. Mesenchymal stem cells protect against TBI-induced pyroptosis in vivo and in vitro through TSG-6. Cell Commun. Signal. 2022, 20, 125. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, Y.; Jiang, Y.; Zhao, L.; Lv, C.; Huang, Q.; Guan, J.; Jin, S. From Hair to Colon: Hair Follicle-Derived MSCs Alleviate Pyroptosis in DSS-Induced Ulcerative Colitis by Releasing Exosomes in a Paracrine Manner. Oxid. Med. Cell Longev. 2022, 2022, 9097530. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Yao, R.; Li, Y.; Wei, Y.; Cao, Y.; Zhang, Z.; Wu, M.; Zhu, H.; Yao, Y.; et al. The role of mesenchymal stem cell-derived extracellular vesicles in inflammation-associated programmed cell death. Nano Today 2023, 50, 101865. [Google Scholar] [CrossRef]
- Lee, K.; Kerner, J.; Hoppel, C.L. Mitochondrial carnitine palmitoyltransferase 1a (CPT1a) is part of an outer membrane fatty acid transfer complex. J. Biol. Chem. 2011, 286, 25655–25662. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Fujita, T.; Usuda, N.; Cook, W.; Qi, C.; Peters, J.M.; Gonzalez, F.J.; Yeldandi, A.V.; Rao, M.S.; Reddy, J.K. Peroxisomal and mitochondrial fatty acid beta-oxidation in mice nullizygous for both peroxisome proliferator-activated receptor alpha and peroxisomal fatty acyl-CoA oxidase. Genotype correlation with fatty liver phenotype. J. Biol. Chem. 1999, 274, 19228–19236. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Vajreswari, A. Stearoyl-CoA desaturase 1: A potential target for non-alcoholic fatty liver disease-perspective on emerging experimental evidence. World. J. Hepatol. 2022, 14, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.D.; Lawrence, R.T.; Healy, M.E.; Dominy, J.E.; Liao, J.A.; Breen, D.S.; Byrne, F.L.; Kenwood, B.M.; Lackner, C.; Okutsu, S.; et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol. Metab. 2014, 3, 419–431. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.H.; Kohli, R.; Gores, G.J. Mechanisms of lipotoxicity in NAFLD and clinical implications. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, D.A.; Davidson, N.O. Functional Relationships between Lipid Metabolism and Liver Regeneration. Int. J. Hepatol. 