Proximal Tubular Lats2 Ablation Exacerbates Ischemia/Reperfusion Injury (IRI)-Induced Renal Maladaptive Repair through the Upregulation of P53
Abstract
1. Introduction
2. Results
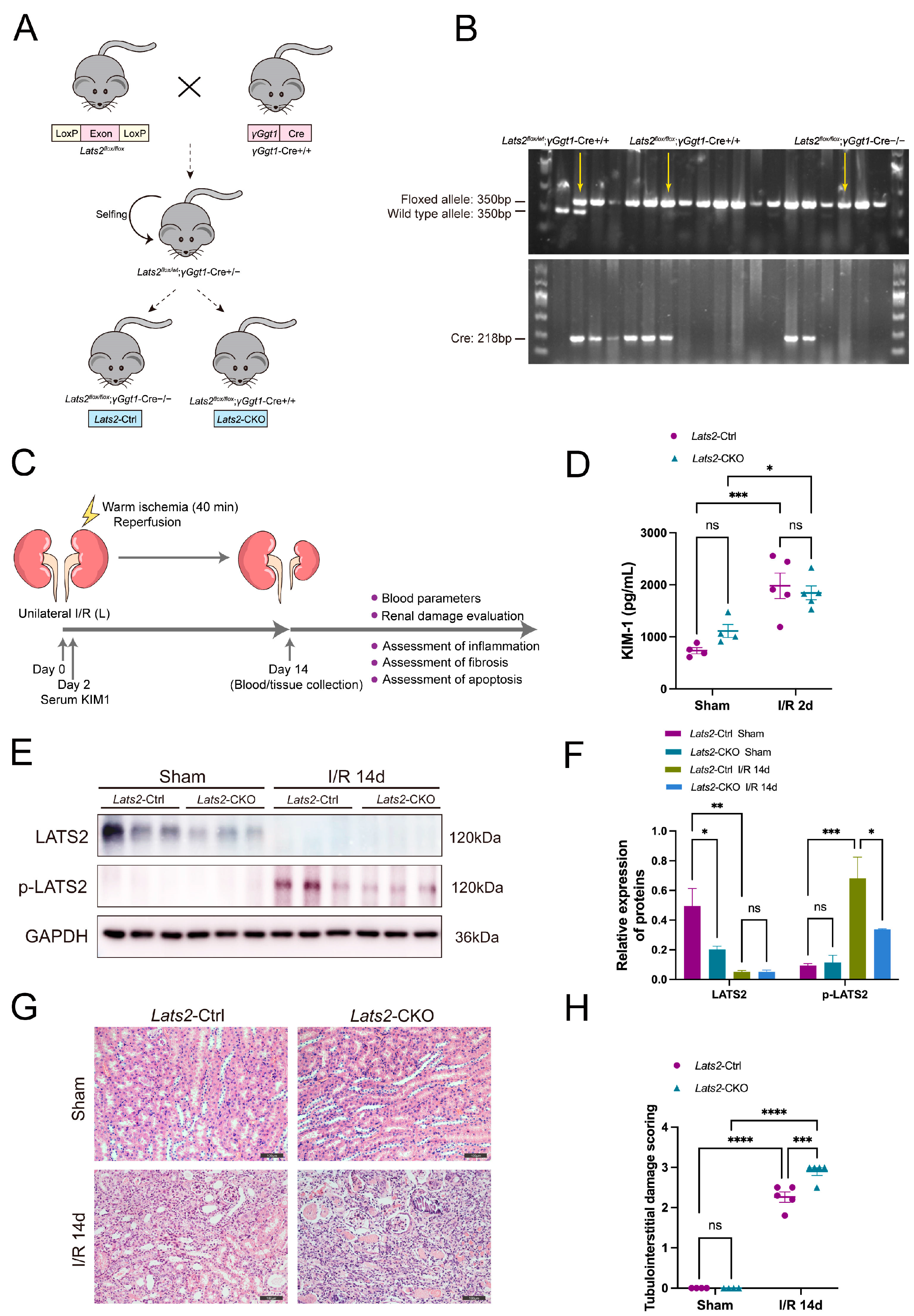
2.1. RNA-seq Analysis Identified Downregulation of Lats2 in Post-IRI Kidneys
2.2. Loss of Lats2 Aggravates Kidney Parenchymal Injury in U-IRI Model
2.3. Proximal Tubule-Specific Knockout of Lats2 Exacerbates Renal Fibrosis after AKI
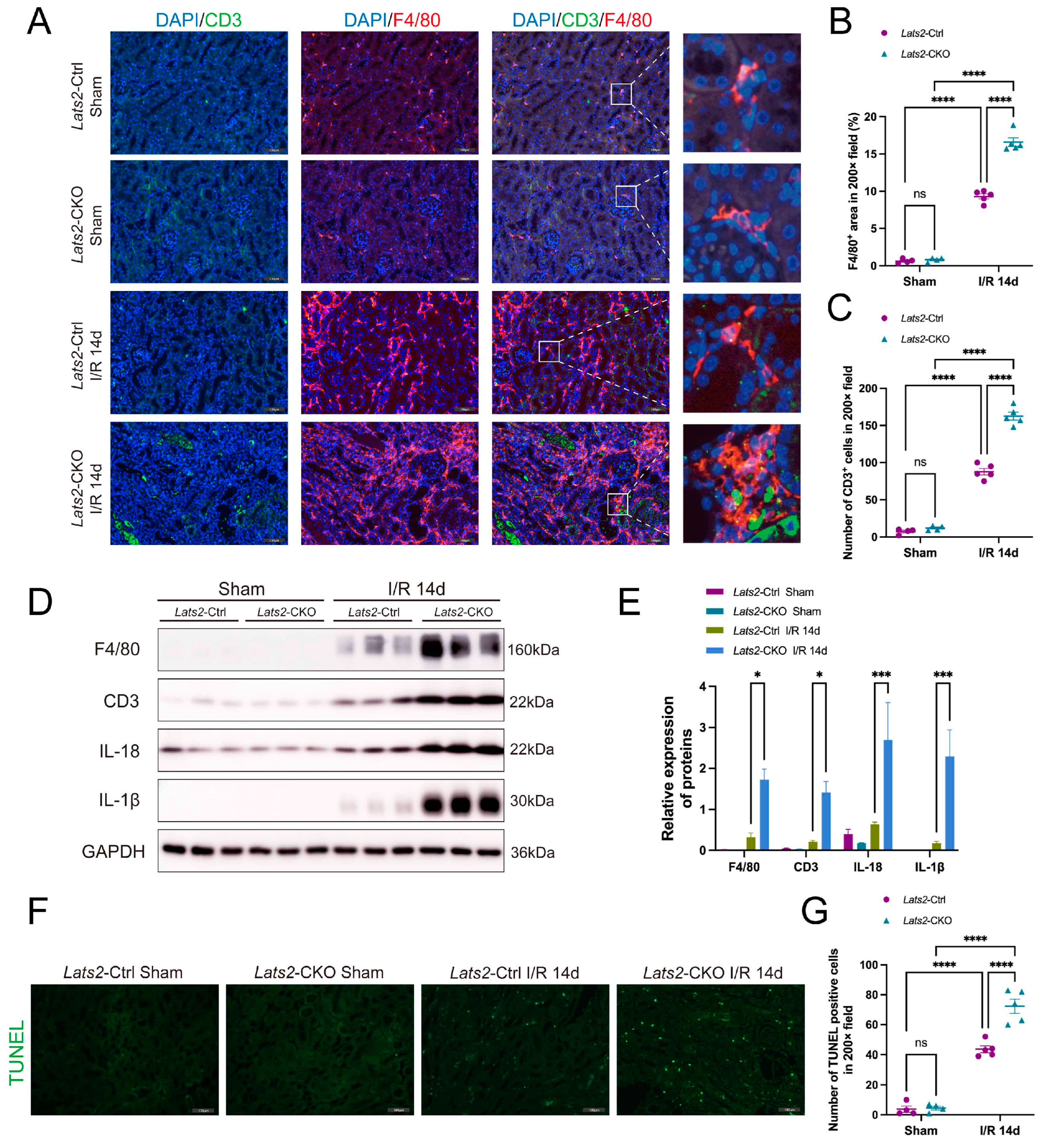
2.4. Lats2 Deficiency Leads to More Renal Inflammatory Cell Infiltration and Apoptosis
2.5. Proximal Tubule-Specific Lats2 Knockout Upregulates p53 Expression in Renal Maladaptive Repair
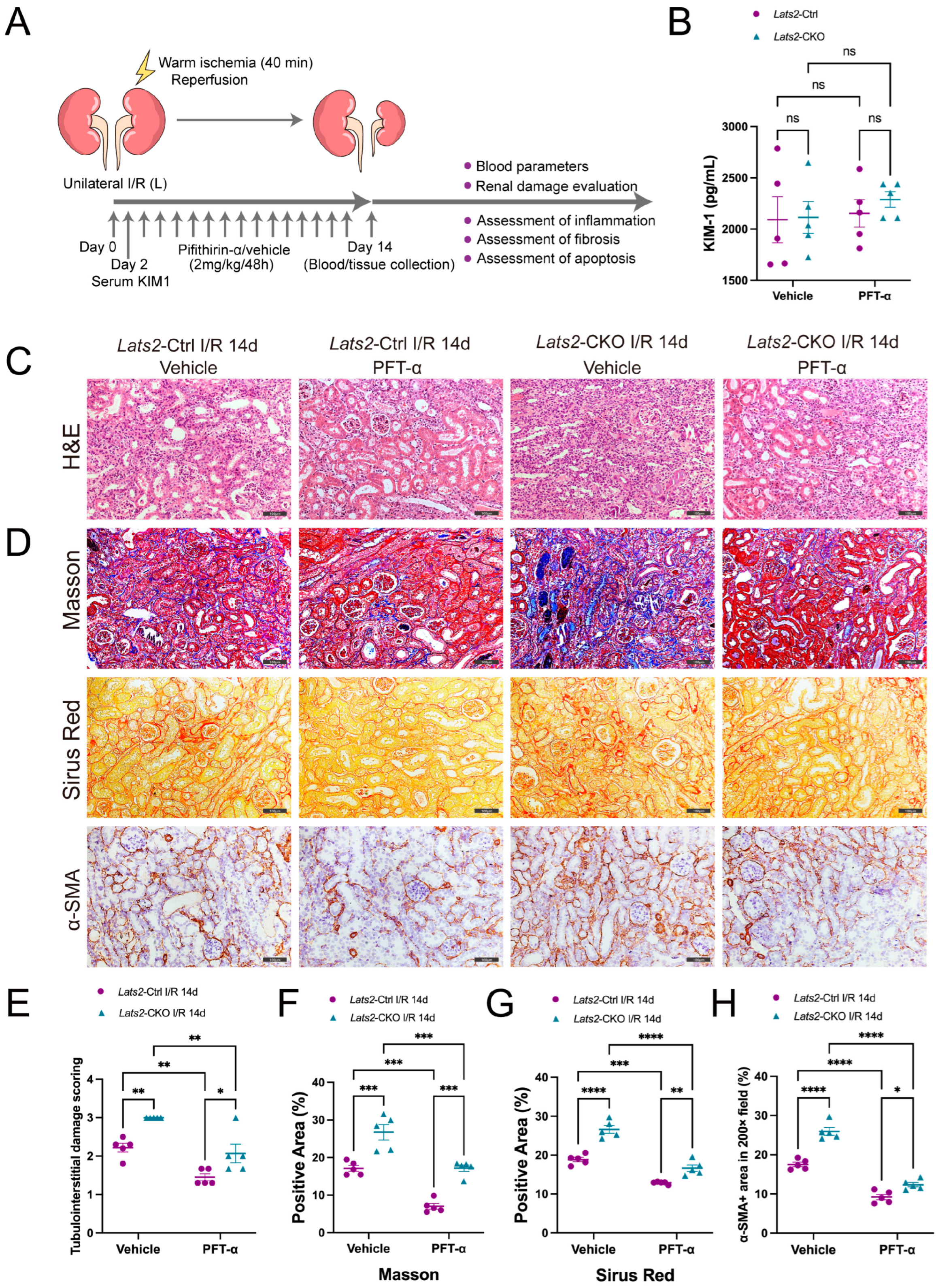
2.6. Pharmacologic Inhibition of p53 Attenuates Lats2 Knockout-Induced Exacerbation of Kidney Parenchymal Injury and Renal Fibrosis
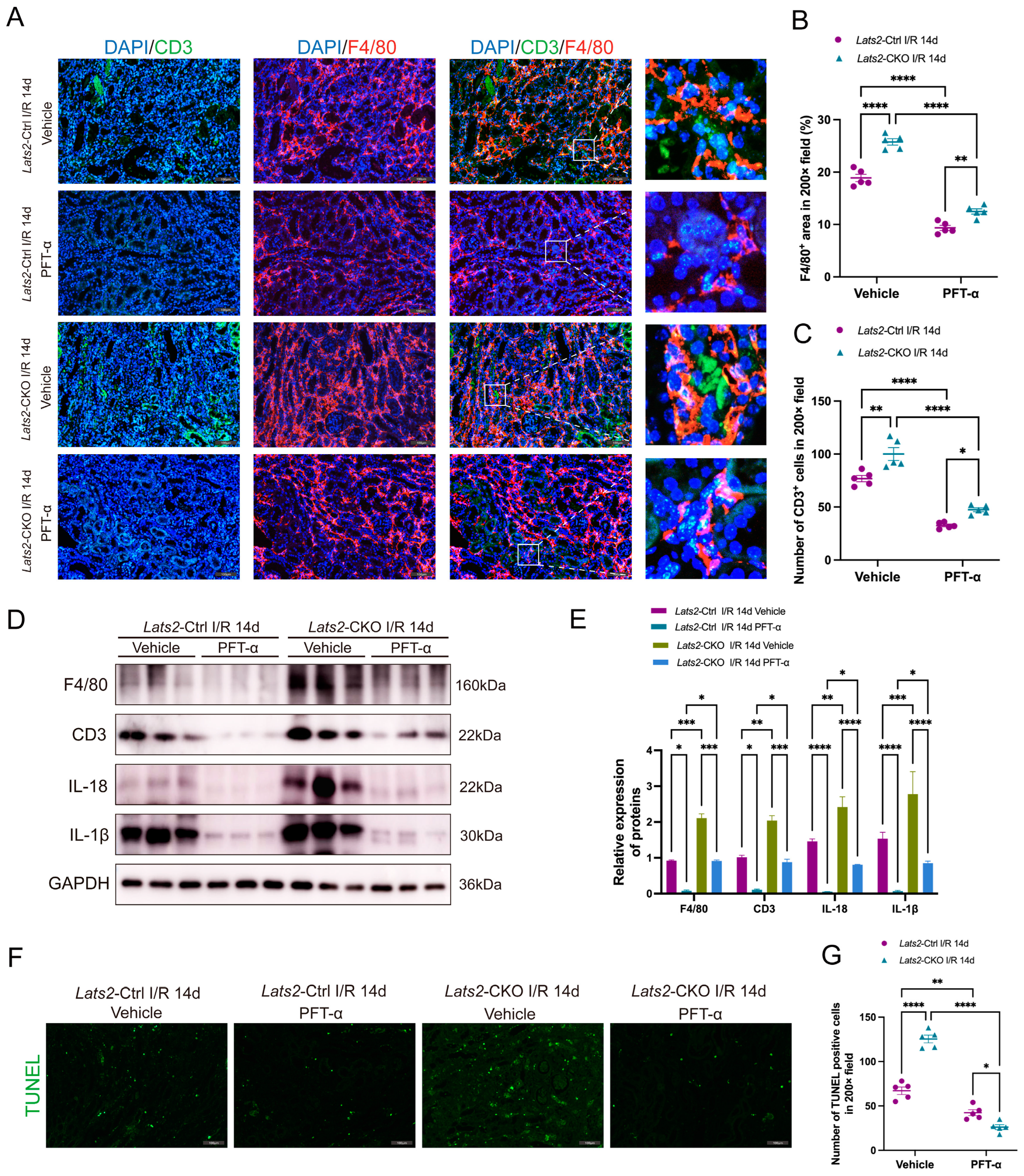
2.7. In Vivo Inhibition of p53 Alleviates the Aggravation of Renal Inflammatory Cell Infiltration and Cell Apoptosis in Response to Lats2 Deficiency after AKI
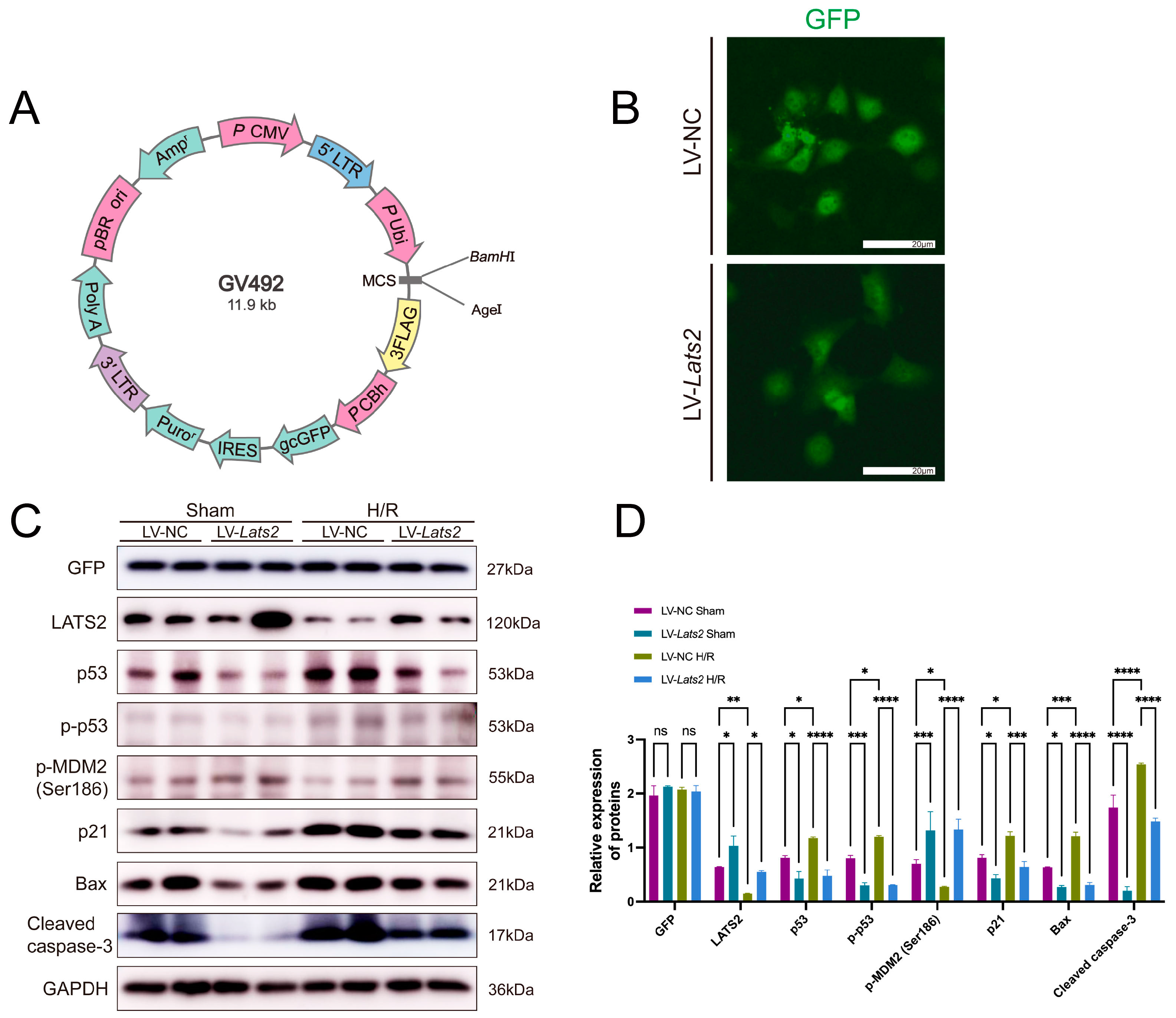
2.8. Lats2 Overexpression Suppressed p53 Expression in TEC Cells Subjected to Sham and Hypoxia/Reoxygenation Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RNA-seq
4.3. Bioinformatic Analysis
4.4. In Vivo Unilateral Renal Ischemia/Reperfusion Model
4.5. TEC Cell Culture and Treatment
4.6. Pharmacological Inhibitor
4.7. Serum Measurements
4.8. Western Blotting
4.9. Kidney Histopathology and TUNEL Assay
4.10. Immunofluorescence and Immunohistochemistry
