Apoptotic Effect of Gallic Acid via Regulation of p-p38 and ER Stress in PANC-1 and MIA PaCa-2 Cells Pancreatic Cancer Cells
Abstract
:1. Introduction
2. Results
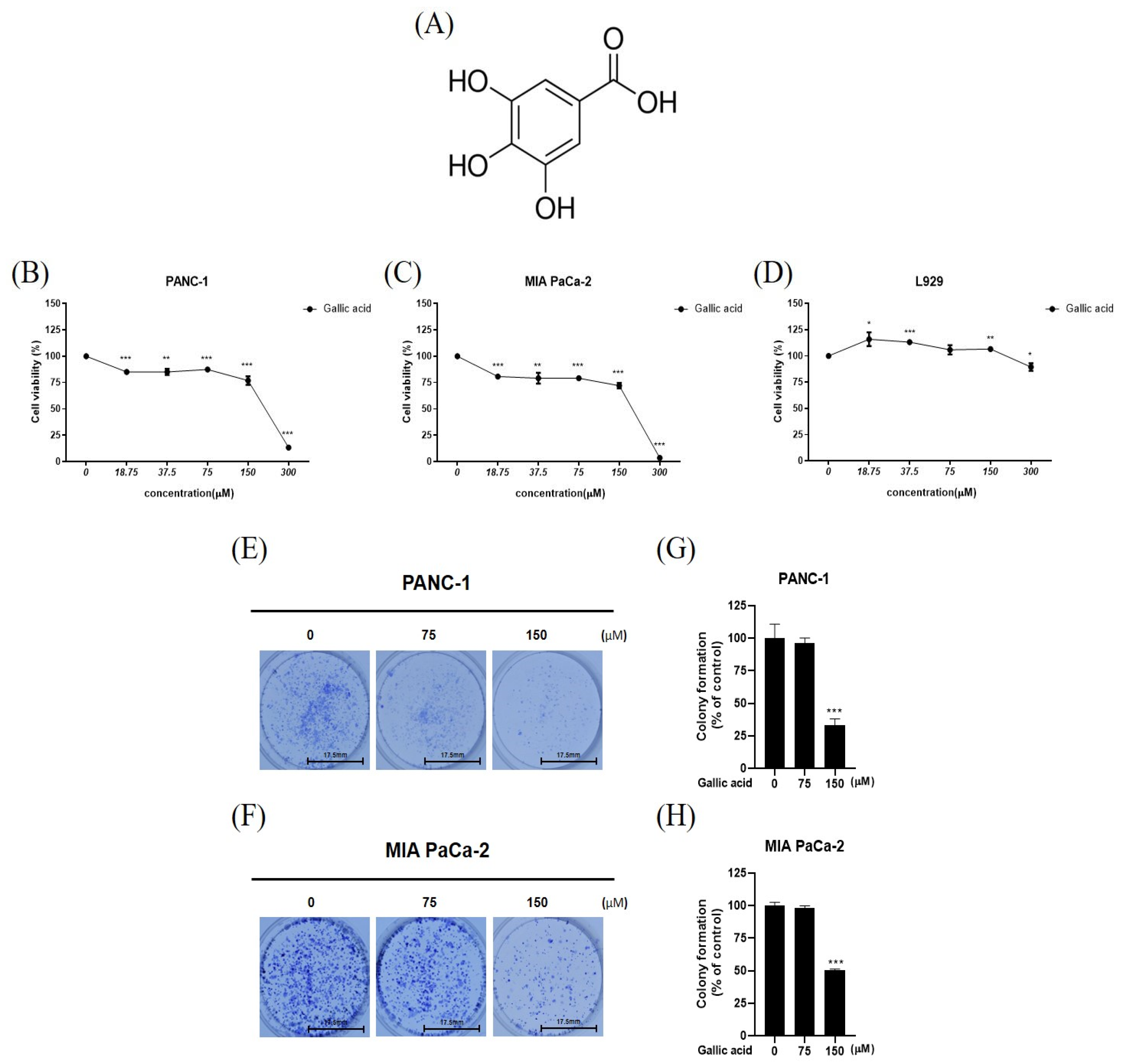
2.1. Gallic Acid Cytotoxic and Antiproliferative in Cancer Cells PANC-1 and MIA PaCa-2 While Not Affecting Normal Cell Line, L-929
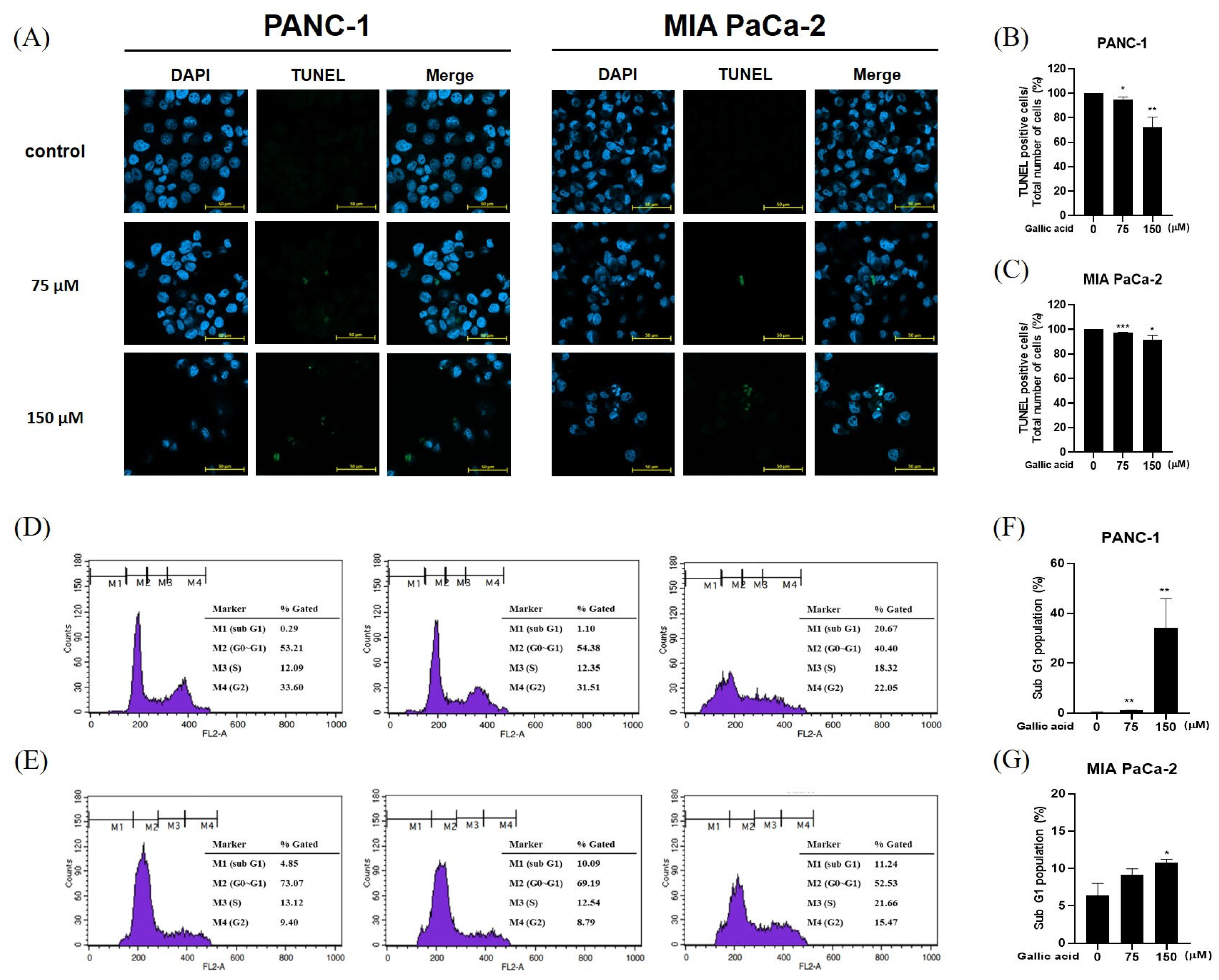
2.2. Gallic Acid Increased the Sub-G1 Ratio and Induced Apoptosis in Pancreatic Cancer Cells
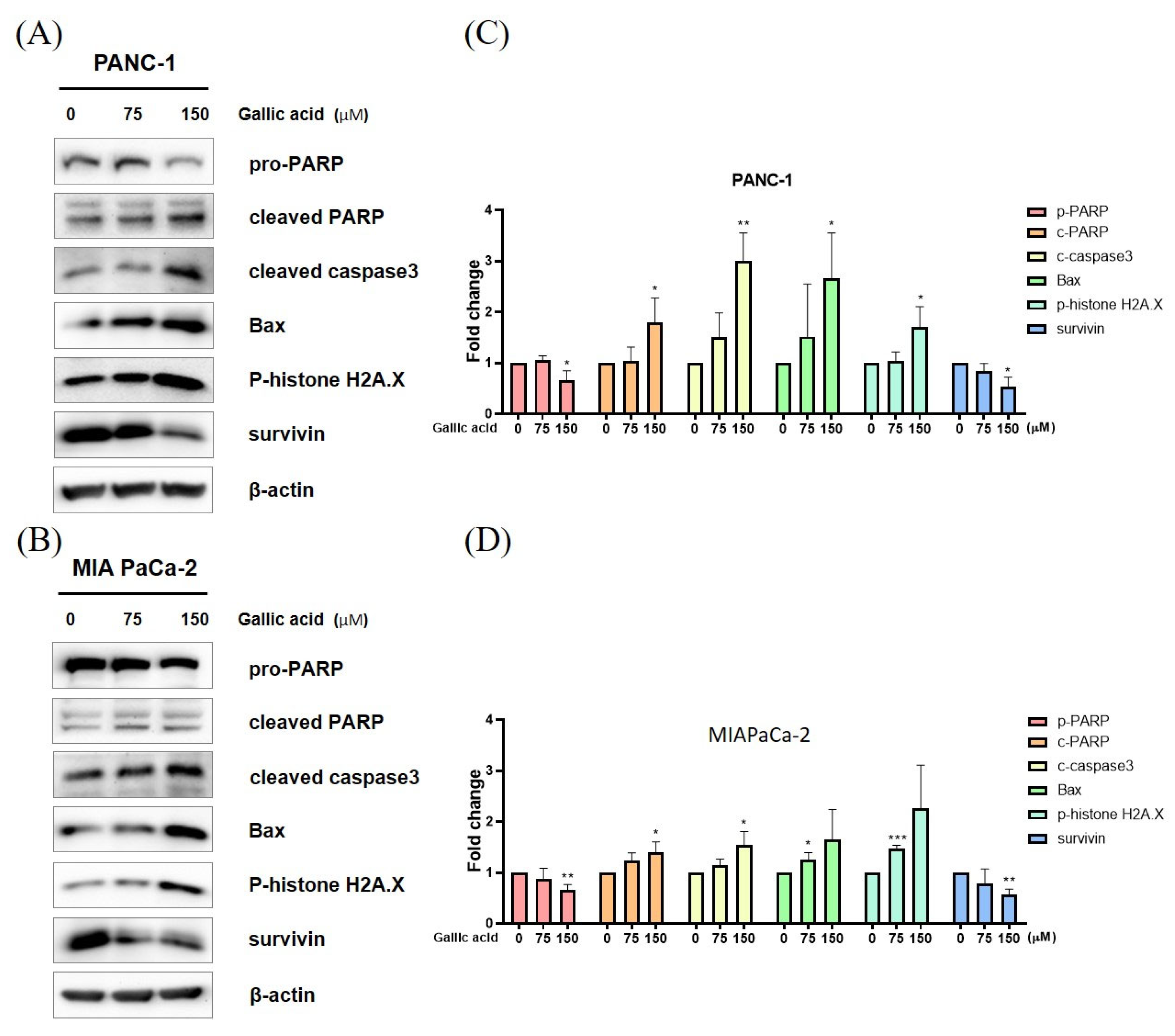
2.3. Gallic Acid Regulated the Expression of Apoptosis-Related Proteins
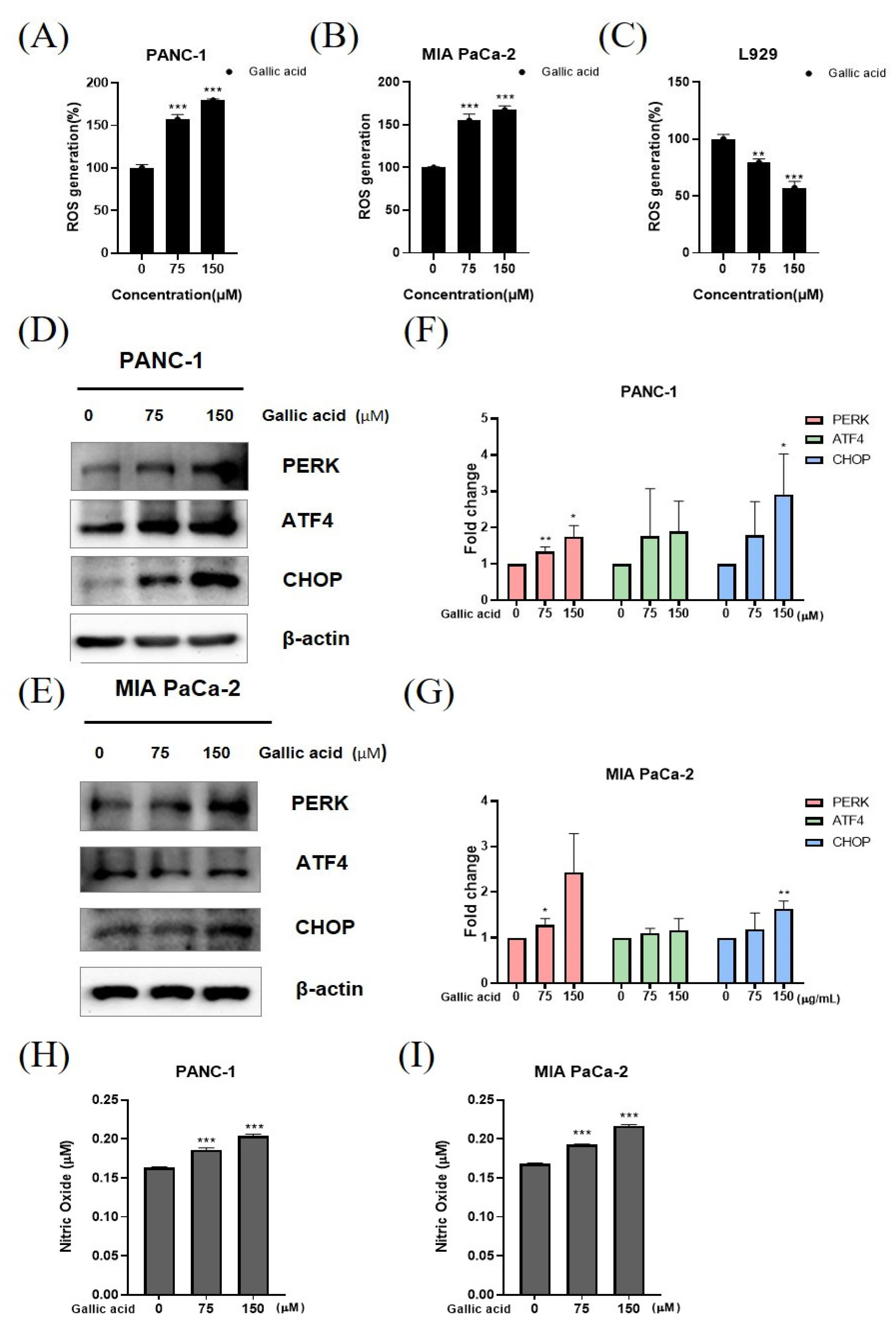
2.4. Gallic Acid Caused ROS Production and ER Stress-Mediated Apoptosis in PANC-1 and MIA PaCa-2 Cells
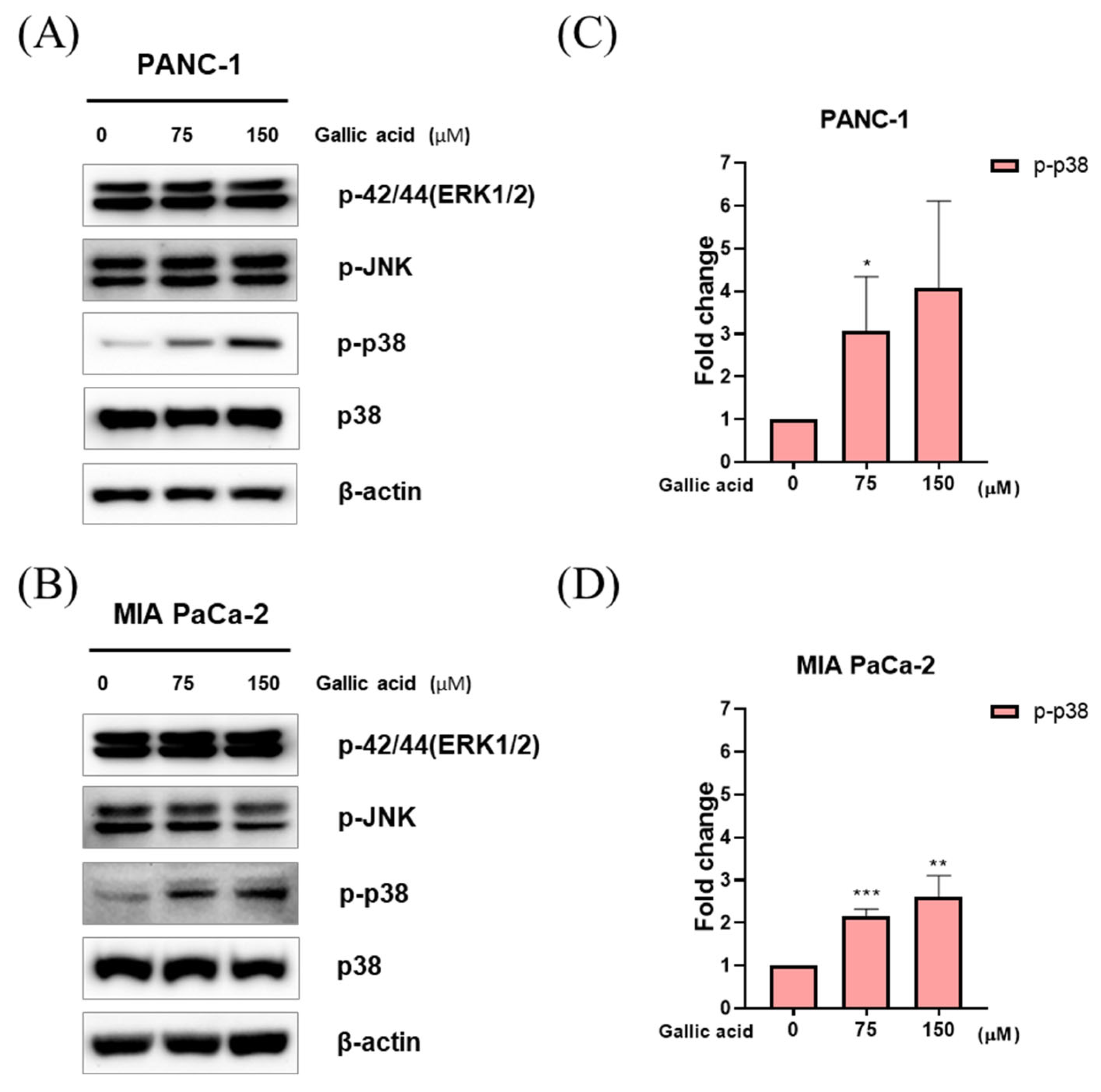
2.5. Gallic Acid Induced p38 Pathways-Mediated Apoptosis in PANC-1 and MIA PaCa-2 Cells
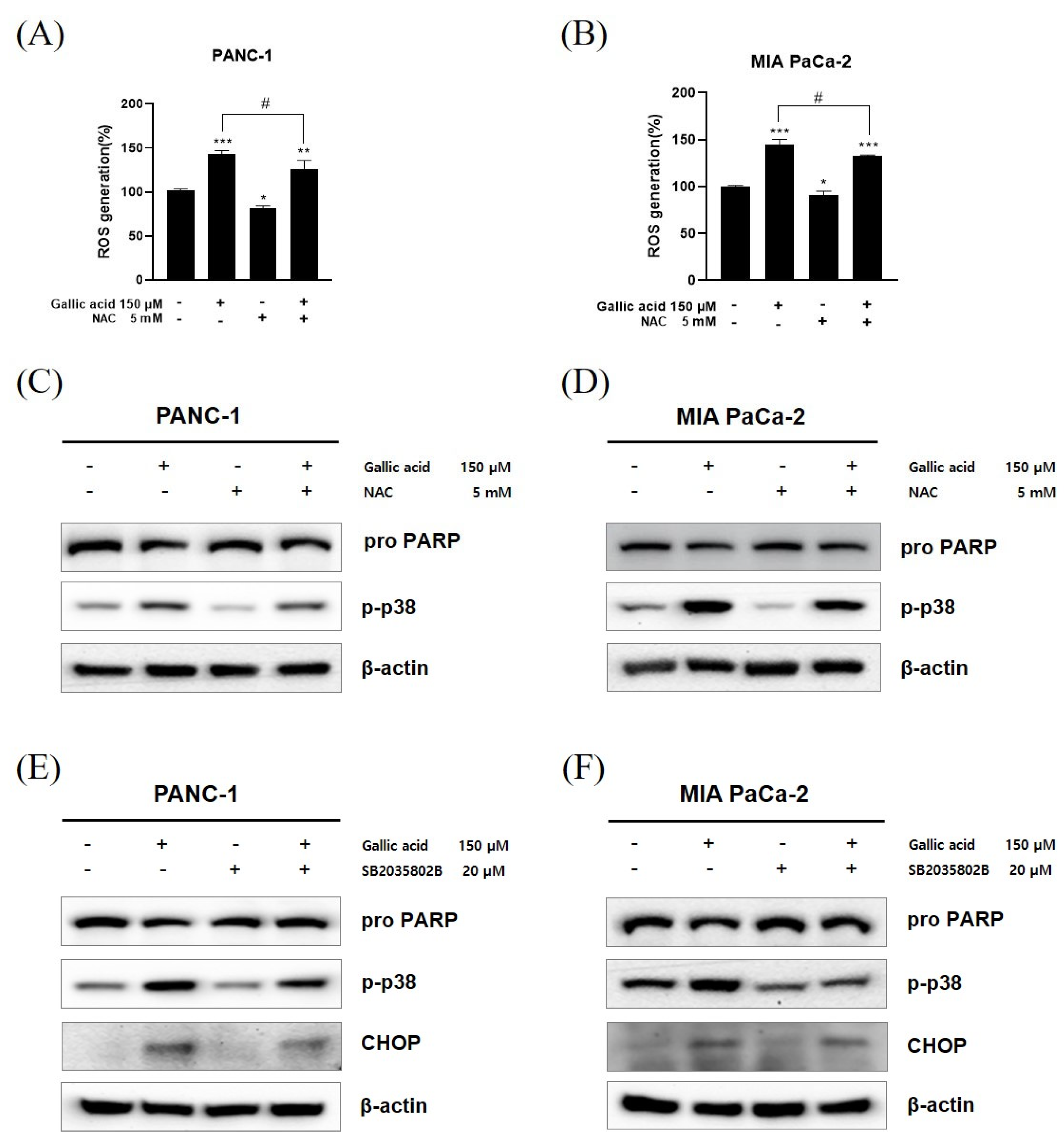
2.6. ROS/p-p38 Signal Pathway Has Pivotal Roles in Gallic Acid Induced Apoptosis
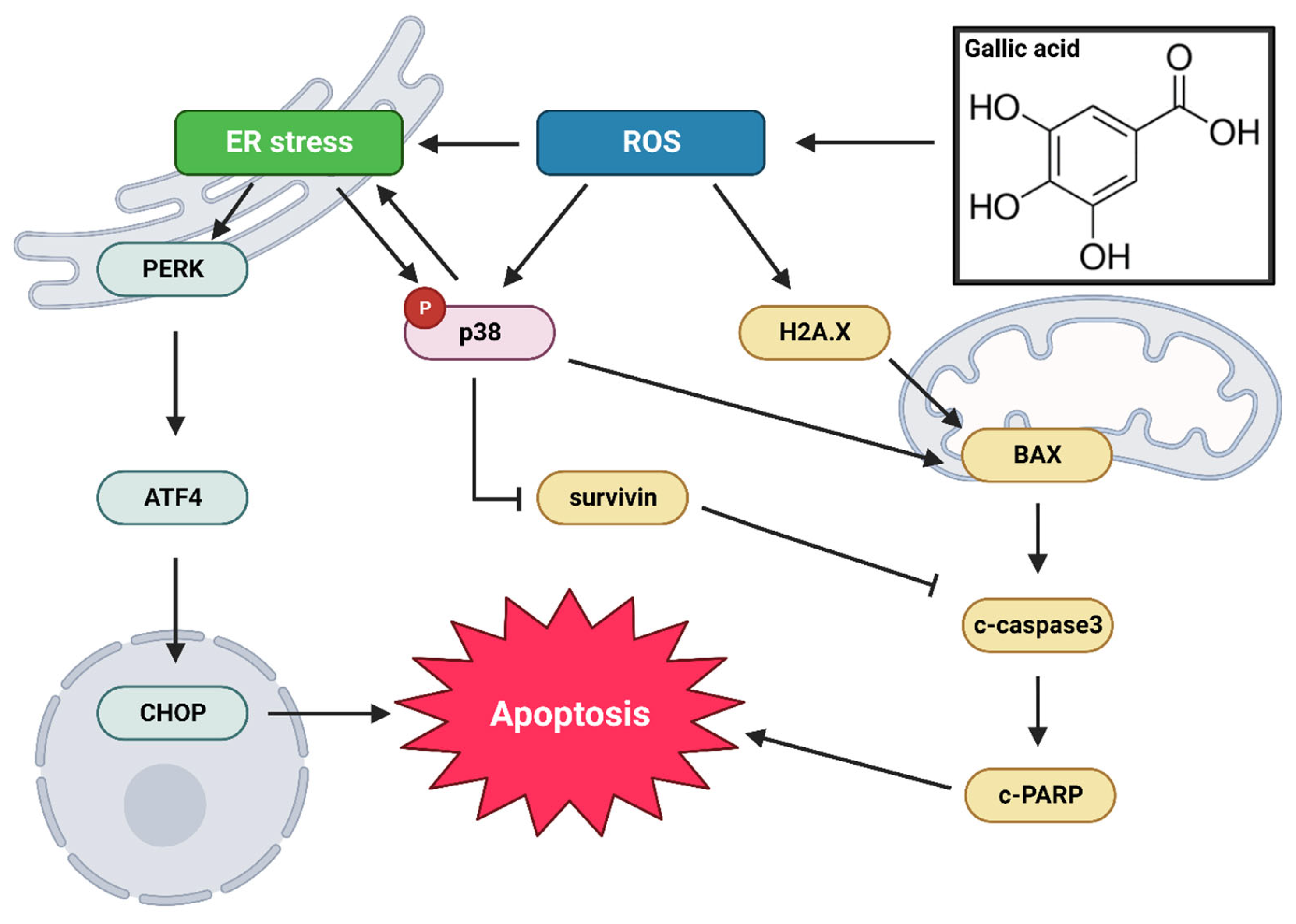
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. TUNEL Assay
4.6. Cell Cycle Analysis
4.7. Western Blot Analysis
4.8. ROS Assay
4.9. Nitric Oxide Assay
4.10. N-Acetylcysteine (NAC) Assay
4.11. N-Acetylcysteine (NAC) Study
4.12. p 38 Inhibitor Study
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Lang, H. Neoadjuvant Therapy of Pancreatic Cancer: Definitions and Benefits. Int. J. Mol. Sci. 2017, 18, 1622. [Google Scholar] [CrossRef] [PubMed]
- Smaglo, B.G.; Pishvaian, M.J. Postresection chemotherapy for pancreatic cancer. Cancer J. 2012, 18, 614–623. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef]
- Yunis, A.A.; Arimura, G.K.; Russin, D.J. Human pancreatic carcinoma (MIA PaCa-2) in continuous culture: Sensitivity to asparaginase. Int. J. Cancer 1977, 19, 128–135. [Google Scholar] [CrossRef]
- Lieber, M.; Mazzetta, J.; Nelson-Rees, W.; Kaplan, M.; Todaro, G. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int. J. Cancer 1975, 15, 741–747. [Google Scholar] [CrossRef]
- Marasini, B.; Sahu, R.P. Natural Anti-Cancer Agents: Implications in Gemcitabine-Resistant Pancreatic Cancer Treatment. Mini Rev. Med. Chem. 2017, 17, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Chia, Y.C.; Rajbanshi, R.; Calhoun, C.; Chiu, R.H. Anti-neoplastic effects of gallic acid, a major component of Toona sinensis leaf extract, on oral squamous carcinoma cells. Molecules 2010, 15, 8377–8389. [Google Scholar] [CrossRef]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Cetera, P.; D’Auria, M.; Mecca, M.; Todaro, L. Gallic acid as main product in the water extractives of Quercus frainetto ten. Nat. Prod. Res. 2019, 33, 2864–2867. [Google Scholar] [CrossRef] [PubMed]