2012, 2012, 549241. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Pagliassotti, M.J.; Kim, P.Y.; Estrada, A.L.; Stewart, C.M.; Gentile, C.L. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metab. Clin. Exp. 2016, 65, 1238–1246. [Google Scholar] [CrossRef]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Comm. 2015, 460, 114–121. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Jiang, S.; Yu, H.; An, W. Enhanced endoplasmic reticulum SERCA activity by overexpression of hepatic stimulator substance gene prevents hepatic cells from ER stress-induced apoptosis. Am. J. Physiol. Cell Physiol. 2014, 306, C279–C290. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Tang, C.; Yang, X.; Yu, N.; Song, Y.; Ding, W.; Sun, Y.; Yan, R.; Wang, S.; Li, X.; et al. Integrated metabolomics and phosphoproteomics reveal the protective role of exosomes from human umbilical cord mesenchymal stem cells in naturally aging mouse livers. Exp. Cell Res. 2023, 427, 113566. [Google Scholar] [CrossRef]
- Law, B.A.; Liao, X.; Moore, K.S.; Southard, A.; Roddy, P.; Ji, R.; Szulc, Z.; Bielawska, A.; Schulze, P.C.; Cowart, L.A. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018, 32, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Kemper, J.K.; Choi, S.E.; Kim, D.H. Sirtuin 1 deacetylase: A key regulator of hepatic lipid metabolism. Vitam. Horm. 2013, 91, 385–404. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cui, Y.; Song, J.; Cui, C.; Wang, L.; Liang, K.; Wang, C.; Sha, S.; He, Q.; Hu, H.; et al. Mesenchymal stem cell-conditioned medium improved mitochondrial function and alleviated inflammation and apoptosis in non-alcoholic fatty liver disease by regulating SIRT1. Biochem. Biophys. Res. Commun. 2021, 546, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Giles, A.; Nakamura, K.; Lee, J.W.; Hou, X.; Donmez, G.; Li, J.; Luo, Z.; Walsh, K.; et al. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011, 25, 1664–1679. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Fiore, D.; Basciani, S.; Persichetti, A.; Contini, S.; Lubrano, C.; Salvatori, L.; Lenzi, A.; Gnessi, L. Plasma levels of SIRT1 associate with non-alcoholic fatty liver disease in obese patients. Endocrine 2015, 49, 711–716. [Google Scholar] [CrossRef]
