Stem Cell Therapy in Children with Traumatic Brain Injury
Abstract
1. Introduction
2. Stem Cells
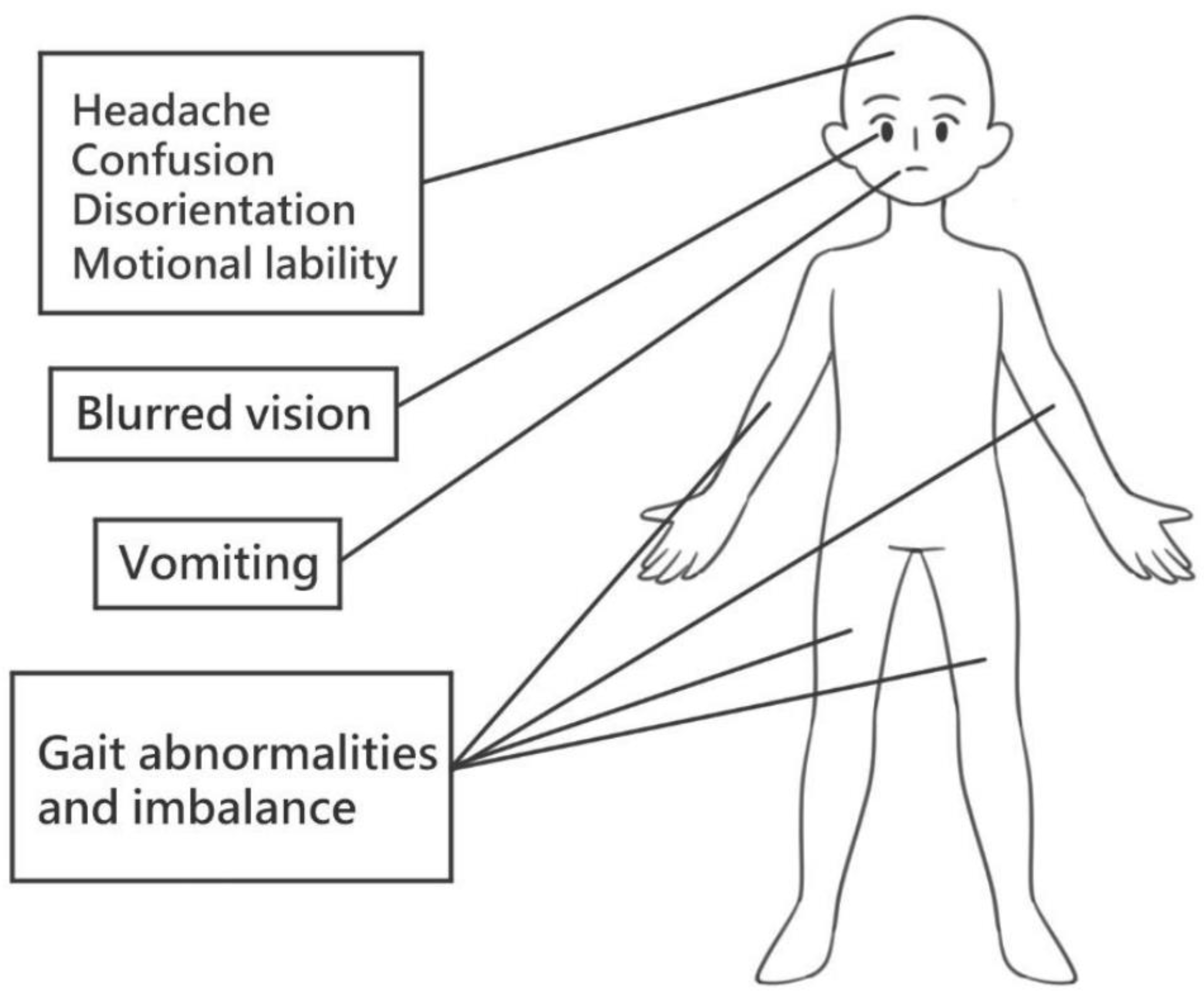
3. Traumatic Brain Injury
4. Stem Cell Therapy and Traumatic Brain Injury
4.1. Neural Stem Cell Response to Injury
4.2. Neuroinflammation Response to Injury
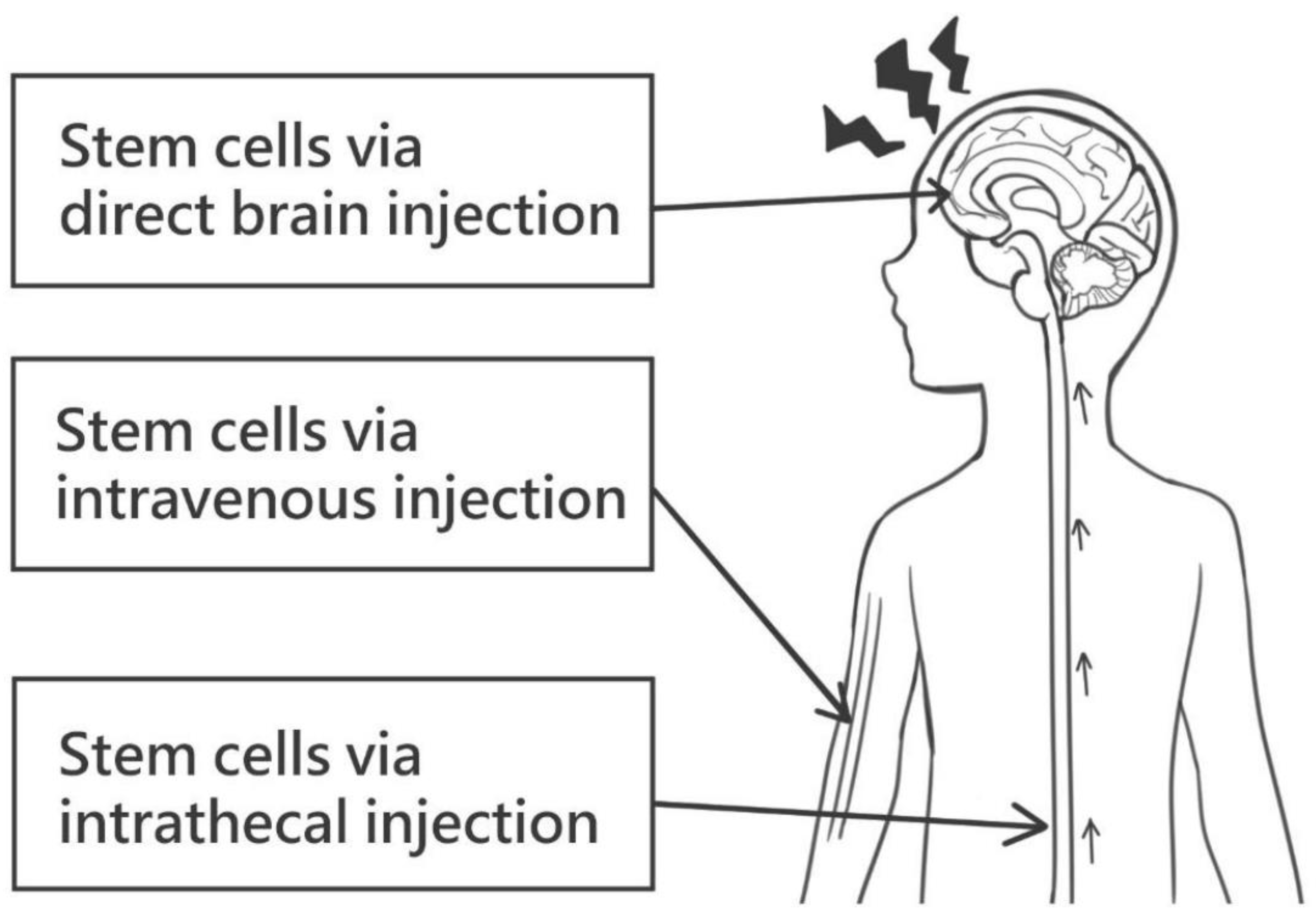
4.3. Stem Cell Therapy
5. Challenges and Future Prospects
6. Limitation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| CP | Cerebral palsy |
| DCX | Doublecortin |
| GCS | Glasgow coma scale |
| IGF-1 | Insulin-like growth factor 1 |
| iPSCs | Induced pluripotent stem cells |
| IQ | Intelligence quotient |
| MSCs | Mesenchymal stem cells |
| NPs | Neural progenitor cells |
| NSCs | Neural stem cells |
| OLGs | Oligodendrocytes |
| OPCs | Oligodendocyte progenitor cell |
| PDGF-AB | Platelet-derived growth factor-AB |
| SCs | Stem cells |
| SVZ | Subventricular zone |
| TBI | Traumatic brain injury |
| TGF-β1 | Transforming growth factor-β1 |
References
- Poliwoda, S.; Noor, N.; Downs, E.; Schaaf, A.; Cantwell, A.; Ganti, L.; Kaye, A.D.; Mosel, L.I.; Carroll, C.B.; Viswanath, O.; et al. Stem cells: A comprehensive review of origins and emerging clinical roles in medical practice. Orthop. Rev. 2022, 14, 37498. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Barzilay, R.; Melamed, E.; Offen, D. Introducing transcription factors to multipotent mesenchymal stem cells: Making transdifferentiation possible. Stem Cells 2009, 27, 2509–2515. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Balistreri, C.R.; De Falco, E.; Bordin, A.; Maslova, O.; Koliada, A.; Vaiserman, A. Stem cell therapy: Old challenges and new solutions. Mol. Biol. Rep. 2020, 47, 3117–3131. [Google Scholar] [CrossRef]
- Newell, L.F.; Cook, R.J. Advances in acute myeloid leukemia. BMJ 2021, 375, n2026. [Google Scholar] [CrossRef]
- Nitkin, C.R.; Bonfield, T.L. Concise Review: Mesenchymal Stem Cell Therapy for Pediatric Disease: Perspectives on Success and Potential Improvements. Stem Cells Transl. Med. 2017, 6, 539–565. [Google Scholar] [CrossRef]
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef]
- Popernack, M.L.; Gray, N.; Reuter-Rice, K. Moderate-to-Severe Traumatic Brain Injury in Children: Complications and Rehabilitation Strategies. J. Pediatr. Health Care 2015, 29, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Deeb, G.; Rahal, R.; Atwi, K.; Mondello, S.; Marei, H.E.; Gali, A.; Sleiman, E. Mesenchymal Stem Cells in the Treatment of Traumatic Brain Injury. Front. Neurol. 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Greve, M.W.; Zink, B.J. Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. 2009, 76, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Marmarou, A.; Lapane, K.; Turf, E.; Wilson, L. A method for reducing misclassification in the extended Glasgow Outcome Score. J. Neurotrauma 2010, 27, 843–852. [Google Scholar] [CrossRef]