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, Y.; Takahashi, M.; Yanagita, M. Pathophysiology of AKI to CKD progression. Semin. Nephrol. 2020, 40, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Zuk, A.; Bonventre, J.V. Recent advances in acute kidney injury and its consequences and impact on chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lameire, N.H.; Bagga, A.; Cruz, D.; De Maeseneer, J.; Endre, Z.; Kellum, J.A.; Liu, K.D.; Mehta, R.L.; Pannu, N.; Van Biesen, W.; et al. Acute kidney injury: An increasing global concern. Lancet 2013, 382, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Bonventre, J.V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 2015, 11, 264–276. [Google Scholar] [CrossRef]
- Yu, S.M.; Bonventre, J.V. Acute kidney injury and maladaptive tubular repair leading to renal fibrosis. Curr. Opin. Nephrol. Hypertens. 2020, 29, 310–318. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Zhao, B.; Tumaneng, K.; Guan, K.L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, C.; Shao, G.; Li, J.; Xu, K.; Zhao, Z.; Zhang, Z.; Liu, J.; Wu, H. Hippo-YAP/MCP-1 mediated tubular maladaptive repair promote inflammation in renal failed recovery after ischemic AKI. Cell Death Dis. 2021, 12, 754. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; He, Q.; Bulus, N.; Fogo, A.B.; Zhang, M.Z.; Harris, R.C. YAP Activation in Renal Proximal Tubule Cells Drives Diabetic Renal Interstitial Fibrogenesis. Diabetes 2020, 69, 2446–2457. [Google Scholar] [CrossRef]
- Chen, J.; You, H.; Li, Y.; Xu, Y.; He, Q.; Harris, R.C. EGF Receptor-Dependent YAP Activation Is Important for Renal Recovery from AKI. J. Am. Soc. Nephrol. 2018, 29, 2372–2385. [Google Scholar] [CrossRef]