- Makihara, H.; Koike, Y.; Ohta, M.; Horiguchi-Babamoto, E.; Tsubata, M.; Kinoshita, K.; Akase, T.; Goshima, Y.; Aburada, M.; Shimada, T. Gallic Acid, the Active Ingredient of Terminalia bellirica, Enhances Adipocyte Differentiation and Adiponectin Secretion. Biol. Pharm. Bull. 2016, 39, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef]
- Deacon, K.; Mistry, P.; Chernoff, J.; Blank, J.L.; Patel, R. p38 Mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol. Biol. Cell 2003, 14, 2071–2087. [Google Scholar] [CrossRef]
- Lin, C.L.; Lee, C.H.; Chen, C.M.; Cheng, C.W.; Chen, P.N.; Ying, T.H.; Hsieh, Y.H. Protodioscin Induces Apoptosis through ROS-Mediated Endoplasmic Reticulum Stress via the JNK/p38 Activation Pathways in Human Cervical Cancer Cells. Cell. Physiol. Biochem. 2018, 46, 322–334. [Google Scholar] [CrossRef]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.J.; Duan, B.Z.; Huang, L.F. Gallic Acid: A Potential Anti-Cancer Agent. Chin. J. Integr. Med. 2022, 28, 661–671. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Choubey, S.; Goyal, S.; Varughese, L.R.; Kumar, V.; Sharma, A.K.; Beniwal, V. Probing Gallic Acid for Its Broad Spectrum Applications. Mini Rev. Med. Chem. 2018, 18, 1283–1293. [Google Scholar] [CrossRef]
- Zhang, P.; Ye, J.; Dai, J.; Wang, Y.; Chen, G.; Hu, J.; Hu, Q.; Fei, J. Gallic acid inhibits osteoclastogenesis and prevents ovariectomy-induced bone loss. Front. Endocrinol. 2022, 13, 963237. [Google Scholar] [CrossRef]
- Durgun, C.; Deveci, E. Gallic acid treatment protects intestinal tissue against ischaemia-reperfusion. Folia Morphol. 2023, 82, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic acid loaded poloxamer gel as new adjuvant strategy for melanoma: A preliminary study. Colloids Surf. B Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Filippin-Monteiro, F.B.; Creczynski-Pasa, T.B. Alkyl esters of gallic acid as anticancer agents: A review. Eur. J. Med. Chem. 2013, 60, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Qu, C.; Chen, L.; Geng, Y.; Cheng, C.; Yu, S.; Wang, D.; Yang, L.; Meng, Z.; et al. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 2021, 21, 396. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Y.; Wang, Y.; Zhang, H.; Wang, X.; Tang, H.; Huang, H.; Zhou, Z.; Chen, B.; Sun, L. Agrimoniin sensitizes pancreatic cancer to apoptosis through ROS-mediated energy metabolism dysfunction. Phytomedicine 2022, 96, 153807. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Wen, Y.; Wang, J.; Zhao, M.; Lv, S.; Chen, N.; Li, Y.; Wan, L.; Zheng, Q.; Mou, Y.; et al. Gallic acid alleviates gastric precancerous lesions through inhibition of epithelial mesenchymal transition via Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2023, 302 Pt A, 115885. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, W.; Gong, J.; Ardelt, B.; Traganos, F.; Darzynkiewicz, Z. The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents. Cancer Res. 1993, 53, 3186–3192. [Google Scholar]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- Babes, R.M.; Tofolean, I.T.; Sandu, R.G.; Baran, O.E.; Cosoreanu, V.; Ilie, M.T.; Duta, A.I.; Ceausescu, M.C.; Ciucur, P.M.; Costache, S.; et al. Simple discrimination of sub-cycling cells by propidium iodide flow cytometric assay in Jurkat cell samples with extensive DNA fragmentation. Cell Cycle 2018, 17, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-H.; Lu, M.-K.; Lo, H.-C.; Chang, C.-C.; Tseng, A.-J.; Chao, C.-H.; Lin, T.-Y. ZnF3, a sulfated polysaccharide from Antrodia cinnamomea, inhibits lung cancer cells via induction of apoptosis and activation of M1-like macrophage-induced cell death. Int. J. Biol. Macromol. 2023, 238, 124144. [Google Scholar] [CrossRef] [PubMed]
- Heiat, M.; Rezaei, E.; Gharechahi, J.; Abbasi, M.; Behroozi, J.; Abyazi, M.A.; Baradaran, B. Knockdown of SIX4 inhibits pancreatic cancer cells via apoptosis induction. Med. Oncol. 2023, 40, 287. [Google Scholar] [CrossRef]
- Demirel, D.; Ozkaya, F.C.; Ebrahim, W.; Sokullu, E.; Sahin, I.D. Aspergillus Carneus metabolite Averufanin induced cell cycle arrest and apoptotic cell death on cancer cell lines via inducing DNA damage. Sci. Rep. 2023, 13, 6460. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Shin, E.A.; Kim, B.; Kim, B.I.; Chitsazian-Yazdi, M.; Iranshahi, M.; Kim, S.H. Auraptene Induces Apoptosis via Myeloid Cell Leukemia 1-Mediated Activation of Caspases in PC3 and DU145 Prostate Cancer Cells. Phytother. Res. 2017, 31, 891–898. [Google Scholar] [CrossRef]
- Huang, H.; Li, L.J.; Zhang, H.B.; Wei, A.Y. Papaverine selectively inhibits human prostate cancer cell (PC-3) growth by inducing mitochondrial mediated apoptosis, cell cycle arrest and downregulation of NF-κB/PI3K/Akt signalling pathway. J. Buon 2017, 22, 112–118. [Google Scholar] [PubMed]
- Han, B.; Wu, J.; Huang, L. Induction of Apoptosis in Lung Cancer Cells by Viburnum grandiflorum via Mitochondrial Pathway. Med. Sci. Monit. 2020, 26, e920265. [Google Scholar] [CrossRef]