- Araki, T.; Yokota, H.; Morita, A. Pediatric Traumatic Brain Injury: Characteristic Features, Diagnosis, and Management. Neurol. Med.-Chir. 2017, 57, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Ryan, N.P.; Wright, D.K.; Caeyenberghs, K.; Semple, B.D. The Impact of Traumatic Injury to the Immature Human Brain: A Scoping Review with Insights from Advanced Structural Neuroimaging. J. Neurotrauma 2020, 37, 724–738. [Google Scholar] [CrossRef]
- Hertz-Pannier, L.; Chiron, C.; Jambaqué, I.; Renaux-Kieffer, V.; Van de Moortele, P.F.; Delalande, O.; Fohlen, M.; Brunelle, F.; Le Bihan, D. Late plasticity for language in a child’s non-dominant hemisphere: A pre- and post-surgery fMRI study. Brain 2002, 125, 361–372. [Google Scholar] [CrossRef]
- Holland, J.N.; Schmidt, A.T. Static and Dynamic Factors Promoting Resilience following Traumatic Brain Injury: A Brief Review. Neural Plast. 2015, 2015, 902802. [Google Scholar] [CrossRef][Green Version]
- Anderson, V.; Jacobs, R.; Spencer-Smith, M.; Coleman, L.; Anderson, P.; Williams, J.; Greenham, M.; Leventer, R. Does early age at brain insult predict worse outcome? Neuropsychological implications. J. Pediatr. Psychol. 2010, 35, 716–727. [Google Scholar] [CrossRef]
- Fay, T.B.; Yeates, K.O.; Taylor, H.G.; Bangert, B.; Dietrich, A.; Nuss, K.E.; Rusin, J.; Wright, M. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2010, 16, 94–105. [Google Scholar] [CrossRef]
- Wade, S.L.; Cassedy, A.; Walz, N.C.; Taylor, H.G.; Stancin, T.; Yeates, K.O. The relationship of parental warm responsiveness and negativity to emerging behavior problems following traumatic brain injury in young children. Dev. Psychol. 2011, 47, 119–133. [Google Scholar] [CrossRef]
- Ganesalingam, K.; Yeates, K.O.; Taylor, H.G.; Walz, N.C.; Stancin, T.; Wade, S. Executive functions and social competence in young children 6 months following traumatic brain injury. Neuropsychology 2011, 25, 466–476. [Google Scholar] [CrossRef]
- Levin, H.S.; Hanten, G.; Li, X. The relation of cognitive control to social outcome after paediatric TBI: Implications for intervention. Dev. Neurorehabilit. 2009, 12, 320–329. [Google Scholar] [CrossRef]
- Chapman, L.A.; Wade, S.L.; Walz, N.C.; Taylor, H.G.; Stancin, T.; Yeates, K.O. Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabil. Psychol. 2010, 55, 48–57. [Google Scholar] [CrossRef]
- Andrews, T.K.; Rose, F.D.; Johnson, D.A. Social and behavioural effects of traumatic brain injury in children. Brain Inj. 1998, 12, 133–138. [Google Scholar] [CrossRef]
- Crowe, L.M.; Catroppa, C.; Babl, F.E.; Rosenfeld, J.V.; Anderson, V. Timing of traumatic brain injury in childhood and intellectual outcome. J. Pediatr. Psychol. 2012, 37, 745–754. [Google Scholar] [CrossRef]