- Niu, Q.; Wang, T.; Wang, Z.; Wang, F.; Huang, D.; Sun, H.; Liu, H. Adipose-derived mesenchymal stem cell-secreted extracellular vesicles alleviate non-alcoholic fatty liver disease via delivering miR-223-3p. Adipocyte 2022, 11, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Johnnidis, J.B.; Harris, M.H.; Wheeler, R.T.; Stehling-Sun, S.; Lam, M.H.; Kirak, O.; Brummelkamp, T.R.; Fleming, M.D.; Camargo, F.D. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature 2008, 451, 1125–9112. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, T.; Lou, G.; Liu, Y. Role of miR-223 in the pathophysiology of liver diseases. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Calvente, C.; Del Pilar, H.; Tameda, M.; Johnson, C.D.; Feldstein, A.E. MicroRNA 223 3p Negatively Regulates the NLRP3 Inflammasome in Acute and Chronic Liver Injury. Mol. Ther. 2020, 28, 653–663. [Google Scholar] [CrossRef]
- Lu, F.B.; Chen, D.Z.; Chen, L.; Hu, E.D.; Wu, J.L.; Li, H.; Gong, Y.W.; Lin, Z.; Wang, X.D.; Li, J.; et al. Attenuation of Experimental Autoimmune Hepatitis in Mice with Bone Mesenchymal Stem Cell-Derived Exosomes Carrying MicroRNA-223-3p. Mol. Cells 2019, 42, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Ansar Ahmed, S. Chapter 9—MicroRNA, an Important Epigenetic Regulator of Immunity and Autoimmunity. In Translating MicroRNAs to the Clinic; Laurence, J., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 223–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, N.; Xu, J.; Kong, B.; Copple, B.; Guo, G.L.; Wang, L. E2F1 is a novel fibrogenic gene that regulates cholestatic liver fibrosis through the Egr-1/SHP/EID1 network. Hepatology 2014, 60, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Ding, H.R.; Pan, C.Y.; Shi, X.L.; Ren, H.Z. Mesenchymal stem cells ameliorate lipid metabolism through reducing mitochondrial damage of hepatocytes in the treatment of post-hepatectomy liver failure. Cell Death Dis. 2021, 12, 111. [Google Scholar] [CrossRef]