- Xie, Y.; Lv, Y.; Zhang, Y.; Liang, Z.; Han, L.; Xie, Y. LATS2 promotes apoptosis in non-small cell lung cancer A549 cells via triggering Mff-dependent mitochondrial fission and activating the JNK signaling pathway. Biomed. Pharmacother. 2019, 109, 679–689. [Google Scholar] [CrossRef]
- Tian, Y.; Lv, W.; Lu, C.; Zhao, X.; Zhang, C.; Song, H. LATS2 promotes cardiomyocyte H9C2 cells apoptosis via the Prx3-Mfn2-mitophagy pathways. J. Recept. Signal Transduct. 2019, 39, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Callus, B.A.; Verhagen, A.M.; Vaux, D.L. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006, 273, 4264–4276. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zha, Z.Y.; Zhou, X.; Zhang, H.; Huang, W.; Zhao, D.; Li, T.; Chan, S.W.; Lim, C.J.; Hong, W.; et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J. Biol. Chem. 2010, 285, 37159–37169. [Google Scholar] [CrossRef]
- Shao, D.; Zhai, P.; Hu, C.; Mukai, R.; Sciarretta, S.; Del Re, D.; Sadoshima, J. Lats2 promotes heart failure by stimulating p53-mediated apoptosis during pressure overload. Sci. Rep. 2021, 11, 23469. [Google Scholar] [CrossRef]
- Furth, N.; Bossel Ben-Moshe, N.; Pozniak, Y.; Porat, Z.; Geiger, T.; Domany, E.; Aylon, Y.; Oren, M. Down-regulation of LATS kinases alters p53 to promote cell migration. Genes Dev. 2015, 29, 2325–2330. [Google Scholar] [CrossRef]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Wei, Q.; Huo, Y.; Liu, K.; Liu, F.; Dong, Z. Tubular p53 regulates multiple genes to mediate AKI. J. Am. Soc. Nephrol. 2014, 25, 2278–2289. [Google Scholar] [CrossRef]
- Tang, C.; Ma, Z.; Zhu, J.; Liu, Z.; Liu, Y.; Liu, Y.; Cai, J.; Dong, Z. P53 in kidney injury and repair: Mechanism and therapeutic potentials. Pharmacol. Ther. 2019, 195, 5–12. [Google Scholar] [CrossRef]