- Anderson, V.; Catroppa, C.; Morse, S.; Haritou, F.; Rosenfeld, J. Recovery of intellectual ability following traumatic brain injury in childhood: Impact of injury severity and age at injury. Pediatr. Neurosurg. 2000, 32, 282–290. [Google Scholar] [CrossRef]
- Keenan, H.T.; Clark, A.E.; Holubkov, R.; Cox, C.S.; Ewing-Cobbs, L. Psychosocial and Executive Function Recovery Trajectories One Year after Pediatric Traumatic Brain Injury: The Influence of Age and Injury Severity. J. Neurotrauma 2018, 35, 286–296. [Google Scholar] [CrossRef]
- Treble-Barna, A.; Schultz, H.; Minich, N.; Taylor, H.G.; Yeates, K.O.; Stancin, T.; Wade, S.L. Long-term classroom functioning and its association with neuropsychological and academic performance following traumatic brain injury during early childhood. Neuropsychology 2017, 31, 486–498. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Tasker, R.C.; Bell, M.J.; Adelson, P.D.; Carney, N.; Vavilala, M.S.; Selden, N.R.; Bratton, S.L.; Grant, G.A.; Kissoon, N.; et al. Management of Pediatric Severe Traumatic Brain Injury: 2019 Consensus and Guidelines-Based Algorithm for First and Second Tier Therapies. Pediatr. Crit. Care Med. 2019, 20, 269–279. [Google Scholar] [CrossRef]
- Weston, N.M.; Sun, D. The Potential of Stem Cells in Treatment of Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2018, 18, 1. [Google Scholar] [CrossRef]
- Niimi, Y.; Levison, S.W. Pediatric brain repair from endogenous neural stem cells of the subventricular zone. Pediatr. Res. 2018, 83, 385–396. [Google Scholar] [CrossRef]
- Clausi, M.G.; Kumari, E.; Levison, S.W. Unmasking the responses of the stem cells and progenitors in the subventricular zone after neonatal and pediatric brain injuries. Neural Regen. Res. 2016, 11, 45–48. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incoorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J. Comp. Neurol. 1966, 126, 337–389. [Google Scholar] [CrossRef]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef]
- De Marchis, S.; Fasolo, A.; Puche, A.C. Subventricular zone-derived neuronal progenitors migrate into the subcortical forebrain of postnatal mice. J. Comp. Neurol. 2004, 476, 290–300. [Google Scholar] [CrossRef]
- Taylor, S.R.; Smith, C.; Harris, B.T.; Costine, B.A.; Duhaime, A.C. Maturation-dependent response of neurogenesis after traumatic brain injury in children. J. Neurosurg. Pediatr. 2013, 12, 545–554. [Google Scholar] [CrossRef]
- Inta, D.; Alfonso, J.; von Engelhardt, J.; Kreuzberg, M.M.; Meyer, A.H.; van Hooft, J.A.; Monyer, H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc. Natl. Acad. Sci. USA 2008, 105, 20994–20999. [Google Scholar] [CrossRef]