- Acosta, J.R.; Tavira, B.; Douagi, I.; Kulyté, A.; Arner, P.; Rydén, M.; Laurencikiene, J. Human-Specific Function of IL-10 in Adipose Tissue Linked to Insulin Resistance. J. Clin. Endocrinol. Metab. 2019, 104, 4552–4562. [Google Scholar] [CrossRef]
- Kalkunte, S.; Nevers, T.; Norris, W.E.; Sharma, S. Vascular IL-10: A protective role in preeclampsia. J. Reprod. Immunol. 2011, 88, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kitamoto, S.; Lian, Q.; Boisvert, W.A. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J. Biol. Chem. 2009, 284, 32950–32958. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Leung, J.C.K.; Chan, L.Y.Y.; Yiu, W.H.; Li, Y.; Lok, S.W.Y.; Liu, W.H.; Chan, K.W.; Tse, H.F.; Lai, K.N.; et al. Amelioration of Endoplasmic Reticulum Stress by Mesenchymal Stem Cells via Hepatocyte Growth Factor/c-Met Signaling in Obesity-Associated Kidney Injury. Stem Cells Transl. Med. 2019, 8, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Li, L.; Liu, X.; Yuan, Y.; Zhu, W.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Mesenchymal stem cells alleviate palmitic acid-induced endothelial-to-mesenchymal transition by suppressing endoplasmic reticulum stress. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E961–E980. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.; Huang, Y.L.; Ezquer, M. New Perspectives to Improve Mesenchymal Stem Cell Therapies for Drug-Induced Liver Injury. Int. J. Mol. Sci. 2022, 23, 2669. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Tsuchiya, A.; Ogawa, M.; Nojiri, S.; Takeuchi, S.; Watanabe, T.; Nakajima, K.; Hara, Y.; Yamashita, J.; Kikuta, J.; et al. Mesenchymal stem cells cultured under hypoxic conditions had a greater therapeutic effect on mice with liver cirrhosis compared to those cultured under normal oxygen conditions. Regen. Ther. 2019, 11, 269–281. [Google Scholar] [CrossRef]