- Marcinkute, M.; Afshinjavid, S.; Fatokun, A.A.; Javid, F.A. Fluoxetine selectively induces p53-independent apoptosis in human colorectal cancer cells. Eur. J. Pharmacol. 2019, 857, 172441. [Google Scholar] [CrossRef]
- Huang, S.S.; Tsai, C.H.; Kuo, C.Y.; Li, Y.S.; Cheng, S.P. ACLY inhibitors induce apoptosis and potentiate cytotoxic effects of sorafenib in thyroid cancer cells. Endocrine 2022, 78, 85–94. [Google Scholar] [CrossRef]
- Tounekti, O.; Belehradek Jr, J.; Mir, L. Relationships between DNA fragmentation, chromatin condensation, and changes in flow cytometry profiles detected during apoptosis. Exp. Cell Res. 1995, 217, 506–516. [Google Scholar] [CrossRef]
- Yu, W.; Chen, G.; Zhang, P.; Chen, K. Purification, partial characterization and antitumor effect of an exopolysaccharide from Rhizopus nigricans. Int. J. Biol. Macromol. 2016, 82, 299–307. [Google Scholar] [CrossRef]
- Leite, A.F.; Bernardo, V.G.; Buexm, L.A.; Fonseca, E.C.; Silva, L.E.; Barroso, D.R.; Lourenço Sde, Q. Immunoexpression of cleaved caspase-3 shows lower apoptotic area indices in lip carcinomas than in intraoral cancer. J. Appl. Oral. Sci. 2016, 24, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Bisen, P.S. Oncoapoptotic signaling and deregulated target genes in cancers: Special reference to oral cancer. Biochim. Biophys. Acta 2013, 1836, 123–145. [Google Scholar] [CrossRef]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef] [PubMed]
- Dozic, B.; Glumac, S.; Boricic, N.; Dozic, M.; Anicic, B.; Boricic, I. Immunohistochemical expression of caspases 9 and 3 in adenoid cystic carcinoma of salivary glands and association with clinicopathological parameters. J. Buon 2016, 21, 152–160. [Google Scholar]
- Van Opdenbosch, N.; Lamkanfi, M. Caspases in Cell Death, Inflammation, and Disease. Immunity 2019, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.M.; Saavedra, V.; Ianez, R.C.F.; de Sousa, E.A.; Gomes, Á.N.; Kelner, N.; Nagai, M.A.; Kowalski, L.P.; Soares, F.A.; Lourenço, S.V.; et al. Apoptotic signaling in salivary mucoepidermoid carcinoma. Head Neck 2019, 41, 2904–2913. [Google Scholar] [CrossRef]
- Suminami, Y.; Nagashima, S.; Vujanovic, N.L.; Hirabayashi, K.; Kato, H.; Whiteside, T.L. Inhibition of apoptosis in human tumour cells by the tumour-associated serpin, SCC antigen-1. Br. J. Cancer 2000, 82, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Zhao, M.; Qiu, H.; Shi, D.; Wang, J.; Tian, Y.; Lin, L.; Deng, W. Effusanin E suppresses nasopharyngeal carcinoma cell growth by inhibiting NF-κB and COX-2 signaling. PLoS ONE 2014, 9, e109951. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, Y.; Herman, B. Caspases, apoptosis and aging. Ageing Res. Rev. 2003, 2, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Festjens, N.; Declercq, W.; Vanden Berghe, T.; Vandenabeele, P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007, 14, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Caspases and Their Substrates. Cold Spring Harb. Perspect. Biol. 2022, 14, a041012. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.; Salvesen, G.S. Human caspases: Activation, specificity, and regulation. J. Biol. Chem. 2009, 284, 21777–21781. [Google Scholar] [CrossRef] [PubMed]
- Park, M.N.; Um, E.-S.; Rahman, M.A.; Kim, J.W.; Park, S.S.; Cho, Y.; Song, H.; Son, S.-R.; Jang, D.S.; Kim, W.; et al. Leonurus japonicus Houttuyn induces reactive oxygen species-mediated apoptosis via regulation of miR-19a-3p/PTEN/PI3K/AKT in U937 and THP-1 cells. J. Ethnopharmacol. 2022, 291, 115129. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.S.; Lindahl, T. Role of poly(ADP-ribose) formation in DNA repair. Nature 1992, 356, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Kessinger, C.; Kobayashi, J.; Chen, B.P.; Chen, D.J.; Chatterjee, A.; Burma, S. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair 2006, 5, 575–590. [Google Scholar] [CrossRef]
- Reed, J.C.; Reed, S.I. Survivin’ cell-separation anxiety. Nat. Cell Biol. 1999, 1, E199–E200. [Google Scholar] [CrossRef] [PubMed]
- Malik, F.; Kumar, A.; Bhushan, S.; Khan, S.; Bhatia, A.; Suri, K.A.; Qazi, G.N.; Singh, J. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis 2007, 12, 2115–2133. [Google Scholar] [CrossRef]
- Park, M.N.; Park, H.; Rahman, M.A.; Kim, J.W.; Park, S.S.; Cho, Y.; Choi, J.; Son, S.R.; Jang, D.S.; Shim, B.S.; et al. BK002 Induces miR-192-5p-Mediated Apoptosis in Castration-Resistant Prostate Cancer Cells via Modulation of PI3K/CHOP. Front. Oncol. 2022, 12, 791365. [Google Scholar] [CrossRef]
- Clarke, H.J.; Chambers, J.E.; Liniker, E.; Marciniak, S.J. Endoplasmic reticulum stress in malignancy. Cancer Cell 2014, 25, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Moghtaderi, H.; Sepehri, H.; Delphi, L.; Attari, F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. Bioimpacts 2018, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Obinata, H.; Konishi, A.; Yamagiwa, N.; Tsuneoka, M. Production of ROS by Gallic Acid Activates KDM2A to Reduce rRNA Transcription. Cells 2020, 9, 2266. [Google Scholar] [CrossRef]
