Adipocytes in the Uterine Wall during Experimental Healing and in Cesarean Scars during Pregnancy
Abstract
:1. Introduction
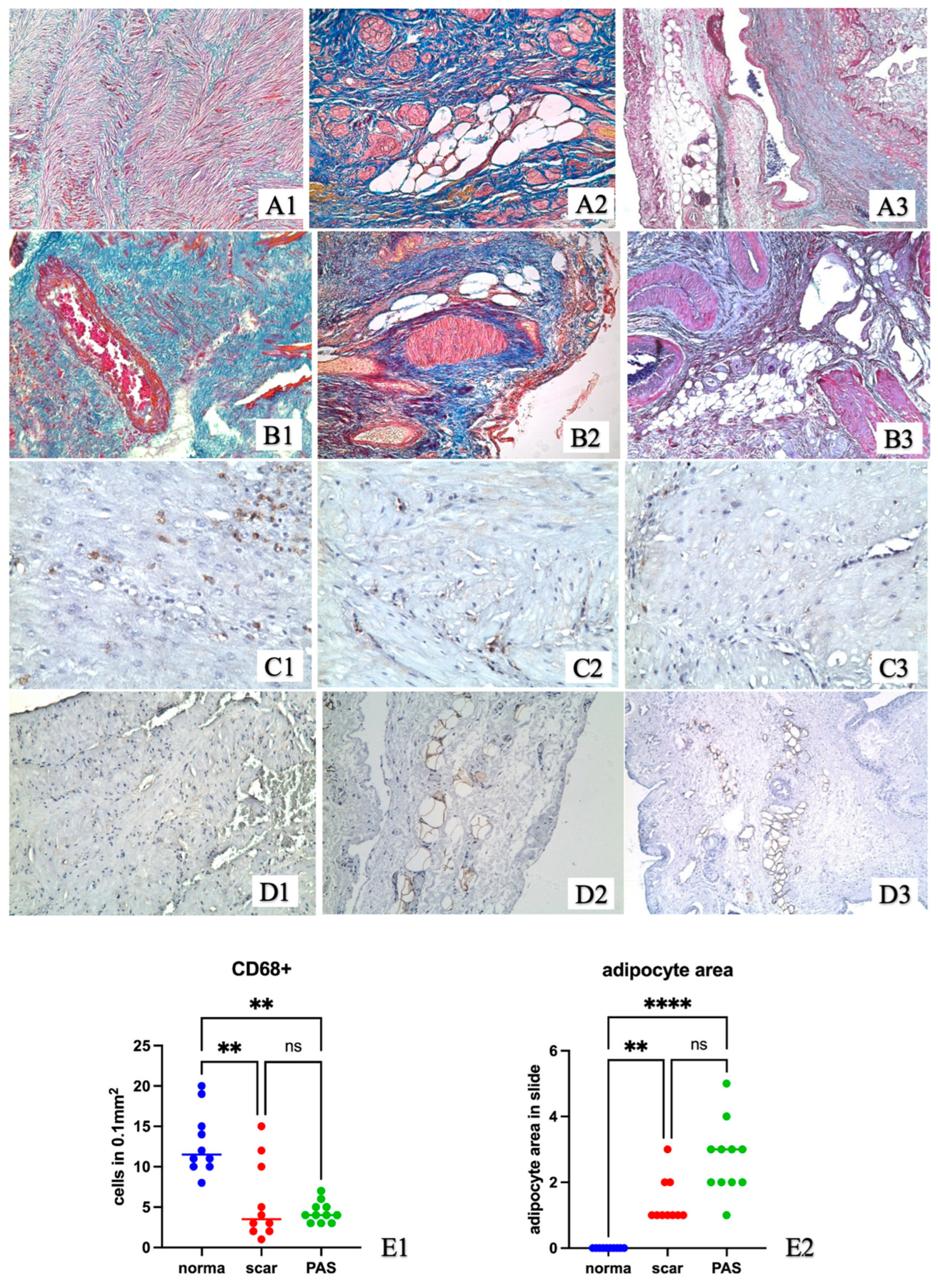
2. Results
2.1. Experimental Study
2.2. Study Samples of Uterine Wall after Caesarean Section from Women
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Dong, H.; Balaz, M.; Slyper, M.; Drokhlyansky, E.; Colleluori, G.; Giordano, A.; Kovanicova, Z.; Stefanicka, P.; Balazova, L.; et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 2020, 587, 98–102. [Google Scholar] [CrossRef]
- Shook, B.A.; Wasko, R.R.; Mano, O.; Rutenberg-Schoenberg, M.; Rudolph, M.C.; Zirak, B.; Rivera-Gonzalez, G.C.; López-Giráldez, F.; Zarini, S.; Rezza, A.; et al. Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell 2020, 26, 880–895.e6. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, F.; Iwaguro, H.; Harada, Y.; Sobajima, S.; Suwabe, T.; Miyamoto, S. Adipose tissue-derived regenerative cells improve implantation of fertilized eggs in thin endometrium. Regen Med. 2020, 15, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Ai, G.; Wang, L.; Qin, J.; Li, Y.; Jiang, H.; Zhang, T.; Zhou, L.; Gao, Z.; Cheng, J.; et al. Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote 2019, 27, 367–374. [Google Scholar] [CrossRef]
- Sun, H.; Lu, J.; Li, B.; Chen, S.; Xiao, X.; Wang, J.; Wang, J.; Wang, X. Partial regeneration of uterine horns in rats through adipose-derived stem cell sheets. Biol. Reprod. 2018, 99, 1057–1069. [Google Scholar] [CrossRef]
- Tikhonova, N.B.; Milovanov, A.P.; Aleksankina, V.V.; Fokina, T.V.; Boltovskaya, M.N.; Aleksankin, A.P.; Artem’eva, K.A. Analysis of Healing of Rat Uterine Wall After Full-Thickness Surgical Incision. Bull. Exp. Biol. Med. 2021, 172, 100–104. [Google Scholar] [CrossRef]
- Tikhonova, N.B.; Milovanov, A.P.; Aleksankina, V.V.; Fokina, T.V.; Boltovskaya, M.N.; Aleksankin, A.P. The role of adipose tissue in the healing of rat uterine wall after a full-thickness surgical incision. Clin. Exp. Morphol. 2021, 10, 72–80. [Google Scholar] [CrossRef]
- Jovanovic, B.; Petrovic, A.; Petrovic, B. Postpartum hysterectomy performed as a consequence of chronic myometritis. Med. Pregl. 2008, 61, 521–524. (In Serbian) [Google Scholar] [CrossRef]
- Alsaif, J.M.; Alali, Z.S.; Elsharkawy, T.; Ahmed, A. Uterine Lipoleiomyoma: A Case Report and Review of Literature. Cureus 2021, 13, e20297. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Zhang, N.; Wang, D. Uterine angiomyolipoma: A clinical analysis of 8 cases and literature review. Arch. Gynecol. Obstet. 2021, 304, 171–177. [Google Scholar] [CrossRef]
- Gálvez, B.G.; Martín, N.S.; Salama-Cohen, P.; Lazcano, J.J.; Coronado, M.J.; Lamelas, M.L.; Alvarez-Barrientes, A.; Eiró, N.; Vizoso, F.; Rodríguez, C. An adult myometrial pluripotential precursor that promotes healing of damaged muscular tissues. In Vivo 2010, 24, 431–441. [Google Scholar] [PubMed]