- Yu, J.; Yin, S.; Zhang, W.; Gao, F.; Liu, Y.; Chen, Z.; Zhang, M.; He, J.; Zheng, S. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res. Ther. 2013, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.J. Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome. Stem Cell Res. Ther. 2015, 6, 75. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Z.; Wang, L.; Bian, Y.; Li, W.; Zhou, H.; Chu, X.; Zhao, Q. Icariin-treated human umbilical cord mesenchymal stem cells decrease chronic liver injury in mice. Cytotechnology 2017, 69, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Tilokani, L.; Nagashima, S.; Paupe, V.; Prudent, J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018, 62, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730, Erratum in Nat. Rev. Mol. Cell Biol. 2018, 19, 746. [Google Scholar] [CrossRef]
- Ramanathan, R.; Ibdah, J.A. Mitochondrial Dysfunction and Acute Fatty Liver of Pregnancy. Int. J. Mol. Sci. 2022, 23, 3595. [Google Scholar] [CrossRef] [PubMed]
- Morio, B.; Panthu, B.; Bassot, A.; Rieusset, J. Role of mitochondria in liver metabolic health and diseases. Cell Calcium 2021, 94, 102336. [Google Scholar] [CrossRef] [PubMed]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial cholesterol accumulation in alcoholic liver disease: Role of ASMase and endoplasmic reticulum stress. Redox Biol. 2014, 3, 100–108. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Tsai, P.J.; Chen, P.H.; Ye, M.; Guo, J.; Su, Z. Mutual interaction between endoplasmic reticulum and mitochondria in nonalcoholic fatty liver disease. Lipids Health Dis. 2020, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Zhang, S.; Li, Y.; Wang, Y.; Peppelenbosch, M.P.; Pan, Q. Mitochondria in the biology, pathogenesis, and treatment of hepatitis virus infections. Rev. Med. Virol. 2019, 29, e2075. [Google Scholar] [CrossRef]
- Mansouri, A.; Gattolliat, C.H.; Asselah, T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology 2018, 155, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Li, D.; Chen, T.; Tian, G.; Zhou, P.; Ju, X. Umbilical Cord MSCs Reverse D-Galactose-Induced Hepatic Mitochondrial Dysfunction via Activation of Nrf2/HO-1 Pathway. Biol. Pharm. Bull. 2017, 40, 1174–1182. [Google Scholar] [CrossRef]