- Qi, R.; Wang, J.; Jiang, Y.; Qiu, Y.; Xu, M.; Rong, R.; Zhu, T. Snai1-induced partial epithelial-mesenchymal transition orchestrates p53-p21-mediated G2/M arrest in the progression of renal fibrosis via NF-kappaB-mediated inflammation. Cell Death Dis. 2021, 12, 44. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, Q.; Dong, G.; Huo, Y.; Dong, Z. DNA damage response in renal ischemia-reperfusion and ATP-depletion injury of renal tubular cells. Biochim. Biophys. Acta 2014, 1842, 1088–1096. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Liu, Y.; Tang, C.; Cai, J.; Chen, G.; Dong, Z. p53/sirtuin 1/NF-κB Signaling Axis in Chronic Inflammation and Maladaptive Kidney Repair After Cisplatin Nephrotoxicity. Front. Immunol. 2022, 13, 925738. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Yang, C. Renal tubular epithelial cells: The neglected mediator of tubulointerstitial fibrosis after injury. Cell Death Dis. 2018, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.L.; Xu, K.X.; Zheng, Z.H.; Cheng, G.Z.; Wu, H.J.; Liu, J. The Hippo pathway and its correlation with acute kidney injury. Zool. Res. 2022, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Havasi, A.; Borkan, S.C. Apoptosis and acute kidney injury. Kidney Int. 2011, 80, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, N.; Okada, N.; Ito, A.; Hosomi, T.; Nishihara, S.; Sasayama, Y.; Fujimori, A.; Okuzaki, D.; Zhao, H.; Ikawa, M.; et al. Lats2 is an essential mitotic regulator required for the coordination of cell division. J. Biol. Chem. 2007, 282, 19259–19271. [Google Scholar] [CrossRef]
- Dagher, P.C. Apoptosis in ischemic renal injury: Roles of GTP depletion and p53. Kidney Int. 2004, 66, 506–509. [Google Scholar] [CrossRef]