- You, B.R.; Kim, S.Z.; Kim, S.H.; Park, W.H. Gallic acid-induced lung cancer cell death is accompanied by ROS increase and glutathione depletion. Mol. Cell. Biochem. 2011, 357, 295–303. [Google Scholar] [CrossRef]
- Subramanian, A.P.; Jaganathan, S.K.; Mandal, M.; Supriyanto, E.; Muhamad, I.I. Gallic acid induced apoptotic events in HCT-15 colon cancer cells. World J. Gastroenterol. 2016, 22, 3952–3961. [Google Scholar] [CrossRef]
- Yeh, R.D.; Chen, J.C.; Lai, T.Y.; Yang, J.S.; Yu, C.S.; Chiang, J.H.; Lu, C.C.; Yang, S.T.; Yu, C.C.; Chang, S.J.; et al. Gallic acid induces G0/G1 phase arrest and apoptosis in human leukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer. Res. 2011, 31, 2821–2832. [Google Scholar] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Yang, M.; Hong, F.; Yang, S. Protein Phosphorylation in Cancer: Role of Nitric Oxide Signaling Pathway. Biomolecules 2021, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Rigas, B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr. Opin. Gastroenterol. 2007, 23, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Mazzon, E.; Krajnovic, T.; Draca, D.; Cavalli, E.; Al-Abed, Y.; Bramanti, P.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Anticancer and Differentiation Properties of the Nitric Oxide Derivative of Lopinavir in Human Glioblastoma Cells. Molecules 2018, 23, 2463. [Google Scholar] [CrossRef]
- Maksimovic-Ivanic, D.; Mijatovic, S.; Miljkovic, D.; Harhaji-Trajkovic, L.; Timotijevic, G.; Mojic, M.; Dabideen, D.; Cheng, K.F.; McCubrey, J.A.; Mangano, K.; et al. The antitumor properties of a nontoxic, nitric oxide-modified version of saquinavir are independent of Akt. Mol. Cancer Ther. 2009, 8, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, S.; Maksimovic-Ivanic, D.; Mojic, M.; Malaponte, G.; Libra, M.; Cardile, V.; Miljkovic, D.; Harhaji, L.; Dabideen, D.; Cheng, K.F.; et al. Novel nitric oxide-donating compound (S,R)-3-phenyl-4,5-dihydro-5-isoxazole acetic acid-nitric oxide (GIT-27NO) induces p53 mediated apoptosis in human A375 melanoma cells. Nitric Oxide 2008, 19, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, S.; Maksimovic-Ivanic, D.; Mojic, M.; Timotijevic, G.; Miljkovic, D.; Mangano, K.; Donia, M.; Di Cataldo, A.; Al-Abed, Y.; Cheng, K.F.; et al. Cytotoxic and immune-sensitizing properties of nitric oxide-modified Saquinavir in iNOS-positive human melanoma cells. J. Cell. Physiol. 2011, 226, 1803–1812. [Google Scholar] [CrossRef]