- Cho, K.A.; Woo, S.Y.; Seoh, J.Y.; Han, H.S.; Ryu, K.H. Mesenchymal stem cells restore CCl4-induced liver injury by an antioxidative process. Cell Biol. Int. 2012, 36, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019, 33, 1695–1710. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Tan, Y.; Zou, S.; Zhang, H.; Mao, F.; Gong, A.; Qian, H.; Xu, W. hucMSC Exosome-Derived GPX1 Is Required for the Recovery of Hepatic Oxidant Injury. Mol. Ther. 2017, 25, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Court, A.C.; Le-Gatt, A.; Luz-Crawford, P.; Parra, E.; Aliaga-Tobar, V.; Bátiz, L.F.; Contreras, R.A.; Ortúzar, M.I.; Kurte, M.; Elizondo-Vega, R.; et al. Mitochondrial transfer from MSCs to T cells induces Treg diferentiation and restricts infammatory response. EMBO Rep. 2020, 21, e48052. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Gao, F.; Zhang, Y.; Wong, D.S.; Li, Q.; Tse, H.F.; Xu, G.; Yu, Z.; Lian, Q. Mitochondrial transfer of mesenchymal stem cells efectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016, 7, e2467. [Google Scholar] [CrossRef]
- Li, C.; Cheung, M.K.H.; Han, S.; Zhang, Z.; Chen, L.; Chen, J.; Zeng, H.; Qiu, J. Mesenchymal stem cells and their mitochondrial transfer: A double-edged sword. Biosci. Rep. 2019, 39, BSR20182417. [Google Scholar] [CrossRef] [PubMed]
- Mohammadalipour, A.; Dumbali, S.P.; Wenzel, P.L. Mitochondrial transfer and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front. Cell Dev. Biol. 2020, 8, 603292. [Google Scholar] [CrossRef]
- Paliwal, S.; Chaudhuri, R.; Agrawal, A.; Mohanty, S. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J. Biomed. Sci. 2018, 25, 31. [Google Scholar] [CrossRef]
- Jiarou, S.; Beibei, N.; Cuiping, L.; Ruixuan, X.; Wenjie, C. Mechanism of human umbilical cord mesenchymal stem cells alleviating ischemia-reperfusion injury of hepatocytes through mitochondrial transfer. Organ Transplant. 2021, 12, 294–301. [Google Scholar] [CrossRef]