- Milovanov, A.P.; Tikhonova, N.B.; Fokina, T.V.; Kulikov, I.A.; Nizyaeva, N.V. Detection of fat cells in uterine scars during normal pregnancy and with placenta accreta spectrum. J. Anat. Histopathol. 2023, 12, 57–64. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Wu, Y.; Zeng, J.; Yuan, X.; Tong, C.; Qi, H. What we know about placenta accreta spectrum (PAS). Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Chantraine, F.; Silver, R.M.; Langhoff-Roos, J.; FIGO Placenta Accreta Diagnosis and Management Expert Consensus Panel. FIGO consensus guidelines on placenta accreta spectrum disorders: Epidemiology. Int. J. Gynaecol. Obstet. 2018, 140, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Roeder, H.A.; Cramer, S.F.; Leppert, P.C. A look at uterine wound healing through a histopathological study of uterine scars. Reprod. Sci. 2012, 19, 463–473. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Mei, Z.; Zhou, J.; Wu, L.; Chiu, W.-H.; Xiao, X. A preliminary study of uterine scar tissue following cesarean section. J. Perinat. Med. 2018, 46, 379–386. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D.; Hussein, A.M.; Burton, G.J. New insights into the etiopathology of placenta accreta spectrum. Am. J. Obstet. Gynecol. 2022, 227, 384–391. [Google Scholar] [CrossRef]
- Tantbirojn, P.; Crum, C.P.; Parast, M.M. Pathophysiology of placenta creta: The role of decidua and extravillous trophoblast. Placenta 2008, 29, 639–645. [Google Scholar] [CrossRef]
- Keag, O.E.; Norman, J.E.; Stock, S.J. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis. PLoS Med. 2018, 15, e1002494. [Google Scholar] [CrossRef]
- Thurn, L.; Lindqvist, P.; Jakobsson, M.; Colmorn, L.; Klungsoyr, K.; Bjarnadóttir, R.; Tapper, A.; Børdahl, P.; Gottvall, K.; Petersen, K.; et al. Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: Results from a large population-based pregnancy cohort study in the Nordic countries. BJOG 2016, 123, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.M.; Landon, M.B.; Rouse, D.J.; Leveno, K.J.; Spong, C.Y.; Thom, E.A.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet. Gynecol. 2006, 107, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Antila-Långsjö, R.M.; Mäenpää, J.U.; Huhtala, H.S.; Tomás, E.I.; Staff, S.M. Cesarean scar defect: A prospective study on risk factors. Am. J. Obstet. Gynecol. 2018, 219, 458.e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Karpathiou, G.; Chauleur, C.; Dridi, M.; Baillard, P.; Corsini, T.; Dumollard, J.M.; Peoc’h, M. Histologic Findings of Uterine Niches. Am. J. Clin. Pathol. 2020, 154, 645–655. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, Y.; Ma, J. Animal models of the placenta accreta spectrum: Current status and further perspectives. Front. Endocrinol. 2023, 14, 1118168. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Guerrero-Juarez, C.F.; Hata, T.; Bapat, S.P.; Ramos, R.; Plikus, M.V.; Gallo, R.L. Innate immunity. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015, 347, 67–71. [Google Scholar] [CrossRef]
- Schmidt, B.A.; Horsley, V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development 2013, 140, 1517–1527. [Google Scholar] [CrossRef]
- Ploner, C.; Rauchenwald, T.; Connolly, C.E.; Joehrer, K.; Rainer, J.; Seifarth, C.; Hermann, M.; Nagl, M.; Lobenwein, S.; Wilflingseder, D.; et al. Oxidant therapy improves adipogenic differentiation of adipose-derived stem cells in human wound healing. Stem. Cell Res. Ther. 2021, 12, 280. [Google Scholar] [CrossRef]