- Mojic, M.; Mijatovic, S.; Maksimovic-Ivanic, D.; Dinic, S.; Grdovic, N.; Miljkovic, D.; Stosic-Grujicic, S.; Tumino, S.; Fagone, P.; Mangano, K.; et al. Saquinavir-NO-targeted S6 protein mediates sensitivity of androgen-dependent prostate cancer cells to TRAIL. Cell Cycle 2012, 11, 1174–1182. [Google Scholar] [CrossRef]
- Mojic, M.; Mijatovic, S.; Maksimovic-Ivanic, D.; Miljkovic, D.; Stosic-Grujicic, S.; Stankovic, M.; Mangano, K.; Travali, S.; Donia, M.; Fagone, P.; et al. Therapeutic potential of nitric oxide-modified drugs in colon cancer cells. Mol. Pharmacol. 2012, 82, 700–710. [Google Scholar] [CrossRef]
- Paskaš, S.; Krajnović, T.; Basile, M.S.; Dunđerović, D.; Cavalli, E.; Mangano, K.; Mammana, S.; Al-Abed, Y.; Nicoletti, F.; Mijatović, S.; et al. Senescence as a main mechanism of Ritonavir and Ritonavir-NO action against melanoma. Mol. Carcinog. 2019, 58, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Paskaš, S.; Mazzon, E.; Basile, M.S.; Cavalli, E.; Al-Abed, Y.; He, M.; Rakocevic, S.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Lopinavir-NO, a nitric oxide-releasing HIV protease inhibitor, suppresses the growth of melanoma cells in vitro and in vivo. Investig. New Drugs 2019, 37, 1014–1028. [Google Scholar] [CrossRef]
- Cicenas, J.; Zalyte, E.; Rimkus, A.; Dapkus, D.; Noreika, R.; Urbonavicius, S. JNK, p38, ERK, and SGK1 Inhibitors in Cancer. Cancers 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.J.; Tenbaum, S.; Perdiguero, E.; Huth, M.; Guerra, C.; Barbacid, M.; Pasparakis, M.; Nebreda, A.R. p38α MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat. Genet. 2007, 39, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Korc, M. p38 MAPK in pancreatic cancer: Finding a protective needle in the haystack. Clin. Cancer Res. 2014, 20, 5866–5868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.-Q.; Zhang, S.-Q.; Bai, J.-X.; Liu, B.; Yung, K.K.-L.; Ko, J.K.-S. Isoliquiritigenin inhibits pancreatic cancer progression through blockade of p38 MAPK-regulated autophagy. Phytomedicine 2022, 106, 154406. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, H.P.; Rodrigues, E.G.; Amorim Reis, A.K.C.; Longo, L.S., Jr.; Ogata, F.T.; Moretti, A.I.S.; da Costa, P.E.; Teodoro, A.C.S.; Toledo, M.S.; Stern, A. Nitric oxide and interactions with reactive oxygen species in the development of melanoma, breast, and colon cancer: A redox signaling perspective. Nitric Oxide 2019, 89, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, M.N.; Jeon, H.W.; Rahman, M.A.; Park, S.S.; Jeong, S.Y.; Kim, K.H.; Kim, S.H.; Kim, W.; Kim, B. Daemonorops draco Blume Induces Apoptosis Against Acute Myeloid Leukemia Cells via Regulation of the miR-216b/c-Jun. Front. Oncol. 