- Azim, K.; Fischer, B.; Hurtado-Chong, A.; Draganova, K.; Cantù, C.; Zemke, M.; Sommer, L.; Butt, A.; Raineteau, O. Persistent Wnt/β-catenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells 2014, 32, 1301–1312. [Google Scholar] [CrossRef]
- Levison, S.W.; Goldman, J.E. Multipotential and lineage restricted precursors coexist in the mammalian perinatal subventricular zone. J. Neurosci. Res. 1997, 48, 83–94. [Google Scholar] [CrossRef]
- Bergmann, O.; Liebl, J.; Bernard, S.; Alkass, K.; Yeung, M.S.; Steier, P.; Kutschera, W.; Johnson, L.; Landén, M.; Druid, H.; et al. The age of olfactory bulb neurons in humans. Neuron 2012, 74, 634–639. [Google Scholar] [CrossRef]
- Neumane, S.; Câmara-Costa, H.; Francillette, L.; Araujo, M.; Toure, H.; Brugel, D.; Laurent-Vannier, A.; Ewing-Cobbs, L.; Meyer, P.; Dellatolas, G.; et al. Functional outcome after severe childhood traumatic brain injury: Results of the TGE prospective longitudinal study. Ann. Phys. Rehabil. Med. 2021, 64, 101375. [Google Scholar] [CrossRef]
- Zhang, Z.; Ishrat, S.; O’Bryan, M.; Klein, B.; Saraswati, M.; Robertson, C.; Kannan, S. Pediatric Traumatic Brain Injury Causes Long-Term Deficits in Adult Hippocampal Neurogenesis and Cognition. J. Neurotrauma 2020, 37, 1656–1667. [Google Scholar] [CrossRef]
- Rizk, M.; Vu, J.; Zhang, Z. Impact of pediatric traumatic brain injury on hippocampal neurogenesis. Neural Regen. Res. 2021, 16, 926–933. [Google Scholar] [CrossRef]
- Vicidomini, C.; Guo, N.; Sahay, A. Communication, Cross Talk, and Signal Integration in the Adult Hippocampal Neurogenic Niche. Neuron 2020, 105, 220–235. [Google Scholar] [CrossRef]
- Willis, E.F.; MacDonald, K.P.A.; Nguyen, Q.H.; Garrido, A.L.; Gillespie, E.R.; Harley, S.B.R.; Bartlett, P.F.; Schroder, W.A.; Yates, A.G.; Anthony, D.C.; et al. Repopulating Microglia Promote Brain Repair in an IL-6-Dependent Manner. Cell 2020, 180, 833–846.e16. [Google Scholar] [CrossRef]
- Clément, T.; Lee, J.B.; Ichkova, A.; Rodriguez-Grande, B.; Fournier, M.L.; Aussudre, J.; Ogier, M.; Haddad, E.; Canini, F.; Koehl, M.; et al. Juvenile mild traumatic brain injury elicits distinct spatiotemporal astrocyte responses. Glia 2020, 68, 528–542. [Google Scholar] [CrossRef]
- Braun, M.; Vaibhav, K.; Saad, N.M.; Fatima, S.; Vender, J.R.; Baban, B.; Hoda, M.N.; Dhandapani, K.M. White matter damage after traumatic brain injury: A role for damage associated molecular patterns. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2614–2626. [Google Scholar] [CrossRef]
- Genc, S.; Anderson, V.; Ryan, N.P.; Malpas, C.B.; Catroppa, C.; Beauchamp, M.H.; Silk, T.J. Recovery of White Matter following Pediatric Traumatic Brain Injury Depends on Injury Severity. J. Neurotrauma 2017, 34, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Androutsellis-Theotokis, A.; Leker, R.R.; Soldner, F.; Hoeppner, D.J.; Ravin, R.; Poser, S.W.; Rueger, M.A.; Bae, S.K.; Kittappa, R.; McKay, R.D. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature 2006, 442, 823–826. [Google Scholar] [CrossRef]