- Lee, J.; Parkm, J.S.; Rohm, Y.S. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch. Pharmacal. Res. 2019, 42, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.H.; Xiao, H.; Wu, J.M.; He, K.M.; Lv, Z.Z.; Li, Z.J.; Xu, M.; Zhang, Y.Y. Mitochondria are transported along microtubules in membrane nanotubes to rescue distressed cardiomyocytes from apoptosis. Cell Death Dis. 2018, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Vignais, M.L.; Caicedo, A.; Brondello, J.M.; Jorgensen, C. Cell connections by tunneling nanotubes: Effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int. 2017, 2017, 6917941. [Google Scholar] [CrossRef]
- Nickel, S.; Christ, M.; Schmidt, S.; Kosacka, J.; Kühne, H.; Roderfeld, M.; Longerich, T.; Tietze, L.; Bosse, I.; Hsu, M.J.; et al. Human Mesenchymal Stromal Cells Resolve Lipid Load in High Fat Diet-Induced Non-Alcoholic Steatohepatitis in Mice by Mitochondria Donation. Cells 2022, 11, 1829. [Google Scholar] [CrossRef]
- Bi, Y.; Guo, X.; Zhang, M.; Zhu, K.; Shi, C.; Fan, B.; Wu, Y.; Yang, Z.; Ji, G. Bone marrow derived-mesenchymal stem cell improves diabetes-associated fatty liver via mitochondria transformation in mice. Stem Cell Res. Ther. 2021, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Heineman, B.D.; Liu, X.; Wu, G.Y. Targeted Mitochondrial Delivery to Hepatocytes: A Review. J. Clin. Transl. Hepatol. 2022, 10, 321–328. [Google Scholar] [CrossRef] [PubMed]
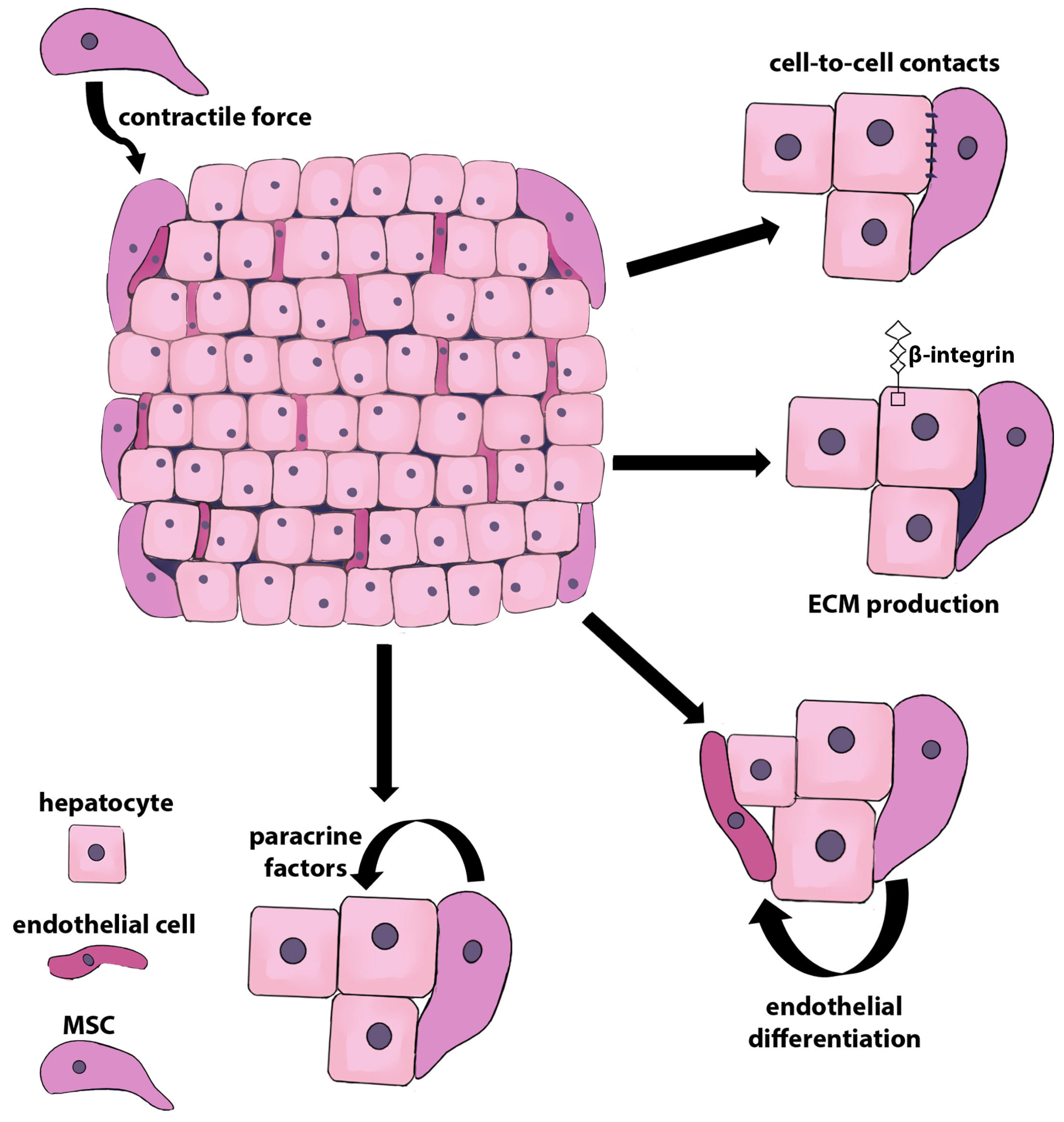
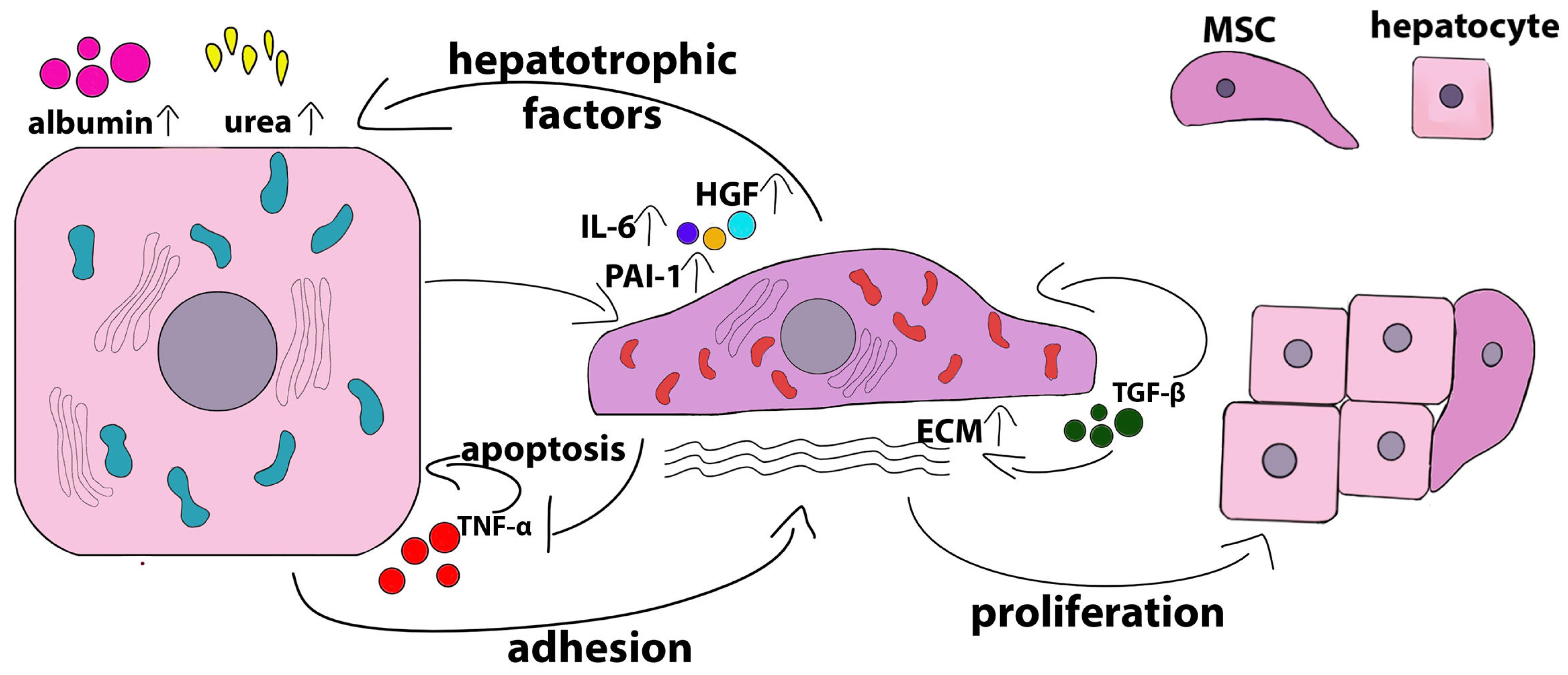
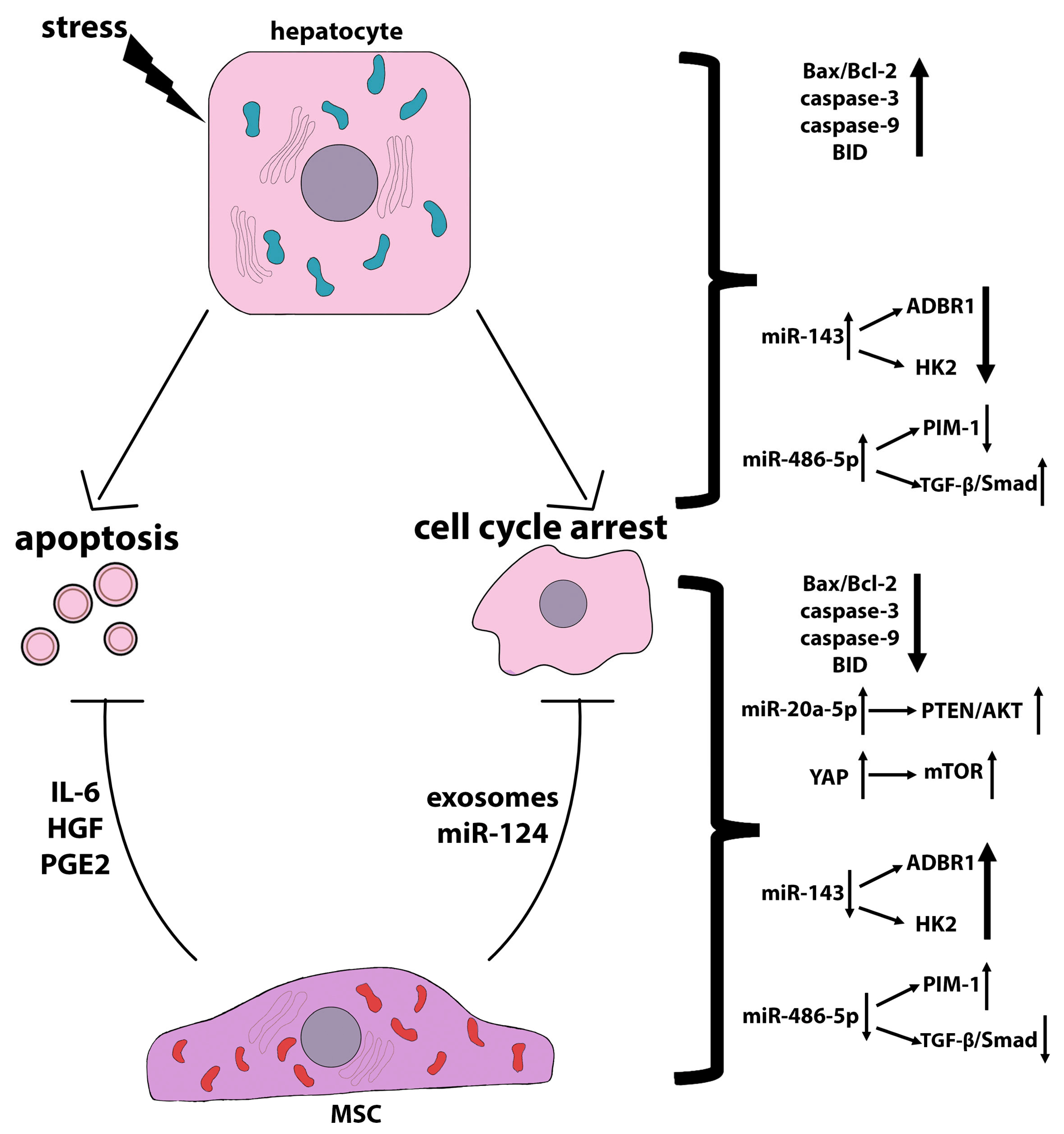

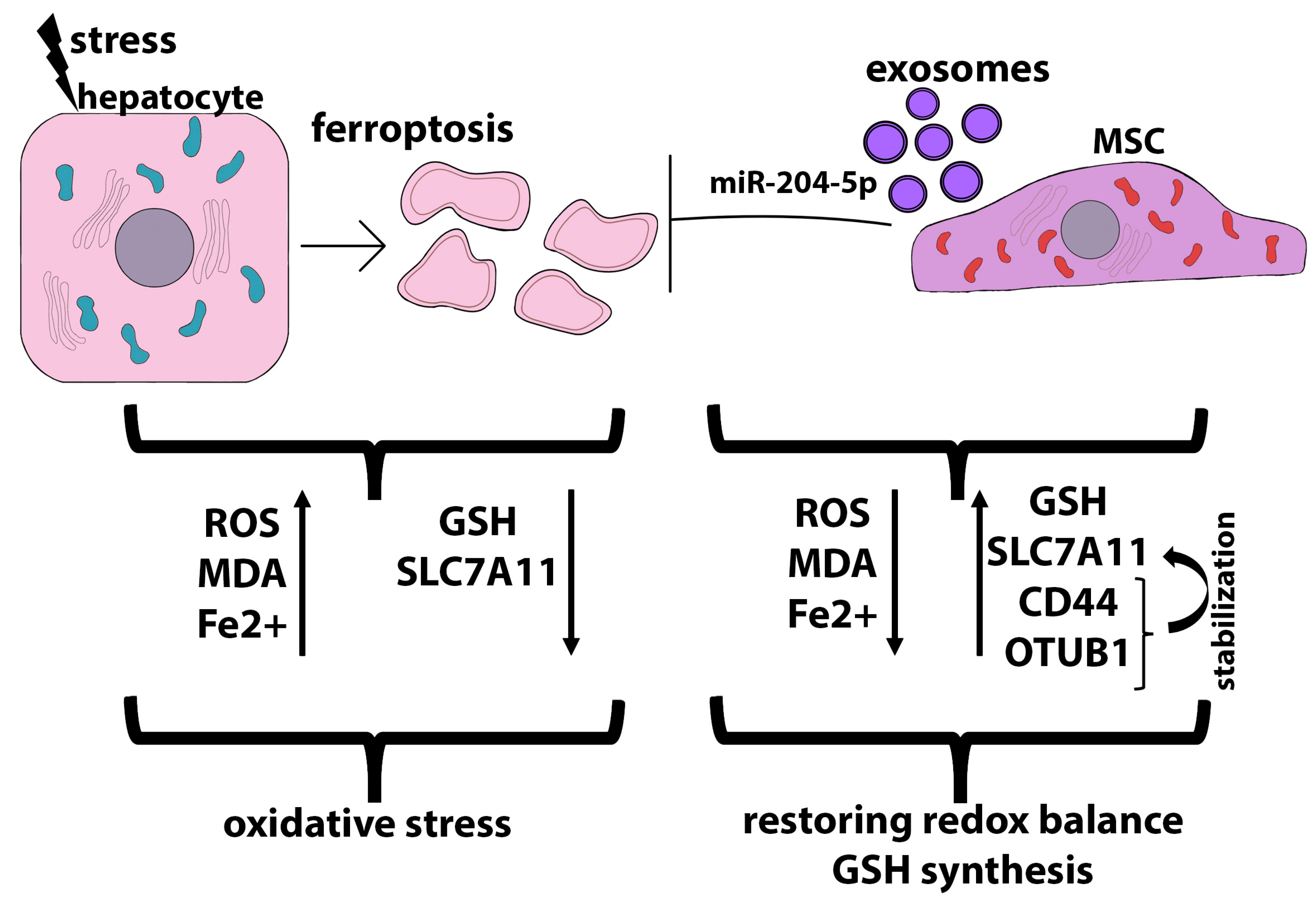
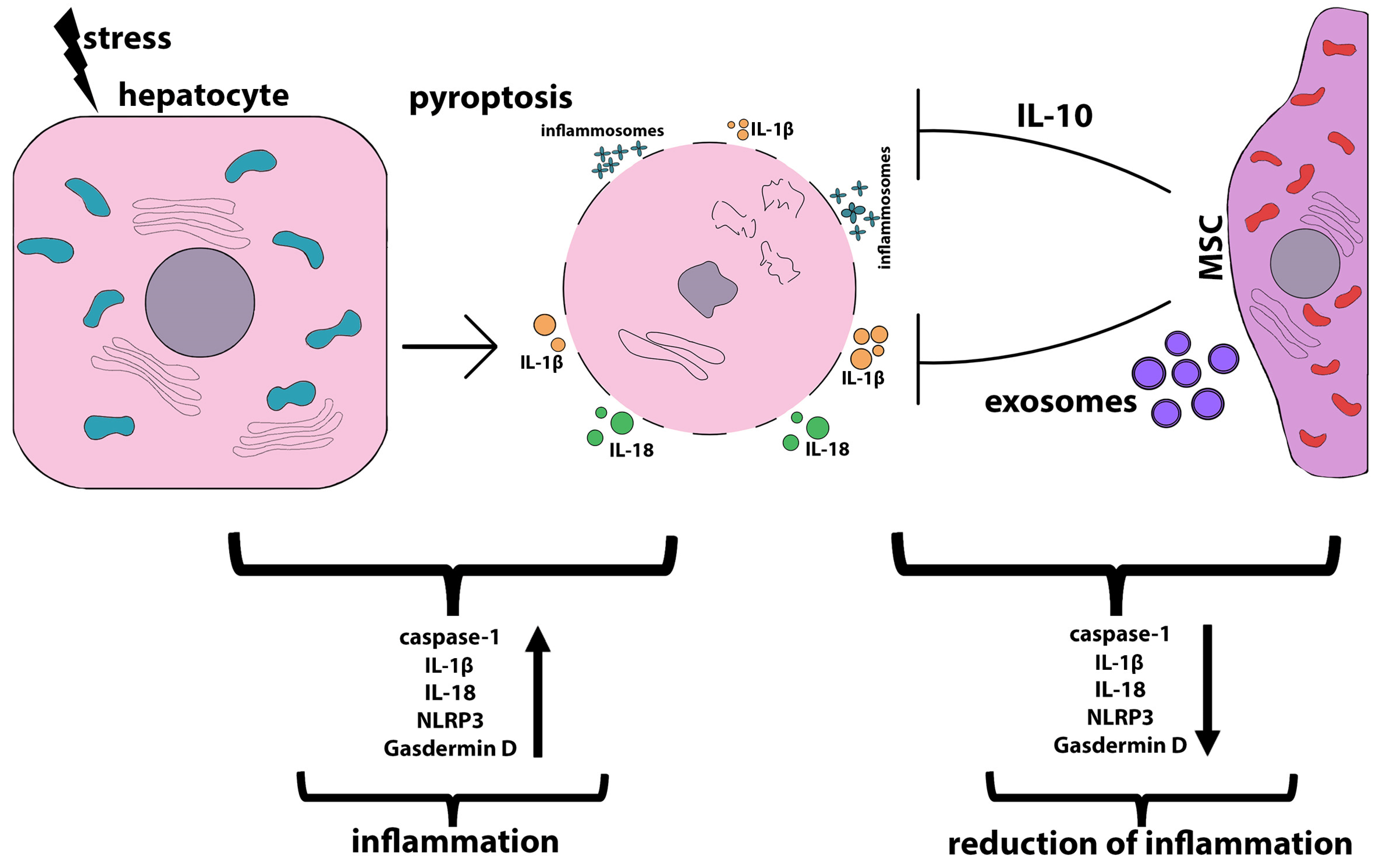
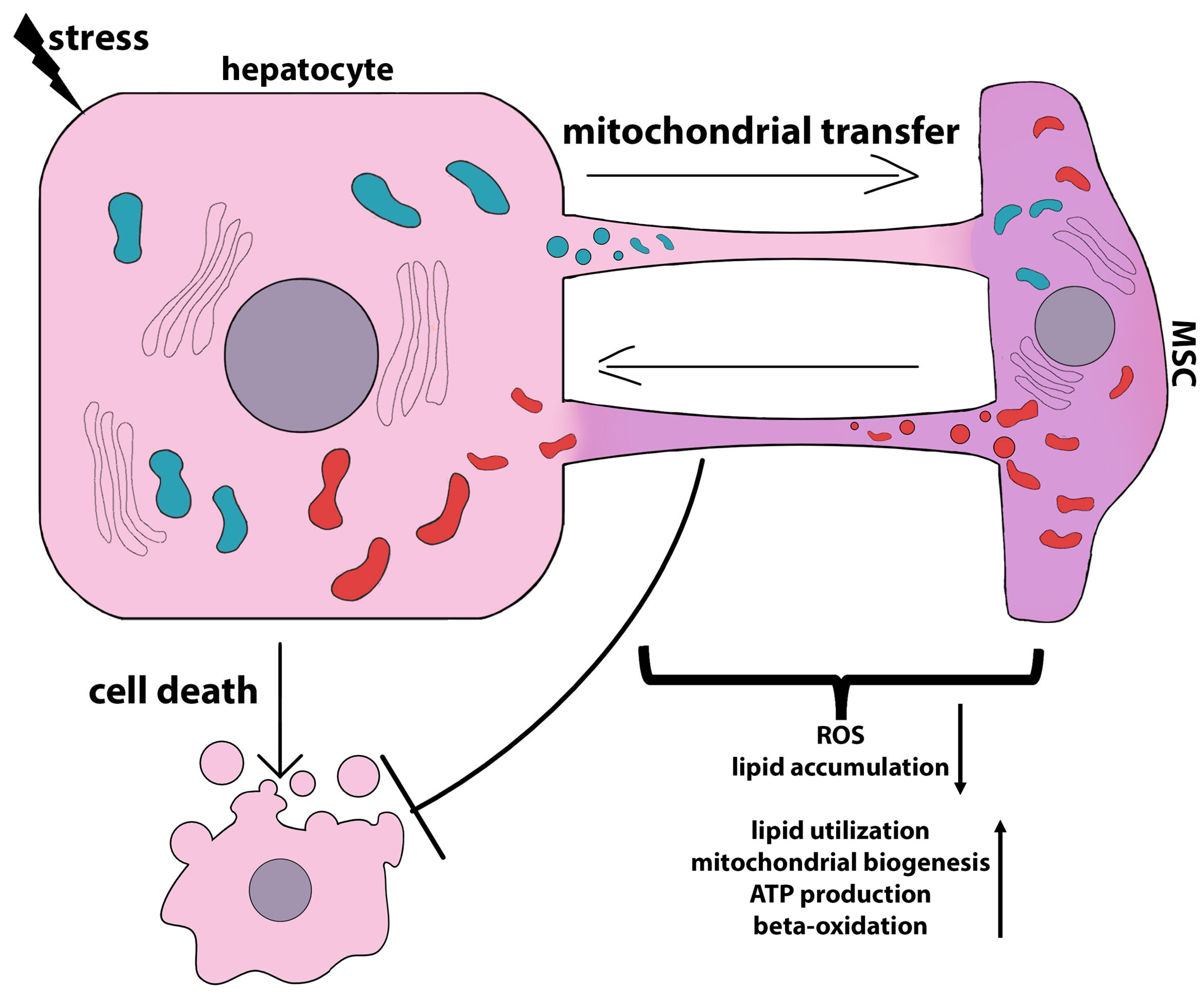
| MSC Tissue Source | Culture Setup and/or Bioactive Agents | Hepatocytes | Anti-Apoptotic Effects and References |
|---|---|---|---|
| BM-MSCs | Exosomes | Primary rat hepatocytes treated with D-galactosamine and lipopolysaccharide | Increase in autophagosome numbers; increase in the expression levels of the autophagy-related proteins LC3II and Beclin-1 and the anti-apoptotic protein Bcl-2; decrease in proapoptotic proteins Bax and cleaved caspase 3 [92] |