- Kelly, K.J.; Plotkin, Z.; Vulgamott, S.L.; Dagher, P.C. P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: Protective role of a p53 inhibitor. J. Am. Soc. Nephrol. 2003, 14, 128–138. [Google Scholar] [CrossRef]
- Megyesi, J.; Udvarhelyi, N.; Safirstein, R.L.; Price, P.M. The p53-independent activation of transcription of p21 WAF1/CIP1/SDI1 after acute renal failure. Am. J. Physiol. 1996, 271, F1211–F1216. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, K.; Padanilam, B.J. p53 target Siva regulates apoptosis in ischemic kidneys. Am. J. Physiol. Ren. Physiol. 2011, 300, F1130–F1141. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Li, R.; Zhang, L.; Zhang, S.; Dong, W.; Chen, Y.; Wang, W.; Li, C.; Ye, Z.; Zhao, X.; et al. p53-cyclophilin D mediates renal tubular cell apoptosis in ischemia-reperfusion-induced acute kidney injury. Am. J. Physiol. Ren. Physiol. 2019, 317, F1311–F1317. [Google Scholar] [CrossRef]
- Dong, L.; Li, L. Lats2-Underexpressing Bone Marrow-Derived Mesenchymal Stem Cells Ameliorate LPS-Induced Acute Lung Injury in Mice. Mediat. Inflamm. 2019, 2019, 4851431. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Hu, Y.; Zeng, M.; Chen, H.; Shi, J.; Jue, H.; Zhao, Z.; Liu, J.; Zhang, Z.; Xu, Y.; et al. Verteporfin inhibits the dedifferentiation of tubular epithelial cells via TGF-beta1/Smad pathway but induces podocyte loss in diabetic nephropathy. Life Sci. 2022, 311, 121186. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Li, F.; Liu, J.; Wang, H.; Tian, Y.; Zhang, Z.; Wang, F.; Zhao, Z.; Chen, J.; Wu, H. Nuclear exclusion of YAP exacerbates podocyte apoptosis and disease progression in Adriamycin-induced focal segmental glomerulosclerosis. Lab. Investig. 2021, 101, 258–270. [Google Scholar] [CrossRef]