- Gonzalez, F.F.; Larpthaveesarp, A.; McQuillen, P.; Derugin, N.; Wendland, M.; Spadafora, R.; Ferriero, D.M. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke 2013, 44, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Wachs, F.P.; Winner, B.; Couillard-Despres, S.; Schiller, T.; Aigner, R.; Winkler, J.; Bogdahn, U.; Aigner, L. Transforming growth factor-β1 is a negative modulator of adult neurogenesis. J. Neuropathol. Exp. Neurol. 2006, 65, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Mashkouri, S.; Crowley, M.G.; Liska, M.G.; Corey, S.; Borlongan, C.V. Utilizing pharmacotherapy and mesenchymal stem cell therapy to reduce inflammation following traumatic brain injury. Neural Regen. Res. 2016, 11, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Lengel, D.; Sevilla, C.; Romm, Z.L.; Huh, J.W.; Raghupathi, R. Stem Cell Therapy for Pediatric Traumatic Brain Injury. Front. Neurol. 2020, 11, 601286. [Google Scholar] [CrossRef]
- Cox, C.S., Jr. Cellular therapy for traumatic neurological injury. Pediatr. Res. 2018, 83, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shao, A.; Xu, W.; Wu, H.; Deng, Y. Advance of Stem Cell Treatment for Traumatic Brain Injury. Front. Cell. Neurosci. 2019, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Dunkerson, J.; Moritz, K.E.; Young, J.; Pionk, T.; Fink, K.; Rossignol, J.; Dunbar, G.; Smith, J.S. Combining enriched environment and induced pluripotent stem cell therapy results in improved cognitive and motor function following traumatic brain injury. Restor. Neurol. Neurosci. 2014, 32, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Lu, D.; Chopp, M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma 2004, 21, 33–39. [Google Scholar] [CrossRef]
- Sekiya, I.; Larson, B.L.; Smith, J.R.; Pochampally, R.; Cui, J.G.; Prockop, D.J. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 2002, 20, 530–541. [Google Scholar] [CrossRef]
- Ghasemi, N. Transdifferentiation of human adipose-derived mesenchymal stem cells into oligodendrocyte progenitor cells. Iran. J. Neurol. 2018, 17, 24–30. [Google Scholar]
- Liao, G.P.; Harting, M.T.; Hetz, R.A.; Walker, P.A.; Shah, S.K.; Corkins, C.J.; Hughes, T.G.; Jimenez, F.; Kosmach, S.C.; Day, M.C.; et al. Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children. Pediatr. Crit. Care Med. 2015, 16, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.S., Jr.; Baumgartner, J.E.; Harting, M.T.; Worth, L.L.; Walker, P.A.; Shah, S.K.; Ewing-Cobbs, L.; Hasan, K.M.; Day, M.C.; Lee, D.; et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery 2011, 68, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, H.; Dai, G.; Wang, X.; Hua, R.; Liu, X.; Wang, P.; Chen, G.; Yue, W.; An, Y. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013, 1532, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Wang, X.; Wang, X.; Wang, L.; Wang, X.; Wu, S.; Wan, Z. Autologous bone marrow mesenchymal stem cell therapy in the subacute stage of traumatic brain injury by lumbar puncture. Exp. Clin. Transplant. 2013, 11, 176–181. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. Traumatic Brain Injury and Stem Cells: An Overview of Clinical Trials, the Current Treatments and Future Therapeutic Approaches. Medicina 2020, 56, 137. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, D.; Schwarz, E.J.; Prockop, D.J.; Black, I.B. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000, 61, 364–370. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef]
- Ponte, A.L.; Marais, E.; Gallay, N.; Langonné, A.; Delorme, B.; Hérault, O.; Charbord, P.; Domenech, J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells 2007, 25, 1737–1745. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Liu, L.; Eckert, M.A.; Riazifar, H.; Kang, D.K.; Agalliu, D.; Zhao, W. From blood to the brain: Can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013, 2013, 435093. [Google Scholar] [CrossRef]
- Matsushita, T.; Kibayashi, T.; Katayama, T.; Yamashita, Y.; Suzuki, S.; Kawamata, J.; Honmou, O.; Minami, M.; Shimohama, S. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci. Lett. 2011, 502, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Steingen, C.; Brenig, F.; Baumgartner, L.; Schmidt, J.; Schmidt, A.; Bloch, W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J. Mol. Cell. Cardiol. 2008, 44, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.R.; Stoutenger, B.R.; Robinson, A.P.; Spees, J.L.; Prockop, D.J. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc. Natl. Acad. Sci. USA 2005, 102, 18171–18176. [Google Scholar] [CrossRef]
- Jensen, A. Cerebral palsy-brain repair with stem cells. J. Perinat. Med. 2023, 51, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhou, L.; Zhang, H.; Han, D.; Luo, Y.; Chen, J.; Li, L.; Zou, Z.; He, Z.; Zhang, M.; et al. Efficacy and safety of stem cell therapy in cerebral palsy: A systematic review and meta-analysis. Front. Bioeng. Biotechnol. 2022, 10, 1006845. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Tajiri, N.; Shinozuka, K.; Ishikawa, H.; Sanberg, P.R.; Sanchez-Ramos, J.; Song, S.; Kaneko, Y.; Borlongan, C.V. Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury. PLoS ONE 2014, 9, e90953. [Google Scholar] [CrossRef]