- Félix, I.; Jokela, H.; Karhula, J.; Kotaja, N.; Savontaus, E.; Salmi, M.; Rantakari, P. Single-Cell Proteomics Reveals the Defined Heterogeneity of Resident Macrophages in White Adipose Tissue. Front. Immunol. 2021, 12, 719979. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Lee, Y.H.; Petkova, A.P.; Granneman, J.G. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab. 2013, 18, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Varghese, M.; Griffin, C.; McKernan, K.; Eter, L.; Lanzetta, N.; Agarwal, D.; Abrishami, S.; Singer, K. Sex differences in inflammatory responses to adipose tissue lipolysis in diet-induced obesity. Endocrinology 2019, 160, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.K.; Li, Y.; Dang, Q.; Yang, F. Monocytes in Rheumatoid Arthritis: Circulating Precursors of Macrophages and Osteoclasts and, Their Heterogeneity and Plasticity Role in RA Pathogenesis. Int. Immunopharmacol. 2018, 65, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Martinez-Pomares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef]
- Lee, S.J.; Evers, S.; Roeder, D.; Parlow, A.F.; Risteli, J.; Risteli, L.; Lee, Y.C.; Feizi, T.; Langen, H.; Nussenzweig, M.C. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 2002, 295, 1898–1901. [Google Scholar] [CrossRef] [PubMed]
- Klessens, C.Q.F.; Zandbergen, M.; Wolterbeek, R.; Bruijn, J.A.; Rabelink, T.J.; Bajema, I.M.; IJpelaar, D.H.T. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol. Dial. Transplant. 2017, 32, 1322–1329. [Google Scholar] [CrossRef]
- Tang, P.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Ooi, G.; Bayliss, J.; O’Brien, P.E.; Schittenhelm, R.B.; Clark, A.K.; Taylor, R.A.; Rodeheffer, M.S.; Burton, P.R.; Watt, M.J. Identification of Metabolically Distinct Adipocyte Progenitor Cells in Human Adipose Tissues. Cell Rep. 2019, 27, 1528–1540.e7. [Google Scholar] [CrossRef]
- Fuseya, T.; Furuhashi, M.; Matsumoto, M.; Watanabe, Y.; Hoshina, K.; Mita, T.; Ishimura, S.; Tanaka, M.; Miura, T. Ectopic Fatty Acid-Binding Protein 4 Expression in the Vascular Endothelium is Involved in Neointima Formation after Vascular Injury. J. Am. Heart Assoc. 2017, 6, e006377. [Google Scholar] [CrossRef]
- Mirza, R.; DiPietro, L.A.; Koh, T.J. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 2009, 175, 2454–2462. [Google Scholar] [CrossRef]
- Keuper, M. On the role of macrophages in the control of adipocyte energy metabolism. Endocr. Connect. 2019, 8, R105–R121. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Morgan-Bathke, M.E.; Jensen, M.D. Adipose tissue macrophage burden, systemic inflammation, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E254–E264. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.; Møller, H.J.; Nielsen, M.J.; Jacobsen, C.; Graversen, J.H.; van den Berg, T.; Moestrup, S.K. Molecular characterization of the haptoglobin·hemoglobin receptor CD163. Ligand binding properties of the scavenger receptor cysteine-rich domain region. J. Biol. Chem. 2004, 279, 51561–51567.e6. [Google Scholar] [CrossRef] [PubMed]