2022, 12, 808174. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, X.; Guo, Y.; Ge, K. Evodiamine induces ROS-Dependent cytotoxicity in human gastric cancer cells via TRPV1/Ca2+ pathway. Chem. Biol. Interact. 2022, 351, 109756. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lee, K.T.; Lim, M.C.; Choi, J.H. TRPV1 Antagonist DWP05195 Induces ER Stress-Dependent Apoptosis through the ROS-p38-CHOP Pathway in Human Ovarian Cancer Cells. Cancers 2020, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Cheng, Y.C.; Lin, Y.C.; Chou, C.H.; Ho, C.T.; Wang, H.K.; Way, T.D. CSC-3436 sensitizes triple negative breast cancer cells to TRAIL-induced apoptosis through ROS-mediated p38/CHOP/death receptor 5 signaling pathways. Environ. Toxicol. 2021, 36, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Zhang, Y.; Ji, W.; Wang, L.; Lee, S.C. Periplogenin Activates ROS-ER Stress Pathway to Trigger Apoptosis via BIP-eIF2α- CHOP and IRE1α-ASK1-JNK Signaling Routes. Anticancer. Agents Med. Chem. 2021, 21, 61–70. [Google Scholar] [CrossRef]
- Bezverkhniaia, E.A.; Ermilova, E.V.; Kadyrova, T.V.; Krasnov, E.A.; Brazovskii, K.S.; Ponkratova, A.O.; Luzhanin, V.G.; Belousov, M.V. Phytochemistry, ethnopharmacology and pharmacology of the genus Empetrum: A review. Adv. Tradit. Med. 2023, 23, 659–672. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.W.; Choi, J.; Park, M.N.; Kim, B. Apoptotic Effect of Gallic Acid via Regulation of p-p38 and ER Stress in PANC-1 and MIA PaCa-2 Cells Pancreatic Cancer Cells. Int. J. Mol. Sci. 2023, 24, 15236. https://doi.org/10.3390/ijms242015236
Kim JW, Choi J, Park MN, Kim B. Apoptotic Effect of Gallic Acid via Regulation of p-p38 and ER Stress in PANC-1 and MIA PaCa-2 Cells Pancreatic Cancer Cells. International Journal of Molecular Sciences. 2023; 24(20):15236. https://doi.org/10.3390/ijms242015236
Chicago/Turabian StyleKim, Jeong Woo, Jinwon Choi, Moon Nyeo Park, and Bonglee Kim. 2023. "Apoptotic Effect of Gallic Acid via Regulation of p-p38 and ER Stress in PANC-1 and MIA PaCa-2 Cells Pancreatic Cancer Cells" International Journal of Molecular Sciences 24, no. 20: 15236. https://doi.org/10.3390/ijms242015236
APA StyleKim, J. W., Choi, J., Park, M. N., & Kim, B. (2023). Apoptotic Effect of Gallic Acid via Regulation of p-p38 and ER Stress in PANC-1 and MIA PaCa-2 Cells Pancreatic Cancer Cells. International Journal of Molecular Sciences, 24(20), 15236. https://doi.org/10.3390/ijms242015236