- Zhong, J.; Ouyang, H.; Zheng, S.; Guo, Z.; Chen, Y.; Zhong, Y.; Zhong, W.; Zuo, L.; Lu, J. The YAP/SERCA2a signaling pathway protects cardiomyocytes against reperfusion-induced apoptosis. Aging 2020, 12, 13618–13632. [Google Scholar] [CrossRef]
- Aylon, Y.; Yabuta, N.; Besserglick, H.; Buganim, Y.; Rotter, V.; Nojima, H.; Oren, M. Silencing of the Lats2 tumor suppressor overrides a p53-dependent oncogenic stress checkpoint and enables mutant H-Ras-driven cell transformation. Oncogene 2009, 28, 4469–4479. [Google Scholar] [CrossRef]
- Aylon, Y.; Michael, D.; Shmueli, A.; Yabuta, N.; Nojima, H.; Oren, M. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 2006, 20, 2687–2700. [Google Scholar] [CrossRef]
- Aylon, Y.; Ofir-Rosenfeld, Y.; Yabuta, N.; Lapi, E.; Nojima, H.; Lu, X.; Oren, M. The Lats2 tumor suppressor augments p53-mediated apoptosis by promoting the nuclear proapoptotic function of ASPP1. Genes Dev. 2010, 24, 2420–2429. [Google Scholar] [CrossRef]
- Pan, W.W.; Moroishi, T.; Koo, J.H.; Guan, K.L. Cell type-dependent function of LATS1/2 in cancer cell growth. Oncogene 2019, 38, 2595–2610. [Google Scholar] [CrossRef]
- Reuven, N.; Adler, J.; Meltser, V.; Shaul, Y. The Hippo pathway kinase Lats2 prevents DNA damage-induced apoptosis through inhibition of the tyrosine kinase c-Abl. Cell Death Differ. 2013, 20, 1330–1340. [Google Scholar] [CrossRef][Green Version]