- Tunc Ata, M.; Turgut, G.; Akbulut, M.; Kocyigit, A.; Karabulut, A.; Senol, H.; Turgut, S. Effect of Erythropoietin and Stem Cells on Traumatic Brain Injury. World Neurosurg. 2016, 89, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Pischiutta, F.; Caruso, E.; Cavaleiro, H.; Salgado, A.J.; Loane, D.J.; Zanier, E.R. Mesenchymal stromal cell secretome for traumatic brain injury: Focus on immunomodulatory action. Exp. Neurol. 2022, 357, 114199. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Mot, Y.Y.; Moses, E.J.; Mohd Yusoff, N.; Ling, K.H.; Yong, Y.K.; Tan, J.J. Mesenchymal Stromal Cells-Derived Exosome and the Roles in the Treatment of Traumatic Brain Injury. Cell. Mol. Neurobiol. 2023, 43, 469–489. [Google Scholar] [CrossRef]
- Cui, L.; Luo, W.; Jiang, W.; Li, H.; Xu, J.; Liu, X.; Wang, B.; Wang, J.; Chen, G. Human umbilical cord mesenchymal stem cell-derived exosomes promote neurological function recovery in rat after traumatic brain injury by inhibiting the activation of microglia and astrocyte. Regen. Ther. 2022, 21, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, Q.; Wei, J.; Zhao, R.C. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 2020, 12, 18274–18296. [Google Scholar] [CrossRef] [PubMed]

| A. Origin of extraction | ||||
| Amniotic cells | Umbilical cord | Bone marrow | Adipose tissue | |
| embryonic stem cells induced pluripotent stem cells human amnion-derived stem cells | hematopoietic stem cell (lymphoid and myeloid lineage) | adipose-derived stem cells | ||
| B. Potency | ||||
| Totipotent/Omnipotent | Pluripotent | Multipotent | Oligopotent | Unipotent |
| form embryonic (embryo) and extra-embryonic tissue (placenta) | form cells arising from all three germ layers (ectoderm, mesoderm, endoderm) | form cells from single germ layer | form two or more cell lineages within a specific tissue | form only one specific cell lineage type |
| Most undifferentiated --------------------------------------------------------------------------------------------------------------------------------> Differentiated | ||||
| Cell Stream Type | Final Designation | Studied Groups |
|---|---|---|
| Rostral migratory stream | Olfactory bulb | Rats |
| Medial migratory stream | Prefrontal cortex | Humans |
| Ventral migratory stream | Nucleus accumbens (islands of calleja) | Mice |
| Ventral migratory stream | Claustrum | Humans |
| Dorsal migratory stream | Occipital cortex | Mice |
| Dorsal migratory stream | Upper-layer glutamargic neurons | Mice |
| Authors (Reference) | Case Numbers | Age | Timing of Intervention | Stem Cell Source | Route and Dose | Outcomes Measured | Improvement | Followed Time of Improvement | Major Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Liao et al. [61] | 10 (19 control) | 5–14 | <48 h of injury | Autologous bone marrow mononuclear cells | Intravenous, 6 × 106 stem cells/kg | Pediatric intensity level of therapy (PILOT score) | Decreased score and treatment intensity for raised intracranial pressure | Day 2 to day 21, significant improvement within week 1 | No |