| hESC-derived HuES9.E1 MSCs | Exosomes | TAMH, an immortalized mouse hepatocyte cell line derived from transgenic MT42 male mice overexpressing TGF-α; THLE-2, an immortalized primary human hepatocyte cell line that expresses phenotypic characteristics of normal adult liver epithelial cells; HuH-7, a well-differentiated human hepatocarcinoma cell line; APAP or H2O2 treatment | Transition from the G0 to G1 phase of the cell cycle; upregulation of TNF-α, IL-6, iNOS, COX-2, and MIP-2; restoration of NF-κB and STAT3 signaling; inhibition of caspase 3; upregulation of anti-apoptotic protein Bcl-xL [93] |
| Rat BM-MSCs | Indirect co-culture or BM-MSC-CM | Rat hepatocytes | Increase in albumin secretion and urea production [99] |
| Human orbital fat-derived stem cells | Direct or indirect co-culture IL-6 | Rat hepatocytes treated with serum from an acute liver failure patient | Increase in cell viability; increase in albumin secretion and urea production [100] |
| Human umbilical cord MSCs | Indirect co-culture HGF, EGF, and IL-6 | Human LO2 cell line treated with acetaminophen | Protection from APAP-induced necrosis; increase in cell viability [110] |
| Mouse BM-MSCs | MSC-CM; prostaglandin E2 | LPS-treated mouse hepatocyte cell line AML12 | Apoptosis inhibition; increase in cell proliferation; YAP activation, suppression of PTEN due to miR-29a-3p, and activation of mTOR signaling [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kholodenko, I.V.; Kholodenko, R.V.; Yarygin, K.N. The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress. Int. J. Mol. Sci. 2023, 24, 15212. https://doi.org/10.3390/ijms242015212
Kholodenko IV, Kholodenko RV, Yarygin KN. The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress. International Journal of Molecular Sciences. 2023; 24(20):15212. https://doi.org/10.3390/ijms242015212
Chicago/Turabian StyleKholodenko, Irina V., Roman V. Kholodenko, and Konstantin N. Yarygin. 2023. "The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress" International Journal of Molecular Sciences 24, no. 20: 15212. https://doi.org/10.3390/ijms242015212
APA StyleKholodenko, I. V., Kholodenko, R. V., & Yarygin, K. N. (2023). The Crosstalk between Mesenchymal Stromal/Stem Cells and Hepatocytes in Homeostasis and under Stress. International Journal of Molecular Sciences, 24(20), 15212. https://doi.org/10.3390/ijms242015212