- Mohanty, S.; Mohapatra, P.; Shriwas, O.; Ansari, S.A.; Priyadarshini, M.; Priyadarsini, S.; Rath, R.; Sultania, M.; Das Majumdar, S.K.; Swain, R.K.; et al. CRISPR-based kinome-screening revealed MINK1 as a druggable player to rewire 5FU-resistance in OSCC through AKT/MDM2/p53 axis. Oncogene 2022, 41, 4929–4940. [Google Scholar] [CrossRef]
- Higgins, S.P.; Tang, Y.; Higgins, C.E.; Mian, B.; Zhang, W.; Czekay, R.P.; Samarakoon, R.; Conti, D.J.; Higgins, P.J. TGF-beta1/p53 signaling in renal fibrogenesis. Cell Signal. 2018, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef]
- Ying, Y.; Kim, J.; Westphal, S.N.; Long, K.E.; Padanilam, B.J. Targeted deletion of p53 in the proximal tubule prevents ischemic renal injury. J. Am. Soc. Nephrol. 2014, 25, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, J.; Mao, L.; Wu, J.; Deng, Z.; He, M.; An, S.; Zeng, Z.; Huang, Q.; Chen, Z. p53 Deacetylation Alleviates Sepsis-Induced Acute Kidney Injury by Promoting Autophagy. Front. Immunol. 2021, 12, 685523. [Google Scholar] [CrossRef] [PubMed]
- Iwano, M.; Plieth, D.; Danoff, T.M.; Xue, C.; Okada, H.; Neilson, E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 2002, 110, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Vittoria, M.A.; Kingston, N.; Kotynkova, K.; Xia, E.; Hong, R.; Huang, L.; McDonald, S.; Tilston-Lunel, A.; Darp, R.; Campbell, J.D.; et al. Inactivation of the Hippo tumor suppressor pathway promotes melanoma. Nat. Commun. 2022, 13, 3732. [Google Scholar] [CrossRef]
- Xu, L.; Sharkey, D.; Cantley, L.G. Tubular GM-CSF Promotes Late MCP-1/CCR2-Mediated Fibrosis and Inflammation after Ischemia/Reperfusion Injury. J. Am. Soc. Nephrol. 2019, 30, 1825–1840. [Google Scholar] [CrossRef]