- Ugocsai, P.; Barlage, S.; Dada, A.; Schmitz, G. Regulation of surface CD163 expression and cellular effects of receptor mediated hemoglobin-haptoglobin uptake on human monocytes and macrophages. Cytom. A 2006, 69, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Schleiffenbaum, B.; Kurrer, M.; Imhof, A.; Bächli, E.; Fehr, J.; Moller, H.J.; Moestrup, S.K.; Schaffner, A. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur. J. Haematol. 2005, 74, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Akahori, H.; Harari, E.; Smith, S.L.; Polavarapu, R.; Karmali, V.; Otsuka, F.; Gannon, R.L.; Braumann, R.E.; Dickinson, M.H.; et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 1106–1124. [Google Scholar] [CrossRef]
- Azad, A.K.; Rajaram, M.V.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1000003. [Google Scholar] [CrossRef]
- Paccola, C.C.; Resende, C.G.; Stumpp, T.; Miraglia, S.M.; Cipriano, I. The rat estrous cycle revisited: A quantitative and qualitative analysis. Anim. Reprod. 2013, 10, 677–683. [Google Scholar]
- Andersson, H.; Rehm, S.; Stanislaus, D.; Wood, C.E. Scientific and regulatory policy committee (SRPC) paper: Assessment of circulating hormones in nonclinical toxicity studies III. female reproductive hormones. Toxicol. Pathol. 2013, 41, 921–934. [Google Scholar] [CrossRef]









| Group | Control (n = 10) | Scar (n = 10) | PAS (n = 11) | |||
|---|---|---|---|---|---|---|
| Me (Q1–Q3) | Min–Max | Me (Q1–Q3) | Min–Max | Me (Q1–Q3) | Min–Max | |
| Age, years | 29.5 (26;37) | 25–41 | 35 (33;38) | 27–41 | 35 (33;38) | 29–42 |
| Number of CS | 1 (1;1) | 1–1 | 2 (2;3) | 2–4 | 2 (2;2) | 2–3 |
| Gestational age, weeks | 39 (39;40) | 39–40 | 39 (38.5;39.5) | 38–40 | 34 (33;37) | 27–38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhonova, N.; Milovanov, A.P.; Aleksankina, V.V.; Kulikov, I.A.; Fokina, T.V.; Aleksankin, A.P.; Belousova, T.N.; Mikhaleva, L.M.; Niziaeva, N.V. Adipocytes in the Uterine Wall during Experimental Healing and in Cesarean Scars during Pregnancy. Int. J. Mol. Sci. 2023, 24, 15255. https://doi.org/10.3390/ijms242015255
Tikhonova N, Milovanov AP, Aleksankina VV, Kulikov IA, Fokina TV, Aleksankin AP, Belousova TN, Mikhaleva LM, Niziaeva NV. Adipocytes in the Uterine Wall during Experimental Healing and in Cesarean Scars during Pregnancy. International Journal of Molecular Sciences. 2023; 24(20):15255. https://doi.org/10.3390/ijms242015255
Chicago/Turabian StyleTikhonova, Natalia, Andrey P. Milovanov, Valentina V. Aleksankina, Ilyas A. Kulikov, Tatiana V. Fokina, Andrey P. Aleksankin, Tamara N. Belousova, Ludmila M. Mikhaleva, and Natalya V. Niziaeva. 2023. "Adipocytes in the Uterine Wall during Experimental Healing and in Cesarean Scars during Pregnancy" International Journal of Molecular Sciences 24, no. 20: 15255. https://doi.org/10.3390/ijms242015255
APA StyleTikhonova, N., Milovanov, A. P., Aleksankina, V. V., Kulikov, I. A., Fokina, T. V., Aleksankin, A. P., Belousova, T. N., Mikhaleva, L. M., & Niziaeva, N. V. (2023). Adipocytes in the Uterine Wall during Experimental Healing and in Cesarean Scars during Pregnancy. International Journal of Molecular Sciences, 24(20), 15255. https://doi.org/10.3390/ijms242015255