| Pediatric logistic organ dysfunction (PELOD score), days of ICP monitoring | Decreased severity of organ injury and days of ICP monitoring | Day 7 to day 21 | |||||||
| Cox et al. [62] | 10 | 5–14 | <48 h of injury | Autologous bone marrow mononuclear cells | Intravenous, 6 × 106 stem cells/kg | Functional outcome
| Significant improvement | 6 months | No |
Neuropsychological outcome
| Significant improvement | ||||||||
| Magnetic resonance imaging (MRI) volumetric study | No significant change in grey matter, white matter or intracranial or CSF volume. | ||||||||
| Wang et al. [63] | 20 (20 controls) | Adults | >1 year after injury | Umbilical cord mesenchymal stem cells | Intrathecal, 1 × 107 stem cells (4 courses) | Fugl-Meyer Assessment (FMA)
| Significant improvement | 6 months | No |
Functional Independence Measures (FIM)
| Significant improvement | ||||||||
| Tian et al. [64] | 97 | - | >1 month after injury | Autologous bone marrow stem cells | Intrathecal, 1 × 106 stem cells | Function of brain (39.2%) | Significant improvement | 14 days | No |
| Consciousness improvement (45.8%) | |||||||||
| Improved motor functions (37%) |
| A: Characteristics | References |
| Differentiate to cell of neuronal lineage | [66,67] |
| Migrate to site of injury | [68] |
| Cross blood–brain barrier | [70,71,72] |
| Modulate neuroinflammatory response; favor regeneration | [73] |
| B: Clinical consideration | References |
| Source Dose Delivery Timing Patient selection Measured outcome | [61,62,63,64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-Y.; Wu, K.-H.; Chen, C.-Y.; Guo, B.-C.; Chang, Y.-J.; Lee, T.-A.; Lin, M.-J.; Wu, H.-P. Stem Cell Therapy in Children with Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 14706. https://doi.org/10.3390/ijms241914706
Lin W-Y, Wu K-H, Chen C-Y, Guo B-C, Chang Y-J, Lee T-A, Lin M-J, Wu H-P. Stem Cell Therapy in Children with Traumatic Brain Injury. International Journal of Molecular Sciences. 2023; 24(19):14706. https://doi.org/10.3390/ijms241914706
Chicago/Turabian StyleLin, Wen-Ya, Kang-Hsi Wu, Chun-Yu Chen, Bei-Cyuan Guo, Yu-Jun Chang, Tai-An Lee, Mao-Jen Lin, and Han-Ping Wu. 2023. "Stem Cell Therapy in Children with Traumatic Brain Injury" International Journal of Molecular Sciences 24, no. 19: 14706. https://doi.org/10.3390/ijms241914706
APA StyleLin, W.-Y., Wu, K.-H., Chen, C.-Y., Guo, B.-C., Chang, Y.-J., Lee, T.-A., Lin, M.-J., & Wu, H.-P. (2023). Stem Cell Therapy in Children with Traumatic Brain Injury. International Journal of Molecular Sciences, 24(19), 14706. https://doi.org/10.3390/ijms241914706