- Komarova, E.A.; Neznanov, N.; Komarov, P.G.; Chernov, M.V.; Wang, K.; Gudkov, A.V. p53 inhibitor pifithrin alpha can suppress heat shock and glucocorticoid signaling pathways. J. Biol. Chem. 2003, 278, 15465–15468. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xu, Y.; Krugel, U.; Schaefer, M.; Grune, T.; Nurnberg, B.; Kohler, M.B.; Gollasch, M.; Tsvetkov, D.; Marko, L. In Vivo Inhibition of TRPC6 by SH045 Attenuates Renal Fibrosis in a New Zealand Obese (NZO) Mouse Model of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 6870. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zheng, Z.; Xu, K.; Cheng, G.; Wu, H.; Liu, J. Proximal Tubular Lats2 Ablation Exacerbates Ischemia/Reperfusion Injury (IRI)-Induced Renal Maladaptive Repair through the Upregulation of P53. Int. J. Mol. Sci. 2023, 24, 15258. https://doi.org/10.3390/ijms242015258
Zhang C, Zheng Z, Xu K, Cheng G, Wu H, Liu J. Proximal Tubular Lats2 Ablation Exacerbates Ischemia/Reperfusion Injury (IRI)-Induced Renal Maladaptive Repair through the Upregulation of P53. International Journal of Molecular Sciences. 2023; 24(20):15258. https://doi.org/10.3390/ijms242015258
Chicago/Turabian StyleZhang, Chi, Zhihuang Zheng, Kexin Xu, Guozhe Cheng, Huijuan Wu, and Jun Liu. 2023. "Proximal Tubular Lats2 Ablation Exacerbates Ischemia/Reperfusion Injury (IRI)-Induced Renal Maladaptive Repair through the Upregulation of P53" International Journal of Molecular Sciences 24, no. 20: 15258. https://doi.org/10.3390/ijms242015258
APA StyleZhang, C., Zheng, Z., Xu, K., Cheng, G., Wu, H., & Liu, J. (2023). Proximal Tubular Lats2 Ablation Exacerbates Ischemia/Reperfusion Injury (IRI)-Induced Renal Maladaptive Repair through the Upregulation of P53. International Journal of Molecular Sciences, 24(20), 15258. https://doi.org/10.3390/ijms242015258




